Abstract
Bacterial conjugation is a key mechanism by which bacteria acquire antibiotic resistance. Therefore, conjugation inhibitors (COINs) are promising compounds in the fight against the spread of antibiotic resistance genes among bacteria. Unsaturated fatty acids (uFAs) and alkynoic fatty acid derivatives, such as 2-hexadecanoic acid (2-HDA), have been reported previously as being effective COINs. The traffic ATPase TrwD, a VirB11 homolog in plasmid R388, is the molecular target of these compounds, which likely affect binding of TrwD to bacterial membranes. In this work, we demonstrate that COINs are abundantly incorporated into Escherichia coli membranes, replacing palmitic acid as the major component of the membrane. We also show that TrwD binds palmitic acid, thus facilitating its interaction with the membrane. Our findings also suggest that COINs bind TrwD at a site that is otherwise occupied by palmitic acid. Accordingly, molecular docking predictions with palmitic acid indicated that it shares the same binding site as uFAs and 2-HDA, although it differs in the contacts involved in this interaction. We also identified 2-bromopalmitic acid, a palmitate analog that inhibits many membrane-associated enzymes, as a compound that effectively reduces TrwD ATPase activity and bacterial conjugation. Moreover, we demonstrate that 2-bromopalmitic and palmitic acids both compete for the same binding site in TrwD. Altogether, these detailed findings open up a new avenue in the search for effective synthetic inhibitors of bacterial conjugation, which may be pivotal for combating multidrug-resistant bacteria.
Keywords: antibiotic resistance, ATPase, drug design, bacterial conjugation, secretion, antibiotic resistance, ATPase inhibitors, bacterial conjugation, drug design, type IV secretion system
Introduction
Bacterial conjugation is one of the main mechanisms whereby bacteria become resistant to antibiotics (1). Therefore, there is major interest in the search for conjugation inhibitors (COINs).4 In conjugation, DNA is transferred between two bacterial cells through a type IV secretion system (T4SS), a multisubunit complex encoded by the conjugative plasmid. Canonical T4SS consists of 11 proteins, named VirB1 to VirB11, after the Agrobacterium tumefaciens T4SS (2, 3). T4SS architecture is well-preserved in most conjugative bacteria, consisting of four distinct sections: the pilus, the core channel complex, the inner membrane platform, and the ATPases that provide the energy for substrate transport and pilus biogenesis (4). COINs have been proposed to target essential components of the T4SS (5). Promising results have been obtained with unsaturated fatty acids (uFAs), which specifically inhibit plasmid conjugation without inhibiting Escherichia coli growth (6, 7). The traffic ATPase TrwD, the VirB11 homolog in plasmid R388, has been shown to be the target for this inhibition by uFAs (8). TrwD contributes to pilus biogenesis and DNA translocation (9), thus working as a molecular switch between pilus synthesis and substrate transport (10). TrwD is specifically inhibited in vitro by the same uFAs and alkynoic fatty acid derivatives (2-aFAs) that inhibit bacterial conjugation in vivo, such as linoleic acid or 2-hexadecynoic acid (2-HDA), respectively. These compounds act as noncompetitive inhibitors, with no effect on the affinity of the protein for ATP or ADP substrates (8). In contrast, saturated fatty acids, such as palmitic acid, show no inhibitory effect in conjugation experiments (6) and in ATPase assays (8).
TrwD belongs to the secretion ATPase superfamily, which also includes members of type II secretion, type IV pilus, and flagellar biogenesis machineries (11). All members of this superfamily are hexameric ATPases, in which each monomer is formed by two domains at the N and C termini (NTD and CTD, respectively), connected by a flexible linker of variable length (11, 12). ATPase catalysis is driven by swapping the NTD over the CTD because of the flexibility of the linker (13, 14). Blind docking predictions suggested a putative binding site for uFAs and 2-aFAs located at the end of the NTD and beginning of the linker region that connects it to the CTD, where the nucleotide binding site is located (8). These predictions are compatible with a model in which the mode of action of the inhibitors consists of preventing the swapping movements between the N- and C-terminal domains that are required in the catalytic cycle of the protein. VirB11 ATPases interact with the cytoplasmic site of the membrane through its N-terminal domain (15, 16). Therefore, it is likely that the inhibitory effect of uFAs and 2-aFAs occurs by affecting the membrane binding capability of TrwD. In this work, we demonstrate that the 2-alkynoic fatty acid 2-HDA incorporates into bacterial membranes, replacing palmitic acid as the major component of the membrane. We also show that TrwD binds palmitic acid, which suggests that uFAs and 2-AFAs act as inhibitors by binding TrwD at a site that is otherwise occupied by palmitic acid. Accordingly, docking predictions with palmitic acid indicate that it shares the same binding site as linoleic acid and 2-HDA.
Interestingly, we also discovered that 2-bromopalmitic acid (2-BP), a proven compound to block palmitate incorporation onto a variety of membrane-associated eukaryotic proteins (17), is also an inhibitor of TrwD ATPase activity and bacterial conjugation. Moreover, we demonstrate that 2-bromopalmitic and palmitic acids compete for the same binding site in TrwD, as shown by the increase in the value of the apparent inhibition constant in the presence of the saturated fatty acid. Recently, tanzawaic acids also appeared as a novel group of bacterial conjugation inhibitors (18). All of these compounds share similar chemical characteristics: a carboxylic group, a long unsaturated aliphatic chain, and the presence of double or triple bonds. The finding of 2-BP as an effective inhibitor allows us to conclude that inhibition does not occur by the presence of double or triple bonds in these compounds but by the conformation these fatty acids acquire upon TrwD binding. These new findings open a new avenue in the search of new and more effective synthetic inhibitors by structure-based drug design methods.
Results
2-Alkynoic fatty acids incorporate into bacterial membranes, where they exert an inhibitory effect on conjugation
uFAs and 2-alkynoic fatty acids specifically inhibit plasmid conjugation without inhibiting Escherichia coli growth (6, 7). Recently, we identified TrwD, the VirB11 homolog in plasmid R388, as their molecular target (8). As VirB11 traffic ATPases transiently interact with the cytoplasmic site of the membrane (19), 2-alkynoic fatty acids, such as 2-HDA, might be incorporated into the bacterial membrane to exert an inhibitory conjugation effect.
