Abstract
Fc γ receptors (FcγRs) bind circulating IgG (IgG1) at the surface of leukocytes. Antibodies clustered at the surface of a targeted particle trigger a protective immune response through activating FcγRs. Three recent reports indicate that the composition of the asparagine-linked carbohydrate chains (N-glycans) of FcγRIIIa/CD16a impacted IgG1-binding affinity. Here we determined how N-glycan composition affected the affinity of the “low-affinity” FcγRs for six homogeneous IgG1 Fc N-glycoforms (G0, G0F, G2, G2F, A2G2, and A2G2F). Surprisingly, CD16a with oligomannose N-glycans bound to IgG1 Fc (A2G2) with a KD = 1.0 ± 0.1 nm. This affinity represents a 51-fold increase over the affinity measured for CD16a with complex-type N-glycans (51 ± 8 nm) and is comparable with the affinity of FcγRI/CD64, the sole “high-affinity” FcγR. CD16a N-glycan composition accounted for increases in binding affinity for the other IgG1 Fc glycoforms tested (10–50-fold). This remarkable sensitivity could only be eliminated by preventing glycosylation at Asn162 with an Asn-to-Gln mutation; mutations at the four other N-glycosylation sites preserved tighter binding in the Man5 glycoform. None of the other low-affinity FcγRs showed more than a 3.1-fold increase upon modifying the receptor N-glycan composition, including CD16b, which differs from CD16a by only four amino acid residues. This result indicates that CD16a is unique among the low-affinity FcγRs, and modifying only the glycan composition of both the IgG1 Fc ligand and receptor provides a 400-fold range in affinities.
Keywords: Fc receptor, Fc-gamma receptor, antibody, N-linked glycosylation, post-translational modification (PTM)
Introduction
Immune cells bind to IgG through six human Fc γ receptors (FcγRs)2 that can induce or suppress a protective but potentially damaging immune response. Although the proteins involved in this recognition are well-described, significant questions remain regarding how each receptor interacts with the enormous diversity of IgG molecules in the serum and how cells of each lineage modify the antibody-binding properties of their receptors (1). Five FcγRs promote immune activation, and one suppresses signaling (FcγRIIb/CD32b). These six receptors are differentially expressed on various leukocytes and are further subdivided into one high-affinity receptor (FcγRI/CD64) and five low-affinity receptors (FcγRIIa,b,c/CD32a,b,c; FcγRIIIa,b/CD16a,b). FcγRs form a fundamental defense against disease and require productive engagement with the majority of therapeutic mAbs to achieve a therapeutic benefit (2, 3).
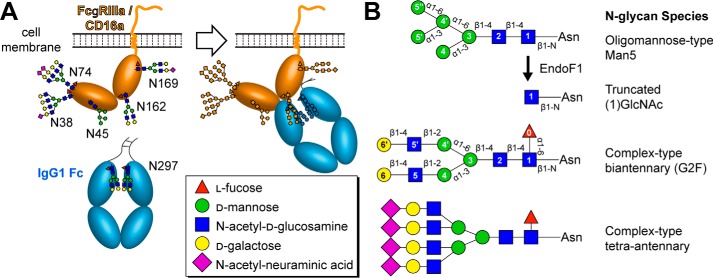
IgG antibodies bind FcγRs through the invariant crystallizable fragment (Fc). However, not all IgG antibodies exhibit the same cytotoxic potential. One variable is the location of the epitope on a given antigen; buried epitopes may lead to Fc sequestration and prevent productive engagement of surface-borne FcγRs. An additional well-known variable is the composition of the asparagine-linked carbohydrate (N-glycan) attached to Asn297 of the IgG1 Fc (Fig. 1) (4, 5). Each IgG molecule contains an Asn297 glycan on both heavy chains, and this modification is required to bind FcγRs (6–8). Furthermore, the composition of this N-glycan, resulting from the template-independent synthesis and glycan remodeling in the Golgi during protein expression, impacts affinity for various FcγRs. IgG1 Fc contains predominantly complex-type biantennary N-glycans with variable incorporation of fucose, galactose, and N-acetylneuraminic acid (Fig. 1) (9). IgG1 Fc glycoforms containing fucose bind with at least 4-fold weaker affinity to CD16a (10–13). Thus, many mAbs are glycoengineered to improve effector functions by preventing fucosylation, which is found on ∼95% of circulating IgG1 (14, 15). The improvement in treatment efficacy by glycoengineered mAbs is likely due to increased affinity for CD16 (16, 17).
Figure 1.
The low-affinity FcγRs are heavily glycosylated and bind the glycosylated Fc region of IgG1. A, the FcγRIIIa/CD16a soluble extracellular domain is sufficient for high-affinity IgG1 Fc binding and serves as a model for the binding of the other low-affinity FcγRs. B, multiple N-glycan species are synthesized on any given protein, and the distribution depends on conditions in the expressing cell among other variables.
Recent reports indicate FcγR N-glycan composition likewise impacts antibody-binding affinity (18–20). In one study, CD16a with minimally processed oligomannose-type Man5 N-glycans bound to IgG1 Fc with 12-fold greater affinity than CD16a with highly processed complex-type N-glycans. The impact of this affinity increase was unclear because it is believed the majority of cell surface glycoproteins, including the FcγRs, display highly processed N-glycans similar to those found on serum glycoproteins. Indeed, a recent report indicated CD16b isolated from human serum contained primarily highly processed complex-type N-glycans, with minimally processed forms found at the Asn45 glycosylation site (21). However, CD16a from primary human natural killer cells contained a large amount of minimally processed N-glycans, which included a significant fraction of hybrid and oligomannose N-glycans (45%) (20, 22).
