Abstract
Background
Several studies suggest that multiple rare genetic variants in genes causing monogenic forms of neurodegenerative disorders interact synergistically to increase disease risk or reduce the age of onset, but these studies have not been validated in large sporadic case series.
Methods
We analysed 980 neuropathologically characterised human brains with Alzheimer’s disease (AD), Parkinson’s disease-dementia with Lewy bodies (PD-DLB), frontotemporal dementia-amyotrophic lateral sclerosis (FTD-ALS) and age-matched controls. Genetic variants were assessed using the American College of Medical Genetics criteria for pathogenicity. Individuals with two or more variants within a relevant disease gene panel were defined as ‘oligogenic’.
Results
The majority of oligogenic variant combinations consisted of a highly penetrant allele or known risk factor in combination with another rare but likely benign allele. The presence of oligogenic variants did not influence the age of onset or disease severity. After controlling for the single known major risk allele, the frequency of oligogenic variants was no different between cases and controls.
Conclusions
A priori, individuals with AD, PD-DLB and FTD-ALS are more likely to harbour a known genetic risk factor, and it is the burden of these variants in combination with rare benign alleles that is likely to be responsible for some oligogenic associations. Controlling for this bias is essential in studies investigating a potential role for oligogenic variation in neurodegenerative diseases.
Keywords: dementia
Background
Genetic variation in over 50 genes contributes to the risk of developing neurodegenerative diseases.1–5 Some of the known risk alleles are common in the general population, raising the possibility that multiple interacting genetic variants might enhance the risk of developing disease or modify the disease phenotype.6–10 In keeping with this, some familial cases of frontotemporal dementia-amyotrophic lateral sclerosis (FTD-ALS) appear to have a greater ‘burden’ of variants when compared with controls,11 which may explain an earlier age of onset.8 However, it is currently not clear whether this also occurs in non-familial FTD-ALS cases or in other neurodegenerative disorders, where previously reported associations could either be due to a single highly penetrant monogenic allele co-associated with benign non-functioning variants, or whether there is a genuine synergistic interaction between two or more functional genetic variants. To address this, we performed comprehensive clinical variant interpretation on exome sequence data and C9Orf72 genotypes in 980 neuropathologically characterised brains from the MRC Brain Bank Network.
Methods
We studied the following: Alzheimer’s disease (AD), n=277; FTD-ALS n=244; Parkinson’s disease-dementia with Lewy bodies (PD-DLB), n=97 and neuropathologically normal controls, n=362,12 with 97.2% of all individuals studied having no family history of a neurodegenerative disorder (online supplementary table 1). Demographic data including the age of disease onset and death, disease duration and family history of disease, together with the antemortem clinical diagnosis and postmortem neuropathological diagnosis were available (table 1).
Table 1.
Clinical and demographic data for the major cohorts within the study
| Phenotype | Number of cases | Male (number) | Female (number) | Mean age onset (years) (SD) | Mean age death (years) (SD) | Number with FH | Cases with highly penetrant allele or RF | Oligogenic cases (N (%)) | Oligogenic cases possessing a penetrant allele or RF (N (%)) | Fisher’s test (P value) |
| Control | 362 | 232 (64.1) | 130 (35.9) | N/A | 63.3 (18.8) | N/A | N/A | |||
| FTD-ALS | 244 | 143 (58.6) | 101 (41.4) | 59.4 (11.8) | 64.6 (11.7) | 14 | 33 | 19 (7.78%) | 11 (57.9%) | 0.0001 |
| AD | 277 | 131 (47.3) | 146 (52.7) | 65.4 (10.2) | 77.7 (11.7) | 11 | 36 | 6 (2.17%) | 6 (100%) | 0.0001 |
| DLB | 58 | 36 (62.1) | 22 (37.9) | 66.7 (8.4) | 76.7 (7.0) | 2 | 16 | 25 (25.78%) | 10 (62.5%) | 0.0007 |
| PD | 39 | 28 (71.8) | 11 (28.2) | 59.9 (10.9) | 72.3 (9.2) |
Oligogenic was defined by the presence of >1 variant within the relevant disease panel at <1% MAF in the Exome Aggregation Consortium database. Monogenic or cases harbouring genetic risk factors were defined as outlined in the supplementary methods.11
AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FH, family history; FTD-ALS, frontotemporal dementia-amyotrophic lateral sclerosis; MAF, Minor allele frequency; N/A, not available; PD, Parkinson’s disease.
jnnp-2017-317234supp001.pdf (1.9MB, pdf)
Exome sequencing was restricted to on-target homozygous, heterozygous and compound heterozygous variants with a minimum read depth of 10, and base quality score of 20 across the 980 subjects, where the variant allele frequency (VAF) was <5% in the Exome Aggregation Consortium (ExAC).13 Ingenuity Variant Analysis was used to study 49 genes known to be associated with neurodegenerative disorders (see online supplementary table 2). The 49 genes were subsequently grouped into six gene panels: AD panel (n=8), PD-DLB panel (n=16), full FTD-ALS panel (n=28), medium FTD-ALS panel based on that previously described3 (n=12) and a small FTD-ALS panel as previously described11 (n=5), together with the entire panel (n=49 genes). All panels were filtered for variants present at VAF <1% and <5% C9orf72 genotypes12 as stated.