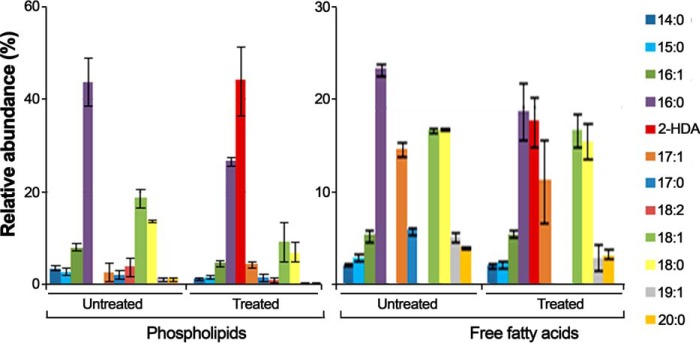
In an attempt to find out whether fatty acid derivatives that act as inhibitors (COINs) do incorporate into membranes, E. coli cells were grown in the presence and absence of the inhibitor 2-HDA. After 18 h of incubation, the composition in esterified fatty acids (fatty acid methyl esters) from membrane phospholipids and free fatty acids was checked by GC-MS. The results revealed that the phospholipid fatty acids present in untreated bacteria are primarily saturated, with palmitic acid (16:0) being the most abundant (almost 50% of the total membrane phospholipids contained palmitic acid) (Fig. 1). When cells were grown in the presence of 2-HDA (50 μg/ml), bacteria incorporated this exogenous fatty acid into their membranes. Moreover, the major component of the total phospholipids in treated bacteria was 2-HDA (44% w/w). The incorporation of 2-HDA into the bacterial membrane was accompanied by a 2-fold decrease in palmitic acid in the phospholipid fraction. In contrast, the percentage of palmitic acid in the free fatty acid fraction was not significantly altered when cells were treated with 2-HDA (Fig. 1). As result of this experiment, we conclude that incorporation of 2-HDA into the bacterial membrane alters the utilization of endogenous fatty acids in the biosynthesis of phospholipids, with a significant amount of palmitic acid being replaced by 2-HDA in the bacterial membrane.
Figure 1.
Composition of phospholipid fatty acids and free fatty acids in E. coli after exposure to 2-HDA. E. coli cells were grown at 37 °C for 18 h in LB broth containing 50 μg/ml 2-HDA (Treated) or in its absence (Untreated). Fatty acids were eluted, converted to methyl esters and analyzed by GC-MS) as described under “Experimental procedures.” Bars represent the relative abundance of different fatty acids obtained from three separated experiments (mean ± S.D.). Fatty acid nomenclature indicates the number of carbons followed by the number of double bonds.
TrwD binds palmitic acid
The surprising effect observed in the previous experiment suggested that such a replacement might, in turn, affect the association of TrwD to the membrane. In contrast to uFAs or 2-HDA, saturated fatty acids inhibit neither bacterial conjugation (6) nor TrwD ATPase activity in vitro (8). Nonetheless, docking predictions with palmitic acid suggested that its binding site is the same as that for uFAs and 2-HDA, albeit adopting a different conformation because of the lack of double or triple bonds (8).
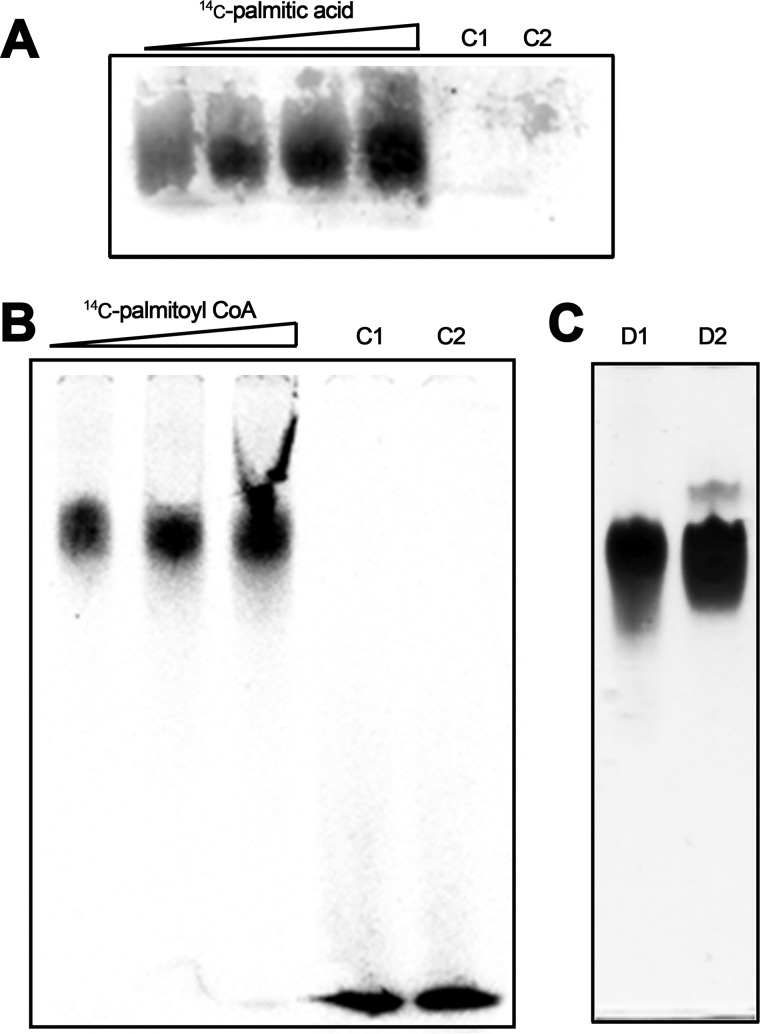
The molecular bases that underlie TrwD binding to the membrane are unknown. Therefore, we investigated a possible lipid–protein association via palmitic acid by studying the binding of TrwD to 14C-labeled palmitic acid. Purified TrwD protein was incubated with palmitic acid at different ratios, and samples were analyzed by PAGE under nondenaturing conditions (Fig. 2A). Radiolabeling of TrwD with palmitic acid was directly related to increasing palmitate/protein ratios. As a control, TrwA protein (a conjugative protein that does not bind to the membrane) was incubated with 14C-labeled palmitic acid at the highest palmitic acid/protein ratio. No radioactivity signal was observed for this control protein. Similar experiments, carried out with 14C-labeled palmitoyl-CoA, gave identical results. In this latter case, it was possible to observe the unbound labeled palmitoyl-CoA running at the front of the gel (Fig. 2B).