From these reports it was clear that a wide range of N-glycan species may be found decorating the low-affinity FcγRs expressed at the surface of human immune cells; however, it was not clear whether the function of each receptor would be affected by N-glycan composition as much as CD16a. Furthermore, the magnitude to which N-glycan composition impacted CD16a remains undefined because only a limited panel of IgG1 Fc glycoforms were used in the prior study. The goal of the experiments described here is to determine the extent to which receptor N-glycan composition impacted affinity for IgG1 Fc in vitro by testing a range of different receptors and Fc glycoforms. We likewise sought to determine whether the glycan at one specific site of the receptor, of the many found on the heavily glycosylated FcγRs, contributes a greater degree toward the receptor sensitivity to N-glycan composition.
Results
Preparation and N-glycan analysis of recombinant Fc γ receptors
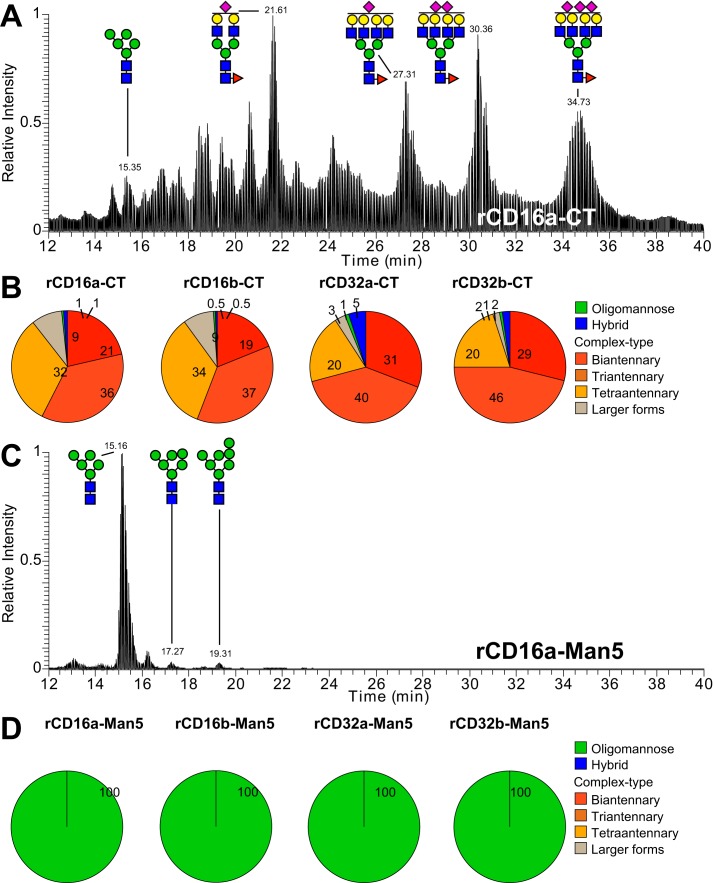
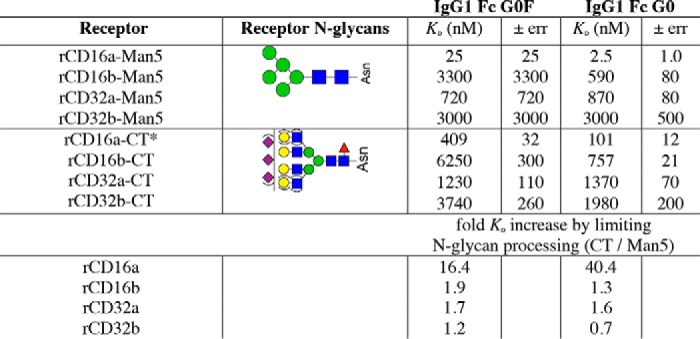
Transiently transfected human embryonic kidney (HEK) 293S cells expressed the soluble, antibody-binding extracellular domains of the low-affinity FcγRs (rCD16a, rCD16b, rCD32a, and rCD32b) to high yields and high purity following elution from a nickel–nitrilotriacetic acid column (80–120 mg liter−1; Fig. S1). The HEK293S (Gnt1-) cell line synthesizes N-glycoproteins with primarily Man5 N-glycans because of a GNT1 gene deletion that prevents the later stages of N-glycan processing (23). LC-MS–based analysis of these recombinant receptors revealed the presence of >99% oligomannose-type N-glycans (Fig. 2). We previously reported the binding affinity of each low-affinity FcγR, expressed in the parent HEK293F cell line, to an array of IgG1 Fc N-glycoforms (12). A parallel analysis of these HEK293F-expressed receptors indicated that >89% of the glycans were of a complex type (CT) (Fig. 2B).
Figure 2.
N-Glycan composition of the recombinant Fc γ receptors. Elution pattern and glycan composition of N-glycans from recombinant Fc γ receptors using hydrophilic interacting chromatography–LC/MS/MS are shown. A–D, expression of receptors using HEK293F cells provides primarily CT N-glycans (A and B) and Man5 N-glycans (C and D) results from expression using HEK293S (Gnt1-) cells. The numbers in the pie charts indicate the percentages of the N-glycan groups.
