ABSTRACT
Both acute and prolonged cold exposure affect cardiovascular responses, which may be modified by an underlying cardiovascular disease. In addition, exercise in a cold environment increases cardiovascular strain further, but its effects among persons with cardiovascular diseases are not well known. Controlled studies employing whole-body or local cold exposure demonstrate comparable or augmented increase in cardiac workload, but aggravated cutaneous vasoconstriction in persons with mild hypertension. A strong sympathetic stimulation of a cold pressor test, increases cardiac workload in persons with coronary artery disease (CAD), but does not markedly differ from those with less severe disease or healthy. However, cold exposure reduces myocardial oxygen supply in CAD, which may lead to ischemia. Exercise in cold often augments cardiac workload in persons with CAD more than when performed in thermoneutral conditions. At the same time, reduced myocardial perfusion may lead to earlier ischemia, angina and impaired performance. Also having a heart failure deteriorates submaximal and maximal performance in the cold. Antianginal medication is beneficial in the cold in lowering blood pressure, but does not affect the magnitude of cold-related cardiovascular responses in hypertension. Similarly, the use of blood pressure lowering medication improves exercise performance in cold both among persons with CAD and heart failure. Both the acute and seasonal effects of cold and added with exercise may contribute to the higher morbidity and mortality of those with cardiovascular diseases. Yet, more controlled studies for understanding the pathophysiological mechanisms behind the adverse cold-related health effects are warranted.
KEYWORDS: cardiovascular diseases, hypertension, coronary artery disease, heart failure, low temperature, exercise, cardiac workload, myocardial oxygen supply
Abbreviations
- AIx
Augmentation index
- BRS
Baroreflex sensitivity
- CPT
Cold pressor test
- CHF
Congestive/chronic heart failure
- CAD
Coronary artery disease
- CBF
Coronary blood flow
- DBP
Diastolic blood pressure
- HR
Heart rate
- HTN
Hypertension
- MBF
Myocardial blood flow
- RPP
Rate pressure product
- SCD
Sudden cardiac death
- SBP
Systolic blood pressure
- TN
Thermoneutral
Introduction
Globally a higher occurrence of cardiovascular morbidity and mortality during the cold season [1–4], or in association with prolonged periods of unusually low temperatures (cold spells) have been documented [5,6]. The adverse cold-related health effects are often related to cardiovascular causes. Hence, wintertime is associated with a higher amount of cardiac symptoms (angina, arrhythmias or dyspnoea) [7] and health events such as hypertensive crisis, deep venous thrombosis, pulmonary embolism, aortic ruptures/dissection, stroke, intracerebral hemorrhage, heart failure (HF), atrial fibrillation, ventricular arrhythmias, angina pectoris, acute myocardial infarctions (AMI) and sudden cardiac deaths (SCD) [2,4,6,8]. Both global [9] and national [10,11] studies have shown that cold-related mortality outnumber the harmful effects of heat. In contrast with the general misconception that adverse health outcomes are mainly observed with cold extremes, the majority of the temperature-related mortality occurs already at milder non-optimal temperatures [9].
Either acute lowering of temperature, or its seasonal effects increases cardiovascular strain in healthy persons through physiological responses targeted to maintain heat balance. However, these may be aggravated in persons with cardiovascular diseases involving altered nervous system, cardiac and circulatory function [2]. Both cold exposure and exercise separately augment cardiovascular strain. Studies from healthy persons suggest that cardiovascular responses are further potentiated during exercise in a cold compared with a warm environment [12]. Having a cardiovascular disease may further aggravate these responses, but has not been comprehensively studied. The higher cardiovascular strain while exercising in the cold may contribute to the adverse health events among those with cardiovascular diseases [13,14]. For example, sudden or intense exercise, such as snow shoveling [15–18] or winter sports [19] increase the risk of AMI's. Previous controlled research examining the acute effects of cold alone, and in combination with exercise, among persons with cardiovascular diseases are scant.