Figure 2.
TrwD binding to 14C-labeled palmitic acid and palmitoyl-CoA. A, TrwD (80 μm) was incubated at increasing 14C-labeled palmitic acid ratios (1:0.25, 1:0.5, 1:0.75, and 1:1 protein:palmitic acid molar ratios, respectively). Lane C1 corresponds to TrwD (80 μm) in the absence of fatty acids. Lane C2 is also a control experiment, in which an alien protein, TrwA (80 μm), was incubated with 14C-labeled palmitic acid at a 1:1 protein:palmitic acid molar ratio. B, TrwD (80 μm) was incubated at increasing 14C-labeled palmitoyl-CoA ratios (1:0.5, 1:0.75, and 1:1 protein:palmitoyl-CoA molar ratios, respectively). Lane C1 corresponds to 14C-labeled palmitoyl-CoA (80 μm) in the absence of any protein. Lane C2, as in A, corresponds to TrwA (80 μm) incubated with 14C-labeled palmitoyl CoA (80 μm). Lane C1 and C2 radioactivity signals at the front of the gel correspond to free 14C-labeled palmitoyl-CoA. In both cases, protein–fatty acid complexes were analyzed by native PAGE, and radiolabeled images were obtained after overnight exposure, as described under “Experimental procedures.” C, TrwD (80 μm, lane D1) and TrwA (80 μm, lane D2) were analyzed by Coomassie Brilliant Blue–stained native PAGE, as described under “Experimental procedures.”
S-palmitoylation, a covalent attachment of fatty acids to cysteine residues of proteins, enhances the surface hydrophobicity and membrane affinity of proteins, playing an important role in modulating protein trafficking (20). TrwD contains five cysteine residues in its sequence, with one of them placed at the N-terminal domain (Cys-44). Although this is a posttranslational modification observed in eukaryotic proteins, we wanted to check whether TrwD was covalently bound to palmitic acid. The association of labeled palmitate with TrwD was sensitive to denaturation induced by SDS gel electrophoresis, which suggests that the binding is noncovalent and that there is no thioester bond involved in such an association.
2-Bromopalmitic acid inhibits TrwD ATPase activity
2-BP is a palmitate analog that acts as inhibitor of many membrane-associated enzymes (17). It is an irreversible inhibitor that blocks palmitate incorporation onto proteins. The exact mechanism responsible for 2-BP–mediated inhibition is not known. It is thought to inhibit protein acyl-transferases (21), but recent reports showed that 2-BP is a nonselective probe with many other different targets (22).
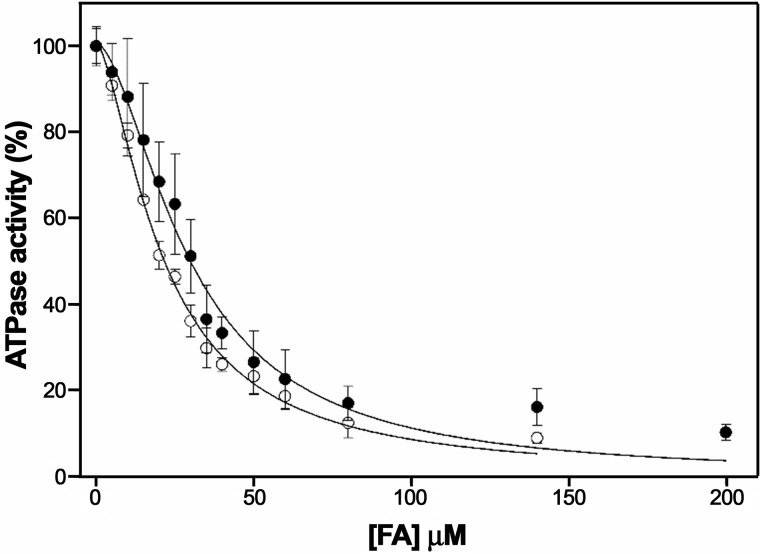
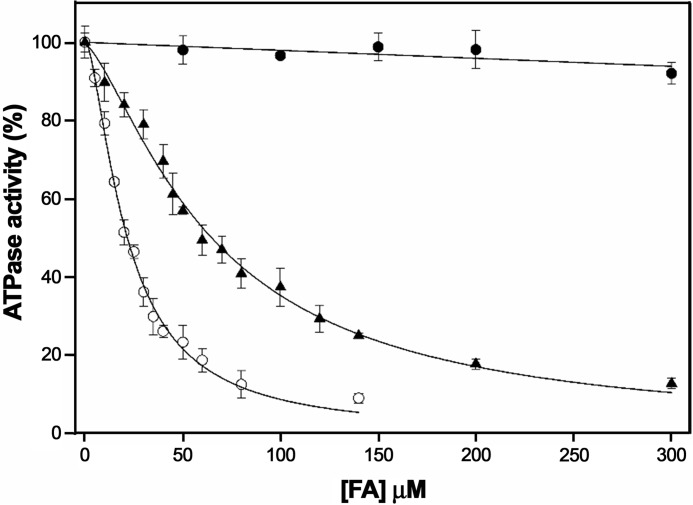
Thus, we analyzed the ATPase activity of TrwD in the presence of increasing concentrations of 2-BP (Fig. 3). Analysis of the kinetics showed an inhibition pattern similar to 2-HDA or unsaturated fatty acids. In all cases, the data did not fit to a Michaelis–Menten inhibition kinetic curve but to a sigmoidal Hill equation, which suggested a cooperative effect in the inhibition kinetics. The apparent inhibition constant (Ki(app)) of 2-bromopalmitic acid was 21.5 ± 1.5 μm, similar to that obtained for 2-HDA (Ki(app) = 29.7 ± 2.1 μm) (Fig. 3).
Figure 3.
Determination of the kinetic parameters of inhibition by 2-bromopalmitic acid. ATP hydrolysis by TrwD (2 μm) was measured at increasing concentrations of 2-bromopalmitic acid (white circles) and compared with the values obtained at increasing concentrations of 2-HDA (black circles) (8). Data were fitted to a Hill inhibition equation (error bars indicate S.D.).