Binding-affinity measurements
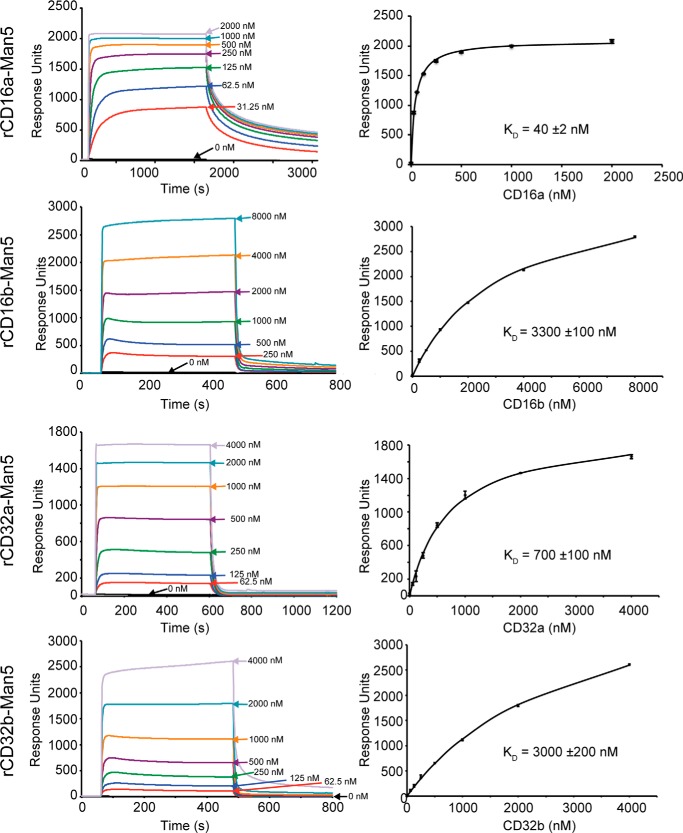
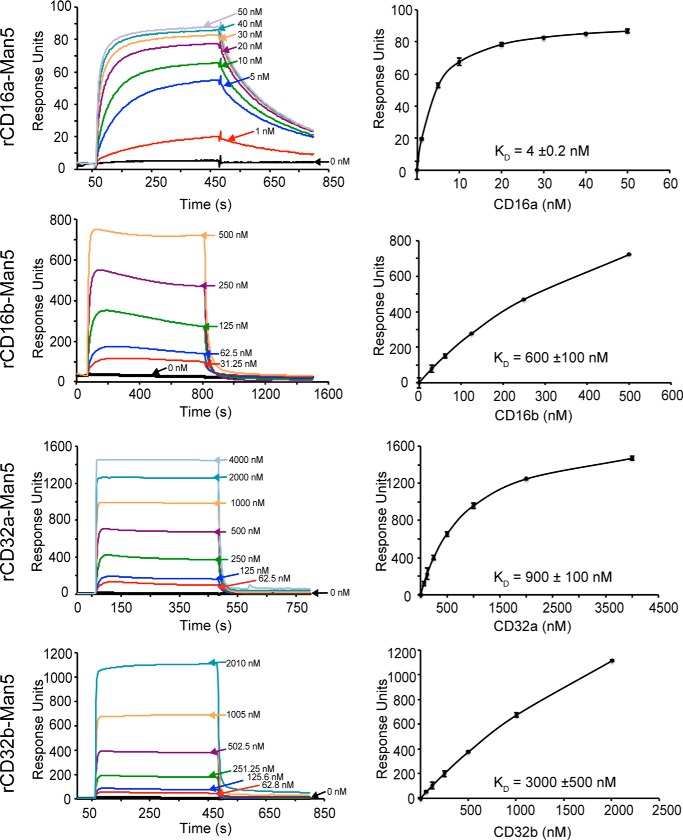
Each of the four low-affinity Fc γ receptors with Man5 N-glycans bound to IgG1 Fc glycovariants immobilized on a surface. These experiments tested binding to six homogeneous IgG1 Fc glycovariants: G0, G0F, G2, G2F, A2G2, and A2G2F with G0 indicating zero galactose residues on the complex-type biantennary N-glycan branches, A2 indicating the presence of two N-acetylneuraminic acid residues on the branches, and F indicating a single core fucose residue. Once binding reached a kinetic equilibrium, the intensity at equilibrium provided a measure of the amount of receptor bound to the single covalently anchored IgG1 Fc glycoform. Fitting a binding isotherm to these equilibrium intensity values provided an estimate for the dissociation constant and is shown with representative data in Figs. 3 and 4. The complete data are compiled in Table 1 and Table S1.
Figure 3.
Representative binding analysis for fucosylated IgG1-Fc (G0F) measured by SPR. The left column shows binding sensograms, and the right column shows fits using response intensity values once a binding equilibrium is reached. Error bars for the binding fits are shown.
Figure 4.
Representative binding analysis for fucosylated IgG1-Fc (G0) measured by SPR. The left column shows binding sensograms, and the right column shows fits using response intensity values once a binding equilibrium is reached. Error bars for the binding fits are shown.
Table 1.
Receptor and IgG1 Fc N-glycan composition impacts binding affinity
* CT values from Ref. 12.
The general patterns of relative affinity among the low-affinity Fc γ receptors with oligomannose-type N-glycans proved comparable with those measured with complex-type N-glycans. rCD16a bound the tightest, followed by rCD32a, rCD32b, and rCD16b when using fucosylated IgG1 Fc. The patterns between receptors with complex-type and oligomannose N-glycans were likewise similar when binding to afucosylated IgG1 Fc. In this case, rCD16a bound the tightest, followed by rCD16b, rCD32a, and rCD32b in descending order of affinity. Thus, both rCD16a-Man5 and rCD16b-Man5 but neither rCD32a-Man5 nor rCD32b-Man5 exhibited a marked sensitivity to IgG1 Fc fucosylation. This relative binding-affinity ranking is consistent with previous results observed using the complex-type glycoforms (12).
