Abstract
Peroxisome proliferator–activated receptor γ (PPARγ) is a member of the nuclear receptor superfamily and polarizes the macrophages into an anti-inflammatory M2 state. Integrins are transmembrane receptors that drive various cellular functions, including monocyte adhesion and foam cell formation. In this study, we first reported that the expression of integrins αV and β5 was up-regulated by PPARγ activation in RAW264.7 cells and human peripheral blood monocytes. Luciferase reporter and ChIP assay revealed that PPARγ directly bound to the potential PPAR-responsive elements sites in the 5′-flanking regions of both murine and human integrin αV and β5 genes, respectively. In addition, we showed that PPARγ augmented the ligation of integrins αV and β5. Knockdown of integrin αVβ5 by siRNA strategy or treatment with cilengitide, a potent inhibitor of integrin αVβ5, attenuated PPARγ-induced expression of Ym1 (chitinase-like protein 3), Arg1 (Arginase1), Fizz1 (resistin-like molecule RELMα), and other M2 marker genes, suggesting that the heterodimers of integrin αVβ5 were involved in PPARγ-induced M2 polarization. In conclusion, these results provided novel evidence that PPARγ-mediated gene expression and the ensuing ligation of integrins αV and β5 are implicated in macrophage M2 polarization.
Keywords: integrin, peroxisome proliferator-activated receptor (PPAR), gene regulation, protein-protein interaction, inflammation, integrin alpha V, integrin beta 5, macrophage polarization
Introduction
Macrophages are markedly heterogenous cells that are responsive to various stimuli in either acute (infection) or chronic (metabolic syndrome) state (1, 2). M1 macrophages, activated upon classical pro-inflammatory cytokines (Toll-like receptor ligands and interferon-γ), are involved in Th1 immune response (3). In the presence of Th2 cytokines (interleukin-4, interleukin-13, and colony-stimulating factor), monocytes undergo an alternative M2 activation, characterized by an increased expression of Ym1, Arg1, Fizz1, and interleukin-10 (IL-10)2 (4). Macrophage polarization is tightly regulated at the transcription level (5, 6). Interferon-regulatory factor 5 and signal transducer and activator of transcription 1 (STAT1) promote M1 polarization, whereas PPARγ cooperates with STAT6 to drive to a M2 phenotype (7).
PPARγ is a member of the nuclear receptor superfamily and a key regulator of lipid metabolism. It has been shown to suppress inflammation by inhibiting nuclear factor κ light-chain enhancer of activated B cells, STAT, and activator protein-1 pathways (8, 9). Constitutive expression of PPARγ in macrophages leads to an anti-inflammatory M2-like phenotype in adipose tissue. In contrast, knockdown of PPARγ impairs M2 polarization and insulin signaling (10). It has been demonstrated that PPARγ-dependent monocyte differentiation into M2 macrophage is beneficial to human carotid atherosclerosis (11). PPARγ activation by pioglitazone regulates M2 macrophage infiltration and stabilizes the neovessels in the infarct border zone, leading to a better outcome for patients after stroke (12). Previous studies showed that Arg1 and IL-10 are regulated by PPARγ and involved in M2 polarization (10, 13). However, the PPARγ-target genes driving the transcriptional program toward M2 polarization remain largely unknown.
Integrins are a family of ubiquitous transmembrane receptor expressed on a variety of cell types. Mammalian integrins comprise 18 α and 8 β subunits that constitute 24 heterodimers. Ligation of α and β subunits leads to a conformational change according to the extracellular ligands presented on the cell surface (14). In macrophages, integrin αMβ2 (15), αDβ2 (16), and α5β1 (17) have been implicated in phagocytosis, M1 polarization, and inflammasome activation. Integrin α4 has recently been found to modulate metabolic inflammation in obesity (18). The absence of integrin β3 favors macrophage polarization into M2a phenotype, which in turn increases fibrosis and impairs muscle regeneration (19). Mice lacking integrin β3 in macrophage lineage cells have enhanced melanoma and breast cancer growth, because of increased tumor-promoting M2 macrophages (20). In addition, ligation of integrin αVβ3 prevents macrophage differentiating into foam cells (21). In this study, we identified integrins αV and β5 as PPARγ target genes, and the heterodimer of integrin αVβ5 was involved in M2 polarization.
Results
PPARγ regulated the expression of integrin αVβ5
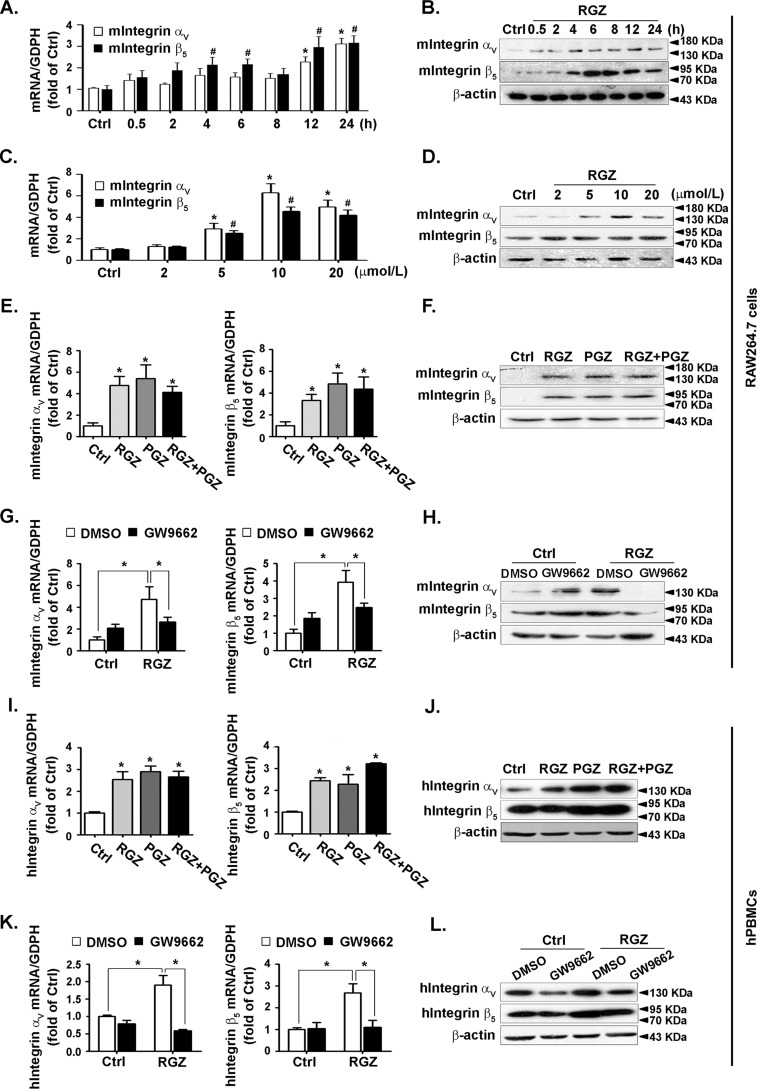
To investigate the activation of PPARγ on different integrins, RAW264.7 cells were treated with rosiglitazone, a PPARγ agonist for 24 h, the mRNA level of integrins αM, αV, α5, α6, αL, αD, αIIb, β1, β2, β3, and β5 was detected with RT–qPCR. Integrins αV, β5, and αM were increased upon rosiglitazone treatment, whereas integrins α5, α6, αL, αIIb, and β1 remained unchanged. Integrin β2 was down-regulated. Integrin β3 was undetectable (Fig. S1).