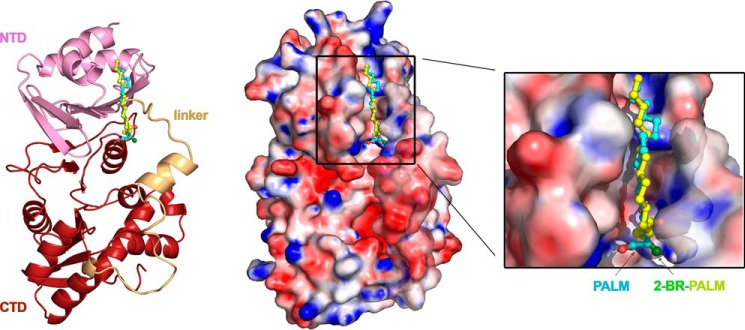
Previously discovered COINs, such as uFAs (oleic and linoleic acids), chemically synthesized 2-HDA and derivatives (7), or tanzawaic acids (18), share similar chemical characteristics: a carboxylic group, a long unsaturated aliphatic chain, and the presence of double or triple bonds. The finding that 2-BP is an effective inhibitor was at first surprising. Nevertheless, the results shown above allow us to conclude that inhibition does not occur by the presence of double or triple bonds in these compounds but, indirectly, by the conformation these fatty acids acquire when bound to TrwD. Docking predictions with 2-BP indicate that the binding site for the palmitic acid analog is also in the same region as linoleic acid, 2-HDA, and palmitic acid (Fig. 4). In this case, the presence of a halogen group (2-bromo) within the saturated fatty acid can result in a similar inhibitory effect but modifying the mode of binding.
Figure 4.
Blind docking of fatty acids into the molecular model of TrwD. Blind docking predictions between a molecular model of monomeric TrwD and fatty acid ligands (palmitic acid (PALM) and 2-bromopalmitic acid (2-BR-PALM)) were performed using the EADock dihedral spacing sampling engine of the SwissDock server (32). Both fatty acids fit into a pocket located at the interface between the NTD (pink) and the linker region (light orange), which connects the NTD with the catalytic CTD (brown). The top left and right panels correspond to the same view in cartoon and surface representation, respectively. The carbon chains of both fatty acids have a similar orientation (blue and yellow for palmitic acid and 2-bromopalmitic acid, respectively). However, the carboxylic group of the 2-bromo derivative is buried in a pocket formed by the NTD and the linker domain of the protein, being the bromine atom (dark green) stabilized by electrostatic basic residues located in the region. On the contrary, the carboxylic group of the palmitic acid (red) is 120° apart, out of the linker region. The right panel shows a zoom of the binding pocket, in which the basic residues surrounding the carboxylic groups of the fatty acids are better appreciated. For more clarity, see also Movie S1.
2-bromopalmitic and palmitic acids compete for the same binding site in TrwD
To determine whether 2-bromopalmitic and palmitic acids compete for the same binding site, we conducted further characterization of the mechanism of inhibition. ATP turnover was measured at increasing concentrations of 2-BP in the presence of palmitic acid (500 μm) (Fig. 5). Under these experimental conditions, the Ki(app) of 2-BP increased 3-fold (from 21.5 ± 1.5 μm to 65.4 ± 1.4 μm) (Fig. 3), suggesting that palmitic acid and 2-BP compete for the same binding site in TrwD.
Figure 5.
2-Bromopalmitic acid and palmitic acid compete for the same TrwD binding site. TrwD ATPase rates (2 μm) were measured as described under “Experimental procedures” at increasing concentrations of palmitic acid (dark circles), 2-bromopalmitic acid (white circles), and mixtures of both fatty acids consisting of a fixed concentration of palmitic acid (500 μm) and increasing concentrations of 2-bromopalmitate (dark triangles). In the latter case, the protein sample was first incubated with palmitic acid for 5 min, and then 2-bromopalmitic acid was added at the indicated concentrations. Data were fitted to a sigmoidal Hill equation for inhibition (error bars indicate S.D.).
2-Bromopalmitic acid inhibits R388 conjugation
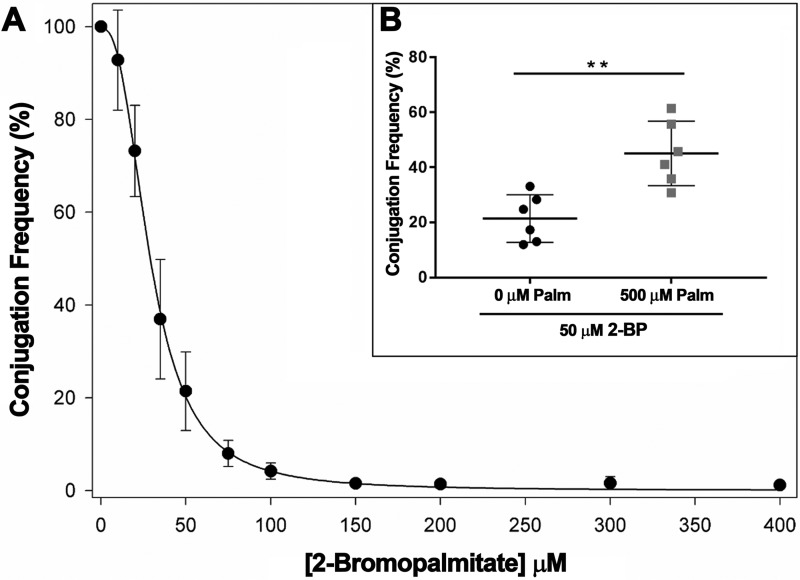
In previous work (8), we observed that 2-alkynoic fatty acids that inhibited bacterial conjugation, such as 2-HDA, 2-octadecynoic acid, or 2,6-hexadecadiynoic acid, also inhibited TrwD ATPase activity in vitro. In contrast, those with no effect in vivo, such as alcohol or tetrahydropyranyl-ether derivatives, did not affect the in vitro TrwD ATPase activity either. Therefore, when we discovered that 2-BP was an effective inhibitor of TrwD ATPase activity, we checked whether this compound was also an inhibitor of bacterial conjugation. R388 conjugation frequencies at increased concentrations of 2-BP are shown in Fig. 6.
Figure 6.