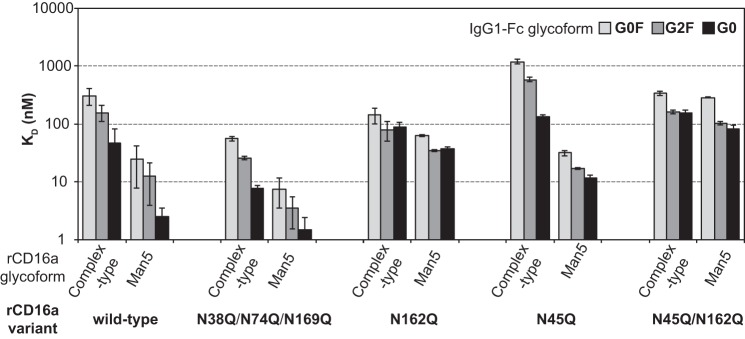
Of the receptors analyzed, only rCD16a with Man5 oligomannose N-glycans showed a dramatic increase in affinity when compared with rCD16a with complex-type N-glycans. rCD16a-Man5 bound to afucosylated IgG1 Fc (G0, G2, and A2G2) with 41–51-fold greater affinity than rCD16a-CT, compared with an increase of 0.7–3.1-fold for rCD16b, rCD32a, and rCD32b (Table 1). The binding affinity to fucosylated IgG1 Fc glycoforms (G0F, G2F, and A2G2F) increased between 10- and 16-fold, compared with a 1.2–2.4-fold increase for rCD16b, rCD32a, and rCD32b.
The affinity of rCD16a-Man5 for afucosylated IgG1 Fc (A2G2) is >400-fold tighter than the affinity of rCD16a-CT binding IgG1 Fc (G0F). This astonishing result indicates that an enormous range of binding affinities are achievable by modifying only the N-glycan composition of the ligand and receptor. Furthermore, the 1.0 ± 0.1 nm affinity measured for the IgG1 Fc (A2G2)–rCD16a-Man5 interaction is comparable with the 1–3 nm binding affinity of the high-affinity Fc γ receptor CD64 (11, 12, 24). Thus, rCD16a is unique among the low-affinity Fc γ receptors with an unprecedented sensitivity to N-glycan composition and the capability to bind afucosylated IgG1 Fc in vitro with affinities comparable to CD64. It is surprising that this unique sensitivity is not shared by rCD16b, which differs by only four amino acid residues in the extracellular antibody-binding domains analyzed (Fig. S2).
The role of individual CD16a N-glycans
Previous reports indicated that two of the five potential CD16a N-glycans contribute to IgG1 Fc-binding affinity (19, 25–27). Indeed, glycans at Asn45 and Asn162 proved essential for the high-affinity interactions described above. Removing the Asn38, Asn74, and Asn169 glycosylation sites through mutation to Gln slightly increased the affinity (Fig. 5 and Table S1). However, removing N-glycosylation sites by mutating either Asn45 or Asn162 mostly reduced affinity. Although the N45Q variant with complex-type N-glycans showed weaker affinity for IgG1 Fc than the N162Q variant, the relative positions were reversed when measuring the affinity of the Man5 receptor glycoforms. Surprisingly, binding affinity measured for the rCD16a-N45Q variant still showed sensitivity to the receptor glycoform: the Man5 glycoform bound more than 10-fold tighter to IgG1 Fc than the CT glycoform. This sensitivity was lost with the rCD16a-N162 variant, which showed comparable binding in either glycoform to IgG1 Fc. Thus, the rCD16a Asn162 glycan mediates high-affinity interactions with IgG1 Fc. This result can be explained by the location of the Asn162 glycan at the interface formed by IgG1 Fc and rCD16 observed in high-resolution structures determined by X-ray crystallography (Fig. S3), although there is considerable disagreement related to the nature of the interactions at this site (19, 28–31).
Figure 5.
rCD16a amino acid substitutions and N-glycan composition affects IgG1 Fc binding affinity. Errors of fit for the dissociation constants are shown.
The role of receptor fucosylation
Multiple laboratories previously demonstrated that fucosylation of the IgG1 Fc core (1)GlcNAc residue reduced the affinity for rCD16a from 4- to 50-fold (10, 12). One major difference between rCD16a-Man5 and rCD16a-CT is that the former glycoform is not fucosylated. We assessed the role of receptor fucosylation by comparing the binding of rCD16a displaying complex-type glycans expressed with or without fucose to determine whether the increased affinity of rCD16a-Man5 could be explained by the missing fucose residue (+fuc or −fuc, respectively). The impact of receptor fucosylation proved minimal for three rCD16a amino acid variants analyzed, with most interactions perturbed by less than a 2-fold change in affinity as compared with a 10–51-fold change upon replacing complex-type N-glycans with Man5 glycans (Fig. 6 and Table S1). One exception to this conclusion is that rCD16a-CT(−fuc) binds IgG1 Fc G0 with 5-fold greater affinity than rCD16a-CT(+fuc), but this affinity increase is still far below the 40-fold increase upon comparing rCD16a-CT and rCD16a-Man5 binding IgG1 Fc G0. Thus, it is unlikely that the predominant contribution to the increased affinity of rCD16a-Man5 for IgG1 Fc can be explained by a lack of CD16a fucose.
Figure 6.
