Abstract
Aim
To identify biomarkers for accurate classification of glioma.
Patients and methods
We evaluated the heat shock protein 27 (Hsp27), phosphorylated Hsp27 (p-Hsp27), ATRX and IDH1R132Hproteins using immunohistochemistry in 421 glioma tissues. The χ2 test was used to assess the relationship between molecular alterations and clinico-pathological parameters. Kaplan-Meier survival curves were constructed, and differences were detected by the log-rank test.
Results
We found that Hsp27 and p-Hsp27 were mainly expressed in aggressive astrocytic gliomas. However, neither Hsp27 nor p-Hsp27 expression was related to survival time for any grade of glioma. Interestingly, p-Hsp27 was mutually exclusive with ATRX loss (ATRX−) and the IDH1R132H mutation, except for one case of anaplastic astrocytoma. We classified glioblastomas (GBMs) into three subtypes: ATRX−/IDH1R132H, high p-Hsp27 expression (p-Hsp27+) and none of these three markers. ATRX-/IDH1R132Hshowed the longest median survival (19.6 months). The prognostic difference between p-Hsp27+ and none of these three markers was significant (15.0 vs 13.1 months, P=0.045). Moreover, p-Hsp27+ predicted better sensitivity for standard therapy among GBMs without the IDH1 mutation and ATRX loss (26.3 vs 15.5 months, P=0.008).
Conclusion
p-Hsp27 is a novel biomarker of glioma and might have important clinical value for further classification of patients with wild-type IDH1 and normal ATRX expression, for evaluating prognosis and for guidance for adjuvant therapy.
Keywords: glioma, Hsp27, IDH1, ATRX, prognosis
Background
Glioma is the most common histological type of primary malignant central nervous system (CNS) tumour. Despite the use of aggressive treatment with surgery, radiation and chemotherapy, the 5-year overall survival rates for WHO grade III and IV gliomas are approximately only 25% and 5%, respectively.1 Thus, there is an urgent need to improve the clinical management of this disease.
Several molecular aberrations have shown importance for the diagnosis and classification of glioma patients.2 The ‘2016 WHO Classification of CNS Tumours’ has combined histological and molecular parameters to define various types of glioma. The mutation status of isocitrate dehydrogenase (IDH) separates diffuse gliomas into two genetically similar entities: IDH wild-type and IDH mutant.3 These two different groups of gliomas have different histopathological features, modes of progression and prognoses.4–7 Specifically, the IDH mutation frequently occurs in low-grade gliomas and secondary glioblastomas, which both grow slowly. Compared with IDH wild-type patients, patients with IDH mutations tend to have a favourable prognosis.6 8 However, prognosis and clinical progression also differ significantly within the same groups. Thus, more accurate molecular subtypes should be identified for gliomas.
Heat shock protein 27 (Hsp27) is an isoform of the heat shock protein family that is induced by heat and other chemical and physical stressors.9 Hsp27 serves as a chaperone, which modulates the activity and half-life of its targets, and is overexpressed in many types of cancer.10 In glioma cell lines, Hsp27 is involved in a malignant phenotype, including proliferation, migration and invasion.11–13 In addition, high Hsp27 expression has previously been mainly observed in astrocytic tumours with a negative correlation with the IDH1R132H mutation in grade II and III gliomas.14 Phosphorylated Hsp27 (p-Hsp27) is a small oligomer that appears to be more active and capable of interacting with substrates than Hsp27 multimers.15 16 Elevated expression of p-Hsp27 has been shown to have clinical significance in hepatocellular carcinoma and pancreatic cancer,17 18 but there are no studies on p-Hsp27 expression in glioma tissues.