The current review summarizes the available evidence from controlled studies investigating the single and combined effects of cold and exercise on cardiovascular responses among persons with cardiovascular diseases. The included studies fulfil the criteria of being a controlled study, involve patients having hypertension (HTN), coronary artery disease (CAD) and heart failure (HF), include some form of exercise (dynamic, isometric) and apply different types of cold exposure (whole-body, local). Results from studies employing the cold pressor test (CPT) are also presented in comparison to other cold stimuli that elicit thermoregulatory responses. Finally, the aim is also to present possible connections to epidemiological studies demonstrating higher wintertime cardiovascular morbidity and mortality.
Acute effects of cold on cardiovascular responses in healthy persons
The type of exposure humans are subjected to include cold air with our without wind, immersion in water, or contact with cold objects. The fundamental factors affecting body heat loss and cardiovascular responses in cold are the exposure itself (type and intensity), the employed physical activity and protection through clothing insulation. In addition, various individual factors, such as ageing, hydration and nutrition status, anthropometry, body composition, previous adaptation, fitness, health status and used medication contributes to the acute responses [20]. Depending on the type of exposure, different forms of cooling occur, which may include whole-body cooling of skin (cold chamber experiments, water-perfusion suits), deeper body areas, or be restricted locally to the facial region (e.g. external application of cold to the front head), hands (cold pressor test) or the respiratory tract (e.g. inhalation of cold air while exercising in the cold). Accordingly, varying cardiovascular responses occur and which are presented below in brief concerning with healthy persons.
Blood pressure
Lowering of skin temperature during cold exposure elicits a reflex activation of sympathetically mediated vasoconstriction [21,22]. This causes vasomotor adjustments mediated by increased sympathetic nerve activity [23] and which results in vasoconstriction of both the peripheral and visceral arteries [24]. Vasoconstriction occurs both in response to reflex and local cooling of skin, as well as a decrease in core temperature [20]. As a result of the higher peripheral resistance systolic (SBP) and diastolic (DBP) blood pressure increase. This occurs with skin cooling involving the whole-body [25–28], face [29–31], local skin areas [27], whole-body cooling excluding the head [32,33], as well as cold air inhalation [34]. These previous studies have demonstrated increases of 5–30 mmHg in SBP and 5–15 mmHg in DBP.
In addition to brachial BP also the non-invasively assessed central aortic BP increases with facial [35,36], skin cooling [32], or whole-body cold exposure [37]. The increase is either comparable [32] or higher [35,37] than the brachial BP response. Ageing results in a greater pressor response towards skin surface cooling and may be mediated by increased levels of central arterial stiffness [32]. The studies assessing central hemodynamics have also reported a robust increase in augmentation index (AIx), an index of arterial stiffness and wave reflection [32,35,38]. In addition, pulse wave velocity, another index of arterial stiffness, increase with whole-body skin or facial cooling [32,37] and especially with aging [32].
Heart rate
Heart rate (HR) responses depend on the type of cold exposure, but are not generally altered much with whole-body cold exposure [39]. Only with the cold pressor test [27,34,40,41] or cold air inhalation [34] is an increase in HR observed which is related to strong activation of the sympathetic nervous system. However, with whole body cooling of skin in cold air or water, and depending on whether the face is exposed or not, a parallel activation of both the sympathetic and parasympathetic nerve branches may occur. Hence, whole-body skin cooling including facial exposure results in either decreased [25,27,28,38,42,43] or unaltered [24,26,44,45] HR. Also skin surface cooling without facial cold exposure demonstrated either reduced [46,47] or no effect on HR [32,33]. Application of cold to the face (ice-packs), on the other hand, stimulates the trigeminal nerve and evokes a non-baroreflex mediated vagal response resembling the diving reflex [29]. Hence, many experimental studies applying facial cooling report reduced HR [25,27,29–31,48,49].