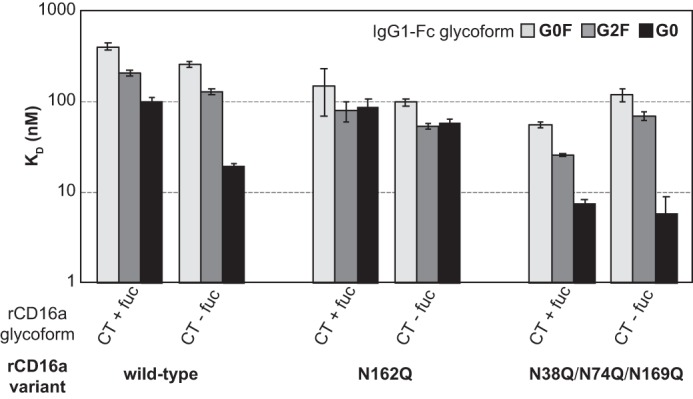
The effect of receptor fucosylation on binding affinity. rCD16a was expressed in the absence (CT + fuc) or presence (CT − fuc) of 2-doexy-2-fluoro-l-fucose. Error bars represent the error of the dissociation constant fit from equilibrium intensity data.
Discussion
These experiments demonstrate CD16a is uniquely sensitive to the composition of its covalently attached N-glycans, as well as the ligand N-glycans in vitro. Of the five possible CD16a N-glycans, Asn162 appears to be primarily responsible for this behavior, and the Asn162 glycan was previously implicated as the primary moiety sensing antibody fucosylation (30, 31). It is remarkable that protein function can be modified by N-glycan composition; however, IgG1 Fc N-glycan composition is a well-described modifier of CD16a binding (5, 6, 12). The surprising aspects of these findings are the magnitude of the modulation and that the sensitivity to N-glycan composition is restricted to a single low-affinity FcγR.
Modifying the biantennary complex-type IgG1 Fc N-glycan with fucose, galactose, and N-acetylneuraminic acid generates an 8-fold range of affinities for rCD16a (12). Modifying receptor N-glycan composition impacts rCD16a-binding affinity by up to 51-fold. These effects combined provide a 400-fold range of affinities for rCD16a, with the predominant impact from receptor N-glycan composition. This wide range of affinities potentially provides the body an opportunity to tune the immune system by modifying N-glycans. A significant amount of evidence indicates IgG1 Fc N-glycans change in response to multiple stressors, including age and disease (including but not limited to Refs. 32–38), but there are very few reports of FcγR glycosylation in native human tissues because of the difficulty in obtaining sufficient material for analysis (20, 21).
It would be appropriate to assume that the low-affinity FcγRs, which share a high degree of structural and sequence homology, share similar functional profiles; however, rCD16a is by far the most sensitive to the composition of its attached N-glycans with up to a 51-fold change in binding affinity, but the highly related rCD16b is only impacted by a 3.1-fold change. This is even more surprising considering that rCD16b shares a similar sensitivity to IgG1 Fc N-glycan composition with an observed 16-fold range of affinities as compared with 8-fold for rCD16a, 1.8-fold for rCD32a, and 2.2-fold for rCD32b (12).
One weakness of this present study is related to the reductionist approach measuring monovalent interactions of the soluble extracellular domains with the IgG1 Fc in vitro. It is unknown how these observed changes in monovalent binding affinity impact the multivalent interactions that hold an opsonized target to the surface of an immune cell; it is likely that the raw affinities measured in this study do not accurately recapitulate affinities for similar interactions in the complex milieu of the serum or peripheral tissues that contain a multitude of factors including 5–15 mg/ml competing antibody and occur at a membrane. However, we expect that relative differences between the receptors we identified are representative of the in vivo activity. It is noteworthy that the modest 4-fold affinity enhancement achieved by preventing antibody fucosylation or the 5-fold increase in affinity observed for the CD16a-V158 v. CD16a-F158 allotype increases patient outcome with therapeutic monoclonal antibodies (11, 39–42). Thus, if modest changes in receptor–antibody interactions provide measurable advantages in patient outcome, then the potential to exploit a larger 51-fold enhancement is noteworthy.
This study highlights the critical need to determine the N-glycan composition of FcγRs from primary, uncultured human tissue and to develop recombinant systems that produce comparable material for in vitro binding studies. Although substantial resources have been previously applied to analyze N-glycans from recombinant receptors (43–45), it is evident that even human cell-based recombinant protein expression systems do not appropriately recapitulate receptor glycosylation because CD16a from primary human natural cells and HEK293F expression are dramatically different (20). CD16a isolated from natural killer cells donated by three older male donors contained smaller complex type N-glycans plus a significant proportion of hybrid and oligomannose types, unlike rCD16a from HEK293F or serum-borne CD16b that contained predominantly large complex-type N-glycans and a small amount of oligomannose forms (20, 21). Therefore, based on these results, the rCD16a used to assess antibody binding imperfectly represents the native CD16a glycoforms and is an inaccurate model of antibody-binding affinities on immune cell surfaces.
Engineering antibody N-glycan composition is proving to be a powerful device to enhance the effector properties of therapeutic monoclonal antibodies (16, 17, 46, 47). Although it is unclear whether the human body modifies antibody N-glycan composition to directly tune sensitivity of the immune system or whether antibody glycosylation changes result from change in the immune system, the clinical evidence supporting the enhanced efficacy of glycoengineered antibodies is substantial. Changes in antibody glycosylation have a much smaller impact on receptor-binding affinity than changes in the receptor glycans and implicate receptor glycosylation as an unexplored device to enhance drug efficacy.
Experimental procedures
Materials
Materials were purchased from Sigma–Aldrich unless otherwise noted.