Due to limited studies on Hsp27 and p-Hsp27 in gliomas and the small number of cases, the relationship between Hsp27 and p-Hsp27 expression and clinico-pathological features is unclear. Further investigation is required to determine if Hsp27 and p-Hsp27 could be used to better classify gliomas. Thus, we investigated the expression of Hsp27 and p-Hsp27, as well as the common glioma biomarkers, the IDH1R132H mutation and ATRX loss, among 421 Chinese gliomas in the present study. We found that p-Hsp27 was mutually exclusive with ATRX loss and the IDH1R132H mutation. Moreover, p-Hsp27, but not Hsp27, may predict better prognosis among glioblastomas without the IDH1 mutation and ATRX loss.
Materials and methods
Patients and tissue samples
A total of 421 adult glioma tissues were procured from the Department of Neurosurgery at the Cancer Hospital, Chinese Academy of Medical Sciences and the Department of Neurosurgery at the Sanbo Brain Hospital. Thirty samples had matched adjacent non-neoplastic tissues collected from the oedema-affected tissues surrounding the high-grade gliomas or resected in the process of obtaining deep-seated gliomas. Two independent neuropathologists diagnosed all specimens according to the WHO classification. A total of 240 men and 181 women were enrolled, ranging in age from 18 to 79, with a mean age of 48.8 years. Grade 2 gliomas accounted for 18.3% of the samples, grade 3 for 15.4% and grade 4 for 66.3%. Written informed consent for sampling and research was obtained from all patients. This study was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (No. NCC2014G-12).
Tissue microarray and immunohistochemistry
The tissue microarray was constructed as described previously.19 For 30 tumours with matched non-neoplastic tissue, three tumour tissue cores and two matched non-neoplastic tissue cores (diameter 1 mm; height 5 mm) were taken from the primary block. For those without paired non-neoplastic tissue, five tumour tissue cores were taken for each case.
Immunohistochemistry was performed as described previously.19 The following antibodies were used: anti-Hsp27 antibody (1:500, 50353, CST), anti-p-Hsp27 (Ser82) antibody (1:100, 9709T, CST), anti-ATRX antibody (1:500, ab188027, Abcam) and anti-IDH1R132H antibody (working solution, ZM0447, ZSGB-BIO).
Evaluation of immunostaining
Slides were scanned using a NanoZoomer (Hamamatsu, Japan) high-resolution scanner. Immunostaining was scored blindly with no information on clinical data. Immunostaining of Hsp27 and p-Hsp27 was evaluated based on the staining intensity and the percentage of immunoreactive cells. Staining intensity was rated as 0 (no staining), 1 (weak staining), 2 (moderate staining) or 3 (strong staining). The percentage of immunoreactive cells was graded as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) or 4 (76–100%). The total score for Hsp27 and p-Hsp27 was calculated by multiplying the intensity by the percentage of immunoreactive tumour cells. The scores for Hsp27 and p-Hsp27 were rated as weak (<4) or strong (≥4). The immuno-scores for ATRX and IDH1R132H were evaluated as previously described.6 8
Statistical analysis
All data were analysed using SPSS Statistics software (V. 22.0). The χ2 test was used to assess the relationship between molecular alterations and clinico-pathological parameters. Kaplan-Meier survival curves were constructed, and differences were detected by the log-rank test. P<0.05 was considered significant for all statistical analyses.