Myocardial work and oxygen supply
Whole-body cold exposure at rest increase both cardiac pre- and afterload, but with no marked changes on inotropy (ventricular contractibility), HR or cardiac output [39]. Furthermore, greater increases in preload and afterload during cooling in older adults contribute to a more considerable increase in the indices of myocardial oxygen demand [46]. The rate pressure product (RPP) is the double product of SBP and HR and considered a surrogate marker for myocardial oxygen demand [13]. Studies involving simultaneous increases in SBP and HR, such as with the cold pressor test [41,50,51] or cold air inhalation [34] show higher RPP as a result of cold stimulation. Also, increased SBP with marginally altered HR may slightly increase myocardial oxygen demand during whole body cold exposure [38] and which is further elevated with ageing [46].
As a result of the higher myocardial oxygen demand, it would be expected to observe an increase in coronary blood flow (CBF) in response to cold [13]. This autoregulation ensures maintaining coronary blood flow at steady state. In fact, the sympathetically driven vasodilation of coronary resistance vessels results in increased myocardial blood flow in response to a cold pressor test [41,52–55]. Especially the structurally and functionally normal coronary arteries dilate in response to this stimulus [55]. In contrast, cold air inhalation impairs the coronary supply-to-demand ratio compared with inhalation of neutral temperature air in healthy persons [34,50]. The combined effects of cold air inhalation and isometric exercise further potentiates RPP and results in less coronary hyperemia [34,50]. It is suggested that in healthy persons cold exposure and isometric exercise influence efferent control of coronary blood flow. In addition, β-adrenergic vasodilation exerts a significant role in coronary regulation when the myocardial oxygen demand is increased [56].
Cardiovascular responses and cold exposure in persons with cardiovascular diseases
Despite of a wealth of evidence demonstrating a link between low ambient temperature and adverse cardiovascular health effects, and this association being especially strong among those with cardiovascular diseases [2], there are only few controlled studies, which examine the possible pathophysiological responses. In this context, evidence is available concerning with hypertension, coronary artery disease and one study of heart failure patients (Table 1). A large share of these have used the cold pressor test for cardiovascular stimulation [40,51,55,57–60]. The reminder of the research included whole-body (with or without facial exposure) skin cooling [38,42,43,61–65], local cooling of skin [66] or face [36], cold water immersion in association with sauna bathing [67], or brief intense whole-body cold exposures (cryostimulation) [68,69]. In the majority, but not all [36,40,62,63,65,70] of the studies cardiovascular responses were compared with healthy controls. Studies examining the effect of medication on cold-related cardiovascular responses often included only a patient group receiving different treatments. The majority of the studied subjects with cardiovascular diseases were middle-aged (mean ca. 50 yrs.), but a few studies examined early disease development and also involved younger (20–35 yrs.) subjects [57,59,63,65,71].
Table 1.
Cold and cardiovascular responses in CVD obtained from controlled (n = 27) studies.
| Subjects | Cold exposure | Measured CV parameters | Results | Conclusion | |
|---|---|---|---|---|---|
| WHOLE-BODY COLD EXPOSURE | |||||
| Greaney et al. (2017) [76] | 7 males and 6 females with HTN (mean 58 yrs.) and 6 normotensive males and 4 females (mean 53 yrs.) | Whole-body cold exposure, water-perfused suit with aim to decrease Tsk to 30 °C | HR, BP, MSNA, BRS | MAP response to skin cooling exaggerated in HTN. MSNA activity (burst frequency and incidence) and BRS higher in HTN than NTN. No effects on HR. | Hypertension alters sympathetic function during whole-body cold exposure, as judged by the higher BP and MSNA response and increased BRS. |