Protein expression and purification
A description of the preparation, purification, in vitro remodeling, and analysis of the batch of human IgG1 Fc (residues 216–447) glycovariants used in this study was published previously because these glycovariants were previously used for binding analyses (12). The low-affinity Fc γ receptors with complex-type N-glycans including recombinant (r)CD16a and associated variants (residues 19–193, Val158 allotype), rCD16b (residues 19–193), rCD32a (residues 43–216, LR (H143) allotype), and rCD32b (residues 43–216) were expressed with HEK293F cells as previously described (12, 48, 49). rCD16a and related variants were also expressed with HEK293F cells in the presence of 250 μm 2-deoxy-2-fluoro-l-fucose (Santa Cruz Biotechnology) to prevent fucosylation (50). Receptors with Man5 oligomannose N-glycans were expressed using HEK293S (Gnt1-) (51). N-Glycans from the low-affinity Fc γ receptors were released, purified, conjugated to procainamide, and analyzed using hydrophilic interacting chromatography–MS on a Q-Exactive mass spectrometer (ThermoFisher) as described previously (20). The spectra were analyzed using Byonic (Protein Metrics) to identify singly, doubly, or triply charged N-glycan species. Each identification was manually validated by analyzing retention time and MS2 spectra using Xcaliber (Thermo Fisher).
Binding-affinity measurements
Fc was coupled onto a CM5 sensor chip on a Biacore T100 instrument (GE Life Sciences). Fc γ receptors were flowed over the Fc-coupled chip as previously described (12). A minimum of two experiments for each receptor/Fc pair was collected on at least two different days, and representative data are reported. Dissociation constants for each experiment were determined by fitting the equilibrium response values at each receptor concentration to the Hill equation. All binding experiments using WT FcγRs with oligomannose-type N-glycans were collected at the same time as the previously reported binding affinity measurements for the WT FcγRs with complex-type N-glycans and are thus directly comparable (12).
Author contributions
G. P. S. and A. W. B. conceptualization; G. P. S. and A. W. B. data curation; G. P. S. and A. W. B. formal analysis; G. P. S. and A. W. B. validation; G. P. S. investigation; G. P. S. and A. W. B. visualization; G. P. S. and A. W. B. methodology; G. P. S. and A. W. B. writing-original draft; G. P. S. and A. W. B. writing-review and editing; A. W. B. supervision; A. W. B. funding acquisition; A. W. B. project administration.
Supplementary Material
This work was supported by NIGMS, National Institutes of Health Grant R01GM115489 and by funds from the Roy J. Carver Department of Biochemistry, Biophysics & Molecular Biology at Iowa State University. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Table S1 and Figs. S1–S3.
- FcγR
- Fc γ receptor
- HEK
- human embryonic kidney
- CT
- complex type.
References
- 1. Oliva K. D., Cavanaugh J. M., and Cobb B. A. (2018) Antibody receptors steal the sweet spotlight. J. Biol. Chem. 293, 3490–3491 10.1074/jbc.H118.001955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nimmerjahn F., Gordan S., and Lux A. (2015) FcγR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 36, 325–336 10.1016/j.it.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 3. Bruhns P. (2012) Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119, 5640–5649 10.1182/blood-2012-01-380121 [DOI] [PubMed] [Google Scholar]
- 4. Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., and Matsuta K. (1985) Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316, 452–457 10.1038/316452a0 [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi Y., Nishimura M., Nagano M., Yagi H., Sasakawa H., Uchida K., Shitara K., and Kato K. (2006) Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim. Biophys. Acta 1760, 693–700 10.1016/j.bbagen.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 6. Lux A., Yu X., Scanlan C. N., and Nimmerjahn F. (2013) Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J. Immunol. 190, 4315–4323 10.4049/jimmunol.1200501 [DOI] [PubMed] [Google Scholar]
- 7. Nose M., and Wigzell H. (1983) Biological significance of carbohydrate chains on monoclonal antibodies. Proc. Natl. Acad. Sci. U.S.A. 80, 6632–6636 10.1073/pnas.80.21.6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Subedi G. P., and Barb A. W. (2015) The structural role of antibody N-glycosylation in receptor interactions. Structure 23, 1573–1583 10.1016/j.str.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menni C., Keser T., Mangino M., Bell J. T., Erte I., Akmacic I., Vuckovic F., Pucic Bakovic M., Gornik O., McCarthy M. I., Zoldos V., Spector T. D., Lauc G., and Valdes A. M. (2013) Glycosylation of immunoglobulin g: role of genetic and epigenetic influences. PLoS One 8, e82558 10.1371/journal.pone.0082558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shields R. L., Lai J., Keck R., O'Connell L. Y., Hong K., Meng Y. G., Weikert S. H., and Presta L. G. (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcγ RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 10.1074/jbc.M202069200 [DOI] [PubMed] [Google Scholar]
- 11. Dekkers G., Treffers L., Plomp R., Bentlage A. E. H., de Boer M., Koeleman C. A. M., Lissenberg-Thunnissen S. N., Visser R., Brouwer M., Mok J. Y., Matlung H., van den Berg T. K., van Esch W. J. E., Kuijpers T. W., Wouters D., et al. (2017) Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front. Immunol. 8, 877 10.3389/fimmu.2017.00877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Subedi G. P., and Barb A. W. (2016) The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fcγ receptor. mAbs 8, 1512–1524 10.1080/19420862.2016.1218586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shinkawa T., Nakamura K., Yamane N., Shoji-Hosaka E., Kanda Y., Sakurada M., Uchida K., Anazawa H., Satoh M., Yamasaki M., Hanai N., and Shitara K. (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 10.1074/jbc.M210665200 [DOI] [PubMed] [Google Scholar]