R388 CF in the presence of increasing concentrations of 2-bromopalmitic acid. Bacterial conjugation experiments were performed by a fluorescence-based assay as described under “Experimental procedures.” A, values represent the mean CF ± S.D. from at least four independent experiments relative to a positive control in the absence of inhibitor (100%). B, the relative CF from individual experiments in the presence of 2-bromopalmitic acid (50 μm, black circles) and a mixture of both palmitic acid (Palm, 500 μm) and 2-bromopalmitic acid (50 μm) (gray squares). Horizontal and vertical bars represent the mean ± S.D. of each group of data. Statistical significance was analyzed by Student's t test (**, p < 0.05).
Bacterial conjugation was monitored by a high-throughput assay based on fluorescence emission by transconjugant cells. Control assays were carried out to discard any effect on bacterial growth or plasmid stability. Addition of 2-BP (0.3 mm) resulted in inhibition of R388 conjugation (Fig. 6). 2-BP inhibited R388 conjugation to levels similar to those obtained with 2-HDA (to about 2% of the control without COIN). Moreover, when palmitic acid (500 μm) was added to the conjugation medium, a higher concentration of 2-BP was required to reach a similar inhibitory effect. This result perfectly correlates with that obtained in vitro regarding TrwD ATPase activity and confirms 2-BP as an effective COIN.
Discussion
VirB11 proteins belong to a large family of hexameric AAA+ traffic ATPases, which includes proteins involved in type II secretion and in type IV pilus and flagellar biogenesis (11). VirB11 from A. tumefaciens localized at the cell pole, as shown by epifluorescence microscopy of a GFP–VirB11 fusion protein (23). The protein required neither ATP nor other VirB proteins for its polar localization (19). In contrast, in the presence of a complete T4SS machinery, VirB11 localized all over the bacterial cell perimeter (24). Accordingly, in vitro experiments have shown previously that TrwD, the VirB11 homolog in plasmid R388, interacts with phospholipids in lipid vesicles, causing lipid vesicle aggregation and intervesicular lipid mixing, supporting the hypothesis that TrwD also behaves as a membrane-associated protein (16). Therefore, although it has been proven that the protein localizes in the bacterial inner membrane, the molecular bases underlying this interaction with membrane lipids were unknown.
Recently, we identified TrwD as the molecular target for fatty acid–mediated inhibition of conjugation (8), showing that uFAs and 2-aFAs that inhibit bacterial conjugation, such as 2-HDA, 2-octadecynoic acid, or 2,6-hexadecadiynoic acid, also inhibit TrwD ATPase activity. In contrast, saturated fatty acids such as palmitic acid did not show an inhibitory effect in conjugation experiments or in ATPase assays. In an attempt to explain how these compounds can act as inhibitors of bacterial conjugation, we investigated the putative interaction of uFAs and aFAs with the bacterial membrane. Indeed, our results revealed that the phospholipid fatty acids present in untreated bacteria are mainly saturated, with palmitic acid being the most abundant. However, when cells were grown in the presence of 2-HDA, this compound was incorporated into the membrane and became the major component of the total phospholipid fraction (Fig. 1). More importantly, a significant amount of palmitic acid was replaced by 2-HDA in the membrane. Therefore, it was reasonable to think that this replacement of palmitic acid by fatty acids with double or triple bonds might be responsible for such an inhibitory effect. Thus, we wondered whether TrwD interaction with the membrane was occurring via palmitic acid under normal growth conditions. Supporting this idea, a blind docking search of the palmitic acid in the TrwD structure revealed the same binding site as for uFAs and 2-HDA, although it differs in the contacts involved in the interaction (8). To confirm the putative binding of saturated fatty acids, purified TrwD protein was incubated with 14C-labeled palmitic acid, and the resulting complex was analyzed in native gels (Fig. 2). Radiolabeling of TrwD with palmitic acid was directly related to increasing palmitate/protein ratios.
A wide variety of proteins associate with the membrane by a covalent attachment to palmitic acid (20). In these palmitoylated proteins, the fatty acid is bound to a cysteine residue through a thioester linkage. This is a reversible process that allows transient binding to the membrane, which is crucial in many biological processes. This lipid modification is common in eukaryotic cells (20) but poorly documented in prokaryotes. To our knowledge, there is only one example of S-palmitoylation for a bacterial protein (25). TrwD presents five cysteine residues. One of them is placed at the NTD, so palmitoylation might be a possible mechanism of interaction of TrwD with the membrane. Therefore, we checked whether the association of radio-labeled palmitate with TrwD was resistant to denaturation induced by SDS gel electrophoresis. No signal of radiolabeled protein was observed in these gels. Samples of purified protein incubated with palmitic acid were also analyzed by MS, but no difference in molecular mass was observed when the sample was compared with a control in the absence of the fatty acid. Therefore, our data indicate that the binding to palmitic acid is noncovalent, excluding the possibility that the association of palmitate with TrwD is due to protein palmitoylation. In the literature, there are also several examples of proteins that associate with the membrane via noncovalent binding to saturated fatty acids, such as Toll-like receptors (26). In this case, the polyunsaturated fatty acid binds to a lipid binding pocket in the protein receptor. Intriguingly, Toll-like receptors are also inhibited by polyunsaturated fatty acids (27), as in the case of TrwD.
To study the binding of palmitic acid to TrwD, we explored the binding capacity of an analog, 2-BP. This fatty acid was found to act as an inhibitor of many membrane-associated enzymes that bind palmitic acid (17). First, we checked whether this palmitate analog also inhibited TrwD activity. We analyzed the ATPase activity of the protein in the presence of increasing concentrations of 2-BP (Fig. 3). The inhibition pattern was similar to that obtained with 2-HDA or uFAs, with an apparent inhibition constant similar to that obtained for 2-HDA. In previous work, we found that the same fatty acid derivatives that were effective inhibitors of bacterial conjugation were also capable of inhibiting TrwD ATPase activity and that, vice versa, those unable to inhibit conjugation also failed to inhibit TrwD activity, with a perfect correlation between the in vivo and in vitro data (8). In the same way, in this work, we demonstrate that 2-BP inhibits R388 conjugation, as in the case of 2-HDA (Fig. 6). Moreover, when palmitic acid was added to the conjugation medium, a higher concentration of 2-BP was required to reach a similar inhibitory effect. This effect is probably due to the incorporation of 2-BP into the bacterial membrane and the consequent replacement of palmitic acid by 2-BP, an effect that has already been observed for 2-HDA (Fig. 1). Therefore, these inhibitors seem to act by displacing the more abundant saturated acid in the membrane.