| Greaney et al. (2017) [61] | 6 males and 8 females with HTN (mean 56 yrs.) and 7 males and 7 females, normotensive (mean 55 yrs.) | Whole-body cold exposure, water-perfused suit with aim to decrease Tsk to 30 °C | HR, BP, MAP, SSNA, CVC | Cooling increased SSNA and reduced skin blood flow in HTN more than NTN. Non-adrenergic sympathetic co-transmitters mediated the vasoconstrictor response to cold in HTN. | Hypertension increases the peripheral cutaneous vasoconstrictor response to cold via greater increases in skin sympathetic outflow coupled with an increased reliance on non-adrenergic neurotransmitters. |
| Missmann et al. (2016) [68] | 23 subjects (8 female, 15 male, 35–69 yrs), divided to treated HTN (n = 5), untreated HTN (n = 5) and normotensives (n = 13) | Cryotherapy/repeated exposures (21 times over 3–4 wks. period) to −110 °C for 3 min each time | SBP, DBP, MAP measured before and after exposure | BP increased modestly following cryotherapy in all groups, no changes in BP over time | Cryotherapy was safe with respect to unwanted BP alterations for adult subjects below 70 yrs. regardless of a pre-existing untreated mild or pharmacologically treated arterial hypertension. |
| Radtke et al (2016) [67] | 37 males, 12 with CHF (62 yrs.), 13 with CAD (61 yrs.) and 12 controls (61 yrs.). | Exposure to Finnish sauna followed by a head-out cold water immersion (CWI) | CO, HR, BP, HRV | CO and HR increased in Sauna and CWI in all patients, but not for CAD after sauna. SBP decreased in sauna and increased in CWI in all groups. No change in HF/LF-ratio in CHF patients. | Finnish sauna and cold-water immersion causes haemodynamic alterations in heart failure patients similarly to control subjects and did not provoke an excessive increase in adrenergic activity or complex arrhythmias. |
| Hintsala et al. (2016) [42] | 24 men with untreated HTN and 17 controls (55–65 yrs.) | Whole body/facial cold exposure (−10 °C) for 15 min, 30 min follow-up | ECG and continuous BP for computing spectral powers of SBP and HRV | Cold exposure resulted in comparable increases in BRS in the HTN and control groups. Instead, a larger increase in LF blood pressure variability occurred in controls. | Untreated HTN does not disturb cold-related cardiovascular protective mechanisms. Blunted response of the peripheral sympathetic modulation may indicate higher tonic sympathetic activity and decreased sympathetic responsiveness to cold in hypertension. |
| Zalewski et al. (2014) [69] | 13 men with HTN (mean 30 yrs.) and 26 normotensive controls (mean 33 yrs.) | Whole-body/cryotherapy (−115 °C to −125 °C, 3 min), measurements before and after, supine position + tilt test | HR, BP, BRS, CO, SV, EDI, TPR, TFC, + cardiac and contractibility parameters (IC, LVET, PEP, ER) | Decreased HR, SBP (HTN group), ER, increased SV, EDI, TFC, LVET, PEP and BRS, mostly similar responses among both groups | Centralization of circulation, increased myocardial preload and venous return in both groups. Less pronounced changes in cardiac contractibility in HTN. |
| Hintsala et al. (2014) [43] | 51 untreated hypertensive and 32 normotensive controls (55–65 yrs.) | Whole body/facial cold exposure (−10 °C) for 15 min, 30 min follow-up | Conduction times and amplitudes, vectorcardiography, arrhythmias, HRV, ectopic beats | Higher T-peak to T-end interval, T-wave amplitude and shortened QTc interval in both groups. LH and HF HRV increased and LF/HF ratio decreased. The amount of ventricular ectopic beats increased slightly during cold exposure. | Altered ventricular repolarization was detected which may be due to altered cardiac autonomic regulation and was unaffected by untreated hypertension. |