- 14. Tai T., Ito S., Yamashita K., Muramatsu T., and Kobata A. (1975) Asparagine-linked oligosaccharide chains of IgG: a revised structure. Biochem. Biophys. Res. Commun. 65, 968–974 10.1016/S0006-291X(75)80480-3 [DOI] [PubMed] [Google Scholar]
- 15. Kapur R., Kustiawan I., Vestrheim A., Koeleman C. A., Visser R., Einarsdottir H. K., Porcelijn L., Jackson D., Kumpel B., Deelder A. M., Blank D., Skogen B., Killie M. K., Michaelsen T. E., de Haas M., et al. (2014) A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood 123, 471–480 10.1182/blood-2013-09-527978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golay J., Da Roit F., Bologna L., Ferrara C., Leusen J. H., Rambaldi A., Klein C., and Introna M. (2013) Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 122, 3482–3491 10.1182/blood-2013-05-504043 [DOI] [PubMed] [Google Scholar]
- 17. Reddy V., Klein C., Isenberg D. A., Glennie M. J., Cambridge G., Cragg M. S., and Leandro M. J. (2017) Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology 56, 1227–1237 10.1093/rheumatology/kex067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayes J. M., Frostell A., Karlsson R., Müller S., Martín S. M., Pauers M., Reuss F., Cosgrave E. F., Anneren C., Davey G. P., and Rudd P. M. (2017) Identification of Fcγ receptor glycoforms that produce differential binding kinetics for rituximab. Mol. Cell. Proteomics 16, 1770–1788 10.1074/mcp.M117.066944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falconer D. J., Subedi G. P., Marcella A. M., and Barb A. W. (2018) Antibody fucosylation lowers FcγRIIIa/CD16a affinity by limiting the conformations sampled by the N162-glycan. ACS Chem. Biol. 13, 2179–2189 10.1021/acschembio.8b00342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel K. R., Roberts J. T., Subedi G. P., and Barb A. W. (2018) Restricted processing of CD16a/Fcγ receptor IIIa N-glycans from primary human NK cells impacts structure and function. J. Biol. Chem. 293, 3477–3489 10.1074/jbc.RA117.001207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yagi H., Takakura D., Roumenina L. T., Fridman W. H., Sautès-Fridman C., Kawasaki N., and Kato K. (2018) Site-specific N-glycosylation analysis of soluble Fcγ receptor IIIb in human serum. Sci. Rep. 8, 2719 10.1038/s41598-018-21145-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edberg J. C., and Kimberly R. P. (1997) Cell type-specific glycoforms of Fcγ RIIIa (CD16): differential ligand binding. J. Immunol. 159, 3849–3857 [PubMed] [Google Scholar]
- 23. Stanley P., Narasimhan S., Siminovitch L., and Schachter H. (1975) Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine–glycoprotein N-acetylglucosaminyltransferase activity. Proc. Natl. Acad. Sci. U.S.A. 72, 3323–3327 10.1073/pnas.72.9.3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bruhns P., Iannascoli B., England P., Mancardi D. A., Fernandez N., Jorieux S., and Daëron M. (2009) Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 113, 3716–3725 10.1182/blood-2008-09-179754 [DOI] [PubMed] [Google Scholar]
- 25. Subedi G. P., Falconer D. J., and Barb A. W. (2017) Carbohydrate-polypeptide contacts in the antibody receptor CD16A identified through solution NMR spectroscopy. Biochemistry 56, 3174–3177 10.1021/acs.biochem.7b00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrara C., Stuart F., Sondermann P., Brünker P., and Umaña P. (2006) The carbohydrate at FcγRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 281, 5032–5036 10.1074/jbc.M510171200 [DOI] [PubMed] [Google Scholar]
- 27. Shibata-Koyama M., Iida S., Okazaki A., Mori K., Kitajima-Miyama K., Saitou S., Kakita S., Kanda Y., Shitara K., Kato K., and Satoh M. (2009) The N-linked oligosaccharide at Fcγ RIIIa Asn-45: an inhibitory element for high Fcγ RIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology 19, 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sondermann P., Huber R., Oosthuizen V., and Jacob U. (2000) The 3.2-A crystal structure of the human IgG1 Fc fragment–FcγRIII complex. Nature 406, 267–273 10.1038/35018508 [DOI] [PubMed] [Google Scholar]
- 29. Sakae Y., Satoh T., Yagi H., Yanaka S., Yamaguchi T., Isoda Y., Iida S., Okamoto Y., and Kato K. (2017) Conformational effects of N-glycan core fucosylation of immunoglobulin G Fc region on its interaction with Fcγ receptor IIIa. Sci. Rep. 7, 13780 10.1038/s41598-017-13845-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrara C., Grau S., Jäger C., Sondermann P., Brünker P., Waldhauer I., Hennig M., Ruf A., Rufer A. C., Stihle M., Umaña P., and Benz J. (2011) Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. U.S.A. 108, 12669–12674 10.1073/pnas.1108455108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mizushima T., Yagi H., Takemoto E., Shibata-Koyama M., Isoda Y., Iida S., Masuda K., Satoh M., and Kato K. (2011) Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells 16, 1071–1080 10.1111/j.1365-2443.2011.01552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park D. I., Stambuk J., Razdorov G., Pucic-Bakovic M., Martins-de-Souza D., Lauc G., and Turck C. W. (2018) Blood plasma/IgG N-glycome biosignatures associated with major depressive disorder symptom severity and the antidepressant response. Sci. Rep. 8, 179 10.1038/s41598-017-17500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Russell A. C., Simurina M., Garcia M. T., Novokmet M., Wang Y., Rudan I., Campbell H., Lauc G., Thomas M. G., and Wang W. (2017) The N-glycosylation of immunoglobulin G as a novel biomarker of Parkinson's disease. Glycobiology 27, 501–510 10.1093/glycob/cwx022 [DOI] [PubMed] [Google Scholar]