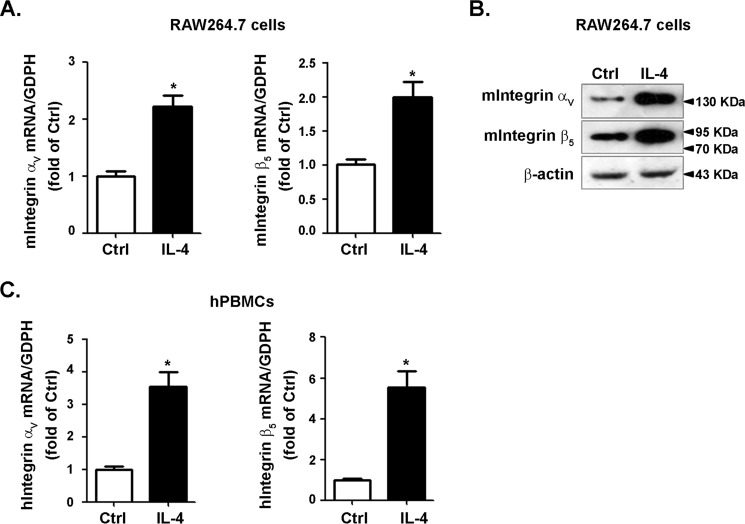
Integrins αV and β5 are the most prominently up-regulated subunits and could be potentially ligated, prompting us to evaluate their precise regulations by PPARγ. To this end, RAW264.7 cells were treated with rosiglitazone for the indicated times or with different doses. Rosiglitazone increased the expression of integrins αV and β5 in a time- (Fig. 1, A and B) and concentration-dependent manner (Fig. 1, C and D). Moreover, they were up-regulated by pioglitazone, another agonist of PPARγ (Fig. 1, E and F). Selective PPARγ antagonists GW9662 abolished the stimulatory effect of rosiglitazone on integrin αV and β5 expressions (Fig. 1, G and H), indicating that the up-regulation by rosiglitazone was PPARγ-specific. Experiments conducted in human peripheral blood monocytes (hPBMCs) confirmed that both integrins αV and β5 could be induced by rosiglitazone and pioglitazone (Fig. 1, I and J). This induction was attenuated by GW9662 (Fig. 1, K and L). Taken together, these data suggested that both integrins αV and β5 could be induced by PPARγ activation. We then tested the expression of integrins αV and β5 in IL-4-promoted M2 macrophages. We found that both integrins αV and β5 could be induced by IL-4 in RAW264.7 cells (Fig. 2, A and B) and hPBMCs (Fig. 2C), demonstrating that integrins αV and β5 might be downstream molecules during M2 polarization.
Figure 1.
Expression of integrins αV and β5 was increased by PPARγ activation. A–D, RAW264.7 cells were incubated with 10 μmol/liter rosiglitazone (RGZ) for the indicated time (A and B) or with the indicated concentrations of RGZ for 24 h (C and D). Cell lysates were analyzed for the level of integrins αV and β5 by using RT–qPCR or immunoblotting. *, p < 0.05 versus control (Ctrl; integrin αV); #, p < 0.05 versus Ctrl (integrin β5). E–J, RAW264.7 cells and hPBMCs were stimulated with RGZ (10 μmol/liter), pioglitazone (PGZ, 10 μmol/liter), or both. The integrin αV and β5 mRNA (E and I) or protein (F and J) levels were examined by RT–qPCR or immunoblotting. G–L, cells were pretreated with or without GW9662 for 1 h and then exposed to RGZ (10 μmol/liter) for 24 h. Cell lysates were analyzed to determine the mRNA (G and K) and protein (H and L) levels of integrin αV and β5. *, p < 0.05.
Figure 2.
Expression of integrins αV and β5 in IL-4–induced M2 macrophages. RAW264.7 cells and hPBMCs were stimulated with IL-4 (10 ng/ml) for 24 h. Cell lysates were analyzed for the levels of integrins αV and β5 using RT–qPCR (A and C) or immunoblotting (B). *, p < 0.05 versus control (Ctrl; integrin αV); #, p < 0.05 versus control (integrin β5).
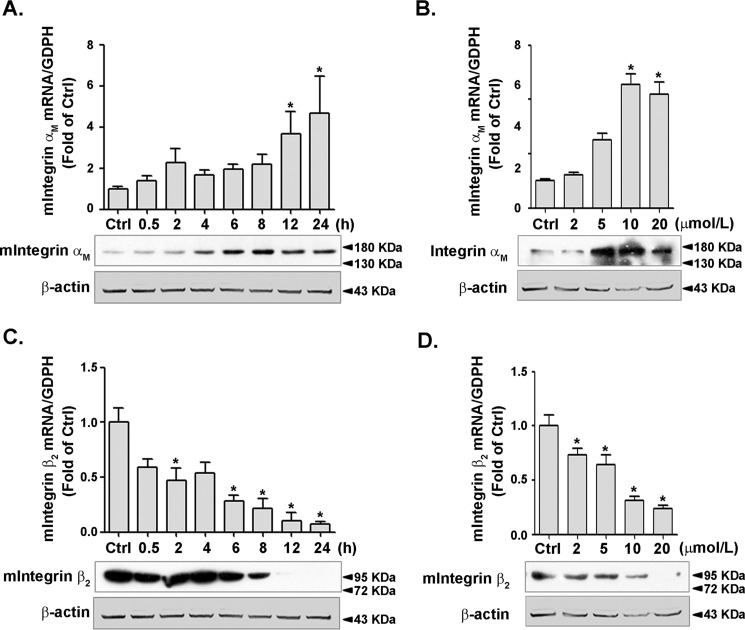
The heterodimer of integrin αMβ2 was shown to mediate the inflammatory response in macrophages. To verify whether PPARγ activation could affect the expression of integrins αM and β2, RAW264.7 cells were treated with rosiglitazone as mentioned above. Expression of integrin αM was increased in a time- (Fig. 3A) and dose-dependent manner (Fig. 3B) by rosiglitazone treatment, whereas integrin β2 expression was significantly decreased (Fig. 3, C and D). Taken together, these results indicated that PPARγ was capable of regulating the expression of integrins αM and β2 in macrophages.