| Hintsala et al. (2014) [38] | 41 untreated hypertensive men and 20 normotensive controls (55–65 yrs.) | Whole body/facial cold exposure (−10 °C) for 15 min, 30 min follow-up | Central aortic blood pressure, AIx, and SEVR measured by radial artery applanation tonometry. | Cold exposure increased the central aortic blood pressure similarly both in both hypertensive men. AIx increased by 12%, whereas SEVR decreased by 10% in both hypertensive and control subjects. | Short-term cold exposure increases central aortic blood pressure. Cardiac workload, and myocardial oxygen demand increases slightly in relation to blood supply in untreated hypertensive men. |
| Seifert et al. (2013) [62] | 53 treated hypertensive subjects (23 males, 30 females, mean 57 yrs.) | Cold chamber, −5 °C for 1 h, winter clothing, with or without protective mask (heat and moisture exchanger) | HR, BP | Increased SBP and MAP in cold, lesser increase with the use of HME mask. | Use of HME mask attenuated the increase in BP in cold and especially in persons above 60 yrs. |
| Komulainen et al. (2004 a) [63] | 7 subjects with borderline HTN (30 ± 9 yrs.) | Whole-body cold exposure to −15 °C for 15 min x three times, testing of metoprolol (beta-blocking agent) | SBP, DBP, MAP, HR | increased SBP, DBP, MAP, decreased HR in both therapy and placebo group. | Metoprolol lowered BP, but did not affect the cold-induced response. |
| Komulainen et al. (2004 b) [64] | 6 subjects (4 females, 2 males, mean 28 yrs.) | Whole-body cold exposure to −15 °C for 15 min x three times, testing of hydrochlorothiazide, HCTZ (diuretic) compared with placebo | SBP, DBP, MAP, HR, PP | SBP, DBP, MAP increased, HR decreased in both the therapy and placebo group. HCTZ did not change CV responses before, during or after cold exposure. | HCTZ did not affect CV responses during whole-body cold exposure in mild HTN. |
| Komulainen et al. (2000) [65] | 10 subjects (2 females, 8 males, 27 ± 8 yrs.) with HTN and 12 normotensive controls (5 females, 7 males, 24 ± 3 yrs.) | Whole-body cold exposure to −15 °C for 15 min x three times, testing of carvedilol (combined alpha- and beta blocking agent) compared with placebo | SBP, DBP, HR, MAP, PP | SBP, DBP and MAP increased and HR decreased in cold. Cardevilol reduces BP in both groups compared with placebo. | Carvedilol reduced BP in both HTN and normotensive subjects, but did not affect the cold-induced rise in BP. |
| LOCAL COLD EXPOSURE | |||||
| Prodel et al. (2017) [36] | 6 males with HTN (mean 51 yrs.) | Icepack for face cooling for 3 min, supine position | HR, BP, MAP, MSNA, aortic pressure waveform parameters (AIx, Tr, AG, Ew) | Higher peripheral and central MAP and sympathetic activity. AIx, AG and EW increased and TR decreased with sympathetic stimulation. | Trigeminal nerve stimulation with facial cooling increases cardiac workload (higher BP, augmentation pressure, AIx) in subjects with HTN simultaneously with increased sympathetic activation. |
| Smith et al. (2013) [66] | 9 subjects of pre- to stage 1 HTN (5 men, 4 women, 50 ± 1 yrs.) and 11 controls (51 ± 1 yrs.) | Local cooling of forearm skin (clamped to 24 °C). Inhibition of ROCK (Rho/Rho-Kinase and adrenoceptors while cooling. | Cutaneous vascular conductance, Rho/Rho-Kinase (ROCK), skin blood flow | Pre to 1-stage HTN subjects demonstrated higher ROCK activity in early and later phases of skin cooling. | The magnitude of vasoconstriction does not differ between HTN and controls, but ROCK activity increases. Functional vasoconstriction is more dependent upon Rho/ROCK mechanisms in HTN. |
| Neill et al. (1974) [84] | total 25 persons (23 males, 2 females, mean 49 yrs); 19 persons with CAD, 6 with chest pain | Application of cold to forehead. Responses compared with pacing tachycardia. | HR, SBP, DBP, CBF, MVO2 | Cutaneous cold increased systemic arterial and left ventricular end-diastolic BP. CBF increased in proportion to increased MVO2. Angina and myocardial ischemia in some patients. Cold intolerance did not alter systemic or coronary circulatory responses. | Myocardial hypoxia is related to restricted coronary reserve, rather than to an effect of cold. |