Pathogenic (P) or likely pathogenic (LP) variants were defined using the American College of Medical Genetics (ACMG) criteria14 as described,12 together with known genetic risk factors. Other variants identified as benign (B), likely benign (LB) or of uncertain significance (US) based on the ACMG criteria, and the remaining variants (VAF 0.5%–5% in monogenic genes, or non-risk factor variants in risk-factor genes) were annotated as unclassified (UC). Oligogenic individuals were defined as those who had two or more non-synonymous, frameshift or stop-loss or gain-inducing point mutations in the relevant panel (as stated), or those who tested positive for the C9orf72 hexanucleotide repeat expansion plus had at least one of the point mutation within the panel.
Results
Across the entire cohort of 980 subjects, we observed a total of 57 genetic variants in the AD gene panel, 141 variants in the primary FTD-ALS gene panel and 140 in the PD-DLB gene panel (table 1). Six AD cases (2.17%) had >1 variant in the AD panel, and 19 cases (7.79%) of primary FTD-ALS had >1 variant in the primary FTD-ALS panel. These proportions were no different to control subjects (control subjects for the AD panel: 5/362, 1.38%, P=0.545; and full FTD-ALS panel: 26/362, 7.18%, P=0.14). In contrast, 23 cases of PD-DLB (23.71%) had >1 variant in PD-DLB genes, which was greater than controls (controls: 37/362, 10.22%, P=0.004, see online supplementary table 3).
Based on ACMG criteria for pathogenicity,12 only three individuals in the entire study (0.30% of n=980) harboured >1 pathogenic, likely pathogenic or known risk factor for a neurodegenerative disease. One patient with DLB (0.1% of n=980) (age of onset in 60s, and death early 70s) had a LRRK2 p.M1646T mutation associated with PD, and a TREM2 p.R62H mutation associated with AD.15 A patient with AD developing in the seventh decade of life had a PSEN2 p.L204I mutation and the TREM2 p.R62H risk factor. A third patient who had early onset PD (onset age fourth decade) due to a compound heterozygous mutation in PARK2 (p.G430D/pR275W) also had the p.R98W TREM2 possible risk factor for AD,16 but displayed no evidence of any amyloid deposition at postmortem (see online supplementary table 8,9).
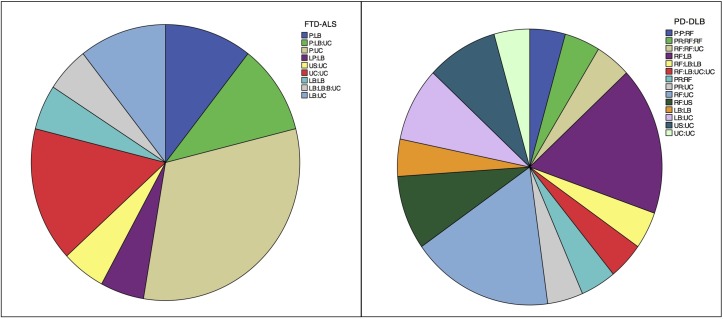
We observed a significant enrichment of highly penetrant alleles or risk factors within ‘oligogenic’ cases in all disease cohorts (see online supplementary table 4). In FTD-ALS, 11 of the 19 oligogenic cases (57.9%) contained one highly penetrant allele or risk factor within the primary panel, giving the presence of oligogenic variation a positive predictive value (PPV) to identify an individual as someone carrying a pathogenic mutation or known risk factor at 57.9% (95% CI 33.5% to 79.8%) (see online supplementary table 5). We subsequently varied the panel size to reflect published approaches,3 11 raised the MAF to 5% within each panel and removed C9orf72 data from the analysis. In all of these permutations, there was a significant over-representation of highly penetrant allele or risk factor carriers within the oligogenic cohort (figure 1, see online supplementary table 4,5). The same enrichment for highly penetrant alleles within ‘oligogenic’ cases was seen in the AD panel at 1% (PPV 100%, 95% CI 54.1% to 100.0%) and PD-DLB panel (PPV 43.5%, 95% CI 23.2% to 65.5%) (see online supplementary table 5). We subsequently combined all data to employ Bayesian mathematical modelling (see online supplementary data file 2), which showed that the presence of >1 variant within a relevant disease gene panel in an affected individual confers an 80% posterior predictive probability that they have a monogenic allele or risk factor for that disease.