Figure 3.
Expression of integrins αM and β2 was modulated by PPARγ activation. RAW264.7 cells were incubated with 10 μmol/liter RGZ for the indicated times (A and C) or with indicated concentrations of RGZ for 24 h (B and D). The cell lysates were analyzed for the level of integrin αM and β2 by using RT–qPCR or immunoblotting. Immunoblots of A and C were from the same representative experiment consecutively used to detect integrin αM, integrin β2, and β-actin. The results were separately presented, but the loading control (β-actin) was identical. The same situation applies to B and D. *, p < 0.05 versus control (Ctrl).
Integrins αV and β5 were direct target genes of PPARγ
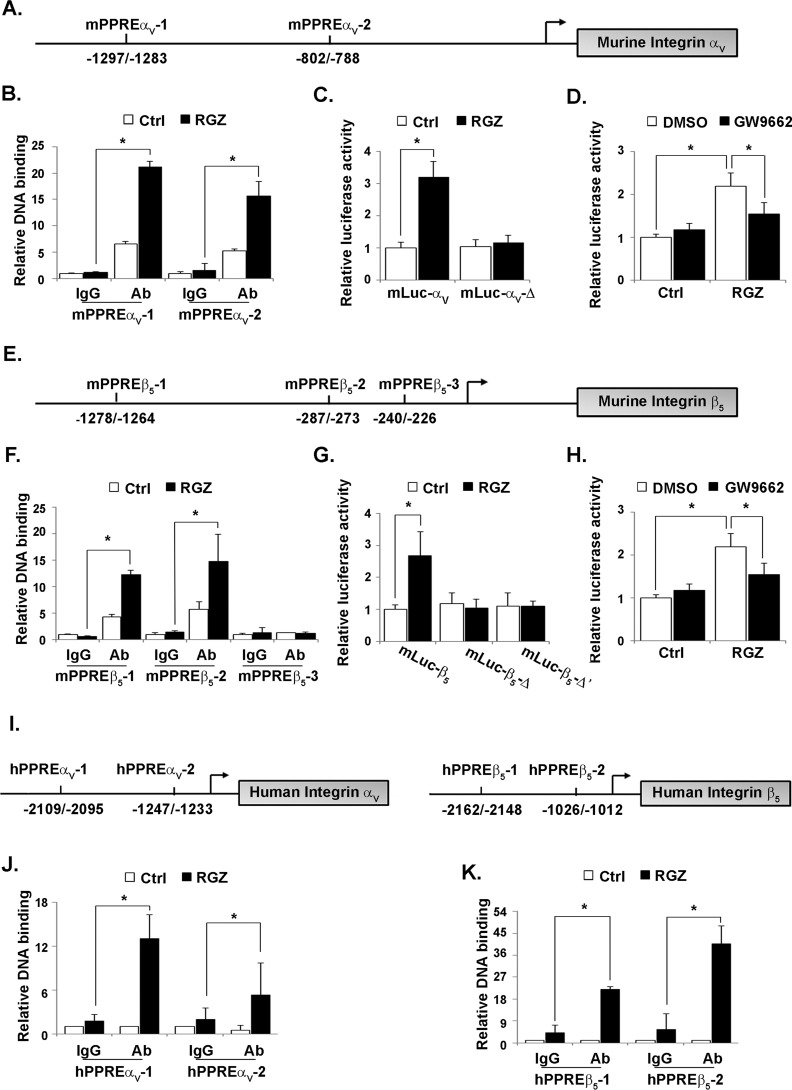
To investigate whether murine integrin αV could be targeted by PPARγ, sequence analysis was performed using PPAR GENE (22) and JASPAR Database (http://jaspar.genereg.net/).3 Integrin αV possesses two potential PPREs within the 2300-bp region upstream of murine integrin αV gene (Fig. 4A). ChIP assay was executed to examine the bindings for PPARγ to the promoter region of integrin αV. PPARγ could directly bind to integrin αV promoter at either mPPRE-αV1 (−1297/−1283) or mPPRE-αV2 (−802/−788). The binding for mPPRE-αV1 was increased with the treatment of rosiglitazone, whereas the binding for mPPRE-αV2 remained unchanged (Fig. 4B). We then created two plasmid constructs containing different upstream regions of the integrin αV promoter fused to the luciferase reporter. HEK 293 cells were treated with rosiglitazone after transfection with mLuc-αV (containing mPPRE-αV1 and mPPRE-αV2) or mLuc-αV-Δ (containing mPPRE-αV2) plasmid. Luciferase activity of mLuc-αV, but not mLuc-αV-Δ, was increased by rosiglitazone treatment (Fig. 4C), suggesting that mPPRE-αV1 could mediate the induction of integrin αV gene by PPARγ. To further confirm the role of PPARγ in integrin αV induction, mLuc-αV vector was transfected into HEK 293 cells and then treated with rosiglitazone in the presence of a PPARγ specific antagonist GW9662 (Fig. 4D). The increase of luciferase activity of mLuc-αV by rosiglitazone were prevented by GW9662, indicating that integrin αV might be a direct target gene of PPARγ.
Figure 4.