| COLD PRESSOR TEST | |||||
| Matthews et al. (2017) [57] | 16 (mean 20 yrs.) women with family history of HTN and 16 (mean 20 yrs.) without family history of HTN | Cold pressor test (cold water for 2 min) | SBP, DBP, MAP, HR CO, TPR | Increased MAP, SBP, DBP, HR and CO, but no effect of family history. | Family history of HTN did not affect CV responses to CPT. |
| Jarvis et al (2015) [40] | 19 mildly hypertensive subjects (females and males 50:50, 66–68 yrs.) | Cold pressor test (water 2–4 °C for 2 min), use of Alisikern (renin inhibitor) or Hydrocholorthiazide (diuretic) for 6 mo., pre-post assessment | HR (ECG), BP, MSNA, hemodynamics | Increased HR, SBP, DBP and MSNA in CPT. Cardiovascular and MSNA responses were similar between pre- and post- treatment. | Antihypertensive medication lowered BP, but did not lower responsiveness to CPT. |
| Greaney et al. (2015) [71] | 14 females with positive history of HTN (22 ± 1 yrs.) and 14 females without history of HTN (22 ± 1 yrs.) | Cold pressor test for 2 min, followed by isometric handgrip | HR, SBP,DBP, MSNA | CPT increased DBP more in those with positive history of HTN; handgrip resulted in more pronounced increase in SBP and DBP, and post-exercise ischemia. MSNA increased more with positive history of HTN. | Reactivity (BP and MSNA response), to stressors increases among young women with a positive history of hypertension. |
| Park et al. (2005) [88] | 14 subjects with vasospastic angina (f:m 5:9, 37–67 yrs.) and 15 controls (f:m 4:11, 23–70 yrs.) | Cold pressor test for 2 min | HR, SBP,DBP, RPP, coronary flow reserve, coronary vascular resistance | Decrease of coronary flow reserve and increased in resistance. | Endothelial dependent vasodilation is impaired at the coronary microvascular and epicardial artery level in those with vasospastic angina. |
| Kudaiberdieva et al. (2003) [70] | 25 men with CAD (50.8 ± 8.1 yrs.) and previous MI. Patients with (n = 10) or without ischemia (n = 15) | Cold pressor test (ice water for 2 min) | HR, SBP, DBP, ST-depression, MBF | Higher SBP, DBP but not HR after CPT. Reduced ESVI and EF%. Patients with ischemia in cold had lower E, prolonged IVRT, shortened DtE, and higher IVRT+ICT/ET index. | Cold pressor test-related vasoconstriction and ischemia was associated with deranged left ventricular myocardial performance manifested as delayed myocardial relaxation, impaired stiffness and contractibility. |
| Sevre & Rostrup (1999) [58] | 20 patients with chest pain, 10 with confirmed CAD (3 females, 7 males, 57 ± 3 yrs.) and 10 without (2 females, 8 males, 52 ± 4 yrs.) | Cold pressor test (0 °C for 1 min), supine | BP, HR | Larger response in SBP and HR in CAD patients with chest pain compared with those with only chest pain. | Higher cardiovascular reactivity in persons with CAD than those with chest pain, but no diagnosis of CAD. |
| Lafleche et al. (1998) [59] | 10 subjects with borderline HTN (8 males, 2 females, mean 33 yrs.) and ten controls (7 males, 2 females, mean 35 yrs.) | Cold pressor test (4 °C for 5 min) | Carotid-brachial pulse-pressure amplification and changes in brachial and carotid distensibility | Increased BP but unaltered HR, HTN does not alter responses. Disappearance of PP amplification due to higher increase in carotid PP. Decrease in carotid aortic distensibility in HTN. | CPT reduces pulse pressure amplification. In subjects with HTN the increase in PP contributed to decreased carotid aortic distensibility. |