Results
Hsp27, p-Hsp27, ATRX and IDH1R132H proteins are associated with glioma grade
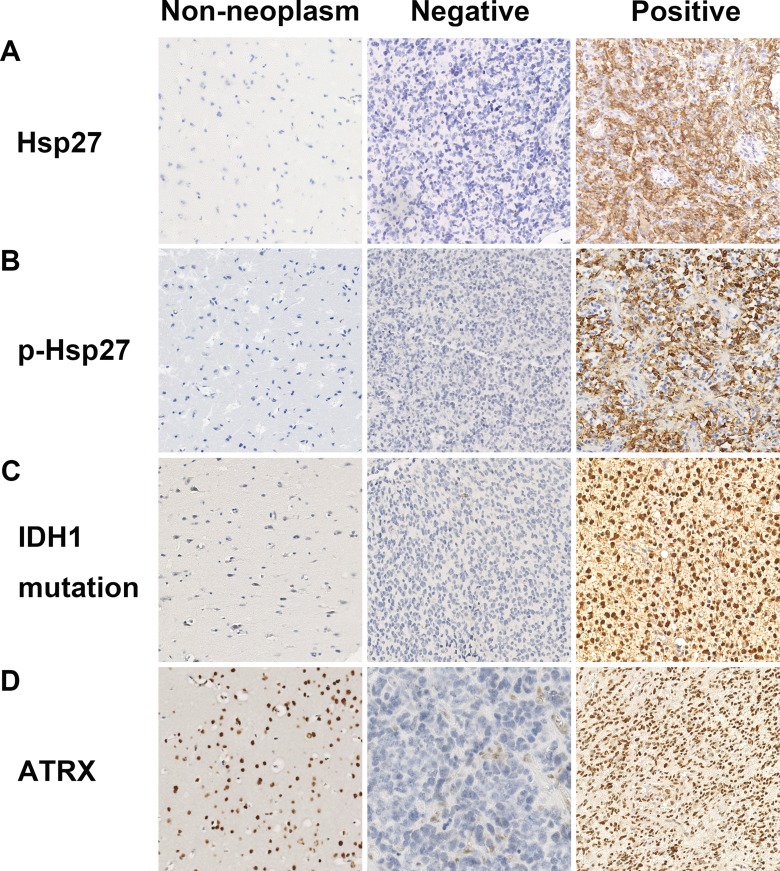
Positive immunostaining of Hsp27 and p-Hsp27 was mainly located in the cytoplasm of tumour cells. However, no Hsp27 or p-Hsp27 immunostaining was observed in non-neoplastic tissue (figure 1A and B). Strong Hsp27 expression was detected in 10.4%, 20.0% and 25.1% of grade 2, 3 and 4 tumours, respectively. Additionally, strong p-Hsp27 immunostaining was detected in 23.1% of grade 3 tumours and 23.7% of grade 4 tumours; however, only 2.6% of grade 2 tumours stained positively. A statistically significant difference was observed between expression of Hsp27 and p-Hsp27 and glioma grade (table 1; P=0.012 and P<0.001, respectively). In addition, elderly patients tended to have high Hsp27 and p-Hsp27 expression (P=0.04 and P=0.011, respectively).
Figure 1.
Representative immunohistochemistry images of Hsp27, p-Hsp27, ATRX and IDH1R132H. Hsp27, p-Hsp27 and IDH1R132H were commonly found in glioma tissue, but no immunostaining was found in non-neoplastic tissue. ATRX is normally expressed in non-neoplastic tissue and endothelial cells, but was often not expressed in glioma tissue.
Table 1.