PPARγ bound and activated the integrin αV and β5 promoters. Potential PPREs located in the regions of murine integrin αV (A) and β5 (E) promoters were schematically presented. RAW264.7 cells were treated with RGZ (10 μmol/liter). The indicated murine PPREs of integrin αV (B) or β5 (F) were analyzed by ChIP assay with the use of anti-PPARγ antibody. IgG was used as an isotype control (Ctrl). HEK 293 cells were transfected with a serial of pGL3 basic vectors in which different fragments of integrin αV (C, mLuc-αV and mLuc-αV-Δ) or β5 (G, mLuc-β5, mLuc-β5-Δ, and mLuc-β5-Δ′) promoter have been cloned. After treatment with RGZ (10 μmol/liter) for 24 h, luciferase activity was measured and normalized to that of β-gal. mLuc-αV (D) or mLuc-β5 (H) transfected HEK293 cells were incubated with RGZ (10 μmol/liter) after pretreatment with GW9662 (20 μmol/liter). Luciferase activity was measured and normalized to that of β-gal. Schematic presentation of PPREs located in the regions of human integrin αV and β5 (I) promoters. hPBMCs were treated with RGZ (10 μmol/liter). The indicated human PPREs of integrin αV (J) or β5 (K) were analyzed by ChIP assay with the use of anti-PPARγ antibody. IgG was used as an isotype control. *, p < 0.05.
Similarly, the promoter region of integrin β5 possesses three PPREs (Fig. 4E). Both mPPRE-β51 (−1278/−1264) and mPPRE-β52 (−287/−273) could be bound to PPARγ, and these bindings were enhanced by rosiglitazone treatment. PPARγ did not bind to mPPRE-β53 (−240/−226) (Fig. 4F). Next, mLuc-β5 (containing mPPRE-β51, mPPRE-β52, and mPPRE-β53), mLuc-β5-Δ (containing mPPRE-β52 and mPPRE-β53), or mLuc-β5-Δ′ (containing PPRE-β53) plasmids were transfected into HEK293 cells. Luciferase activity of mLuc-β5, but not mLuc-β5-Δ or mLuc-β5-Δ′, was increased by rosiglitazone treatment (Fig. 4G), suggesting that mPPRE-β51 could mediate the induction of integrin β5 gene by rosiglitazone. Meanwhile, the increase of luciferase activity of mLuc-β5 by rosiglitazone was prevented by GW9662 (Fig. 4H), indicating that integrin β5 might be direct target gene of PPARγ as well.
In addition, we also identified cognate PPARγ motifs in the regulation region of the human integrin αV and β5 genes. By using ChIP assay, we confirmed that PPARγ could bind to the promoter regions for human integrin αV at hPPRE-αV1 (−2109/−2095) or hPPRE-αV2 (−1247/−1233) (Fig. 4I). Moreover, either the binding for hPPRE-αV1 or for hPPRE-αV2 was increased by rosiglitazone (Fig. 4J). Similarly, the functionality of PPRE in the human integrin β5 gene was also confirmed (Fig. 4K).
Ligation of integrin αVβ5 were increased by PPARγ activation
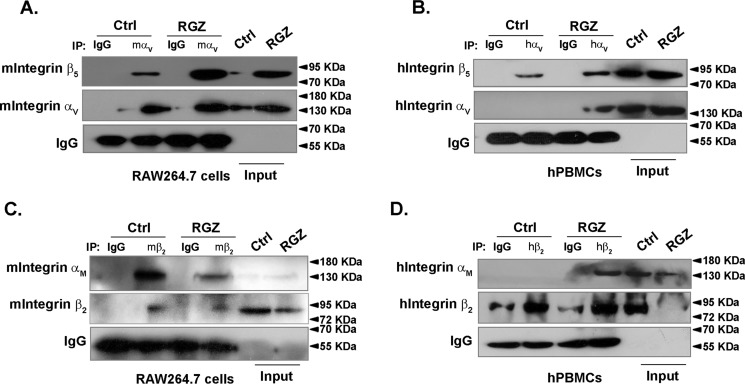
Given that integrins are obligate heterodimers, immunoprecipitation analysis was performed to examine the effect of rosiglitazone on αVβ5 ligation. Consistent with the up-regulation of integrin αV and β5 expression by rosiglitazone, their ligation was significantly enhanced in RAW264.7 cells and hPBMCs (Fig. 5, A and B). Although integrin αM was slightly increased, integrin αMβ2 ligation was attenuated when treated with rosiglitazone in RAW264.7 cells and hPBMCs (Fig. 5, C and D). These results suggested that PPARγ activation might shift the ligation of different integrins, leading to a distinct downstream signaling pathway in macrophages.
Figure 5.
Rosiglitazone increased the formation of integrin αVβ5 heterodimer. RAW264.7 cells and hPBMCs were treated with RGZ for 24 h. Immunoblotting of anti-integrin αV (A and B) or anti-integrin β2 (C and D) immunoprecipitates were performed. IgG was used as a negative control. Ctrl, control; IP, immunoprecipitation.
Integrin αVβ5-mediated rosiglitazone-induced M2 macrophage polarization
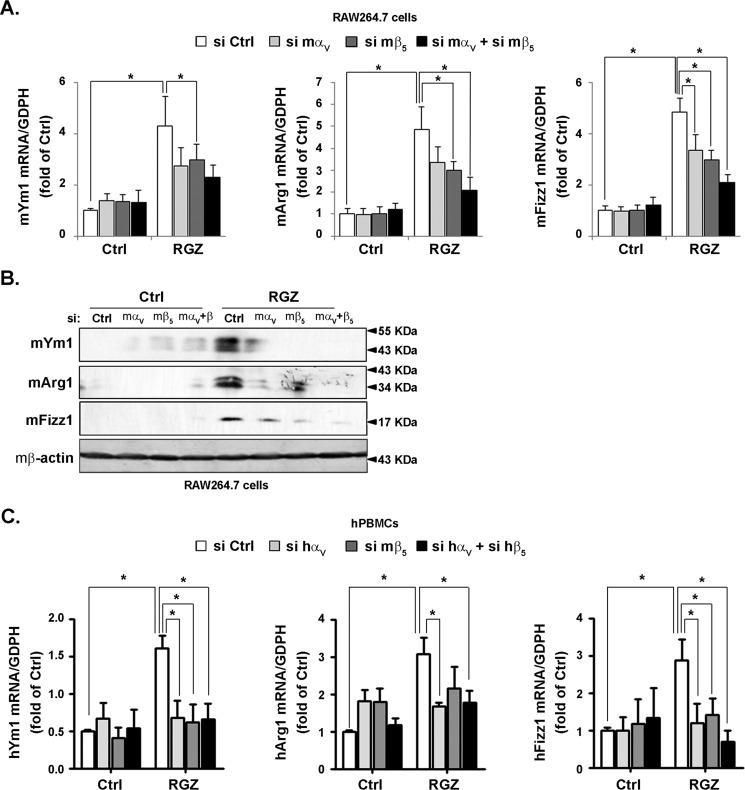
To investigate the participation of integrin αVβ5 in PPARγ-induced M2 polarization, siRNA strategy was first used following a treatment with rosiglitazone. Rosiglitazone induced mRNA and protein levels of M2 marker genes, including Ym1, Arg1, and Fizz1. Importantly, either siRNA against integrin αV or β5 attenuated the induction of M2 marker genes by rosiglitazone. Combination of siRNA against integrins αV and β5 abrogated this augmentation in RAW264.7 cells (Fig. 6, A and B) and hPBMCs (Fig. 6C). Rosiglitazone exhibited an anti-inflammatory effect in monocytes/macrophages by reducing the expression of M1 marker genes such as iNOS, TNFα, and IL-6. Neither siRNA against integrin αV/β5 nor their combination could reverse the reduced expression of iNOS, TNFα and IL-6 expressions (Fig. S2, A and B).