| Benetos & Safar (1991) [60] | 73 patients with HTN (24 females, 49 males, 45 ± 1 yrs.) and 49 normotensive (17 females, 32 males, 42 ± 2 yrs.) | Cold pressor test (4–5 °C water for 90 sec.) | SBP,DBP, MBP, HR | Aggravated BP response in HTN to CPT. Slightly higher (ns.) HR response in HTN. Active renin was positively related to mean BP increase during CPT in HTN. | Increased BP response in HTN reflects an age-independent sympathetic overactivity. |
| Zeiher et al. (1989) [51] | 32 patients (17 females, 15 males, 45.2 yrs.) without CAD and 55 patients (10 females, 45 males, 54.3 yrs.) with CAD | Cold pressor test (ice water for 90 sec.) | HR, SBP RPP, CBF | The dilation of normal and the constriction of atherosclerotic coronary arteries with CPT. | Endothelial dysfunction in coronary atherosclerosis resulted in a loss of normal dilator function and permitted vasoconstrictor responses to sympathetic stimulation. |
| Nabel et al. (1988) [54] | 9 patients with normal coronary arteries (5 males, 3 females, mean 37 yrs.), 9 with mild atherosclerosis (six males, three women, mean 48 yrs.), 13 with advanced stenosis 10 males, 3 females, mean 59 yrs.) | Cold pressor test (ice water for 90 sec.) | HR, BP, CBF velocity, β-blockade studies, quantitative coronary angiography | The majority of coronary arteries dilated in those with normal arteries, but constrict more often with advancing stenosis. CBF increased by 65% in those with normal arteries, 15% in mild atherosclerosis, but decreased by 39% with advanced stenosis. | Normal coronary arteries dilate in response to the cold pressor test, in part related to beta-adrenoreceptor stimulation and possibly flow-mediated endothelial dilation or alpha 2-adrenergic activity. The vasoconstrictor response in atherosclerosis may relate to altered catecholamine sensitivity and/or a defect in endothelial vasodilator function. |
| Rodger et al. (1984) [85] | 12 patients with CAD (3 females, 9 males, 46 ± 7 yrs.), 9 subjects with chest pain (5 females, 4 males, 45 ± 6 yrs.) and 10 controls (10 men, 27 ± 5 yrs.) | Cold pressor test (both hands immersed in ice water for 2 min) | HR, BP, thallium scintigraphy | Increased HR, BP and RPP, but no differences between groups. Transient perfusion defects in response to CPT were observed in some persons from all study groups. | Cold can provoke transient abnormalities in myocardial perfusion not only in persons with coronary heart diseases but also in healthy subjects. |
| Mudge et al. (1976) [55] | 12 patient with CAD (9 males, 3 females, 47 ± 1 yrs.) and 12 controls (6 males and 6 females, 46 ± 2 yrs.), | Cold pressor test (cold water for 1 min) | Cardiac catheterization (assessment of BP and CBF) | BP increases to similar extent in CAD and controls. Increased coronary resistance and decreased BF in CAD. Application of phentolamine (adrenergic blocking agent) eliminated the increase in coronary vascular resistance. | Higher coronary vascular resistance and reduced BF during the cold pressor test in patients with CAD. |
Abbreviations: AIx = augmentation index), AG = augmented pressure of reflected wave, BP = blood pressure, BRS = baroreflex sensitivity, CAD = coronary artery disease, CHF = chronic heart failure, CO = cardiac output, CPT = cold pressor test, CVC = cutaneous vascular conductance, CWI = cold water immersion, DBP = diastolic blood pressure, EDI = end diastolic index, EW = wasted left ventricle pressure, ER = ejection rate, HF = high frequency spectral power, HTN = hypertension, HR = heart rate, HRV = heart rate variability, LF = low frequency spectral power, LVET = left ventricular ejection time, MAP = mean arterial pressure, MBF = myocardial blood flow, MSNA = muscle sympathetic nerve activity, NTN = normotensive, PEP = pre-ejection period, ROCK = Rho/Rho-Kinase, RPP = rate pressure product, SBP = systolic blood pressure, SEVR = subendocardial viability ratio, SSNA = skin sympathetic nerve activity, SV = stroke volume, Tr = systolic duration of reflected wave, TFC = thoracic fluid content, TPR = total peripheral resistance.