Baseline information on selected gliomas by Hsp27, p-Hsp27, IDH1 mutation and ATRX loss
| Variable | No. | Hsp27 | High p-Hsp27 | IDH1 mutation | ATRX loss |
| Gender | |||||
| Male | 240 | 45 (18.8%) | 42 (17.5%) | 75 (31.3%) | 47 (19.6%) |
| Female | 181 | 46 (25.4%) | 41 (22.7%) | 48 (26.5%) | 36 (19.9%) |
| P value | 0.100 | 0.188 | 0.291 | 0.938 | |
| Age (years) | |||||
| ≤50 | 225 | 40 (17.8%) | 34 (15.1%) | 92 (40.9%) | 66 (29.3%) |
| >50 | 196 | 51 (26.0%) | 49 (25.0%) | 31 (15.8%) | 17 (8.7%) |
| P value | 0.040 | 0.011 | <0.001 | <0.001 | |
| KPS | |||||
| ≤60 | 87 | 20 (23.0%) | 17 (19.5%) | 25 (28.7%) | 14 (16.1%) |
| >60 | 215 | 46 (21.4%) | 46 (21.4%) | 62 (28.8%) | 49 (22.8%) |
| NA | 119 | 25 (21.0%) | 20 (16.8%) | 36 (30.3%) | 20 (16.8%) |
| P value | 0.938 | 0.600 | 0.958 | 0.267 | |
| Grade | |||||
| 2 | 77 | 8 (10.4%) | 2 (2.6%) | 32 (41.6%) | 24 (31.2%) |
| 3 | 65 | 13 (20.0%) | 15 (23.1%) | 27 (41.5%) | 16 (24.6%) |
| 4 | 279 | 70 (25.1%) | 66 (23.7%) | 64 (22.9%) | 43 (15.4%) |
| P value | 0.012 | <0.001 | <0.001 | 0.005 | |
| Pathology | |||||
| A | 52 | 6 (11.5%) | 1 (2.0%) | 17 (32.7%) | 19 (36.5%) |
| O | 16 | 2 (12.5%) | 1 (6.3%) | 8 (50.0%) | 2 (12.5%) |
| OA | 9 | 0 (0.0%) | 0 (0.0%) | 7 (77.8%) | 3 (33.3%) |
| AA | 39 | 11 (28.2%) | 12 (30.8%) | 11 (28.2%) | 11 (28.2%) |
| AO | 15 | 1 (6.7%) | 1 (6.7%) | 10 (66.7%) | 1 (6.7%) |
| AOA | 11 | 1 (9.9%) | 2 (18.2%) | 6 (54.5%) | 4 (36.4%) |
| pGBM | 233 | 67 (28.8%) | 63 (27.0%) | 32 (13.7%) | 28 (12.0%) |
| sGBM | 46 | 3 (6.5%) | 3 (6.5%) | 32 (69.6%) | 15 (32.6%) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
A, astrocytoma; AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; KPS, Karnofsky Performance Status; O, oligodendroglioma; OA, oligoastrocytoma; pGBM, primary glioblastoma; sGBM, secondary glioblastoma.
ATRX expression occurred in normal brain tissue cells. However, ATRX loss (ATRX−) was found in 19.3% of all tumours, with loss in 32.4%, 24.2% and 14.7% of grade 2, 3 and 4 tumours, respectively. Mutated IDH1R132H protein was observed in 28.4% of tumours, including 43.2% of grade 2, 40.9% of grade 3, and 21.5% of grade 4 tumours, with the highest frequency observed in oligoastrocytomas (77.8%) (figure 1C and D). Younger patients (≤50 years) presented with a higher frequency of ATRX− and the IDH1R132H mutation than older patients (>50 years) (P<0.001) (table 1).
Hsp27 and p-Hsp27 are predominately expressed in WHO grade III/IV astrocyte-originating gliomas
To further explore the significance of Hsp27 and p-Hsp27 for pathological diagnosis, we investigated the expression of both markers in all glioma types. High Hsp27 expression was found in 28.2% and 28.8% of anaplastic astrocytomas (AAs) and primary glioblastomas (pGBMs), respectively. In addition, p-Hsp27 was strongly expressed in 30.8% and 27.0% of AAs and pGBMs, respectively (table 1). However, high expression of Hsp27 and p-Hsp27 was found in 20% or less of other pathological types, especially in oligodendroglioma (O), with rates of 12.5% and 6.7%, respectively, and anaplastic oligodendroglioma (AO), with rates of 6.3% and 6.7%, respectively. The differences between the expression of the two biomarkers and histopathological types were statistically significant (P<0.001).
p-Hsp27 is mutually exclusive with ATRX- and the IDH1R132H mutation
The relationships between the four selected biomarkers among the 421 gliomas are shown in table 2 and figure 2. Expression of Hsp27 and p-Hsp27 was negatively associated with ATRX– and IDH1R132H mutation status. Moreover, almost all tumours with high p-Hsp27 expression showed neither the IDH1R132H mutation nor ATRX−, except for one AA sample with expression of the IDH1R132H protein (P<0.001). However, in tumours with ATRX−, 60.5% were accompanied by the IDH1R132H mutation. Thus, we classified tumours with the IDH1R132H mutation or ATRX- in a category called ‘ATRX–/IDH1R132H mutation’ in the following analysis.