In an attempt to determine whether 2-BP and palmitic acid compete for the same binding site in TrwD, ATP turnover was measured at increasing concentrations of 2-BP in the presence of palmitic acid (Fig. 5). The Ki(app) of 2-BP increased from 21.5 ± 1.5 μm to 65.4 ± 1.4 μm (Fig. 3), which suggests that both fatty acids (palmitic acid and 2-BP) compete for the same binding site in TrwD. Although it is generally accepted that 2-BP blocks S-palmitoylation by inhibiting protein acyl transferases (21), recent work has shown that 2-BP has many other targets, leading the authors to suggest that the compound might exert the inhibition by direct competition with palmitic acid (22). The results obtained with TrwD in this work agree with this hypothesis and might be extended to proteins that associate with the inner membrane via interaction with palmitic acid.
In summary, in this work, we describe 2-BP, a palmitate analog, as a new inhibitor of bacterial conjugation. As previously described inhibitors, which are also fatty acid derivatives, 2-BP acts on the traffic ATPase TrwD as its molecular target. The results presented here demonstrate that inhibitors such as 2-HDA incorporate into the bacterial membrane and replace a significant fraction of palmitic acid. Although it is known that VirB11 proteins localize in the bacterial inner membrane, the molecular bases of this interaction has not been identified. We have proven that TrwD interacts with palmitic acid, which suggests that TrwD interacts with the membrane by binding this fatty acid. This work shows that COINs act by competing with palmitic acid for the same binding site in TrwD. Moreover, fatty acid derivatives lacking the carboxylic group do not inhibit the protein but are still predicted to bind to the same pocket, albeit with different protein contacts (8), reinforcing the importance of the stereospecificity of the binding to exert the inhibition. These differences in the mode of binding might explain the different behavior of palmitic acid and the fatty acid derivatives that act as inhibitors, such as 2-BP (Fig. 4). In the latter case, the bromine substituent induces a different conformation in the carboxylic group, which is forced to move 120° apart, creating closer contacts with basic residues of the flexible linker. This linker plays an essential role in ATP catalysis, allowing movement of the N-terminal domain (NTD) over the C-terminal domain (CTD) (13, 14). In contrast, binding predictions for palmitic acid locate its carboxylic group in the opposite direction, out of the linker region. These differences might explain why the presence of the bromide ion or double and triple bonds in the fatty acid derivatives that act as inhibitors affect the catalytic mechanism of TrwD.
It is worth noting that uFAs and 2-aFAs are effective inhibitors of IncW plasmids such as R388, but other plasmid groups, such as IncN and IncP, are not affected by them (6, 7). In contrast to TrwD, TrbB, its homolog in the RP4 plasmid, contains a much shorter linker, being more similar to HP0525 (Helicobacter pylori) than to VirB11 (10). Likewise to HP0525, TrbB does not have a pocket between the linker and the NTD, and, therefore, it is not surprising to observe a lack of effect of COINs in this system. By contrast, VirB11 homologs from IncN plasmids are close TrwD relatives. However, plasmids from this group, such as pKM101, are not inhibited by these COINs either. A close inspection of TraG structure, the VirB11 homolog in plasmid pKM101, revealed substantial differences with TrwD. The equivalent binding site in TraG presents a different global charge balance and is occluded by two hydrophobic residues, which gives no room for fatty acid binding (Fig. S1). Moreover, docking predictions in TraG with 2-BP and 2-HDA indicate that the binding site for these fatty acids is in a different region (Fig. S2). This binding site is also at the NTD, but it does not involve the linker, and, therefore, it does not affect the catalytic mechanism of the enzyme, which might explain the lack of inhibition of TraG by these fatty acid derivatives. These new findings will allow us to optimize the chemical structure of these compounds by structure-based drug design methods to design new and more effective derivatives that act as COINs.
Experimental procedures
Proteins and fatty acids
Cloning, overexpression, and purification of TrwD and TrwA proteins were carried out as described previously (28, 29). Palmitic acid and 2-bromopalmitic acid were purchased from Sigma-Aldrich. Palmitic acid derivatives, such as [14C(U)]palmitic acid and [palmitoyl-1-14C]palmitoyl CoenzymeA, were purchased from PerkinElmer Life Sciences. 2-HDA was synthesized as described previously (30).
GC-MS
E. coli ATCC 25922 (American Type Culture Collection, Manassas, VA) was grown in Luria–Bertani (LB) broth (Lennox, Fisher Scientific, Fair Lawn, NJ) medium in the presence and absence of 2-HDA (50 μg/ml) at 37 °C for 18 h. Then cells were collected, extracted by using 15 ml of chloroform:methanol (2:1, v/v), shaken (Labnet, Edison, NJ) for 30 min at 200 rpm, and sonicated into an ultrasonic cleaner (Fisher Brand, FB11201) at 37 kHz (100% power, 390 watt) for 15 min at room temperature. Phospholipids and free fatty acids were separated from total lipids by using silica gel column chromatography. Free fatty acids were eluted by adding 15 ml of acetone, whereas phospholipids were eluted by adding 15 ml of hexane. Solvents from both the acetone and hexane fractions were removed by rotoevaporation (Büchi, Rotavapor R-114), and the resulting lipid content was treated with 3 ml of methanol and 150 μl of 12 m HCl and subsequently refluxed for 3 h. When the transesterification reaction was completed, methanol was removed by rotoevaporation and fatty acid methyl esters were analyzed by using GC-MS (Hewlett Packard Series II MS ChemsStation coupled to a Hewlett Packard 5972 Series Mass Selective Detector equipped with a 30 m × 0.25 mm special performance capillary column, HP-5MS, of polymethyl siloxane cross-linked with 5% phenyl methylpolysiloxane). The selected temperature method was as follows: 120 °C initial temperature, 5 °C/min temperature rate, and 260 °C final temperature. The split ratio was set at 10:1 for all analyses. All fatty acids were identified based on their molecular ion, base peak, fragmentation pattern, and retention time and then expressed as a percentage of total FAs.
Radiolabeling assays
Purified proteins were incubated with 14C-labeled fatty acids for 10 min at room temperature. Then samples were analyzed by native PAGE, following a protocol described previously (31). 4.5% polyacrylamide gels were run at pH 8.5 for 140 min in an ice bath with a constant amperage (30 mA). Then gels were dried, exposed overnight, and visualized using a Fluoro Image Analyzer (FLA-5100, FujiFilm) to detect radiolabeled proteins on electrophoretic bands.