Figure 1.
American College of Medical Genetics (ACMG) variant criteria for all cases within the frontotemporal dementia-amyotrophic lateral sclerosis (FTD-ALS) and Parkinson’s disease-dementia with Lewy bodies (PD-DLB) cohorts that had >1 variant within their respective disease panels at 1% MAF. The relative combination of alleles can be seen in the top right of each cohort’s chart. Key references to ACMG classification of each variant: P, pathogenic; LP, likely pathogenic; RF, risk factor; PR, pathogenic in the recessive state (but considered likely benign in the heterozygous state); LB, likely benign; US, uncertain significance; UC, uncategorised.
jnnp-2017-317234supp002.pdf (158.9KB, pdf)
We then investigated whether the enrichment of monogenic alleles or risk factors within oligogenic cases was due to a greater overall background mutation rate in these individuals as previously suggested in some genotypes of PD,17 but found no evidence of such an association (see online supplementary table 6).
Finally, we removed all cases possessing a highly penetrant allele or risk factor, and compared remaining oligogenic cases of PD-DLB and FTD-ALS with controls (n=362). Based on this analysis, there was no difference in either the proportion of ‘oligogenic’ cases or the mean pathogenicity defined by both SIFT or Polyphen2 score (see online supplementary table 7-9, figures 1−6) between any study group. We also observed no difference in the age of onset, age of death or disease duration between remaining oligogenic cases compared with those with <2 variants (see online supplementary figures 7,8), including the C9orf72 expansion in the presence of additional variants (see online supplementary figure 9).
Discussion
With ever more comprehensive panels of genetic testing in neurodegenerative disorders, the possibility of detecting more than one rare variant in an individual will become increasingly likely, posing significant diagnostic challenges and difficulties for genetic counselling. Our data show the observed frequency of ‘oligogenic’ variation is linked to the size of the gene panel and MAF threshold, ranging from 1.4% (AD panel) to 13.3% (PD-DLB panel) in both affected and unaffected individuals (see online supplementary table 3). This highlights that, while each allele is in itself rare, it is not uncommon for any individual to have more than one rare variant across a small disease gene panel. This should be borne in mind when investigating the possibility of an oligogenic mechanism, particularly given the increasing number of genes identified as causing or contributing to neurodegenerative disorders.
Why are our conclusions different to previous studies that were of a similar size? In order to be defined as ‘oligogenic’, an individual must have >1 variant in a known relevant risk gene. This introduces a systematic bias, whereby affected individuals are more likely to harbour one of these alleles than healthy aged individuals. This has been seen previously,8 where 10/18 (55.6%) of the ‘oligogenic’ cases had an established highly penetrant allele already known to cause an earlier onset disease (see online supplementary data file 1). The presence of these known risk alleles, in conjunction with a background rate of polymorphic variation, inevitably results in individuals with a known highly penetrant allele or risk factor being more likely to fall into the ‘oligogenic’ group. In keeping with this, our analysis shows that the vast majority of individuals defined as having ‘oligogenic’ variation do indeed have a known risk allele or highly penetrant variant, explaining the initial association we observed between oligogenic variants and PD-DLB. Importantly, after excluding the known major variant in individual cases there was no association between the benign oligogenic variation and neurodegenerative disease or the age of onset.
This same systematic bias will lead to the apparent enrichment of ‘oligogenic’ variants in familial cases. By being familial, these individuals are more likely to harbour a known risk genetic factor, which when combined with the background variant carrier rate, makes them more likely to be classified as oligogenic than healthy controls. Thus, a priori, being a familial case will make it more likely for an individual to have oligogenic variants. This does not necessarily mean that the additional variants are having an effect on the risk of being a familial case. Given the frequency of any individual harbouring two or more variants, and the likely diminishing impact of each variant on the phenotype and disease risk, substantially larger datasets (eg, n>10 000) will be required to definitively resolve this complex issue with robust variant pathogenicity interpretation.