Table 2.
Relationship between the four selected biomarkers among all gliomas
| Variable | IDH1 | P value | ATRX expression | P value | ||
| Mutant | Wild-type | Loss | Normal | |||
| Hsp27 expression | ||||||
| High | 6 | 85 | <0.001 | 3 | 88 | <0.001 |
| Low | 117 | 213 | 80 | 250 | ||
| p-HSP27 expression | ||||||
| High | 1 | 82 | <0.001 | 0 | 83 | <0.001 |
| Low | 122 | 216 | 83 | 255 | ||
Figure 2.
Distribution of the four selected biomarkers in 421 gliomas. p-Hsp27 was mutually exclusive with the IDH1R132H mutation or ATRX loss, except for one anaplastic astrocytoma sample with expression of the IDH1R132H protein. Each column represents a glioma patient.
Prognostic value of the Hsp27, p-Hsp27, ATRX and IDH1R132H proteins in glioma patients
In the present study, we acquired follow-up information for 385 of the 421 glioma patients, 285 of whom received postoperative adjuvant radiotherapy and/or chemotherapy with temozolomide (TMZ). We analysed the prognostic relevance of the studied proteins in all patients. The results showed that patients with ATRX−/IDH1R132H mutation tended to have favourable survival in both low-grade and high-grade gliomas (P=0.005 and P=0.06, respectively). However, neither Hsp27 nor p-Hsp27 expression was related to longer survival time after surgery in any grade of glioma. Even patients with high Hsp27 or p-Hsp27 expression did not benefit more from adjuvant therapy.
p-Hsp27 predicts better prognosis among glioblastomas without the IDH1 mutation or ATRX loss
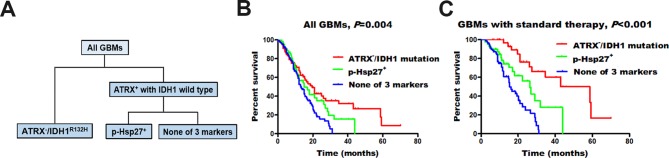
Based on the mutually exclusive relationship between p-Hsp27 and the IDH1R132H mutation or ATRX loss, we stratified glioblastomas (GBMs) into three groups: ATRX−/IDH1R132H, high p-Hsp27 expression (p-Hsp27+) and none of these three markers (figure 3A). We found that ATRX−/IDH1R132Hhad the longest median survival (19.6 months). Moreover, patients with high p-Hsp27 expression had a better prognosis than those without any alteration in the three proteins (15.0 vs 13.1 months, P=0.045; figure 3B). We further analysed the prognostic value of p-Hsp27 among GBMs with adjuvant standard therapy. The results showed that patients with high p-Hsp27 expression survived longer than patients with low p-Hsp27 expression among the 159 IDH1 wild-type individuals without ATRX loss (none of these three markers) (26.3 vs 15.5 months, P=0.008; figure 3C), which demonstrates that gliomas with high p-Hsp27 expression were more sensitive to standard management with chemo-radiation. In contrast, Hsp27 was not related to the prognosis of GBMs regardless of the status of IDH1 and ATRX.
Figure 3.
Molecular classification of glioblastomas (GBMs). We separated GBMs into three groups: ATRX−/IDH1R132H, high p-Hsp27 expression (p-Hsp27+) and none of these three markers. Individuals with ATRX−/IDH1R132Hshowed the longest median survival, those with high p-Hsp27 expression had an intermediate prognosis, and those without any alteration in the three proteins had the poorest survival rates.