Figure 6.
Integrin αVβ5 knockdown abolished rosiglitazone-promoted M2 polarization. RAW264.7 cells and hPBMCs were transfected with murine and human si control (Ctrl), si αV, si β5 or si αV+β5, respectively. Then the cells were treated with RGZ (10 μmol/liter) for 24 h. The cell lysates were analyzed for the level of Ym1, Arg1, and Fizz1 by using RT–qPCR (A and C) or immunoblotting (B).
Furthermore, pharmacological blockage with cilengitide, a potent inhibitor blocking the accessibility of integrin αVβ5 to their ligands (23), effectively abolished rosiglitazone-induced Ym1, Arg1, and Fizz1 in RAW264.7 cells (Fig. 7, A and B) and hPBMCs (Fig. 7C). In contrast, cilengitide had no effect on rosiglitazone-decreased expression of M1 marker genes (Fig. S3, A and B). These data suggested that the heterodimer of integrin αVβ5 was required in PPARγ-induced M2 polarization.
Figure 7.
Cilengitide partially inhibited rosiglitazone-induced M2 polarization. RAW264.7 cells and hPBMCs were pretreated with cilengitide (Cilen, 1μmol/liter) for 30 min and then incubated with RGZ (10 μmol/liter) for 24 h. Lysates were analyzed for the level of Ym1, Arg1, and Fizz1 by using RT–qPCR (A and C) or immunoblotting (B). Ctrl, control.
Discussion
In this present study, we demonstrated a novel mechanism by which PPARγ regulates macrophage polarization via integrin αVβ5 induction. These results also provided evidence that a specific integrin heterodimer plays an important role in M2 polarization.
Integrins are important signaling receptors that mediate the interactions of the cells with extracellular matrix (24). They are involved in multiple inflammatory responses, including coronary atherosclerosis, obesity, etc. (25, 26). However, the gene regulation of specific integrin subunits in macrophages has not been well-characterized. Here we showed that PPARγ transcriptionally activated integrins αV and β5. GW9662, an antagonist of PPARγ, attenuated the up-regulation of integrins αV and β5 by rosiglitazone. It is noticed that GW9662 elevated the basal level of integrins αV and β5 by an unrecognized mechanism, which has been reported in the case of the other nuclear receptor antagonist (27). We identified murine PPRE-αV1 (−1297/−1283) and murine PPRE-β51 (−1278/−1264) as functional binding sites to trigger integrin αV and β5 transcription, respectively. Meanwhile, we found that the expression of inflammatory integrins αM and β2 was regulated by PPARγ as well.
Integrin is strictly heterodimer of α and β subunits, which are ligated by extracellular stimuli and required for its signaling transduction (24). The mechanism of the formation of integrin heterodimer has not been well-understood. The heterodimer of integrin α2β1 ligated by C1q-containing immune complexes is required for peritoneal mast cells activation during innate immunity (28). Adhesion of monocytes to the endothelium results in integrin αVβ3 ligation and prevents macrophage transition into foam cells (21). Here, we found that a signaling pathway initiated by integrin αVβ5 ligation could participate in macrophage M2 polarization. Meanwhile, ligation of integrin αMβ2, which has been implicated in inflammatory response (26), was decreased upon PPARγ activation. This is the first evidence that PPARγ switches the ligation of specific integrin subunits, leading to distinct cellular functions in macrophages.
PPARγ has been shown to function as insulin sensitizers and thus improve hyperglycemia in patients with type 2 diabetic mellitus (29). Knockdown of PPARγ in immune cells reduces insulin sensitivity by decreasing the infiltration of macrophages into white adipose tissue (30). Dominant mutations in PPARγ cause insulin resistance accompanied by early onset of severe hypertension (31, 32). The aortas from troglitazone-treated mice show decreased accumulation of macrophages in atherosclerotic lesions and attenuated expression of numerous inflammatory markers such as TNFα and iNOS (33), which is consistent with our results that the expression and ligation of inflammatory integrin αMβ2 were decreased by PPARγ activation in macrophages. Alternation of macrophage polarization and function requires precise regulation of master factors, including cytokines (IL-4 and IL-13) and transcription factors like PPARγ. PPARγ activates anti-inflammatory gene expressions, such as Arg-1 and IL-10 through the PPREs at their promoter regions (10, 13). In this study, we found that integrins αV and β5 were PPARγ target genes and necessary to PPARγ-induced M2 polarization. To the best of our knowledge, this is the first evidence implicating a specific integrin heterodimer in M2 macrophages.
Integrins are transmembrane receptors that respond to extracellular stimuli. Ligation of two integrin subunits is not sufficient to induce the downstream signaling; thus it is important to identify the ligands of integrin αVβ5 during M2 polarization, especially on the basis of physiopathological context. Integrin αVβ5 binds to a variety of extracellular matrix proteins, including osteopontin, fibronectin, vitronection, von Willebrand factor, and thrombospondin. An in vitro study demonstrated that IL-10 acts synergistically with IL-18 to amplify the production of osteopontin, thereby augmenting M2 polarization of macrophage (34). The osteopontin-generated M2 macrophages exhibit a protective effect in vascular calcification of patients with hypertension (35). Although the culture of macrophage on fibronectin-coated surface does not induce a M2 phenotype (36), the production and deposition of fibronectin are common features during M2 polarization (37, 38), which is believed to govern the remodeling process after tissue damage (39). Moreover, the downstream signaling of integrin αVβ5 in macrophages should be implemented in our further studies. It has been previously shown that the selective inhibitor of Rok2 (Rho-associated kinase 2) decreases M2-like macrophages (40). As an upstream receptor of Rho, integrin αVβ5 activation on Rok2 could explain how it participated in M2 polarization. Furthermore, because macrophages exhibit a longer shape when differentiated into M2 phenotypes (41), the effect of integrin αVβ5 on cytoskeleton rearrangement (42) could be another hypothesis to elucidate the involving mechanism. Taken together, our results suggested a novel molecular mechanism with which the induction and ligation of integrin αVβ5 participate in PPARγ-induced M2 polarization, whereas PPARγ represses the inflammation by targeting the expression and the ligation of integrin αMβ2 (Fig. 8), which may provide a potent target against inflammatory diseases.