ATP hydrolysis assays
Steady-state ATP hydrolysis activity in vitro was measured with the EnzCheckTM Kit (Invitrogen) in a UV-1800 spectrophotometer (Shimadzu), as described previously (28). Pi released after ATP hydrolysis was monitored as an increase of absorption at 360 nm for 10 min following the manufacturer's instructions and components: 0.2 mm 2-amino-6-mercapto-7-methylpurine riboside and 1 unit/ml of purine nucleoside phosphorylase. Fatty acids diluted in DMSO were added to the reaction buffer, consisting of 50 mm Tris-HCl (pH 8.5), 75 mm potassium acetate, 10 μm magnesium acetate, 1 mm ATP, and 10% glycerol (w/v). Reactions were started by addition of the ATPase TrwD (2 μm).
Conjugation assays
Conjugation frequencies (CFs) were obtained with a fluorescence-based assay described previously (7). Strain DH5α carrying the conjugative plasmid pJC01, which expresses GFP under the control of the T7 promoter, was used as a donor. A streptomycin-resistant derivative of E. coli BL21 (DE3), expressing T7 RNA polymerase from the inserted phage, was used as the recipient strain. Both strains at stationary phase were concentrated 4-fold and mixed at a 1:1 ratio. Mixture samples (10 μl) were spotted onto 96-well microtiter plates (Bioster a.s.) containing 150 μl of LB broth, 1% agar, 1 mm isopropyl 1-thio-β-d-galactopyranoside, and different concentrations of fatty acids with the help of a Biomek 3000 liquid-handling robot (Beckman Coulter). After incubation at 37 °C for 6 h to allow conjugation, cells were resuspended in PBS and transferred to a new plate. The optical density at 600 nm (A600) and GFP emission were measured in a Victor3 multilabel counter (PerkinElmer Life Sciences). CF was calculated as the ratio of absolute fluorescence emitted by transconjugant cells and the total number of cells (A600). Relative CF in the presence of a compound was determined as a fraction of the CF observed in controls performed in the absence of the inhibitor. To reproduce the conditions, equivalent volumes of solvent (DMSO) were added to control samples.
Molecular modeling and ligand docking
An atomic model of TrwD was generated by molecular threading using as a template the atomic coordinates of Brucella suis VirB11 (PDB code 2GZA) (14), as described previously (28). The structural coordinates of palmitic acid, 2-HDA, and 2-BP acid were retrieved from the PubChem database and prepared for docking as described previously (8). Files containing the atomic coordinates of the TrwD model and the fatty acids were submitted to the SwissDock server (http://www.swissdock.ch/)5 to run blind docking. The results were examined with UCSF Chimera. Binding poses with the best full fitness score and minimal energy were finally selected.
Author contributions
Y. G.-C., D. J. S.-R., F. d. l. C., I. A., and E. C. formal analysis; Y. G.-C., M. G., N. M. C., and E. C. investigation; Y. G.-C., M. G., D. J. S.-R., and I. A. methodology; I. A. resources; I. A. and E. C. supervision; I. A. and E. C. funding acquisition; I. A. and E. C. writing-original draft; I. A. and E. C. writing-review and editing; E. C. conceptualization; E. C. project administration.
Supplementary Material
This work was supported by Spanish Ministerio de Economia y Competitividad (MINECO) Grants BFU2016-78521-R (to E. C. and I. A.) and BFU2014-55534 (to F. d. l. C.) and by Grant P20GM103475-16 from the National Center for Research Resources and NIGMS, National Institutes of Health (to D. S. R.). The authors declare that they have no conflicts of interest withthe contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Figs. S1 and S2 and Movie S1.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party–hosted site.
- COIN
- conjugation inhibitor
- T4SS
- type IV secretion system
- uFA
- unsaturated fatty acid
- 2-aFA
- alkynoic fatty acid
- 2-HDA
- 2-hexadecanoic acid
- NTD
- N-terminal domain
- CTD
- C-terminal domain
- 2-BP
- 2-bromopalmitic acid
- LB
- Luria–Bertani
- CF
- conjugation frequency.
References
- 1. Mazel D., and Davies J. (1999) Antibiotic resistance in microbes. Cell Mol. Life Sci. 56, 742–754 10.1007/s000180050021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christie P. J., Atmakuri K., Krishnamoorthy V., Jakubowski S., and Cascales E. (2005) Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59, 451–485 10.1146/annurev.micro.58.030603.123630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christie P. J., Whitaker N., and González-Rivera C. (2014) Mechanism and structure of the bacterial type IV secretion systems. Biochim. Biophys. Acta 1843, 1578–1591 10.1016/j.bbamcr.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cabezón E., Ripoll-Rozada J., Peña A., de la Cruz F., and Arechaga I. (2015) Towards an integrated model of bacterial conjugation. FEMS Microbiol. Rev. 39, 81–95 [DOI] [PubMed] [Google Scholar]
- 5. Cabezón E., de la Cruz F., and Arechaga I. (2017) Conjugation inhibitors and their potential use to prevent dissemination of antibiotic resistance genes in bacteria. Front. Microbiol. 8, 2329 10.3389/fmicb.2017.02329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernandez-Lopez R., Machón C., Longshaw C. M., Martin S., Molin S., Zechner E. L., Espinosa M., Lanka E., and de la Cruz F. (2005) Unsaturated fatty acids are inhibitors of bacterial conjugation. Microbiology 151, 3517–3526 10.1099/mic.0.28216-0 [DOI] [PubMed] [Google Scholar]