Discussion
Heat shock proteins (Hsps) are a widespread and diverse class of molecular chaperones which are upregulated in response to endogenous and exogenous stress, including heat and other chemical and physical stressors.20 Many studies have evaluated the clinical significance and biological mechanisms of Hsps, including Hsp27, in cancers.10 21 Similar to previous studies,14 22 we found that Hsp27 was highly expressed in some gliomas, but the positive rate was low. Possible reasons are differences in the genetic background of the selected glioma patients and discrepancies in immunostaining evaluation standards. In addition, related studies demonstrated that Hsp27 was positively associated with high-grade, astrocyte-originating gliomas and negatively related to ATRX loss and the IDH1 mutation, which suggests that Hsp27 is an indicator of glioma origin and progression. However, studies have shown that Hsp27 is not useful as a prognostic factor for all grades of gliomas. Thus, further analysis of Hsp27 and inter-related aberrations of Hsp27 in glioma subtypes would be helpful for investigating the clinical value of Hsp27.
Phosphorylated Hsp27 loses its amino-terminal interactions, resulting in a shift from large dimers to small oligomers, thereby gaining some novel activities.23 24 In the present study, p-Hsp27 had a similar association with high-grade, astrocyte-originating gliomas with ATRX loss and the IDH1 mutation. More importantly, we found that p-Hsp27 (but not Hsp27) was mutually exclusive with ATRX loss and the IDH1R132H mutation in aggressive astrocytic gliomas. ATRX interacts with DAXX and results in the formation of a histone chaperone complex, which plays a critical role in the stabilisation of chromosomes.25 Any aberrations of ATRX and DAXX may disrupt the ATRX/DAXX complex in cancer cells. Interestingly, p-Hsp27 can interact with DAXX and blocks DAXX-mediated activity in vivo.26 Thus, p-Hsp27 might have the ability to induce chromosome instability, as do aberrations of ATRX and DAXX in cancer cells. As previous studies reported, IDH mutations could confer intrinsic double-strand break repair defects in cancer cells due to overproduction of 2-hydroxyglutarate.27 The observation that p-Hsp27 is mutually exclusive with ATRX loss and the IDH1R132H mutation was consistent with their shared function of impairing the stability of chromosomes. Further studies may interrogate the shared mechanisms and relationship between p-Hsp27 and ATRX loss and the IDH1 mutation.
We tried to classify GBMs into three subtypes according to mutually exclusive associations between p-Hsp27, ATRX loss and the IDH1R132H mutation. ATRX−/IDH1R132H cases presented with longer survival among GBM patients receiving standard therapy. In fact, an increasing number of studies have demonstrated that patients with the IDH mutation or ATRX loss are more sensitive to radiation and the DNA-damaging agent TMZ.28 29 Although p-Hsp27 was not related to overall survival time in high-grade gliomas, patients with high p-Hsp27 levels tended to survive longer than patients with low p-Hsp27 expression and normal ATRX expression and IDH1 wild-type, which indicated that p-Hsp27 was more sensitive to radiation and TMZ chemotherapy. These results all indicated that p-Hsp27 might have great clinical value in molecular classification, evaluation of prognoses and guidance for adjuvant therapy.
Several studies have demonstrated that cancer cells with the IDH1 mutation or ATRX loss are sensitive to poly (ADP ribose)-polymerase (PARP) inhibitors and ATR inhibitors in vitro.27 30 ATRX−/IDH1R132H cases may benefit more from PARP inhibitors and/or ATR inhibitors, in addition to standard therapy. Notably, two inhibitors, SB203580 and KRIBB3, demonstrated an ability to downregulate the phosphorylation of Hsp27 in glioma cell lines.31 Thus, these inhibitors may be effective for the treatment of gliomas with high p-Hsp27 expression. For patients without alteration in these three proteins, more studies may be required to explore potential targets and inhibitors.