Footnotes
Contributors: MJK: study concept and design, data acquisition, analysis and interpretation of data, drafting/revising the manuscript. WW: data generation and analysis. JA: data interpretation, mathematical analysis, drafting the manuscript. IW: data generation. JC: study design, coordination, data acquisition. MRT: data acquisition and interpretation. CAMcK: data acquisition and interpretation. CT: data acquisition and interpretation. JA: data acquisition and interpretation. CS: data acquisition and interpretation. SAlS: data acquisition and interpretation. CMM: data acquisition and interpretation. OA: data acquisition and interpretation. SP-B: data acquisition and interpretation. NJ: mathematical analysis and interpretation. JWI: study supervision, interpretation. PFC: study supervision, drafting the manuscript.
Funding: MJK is a Wellcome Trust Clinical Research Training Fellow (103396/Z/13/Z). PFC is a Wellcome Trust Senior Fellow in Clinical Science (101876/Z/13/Z), and a UK NIHR Senior Investigator, who receives support from the Medical Research Council Mitochondrial Biology Unit (MC_UP_1501/2) and the National Institute for Health Research Biomedical Research Centre for Ageing and Age-Related Disease award to the Newcastle upon Tyne Hospitals National Health Service Foundation Trust.
Competing interests: None declared.
Ethics approval: Approved in each centre before DNA was extracted.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data will be made publicly available through the European Genome Archive.
References
- 1.Tsuji S. Genetics of neurodegenerative diseases: insights from high-throughput resequencing. Hum Mol Genet 2010;19(R1):R65–R70. 10.1093/hmg/ddq162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerreiro R, Brás J, Hardy J. Snapshot: genetics of ALS and FTD. Cell 2015;160:798 e1 10.1016/j.cell.2015.01.052 [DOI] [PubMed] [Google Scholar]
- 3.Singleton A, Hardy J. The evolution of genetics: alzheimer’s and Parkinson’s diseases. Neuron 2016;90:1154–63. 10.1016/j.neuron.2016.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner MR, Al-Chalabi A, Chio A, et al. Genetic screening in sporadic ALS and FTD. J Neurol Neurosurg Psychiatry 2017;88:1042–4. 10.1136/jnnp-2017-315995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari R, Wang Y, Vandrovcova J, et al. Genetic architecture of sporadic frontotemporal dementia and overlap with Alzheimer’s and Parkinson’s diseases. J Neurol Neurosurg Psychiatry 2017;88:152–64. 10.1136/jnnp-2016-314411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo JF, Li K, Yu RL, et al. Polygenic determinants of Parkinson’s disease in a Chinese population. Neurobiol Aging 2015;36:1765.e1–1765.e6. 10.1016/j.neurobiolaging.2014.12.030 [DOI] [PubMed] [Google Scholar]
- 7.Escott-Price V, Sims R, Bannister C, et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain 2015;138(Pt 12):3673–84. 10.1093/brain/awv268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cady J, Allred P, Bali T, et al. Amyotrophic lateral sclerosis onset is influenced by the burden of rare variants in known amyotrophic lateral sclerosis genes. Ann Neurol 2015;77:100–13. 10.1002/ana.24306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lubbe SJ, Escott-Price V, Gibbs JR, et al. Additional rare variant analysis in Parkinson’s disease cases with and without known pathogenic mutations: evidence for oligogenic inheritance. Hum Mol Genet 2016;25:5483–9. 10.1093/hmg/ddw348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dols-Icardo O, García-Redondo A, Rojas-García R, et al. Analysis of known amyotrophic lateral sclerosis and frontotemporal dementia genes reveals a substantial genetic burden in patients manifesting both diseases not carrying the C9orf72 expansion mutation. J Neurol Neurosurg Psychiatry 2018;89:162–8. 10.1136/jnnp-2017-316820 [DOI] [PubMed] [Google Scholar]
- 11.van Blitterswijk M, van Es MA, Hennekam EA, et al. Evidence for an oligogenic basis of amyotrophic lateral sclerosis. Hum Mol Genet 2012;21:3776–84. 10.1093/hmg/dds199 [DOI] [PubMed] [Google Scholar]
- 12.Keogh MJ, Wei W, Wilson I, et al. Genetic compendium of 1511 human brains available through the UK medical research council brain banks network resource. Genome Res 2017;27:165–73. 10.1101/gr.210609.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–91. 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 2015;17:405–23. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome Res 2009;19:1553–61. 10.1101/gr.092619.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med 2013;368:117–27. 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubbe SJ, Escott-Price V, Gibbs JR, et al. Additional rare variant analysis in Parkinson’s disease cases with and without known pathogenic mutations: evidence for oligogenic inheritance. Hum Mol Genet 2016;25:ddw348 10.1093/hmg/ddw348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2017-317234supp001.pdf (1.9MB, pdf)
jnnp-2017-317234supp002.pdf (158.9KB, pdf)