Figure 8.
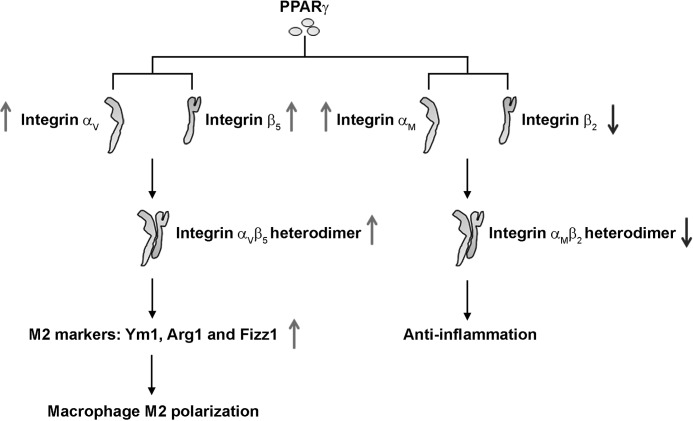
Mechanism of integrin in PPARγ-induced M2 macrophage polarization. PPARγ activates the expression of integrin αV and β5 by targeting the PPREs in their promoter regions. The formation of integrin αVβ5 heterodimer is therewith increased, leading to M2 macrophages polarization. Meanwhile, PPARγ activation regulates the expression and the ligation of integrin αM and β2, leading to a reduced inflammatory phenotype in macrophages.
Experimental procedures
Reagents
Rosiglitazone, GW9662, and recombinant IL-4 were from Sigma–Aldrich. Pioglitazone and cilengitide were from Selleck Chemicals (Houston, TX). Antibodies against Arg1, β-actin, integrin αM, and integrin β2 were from Santa Cruz Biotechnology (Dallas, TX). Antibodies against integrins αV and β5 were from Cell Signaling (Danvers, MA). Antibodies against Ym1 and Fizz1 were from Abcam (Cambridge, MA).
Cell culture, isolation, and transfection
Murine monocytic cell line RAW264.7 and HEK 293 cells were obtained from ATCC (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum in a humidified 5% CO2 atmosphere at 37 °C. Human PBMCs were isolated from healthy donors by Ficoll–Hypaque density centrifugation. After washing three times, hPBMCs were incubated in RPMI 1640 medium supplemented with 10% fetal bovine for 4 days. The experiments were approved by the institutional ethics review board of Xi'an Jiaotong University (approval XJTULAC2018-497) and performed in accordance with the National Institutes of Health guidelines.
RNA extraction and reverse transcriptase–PCR (RT–qPCR)
Total RNA was extracted from RAW264.7 cells and hPBMCs by using TRIzol (Invitrogen). Quantitative RT–qPCR was performed using the SYBR Green technique (Promega, Madison, WI). Primer sequences were described in Table S1. GAPDH was used as a housekeeping gene.
Plasmids and luciferase reporter assay
mLuc-αV (−1383/+109), mLuc-αV-Δ (−831/+109), Luc-β5 (−1346/+40), mLuc-β5-Δ (−443/+40), and mLuc-β5-Δ′ (−270/+40) plasmids were made by PCR cloning into the pGL3 basic luciferase-reporter plasmid. The cells were co-transfected with a reporter gene and a β-gal plasmid. Luciferase and the β-gal activities were measured as previously described (43).
ChIP assay
The cells were cross-linked with 0.75% formaldehyde before harvesting. Sheared chromatin was immunoprecipitated with an anti-PPARγ antibody (or control IgG) and pulled down with protein A/G–Sepharose beads (Santa Cruz). The eluted immunoprecipitates were digested with proteinase K to reverse the cross-link between DNA and proteins. DNA was extracted and subjected to PCR experiment with specific primers flanking the putative PPARγ binding motifs. Primer sequences were described in Table S2.
Immunoblotting and immunoprecipitation
Proteins were extracted in RIPA buffer supplemented with protease inhibitors. Protein concentrations were measured using the BCA protein assay. Immunoblotting was performed with appropriate primary antibodies and horseradish peroxidase-conjugated secondary antibodies followed by ECL detection. Immunoblots shown were representative of three independent experiments.
For immunoprecipitation, cell lysates were incubated with the appropriate antibodies or control IgG at 4 °C overnight followed by incubation with protein A/G-Sepharose beads. Immunoprecipitates were washed with NETN buffer (20 mm Tris, pH 8.0, 100 mm NaCl, 1 mm EDTA, and 0.5% NP-40). Immunoblots shown were representative of three independent experiments.
siRNA and transfection
RAW264.7 cells or hPBMCs were transfected with adequate species of integrin αV, integrin β5, or scrambled siRNA (si Ctrl). Sequences were described in Table S3. Experiments using these cells were executed at 24 h after transfection.
Statistical analysis
The results are reported as means ± S.D. Comparisons within and between groups were performed using analysis of variance and Mann–Whitney U test, respectively. p < 0.05 was considered significant.
Author contributions
Q. Y. and N. W. conceptualization; Q. Y., J. L., Z. Z., C. Z., and N. W. data curation; Q. Y. and Z. Z. formal analysis; Q. Y. and N. W. supervision; Q. Y., J. L., L. X., and N. W. funding acquisition; Q. Y., J. L., Z. Z., and N. W. validation; Q. Y., J. L., F.L., C. Z., and N. W. investigation; Q. Y. and N. W. visualization; Q. Y., J. L., F.L., C. Z., B.L., L. X., and N. W. methodology; Q. Y. and N. W. writing-original draft; Q. Y. and N. W. writing-review and editing; N. W. resources.