Conclusions
The present study demonstrated that p-Hsp27 is a novel biomarker for glioma and has a mutually exclusive relationship with the IDH1R132H mutation and ATRX loss. Additionally, p-Hsp27 may predict better prognosis among glioblastomas without the IDH1 mutation and ATRX loss. In summary, p-Hsp27 may have great clinical value in molecular classification, evaluation of prognoses and guidance for adjuvant therapy.
Take home messages.
Hsp27 and p-Hsp27 are positively related to glioma grade and predominately expressed in WHO grade III/IV astrocyte-originating gliomas.
Expression of Hsp27 and p-Hsp27 is negatively associated with ATRX loss and IDH1R132H mutation status.
p-Hsp27, but not Hsp27, is mutually exclusive with ATRX loss and the IDH1R132H mutation.
Glioblastomas were classified into three subtypes with significantly different overall survival times: ATRX-/IDH1R132H, high p-Hsp27 expression (p-Hsp27+) and none of these three markers.
jclinpath-2018-205000supp001.pdf (107.9KB, pdf)
Footnotes
Handling editor: Runjan Chetty.
Contributors: J-HW, M-RW and C-XY designed the study, analyzed the data, and revised the manuscript. H-QC designed the study, performed the experiments, analyzed the data and drafted the manuscript. P-FW contributed materials and performed the experiments. H-PZ, Z-JC, S-WL, JH, YZ and J-JH contributed materials, collected clinical information, designed the experiments and analyzed the data.
Funding: This study was supported by the National Natural Science Foundation of China (81470112) and the CAMS Innovation Fund for Medical Sciences (2016-I2M-1-001).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: This study was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (No. NCC2014G-12). Each patient provided informed consent for their tissue to be used for scientific research.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Chien LN, Gittleman H, Ostrom QT, et al. Comparative brain and central nervous system tumor incidence and survival between the United States and Taiwan based on population-based registry. Front Public Health 2016;4:151 10.3389/fpubh.2016.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reifenberger G, Wirsching HG, Knobbe-Thomsen CB, et al. Advances in the molecular genetics of gliomas - implications for classification and therapy. Nat Rev Clin Oncol 2017;14:434–52. 10.1038/nrclinonc.2016.204 [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016;131:803–20. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 4. Hattori N, Hirose Y, Sasaki H, et al. World Health Organization grade II-III astrocytomas consist of genetically distinct tumor lineages. Cancer Sci 2016;107:1159–64. 10.1111/cas.12969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohba S, Mukherjee J, Johannessen TC, et al. Mutant IDH1 expression drives TERT promoter reactivation as part of the cellular transformation process. Cancer Res 2016;76:6680–9. 10.1158/0008-5472.CAN-16-0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Yu T, Gong J, et al. IDH1/2 gene hotspot mutations in central nervous system tumours: analysis of 922 Chinese patients. Pathology 2016;48:675–83. 10.1016/j.pathol.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 7. Bai H, Harmancı AS, Erson-Omay EZ, et al. Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat Genet 2016;48:59–66. 10.1038/ng.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ebrahimi A, Skardelly M, Bonzheim I, et al. ATRX immunostaining predicts IDH and H3F3A status in gliomas. Acta Neuropathol Commun 2016;4:60 10.1186/s40478-016-0331-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet 1988;22:631–77. 10.1146/annurev.ge.22.120188.003215 [DOI] [PubMed] [Google Scholar]
- 10. Ciocca DR, Arrigo AP, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch Toxicol 2013;87:19–48. 10.1007/s00204-012-0918-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golembieski WA, Thomas SL, Schultz CR, et al. HSP27 mediates SPARC-induced changes in glioma morphology, migration, and invasion. Glia 2008;56:1061–75. 10.1002/glia.20679 [DOI] [PubMed] [Google Scholar]