- 34. Pezer M., Stambuk J., Perica M., Razdorov G., Banic I., Vuckovic F., Gospic A. M., Ugrina I., Vecenaj A., Bakovic M. P., Lokas S. B., Zivkovic J., Plavec D., Devereux G., Turkalj M., et al. (2016) Effects of allergic diseases and age on the composition of serum IgG glycome in children. Sci. Rep. 6, 33198 10.1038/srep33198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vuckovic F., Theodoratou E., Thaçi K., Timofeeva M., Vojta A., Stambuk J., Pucic-Bakovic M., Rudd P. M., Derek L., Servis D., Wennerström A., Farrington S. M., Perola M., Aulchenko Y., Dunlop M. G., et al. (2016) IgG glycome in colorectal cancer. Clin. Cancer Res. 22, 3078–3086 10.1158/1078-0432.CCR-15-1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kristic J., Vuckovic F., Menni C., Klaric L., Keser T., Beceheli I., Pucic-Bakovic M., Novokmet M., Mangino M., Thaqi K., Rudan P., Novokmet N., Sarac J., Missoni S., Kolcic I., et al. (2014) Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. A Biol. Sci. Med. Sci. 69, 779–789 10.1093/gerona/glt190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu D., Zhao Z., Wang A., Ge S., Wang H., Zhang X., Sun Q., Cao W., Sun M., Wu L., Song M., Zhou Y., Wang W., and Wang Y. (2018) Ischemic stroke is associated with the pro-inflammatory potential of N-glycosylated immunoglobulin G. J. Neuroinflammation 15, 123 10.1186/s12974-018-1161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guu S. Y., Lin T. H., Chang S. C., Wang R. J., Hung L. Y., Fang P. J., Tang W. C., Yu P., and Chang C. F. (2017) Serum N-glycome characterization and anti-carbohydrate antibody profiling in oral squamous cell carcinoma patients. PLoS One 12, e0178927 10.1371/journal.pone.0178927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stork A. C., Notermans N. C., van den Berg L. H., Schellevis R. D., Niermeijer J. M., Nederend M., Leusen J. H., and van der Pol W. L. (2014) Fcγ receptor IIIA genotype is associated with rituximab response in antimyelin-associated glycoprotein neuropathy. J. Neurol. Neurosurg. Psychiatry 85, 918–920 10.1136/jnnp-2013-306958 [DOI] [PubMed] [Google Scholar]
- 40. Cartron G., Dacheux L., Salles G., Solal-Celigny P., Bardos P., Colombat P., and Watier H. (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99, 754–758 10.1182/blood.V99.3.754 [DOI] [PubMed] [Google Scholar]
- 41. Weng W. K., and Levy R. (2003) Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 10.1200/JCO.2003.05.013 [DOI] [PubMed] [Google Scholar]
- 42. Dall'Ozzo S., Tartas S., Paintaud G., Cartron G., Colombat P., Bardos P., Watier H., and Thibault G. (2004) Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 64, 4664–4669 10.1158/0008-5472.CAN-03-2862 [DOI] [PubMed] [Google Scholar]
- 43. Hayes J. M., Frostell A., Cosgrave E. F., Struwe W. B., Potter O., Davey G. P., Karlsson R., Anneren C., and Rudd P. M. (2014) Fcγ receptor glycosylation modulates the binding of IgG glycoforms: a requirement for stable antibody interactions. J. Proteome Res 13, 5471–5485 10.1021/pr500414q [DOI] [PubMed] [Google Scholar]
- 44. Takahashi N., Cohen-Solal J., Galinha A., Fridman W. H., Sautès-Fridman C., and Kato K. (2002) N-Glycosylation profile of recombinant human soluble Fcγ receptor III. Glycobiology 12, 507–515 10.1093/glycob/cwf063 [DOI] [PubMed] [Google Scholar]
- 45. Zeck A., Pohlentz G., Schlothauer T., Peter-Katalinic J., and Regula J. T. (2011) Cell type-specific and site directed N-glycosylation pattern of FcγRIIIa. J. Proteome Res. 10, 3031–3039 10.1021/pr1012653 [DOI] [PubMed] [Google Scholar]
- 46. Peschke B., Keller C. W., Weber P., Quast I., and Lünemann J. D. (2017) Fc-galactosylation of human immunoglobulin γ isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front. Immunol. 8, 646 10.3389/fimmu.2017.00646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Estupina P., Fontayne A., Barret J. M., Kersual N., Dubreuil O., Le Blay M., Pichard A., Jarlier M., Pugnière M., Chauvin M., Chardès T., Pouget J. P., Deshayes E., Rossignol A., Abache T., et al. (2017) The anti-tumor efficacy of 3C23K, a glyco-engineered humanized anti-MISRII antibody, in an ovarian cancer model is mainly mediated by engagement of immune effector cells. Oncotarget 8, 37061–37079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Subedi G. P., Hanson Q. M., and Barb A. W. (2014) Restricted motion of the conserved immunoglobulin G1 N-glycan is essential for efficient FcγRIIIa binding. Structure 22, 1478–1488 10.1016/j.str.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Subedi G. P., Johnson R. W., Moniz H. A., Moremen K. W., and Barb A. (2015) High yield expression of recombinant human proteins with the transient transfection of HEK293 cells in suspension. J. Vis. Exp. e53568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okeley N. M., Alley S. C., Anderson M. E., Boursalian T. E., Burke P. J., Emmerton K. M., Jeffrey S. C., Klussman K., Law C. L., Sussman D., Toki B. E., Westendorf L., Zeng W., Zhang X., Benjamin D. R., et al. (2013) Development of orally active inhibitors of protein and cellular fucosylation. Proc. Natl. Acad. Sci. U.S.A. 110, 5404–5409 10.1073/pnas.1222263110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reeves P. J., Callewaert N., Contreras R., and Khorana H. G. (2002) Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. U.S.A. 99, 13419–13424 10.1073/pnas.212519299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.