- 7. Getino M., Sanabria-Ríos D. J., Fernández-López R., Campos-Gómez J., Sánchez-López J. M., Fernández A., Carballeira N. M., and de la Cruz F. (2015) Synthetic fatty acids prevent plasmid-mediated horizontal gene transfer. mBio 6, e01032–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ripoll-Rozada J., García-Cazorla Y., Getino M., Machón C., Sanabria-Ríos D., de la Cruz F., Cabezón E., and Arechaga I. (2016) Type IV traffic ATPase TrwD as molecular target to inhibit bacterial conjugation. Mol. Microbiol. 100, 912–921 10.1111/mmi.13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atmakuri K., Cascales E., and Christie P. J. (2004) Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 54, 1199–1211 10.1111/j.1365-2958.2004.04345.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ripoll-Rozada J., Zunzunegui S., de la Cruz F., Arechaga I., and Cabezón E. (2013) Functional interactions of VirB11 traffic ATPases with VirB4 and VirD4 molecular motors in type IV secretion systems. J. Bacteriol. 195, 4195–4201 10.1128/JB.00437-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Planet P. J., Kachlany S. C., DeSalle R., and Figurski D. H. (2001) Phylogeny of genes for secretion NTPases: identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc. Natl. Acad. Sci. U.S.A. 98, 2503–2508 10.1073/pnas.051436598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peña A., and Arechaga I. (2013) Molecular motors in bacterial secretion. J. Mol. Microbiol. Biotechnol. 23, 357–369 10.1159/000351360 [DOI] [PubMed] [Google Scholar]
- 13. Savvides S. N., Yeo H. J., Beck M. R., Blaesing F., Lurz R., Lanka E., Buhrdorf R., Fischer W., Haas R., and Waksman G. (2003) VirB11ATPases are dynamic hexameric assemblies: new insights into bacterial type IV secretion. EMBO J. 22, 1969–1980 10.1093/emboj/cdg223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hare S., Bayliss R., Baron C., and Waksman G. (2006) A large domain swap in the VirB11 ATPase of Brucella suis leaves the hexameric assembly intact. J. Mol. Biol. 360, 56–66 10.1016/j.jmb.2006.04.060 [DOI] [PubMed] [Google Scholar]
- 15. Yeo H. J., Savvides S. N., Herr A. B., Lanka E., and Waksman G. (2000) Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol. Cell 6, 1461–1472 10.1016/S1097-2765(00)00142-8 [DOI] [PubMed] [Google Scholar]
- 16. Machón C., Rivas S., Albert A., Goñi F. M., and de la Cruz F. (2002) TrwD, the hexameric traffic ATPase encoded by plasmid R388, induces membrane destabilization and hemifusion of lipid vesicles. J. Bacteriol. 184, 1661–1668 10.1128/JB.184.6.1661-1668.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coleman R. A., Rao P., Fogelsong R. J., and Bardes E. S. (1992) 2-Bromopalmitoyl-CoA and 2-bromopalmitate: promiscuous inhibitors of membrane-bound enzymes. Biochim. Biophys. Acta 1125, 203–209 10.1016/0005-2760(92)90046-X [DOI] [PubMed] [Google Scholar]
- 18. Getino M., Fernández-López R., Palencia-Gándara C., Campos-Gómez J., Sánchez-López J. M., Martínez M., Fernández A., and de la Cruz F. (2016) Tanzawaic acids, a chemically novel set of bacterial conjugation inhibitors. PLoS ONE 11, e0148098 10.1371/journal.pone.0148098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Judd P. K., Kumar R. B., and Das A. (2005) Spatial location and requirements for the assembly of the Agrobacterium tumefaciens type IV secretion apparatus. Proc. Natl. Acad. Sci. U.S.A. 102, 11498–11503 10.1073/pnas.0505290102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Linder M. E., and Deschenes R. J. (2007) Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8, 74–84 10.1038/nrm2084 [DOI] [PubMed] [Google Scholar]
- 21. Jennings B. C., Nadolski M. J., Ling Y., Baker M. B., Harrison M. L., Deschenes R. J., and Linder M. E. (2009) 2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro. J. Lipid Res. 50, 233–242 10.1194/jlr.M800270-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davda D., El Azzouny M. A., Tom C. T., Hernandez J. L., Majmudar J. D., Kennedy R. T., and Martin B. R. (2013) Profiling targets of the irreversible palmitoylation inhibitor 2-bromopalmitate. ACS Chem. Biol. 8, 1912–1917 10.1021/cb400380s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Das A., and Das A. (2014) Delineation of polar localization domains of Agrobacterium tumefaciens type IV secretion apparatus proteins VirB4 and VirB11. Microbiologyopen 3, 793–802 10.1002/mbo3.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aguilar J., Cameron T. A., Zupan J., and Zambryski P. (2011) Membrane and core periplasmic Agrobacterium tumefaciens virulence type IV secretion system components localize to multiple sites around the bacterial perimeter during lateral attachment to plant cells. mBio 2, e00218–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quevillon-Cheruel S., Leulliot N., Muniz C. A., Vincent M., Gallay J., Argentini M., Cornu D., Boccard F., Lemaître B., and van Tilbeurgh H. (2009) Evf, a virulence factor produced by the Drosophila pathogen Erwinia carotovora, is an S-palmitoylated protein with a new fold that binds to lipid vesicles. J. Biol. Chem. 284, 3552–3562 10.1074/jbc.M808334200 [DOI] [PubMed] [Google Scholar]
- 26. Jin M. S., and Lee J. O. (2008) Structures of the Toll-like receptor family and its ligand complexes. Immunity 29, 182–191 10.1016/j.immuni.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 27. Hwang D. H., Kim J. A., and Lee J. Y. (2016) Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharmacol. 785, 24–35 10.1016/j.ejphar.2016.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ripoll-Rozada J., Peña A., Rivas S., Moro F., de la Cruz F., Cabezón E., and Arechaga I. (2012) Regulation of the type IV secretion ATPase TrwD by magnesium: implications for catalytic mechanism of the secretion ATPase superfamily. J. Biol. Chem. 287, 17408–17414 10.1074/jbc.M112.357905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moncalián G., Grandoso G., Llosa M., and de la Cruz F. (1997) oriT-processing and regulatory roles of TrwA protein in plasmid R388 conjugation. J. Mol. Biol. 270, 188–200 10.1006/jmbi.1997.1082 [DOI] [PubMed] [Google Scholar]
- 30. Carballeira N. M., Sanabria D., Cruz C., Parang K., Wan B., and Franzblau S. (2006) 2,6-hexadecadiynoic acid and 2,6-nonadecadiynoic acid: novel synthesized acetylenic fatty acids as potent antifungal agents. Lipids 41, 507–511 10.1007/s11745-006-5124-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arndt C., Koristka S., Bartsch H., and Bachmann M. (2012) Native polyacrylamide gels. Methods Mol. Biol. 869, 49–53 10.1007/978-1-61779-821-4_5 [DOI] [PubMed] [Google Scholar]
- 32. Grosdidier A., Zoete V., and Michielin O. (2011) SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 39, W270–W277 10.1093/nar/gkr366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.