Supplementary Material
This work was supported by National Science Foundation of China Grants 81830015, 81500345, 31430045, 81470373, 81600389, and 81770497; China Postdoctoral Science Foundation General Financial Grant 2015M582674; China Postdoctoral Science Foundation Special Financial Grant 2016T90928; and a Shaanxi Postdoctoral Science Foundation general financial grant. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1–S3 and Figs. S1–S3.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- IL
- interleukin
- PPAR
- peroxisome proliferator–activated receptor
- PPRE
- potential PPAR-responsive elements
- RT–qPCR
- reverse transcriptase–polymerase chain reaction
- hPBMC
- human peripheral blood monocyte
- PPRE
- PPAR-responsive element
- STAT
- signal transducers and activators of transcription
- iNOS
- inducible nitric-oxide synthase
- TNF
- tumor necrosis factor
- RGZ
- rosiglitazone.
References
- 1. Pello O. M. (2016) Macrophages and c-Myc cross paths. Oncoimmunology 5, e1151991 10.1080/2162402X.2016.1151991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., and Tsatsanis C. (2017) Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 198, 1006–1014 10.4049/jimmunol.1601515 [DOI] [PubMed] [Google Scholar]
- 3. Covarrubias A. J., Aksoylar H. I., and Horng T. (2015) Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin. Immunol. 27, 286–296 10.1016/j.smim.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orihuela R., McPherson C. A., and Harry G. J. (2016) Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173, 649–665 10.1111/bph.13139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo X., Li T., Xu Y., Xu X., Zhu Z., Zhang Y., Xu J., Xu K., Cheng H., Zhang X., and Ke Y. (2017) Increased levels of Gab1 and Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2 macrophage-driven pulmonary fibrosis in mice. J. Biol. Chem. 292, 14003–14015 10.1074/jbc.M117.802066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steiger S., Kumar S. V., Honarpisheh M., Lorenz G., Günthner R., Romoli S., Gröbmayr R., Susanti H. E., Potempa J., Koziel J., and Lech M. (2017) Immunomodulatory molecule IRAK-M balances macrophage polarization and determines macrophage responses during renal fibrosis. J. Immunol. 199, 1440–1452 10.4049/jimmunol.1601982 [DOI] [PubMed] [Google Scholar]
- 7. Lawrence T., and Natoli G. (2011) Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 10.1038/nri3088 [DOI] [PubMed] [Google Scholar]
- 8. Delerive P., Martin-Nizard F., Chinetti G., Trottein F., Fruchart J. C., Najib J., Duriez P., and Staels B. (1999) Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ. Res. 85, 394–402 10.1161/01.RES.85.5.394 [DOI] [PubMed] [Google Scholar]
- 9. Ricote M., Li A. C., Willson T. M., Kelly C. J., and Glass C. K. (1998) The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391, 79–82 10.1038/34178 [DOI] [PubMed] [Google Scholar]
- 10. Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A. W., and Chawla A. (2007) Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 10.1038/nature05894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bermudez B., Dahl T. B., Medina I., Groeneweg M., Holm S., Montserrat-de la Paz S., Rousch M., Otten J., Herias V., Varela L. M., Ranheim T., Yndestad A., Ortega-Gomez A., Abia R., Nagy L., et al. (2017) Leukocyte overexpression of intracellular NAMPT attenuates atherosclerosis by regulating PPARγ-dependent monocyte differentiation and function. Arterioscler. Thromb. Vasc. Biol. 37, 1157–1167 10.1161/ATVBAHA.116.308187 [DOI] [PubMed] [Google Scholar]
- 12. Gliem M., Klotz L., van Rooijen N., Hartung H. P., and Jander S. (2015) hyperglycemia and PPARγ antagonistically influence macrophage polarization and infarct healing after ischemic stroke. Stroke 46, 2935–2942 10.1161/STROKEAHA.115.010557 [DOI] [PubMed] [Google Scholar]
- 13. Bouhlel M. A., Derudas B., Rigamonti E., Dièvart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., Staels B., and Chinetti-Gbaguidi G. (2007) PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143 10.1016/j.cmet.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 14. Park Y. K., and Goda Y. (2016) Integrins in synapse regulation. Nat. Rev. Neurosci. 17, 745–756 10.1038/nrn.2016.138 [DOI] [PubMed] [Google Scholar]
- 15. Rotty J. D., Brighton H. E., Craig S. L., Asokan S. B., Cheng N., Ting J. P., and Bear J. E. (2017) Arp2/3 complex is required for macrophage integrin functions but is dispensable for FcR phagocytosis and in vivo motility. Dev. Cell 42, 498–513.e6 10.1016/j.devcel.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aziz M. H., Cui K., Das M., Brown K. E., Ardell C. L., Febbraio M., Pluskota E., Han J., Wu H., Ballantyne C. M., Smith J. D., Cathcart M. K., and Yakubenko V. P. (2017) The upregulation of integrin αDβ2 (CD11d/CD18) on inflammatory macrophages promotes macrophage retention in vascular lesions and development of atherosclerosis. J. Immunol. 198, 4855–4867 10.4049/jimmunol.1602175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. St-Pierre J., Moreau F., Cornick S., Quach J., Begum S., Aracely Fernandez L., Gorman H., and Chadee K. (2017) The macrophage cytoskeleton acts as a contact sensor upon interaction with Entamoeba histolytica to trigger IL-1β secretion. PLoS Pathogens 13, e1006592 10.1371/journal.ppat.1006592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chung K. J., Chatzigeorgiou A., Economopoulou M., Garcia-Martin R., Alexaki V. I., Mitroulis I., Nati M., Gebler J., Ziemssen T., Goelz S. E., Phieler J., Lim J. H., Karalis K. P., Papayannopoulou T., Blüher M., et al. (2017) A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat. Immunol. 18, 654–664 10.1038/ni.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L., Dong Y., Dong Y., Cheng J., and Du J. (2012) Role of integrin-β3 protein in macrophage polarization and regeneration of injured muscle. J. Biol. Chem. 287, 6177–6186 10.1074/jbc.M111.292649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su X., Esser A. K., Amend S. R., Xiang J., Xu Y., Ross M. H., Fox G. C., Kobayashi T., Steri V., Roomp K., Fontana F., Hurchla M. A., Knolhoff B. L., Meyer M. A., Morgan E. A., et al. (2016) Antagonizing integrin β3 increases immunosuppression in cancer. Cancer Res. 76, 3484–3495 10.1158/0008-5472.CAN-15-2663, 10.1158/1538-7445.AM2016-3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Antonov A. S., Kolodgie F. D., Munn D. H., and Gerrity R. G. (2004) Regulation of macrophage foam cell formation by αVβ3 integrin: potential role in human atherosclerosis. Am. J. Pathol. 