- 12. Castro GN, Cayado-Gutiérrez N, Zoppino FC, et al. Effects of temozolomide (TMZ) on the expression and interaction of heat shock proteins (HSPs) and DNA repair proteins in human malignant glioma cells. Cell Stress Chaperones 2015;20:253–65. 10.1007/s12192-014-0537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ye H, Huang H, Cao F, et al. HSPB1 enhances SIRT2-mediated G6PD activation and promotes glioma cell proliferation. PLoS One 2016;11:e0164285 10.1371/journal.pone.0164285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mäkelä KS, Haapasalo JA, Ilvesaro JM, et al. Hsp27 and its expression pattern in diffusely infiltrating astrocytomas. Histol Histopathol 2014;29:1161–8. doi:10.14670/HH-29.1161 [DOI] [PubMed] [Google Scholar]
- 15. Mounier N, Arrigo A-P. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 2002;7:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knapinska AM, Gratacós FM, Krause CD, et al. Chaperone Hsp27 modulates AUF1 proteolysis and AU-rich element-mediated mRNA degradation. Mol Cell Biol 2011;31:1419–31. 10.1128/MCB.00907-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eto D, Hisaka T, Horiuchi H, et al. Expression of HSP27 in hepatocellular carcinoma. Anticancer Res 2016;36:3775–9. [PubMed] [Google Scholar]
- 18. Okuno M, Yasuda I, Adachi S, et al. The significance of phosphorylated heat shock protein 27 on the prognosis of pancreatic cancer. Oncotarget 2016;7:14291–9. doi:10.18632/oncotarget.7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng YB, Lin DC, Shi ZZ, et al. Overexpression of PLK1 is associated with poor survival by inhibiting apoptosis via enhancement of survivin level in esophageal squamous cell carcinoma. Int J Cancer 2009;124:578–88. 10.1002/ijc.23990 [DOI] [PubMed] [Google Scholar]
- 20. Carra S, Alberti S, Arrigo PA, et al. The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperones 2017;22:601–11. 10.1007/s12192-017-0787-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu J, Liu T, Rios Z, et al. Heat shock proteins and cancer. Trends Pharmacol Sci 2017;38:226–56. 10.1016/j.tips.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 22. Gimenez M, Marie SK, Oba-Shinjo S, et al. Quantitative proteomic analysis shows differentially expressed HSPB1 in glioblastoma as a discriminating short from long survival factor and NOVA1 as a differentiation factor between low-grade astrocytoma and oligodendroglioma. BMC Cancer 2015;15:481 10.1186/s12885-015-1473-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paul C, Simon S, Gibert B, et al. Dynamic processes that reflect anti-apoptotic strategies set up by HspB1 (Hsp27). Exp Cell Res 2010;316:1535–52. 10.1016/j.yexcr.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 24. Rogalla T, Ehrnsperger M, Preville X, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor α by phosphorylation. J Biol Chem 1999;274:18947–56. 10.1074/jbc.274.27.18947 [DOI] [PubMed] [Google Scholar]
- 25. Goldberg AD, Banaszynski LA, Noh KM, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 2010;140:678–91. 10.1016/j.cell.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Charette SJ, Lavoie JN, Lambert H, et al. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 2000;20:7602–12. 10.1128/MCB.20.20.7602-7612.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sulkowski PL, Corso CD, Robinson ND, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med 2017;9:eaal2463 10.1126/scitranslmed.aal2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tran AN, Lai A, Li S, et al. Increased sensitivity to radiochemotherapy in IDH1 mutant glioblastoma as demonstrated by serial quantitative MR volumetry. Neuro Oncol 2014;16:414–20. 10.1093/neuonc/not198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol 2014;32:783–90. 10.1200/JCO.2013.49.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koschmann C, Calinescu AA, Nunez FJ, et al. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci Transl Med 2016;8:328ra28 10.1126/scitranslmed.aac8228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li J, Hu W, Lan Q. The apoptosis-resistance in t-AUCB-treated glioblastoma cells depends on activation of Hsp27. J Neurooncol 2012;110:187–94. 10.1007/s11060-012-0963-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jclinpath-2018-205000supp001.pdf (107.9KB, pdf)