165, 247–258 10.1016/S0002-9440(10)63293-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fang L., Zhang M., Li Y., Liu Y., Cui Q., and Wang N. (2016) PPARgene: a database of experimentally verified and computationally predicted PPAR target genes. PPAR Res. 2016, 6042162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim K. H., Chen C. C., Alpini G., and Lau L. F. (2015) CCN1 induces hepatic ductular reaction through integrin αvβ5-mediated activation of NF-κB. J. Clin. Invest. 125, 1886–1900 10.1172/JCI79327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vitillo L., and Kimber S. J. (2017) Integrin and FAK regulation of human pluripotent stem cells. Curr. Stem Cell Rep. 3, 358–365 10.1007/s40778-017-0100-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoshiga M., Alpers C. E., Smith L. L., Giachelli C. M., and Schwartz S. M. (1995) αvβ3 integrin expression in normal and atherosclerotic artery. Circ. Res. 77, 1129–1135 10.1161/01.RES.77.6.1129 [DOI] [PubMed] [Google Scholar]
- 26. Schittenhelm L., Hilkens C. M., and Morrison V. L. (2017) β2 integrins as regulators of dendritic cell, monocyte, and macrophage function. Front. Immunol. 8, 1866 10.3389/fimmu.2017.01866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao L., Zhang Z., Luo X., Yang H., Li F., and Wang N. (2016) Retinoid acid receptor-related orphan receptor α (RORα) regulates macrophage M2 polarization via activation of AMPKα. Mol. Immunol. 80, 17–23 10.1016/j.molimm.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 28. McCall-Culbreath K. D., Li Z., and Zutter M. M. (2008) Crosstalk between the α2β1 integrin and c-met/HGF-R regulates innate immunity. Blood 111, 3562–3570 10.1182/blood-2007-08-107664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kahn C. R., Chen L., and Cohen S. E. (2000) Unraveling the mechanism of action of thiazolidinediones. J. Clin. Invest. 106, 1305–1307 10.1172/JCI11705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guri A. J., Hontecillas R., Ferrer G., Casagran O., Wankhade U., Noble A. M., Eizirik D. L., Ortis F., Cnop M., Liu D., Si H., and Bassaganya-Riera J. (2008) Loss of PPARγ in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue. J. Nutr. Biochem. 19, 216–228 10.1016/j.jnutbio.2007.02.010 [DOI] [PubMed] [Google Scholar]
- 31. Halabi C. M., Beyer A. M., de Lange W. J., Keen H. L., Baumbach G. L., Faraci F. M., and Sigmund C. D. (2008) Interference with PPARγ function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 7, 215–226 10.1016/j.cmet.2007.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ketsawatsomkron P., Pelham C. J., Groh S., Keen H. L., Faraci F. M., and Sigmund C. D. (2010) Does peroxisome proliferator-activated receptor-γ (PPARγ) protect from hypertension directly through effects in the vasculature? J. Biol. Chem. 285, 9311–9316 10.1074/jbc.R109.025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Z., Ding X., Jin S., Pitt B., Zhang L., Billiar T., and Li Q. (2016) WISP1-αvβ3 integrin signaling positively regulates TLR-triggered inflammation response in sepsis induced lung injury. Sci. Rep. 6, 28841 10.1038/srep28841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kobori T., Hamasaki S., Kitaura A., Yamazaki Y., Nishinaka T., Niwa A., Nakao S., Wake H., Mori S., Yoshino T., Nishibori M., and Takahashi H. (2018) Interleukin-18 amplifies macrophage polarization and morphological alteration, leading to excessive angiogenesis. Front. Immunol. 9, 334 10.3389/fimmu.2018.00334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ge Q., Ruan C. C., Ma Y., Tang X. F., Wu Q. H., Wang J. G., Zhu D. L., and Gao P. J. (2017) Osteopontin regulates macrophage activation and osteoclast formation in hypertensive patients with vascular calcification. Sci. Rep. 7, 40253 10.1038/srep40253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ploeger D. T., van Putten S. M., Koerts J. A., van Luyn M. J., and Harmsen M. C. (2012) Human macrophages primed with angiogenic factors show dynamic plasticity, irrespective of extracellular matrix components. Immunobiology 217, 299–306 10.1016/j.imbio.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 37. Rahal O. M., Wolfe A. R., Mandal P. K., Larson R., Tin S., Jimenez C., Zhang D., Horton J., Reuben J. M., McMurray J. S., and Woodward W. A. (2018) Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 100, 1034–1043 10.1016/j.ijrobp.2017.11.043 [DOI] [PubMed] [Google Scholar]
- 38. Wang D., Xiong M., Chen C., Du L., Liu Z., Shi Y., Zhang M., Gong J., Song X., Xiang R., Liu E., and Tan X. (2018) Legumain, an asparaginyl endopeptidase, mediates the effect of M2 macrophages on attenuating renal interstitial fibrosis in obstructive nephropathy. Kidney Int. 94, 91–101 10.1016/j.kint.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 39. Cory T. J., Birket S. E., Murphy B. S., Hayes D. Jr, Anstead M. I., Kanga J. F., Kuhn R. J., Bush H. M., and Feola D. J. (2014) Impact of azithromycin treatment on macrophage gene expression in subjects with cystic fibrosis. J. Cyst. Fibros. 13, 164–171 10.1016/j.jcf.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zandi S., Nakao S., Chun K. H., Fiorina P., Sun D., Arita R., Zhao M., Kim E., Schueller O., Campbell S., Taher M., Melhorn M. I., Schering A., Gatti F., Tezza S., et al. (2015) ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Reports 10, 1173–1186 10.1016/j.celrep.2015.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McWhorter F. Y., Wang T., Nguyen P., Chung T., and Liu W. F. (2013) Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. U.S.A. 110, 17253–17258 10.1073/pnas.1308887110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee J., Abdeen A. A., Wycislo K. L., Fan T. M., and Kilian K. A. (2016) Interfacial geometry dictates cancer cell tumorigenicity. Nat. Materials 15, 856–862 10.1038/nmat4610 [DOI] [PubMed] [Google Scholar]
- 43. Tian J., Wong W. T., Tian X. Y., Zhang P., Huang Y., and Wang N. (2010) Rosiglitazone attenuates endothelin-1-induced vasoconstriction by upregulating endothelial expression of endothelin B receptor. Hypertension 56, 129–135 10.1161/HYPERTENSIONAHA.110.150375 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.