Hypertension
Hypertension appears globally and the age-standardized prevalence of raised BP was 24% of men and 20% of women [72]. The major pathophysiologic mechanism includes changes in renal (e.g. excess sodium intake), neural (e.g. sympathetic over activity), vascular (endothelial dysfunction, vascular remodeling) and hormonal (activation of the renin-angiontensin-aldosterone system, RAAS) function [73]. The consequences of hypertension may include both subtle end-organ damage, as well as serious events, such as stroke, MI, renal failure and dementia. Both the acute cold-induced rise and sustained higher BP during the cold season could further modify cardiovascular dysregulation of hypertension and increase the risk of cold-related cardiovascular events [4,74] (Figure 1).
Figure 1.
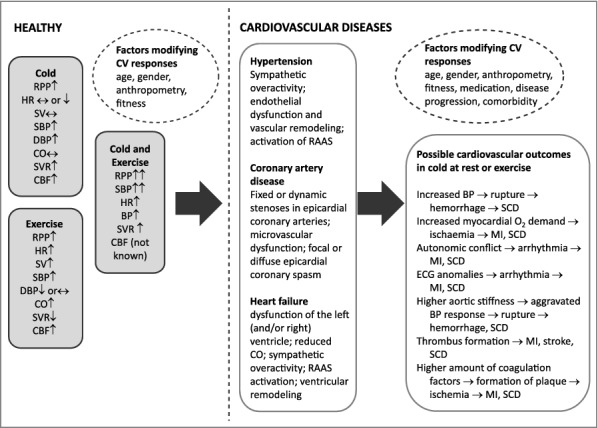
Effects of cold and exercise and their combination on cardiovascular responses in healthy subjects [13,39]. In addition, potential mechanisms explaining cardiovascular events [2,4,83] in both healthy and those with hypertension [73], coronary artery disease [80] and heart failure [91] are presented. Abbreviations: CBF = coronary blood flow, CO = cardiac output, DBP = diastolic blood pressure, HR = heart rate, MI = myocardial infarction, RAAS = renin-angiotensin system, RPP = rate pressure product, SBP = systolic blood pressure, SCD = sudden cardiac death, SV = stroke volume, SVR = systemic vascular resistance.
Whole body cold exposure
One could speculate that hypertension would result in exaggerated cardiovascular responses related to acute cold exposure resulting from the impaired autonomic cardiovascular control, and characteristic sympathetic over activity. Hypertension also relates to increased arterial stiffness [73,75], as well as endothelial dysfunction which contribute to the basal vascular tone, but possibly also to the cold-related pressor response. Furthermore, ageing associated with hypertension may itself contribute to the increased central arterial stiffness and pressor response [32]. Indeed, Greaney et al. [76] demonstrated a greater increase in BP, as well as muscle sympathetic nerve activity (MSNA) among hypertensive compared with normotensive controls. Alongside these findings they investigated baroreflex sensitivity (BRS), which is a dominant short-term control mechanism for BP and may be impaired in hypertension [73]. Their study detected cold-related increase in BRS among hypertensive, but not normotensive subjects [76] and related their findings to functional buffering of cooling-induced increases in BP. The results further showed an aggravated increase in skin sympathetic nervous activity (SSNA) and peripheral cutaneous vasoconstrictor response to skin cooling among hypertensive compared with normotensive subjects [61]. Interestingly, differences in cutaneous adrenergic sensitivity did not contribute to the observed exaggerated vasoconstriction. Hence, the authors suggested this to occur via greater increases in skin sympathetic outflow coupled with an increased reliance on non-adrenergic neurotransmitters [61]. The previously mentioned studies have been performed employing whole-body cooling of large skin areas (water perfusion suit), but excluding the head.
In contrast, studies employing whole-body cooling that includes facial cooling, and where larger skin areas are protected with winter clothing, have detected comparable cardiovascular responses between hypertensive and normotensive subjects [38,42,43,63,65]. This form of cold exposure involves autonomic co-activation, as judged by the simultaneously increased BP and heart rate variability and decreased HR both among untreated [38] and treated [63,65] hypertensive compared with normotensive subjects. In addition, it was demonstrated that hypertension do not modify the cold-related increase in augmentation index (surrogate marker of aortic stiffness), or decrease in subendocardial viability ratio (an index of myocardial oxygen supply/demand-ratio) [38]. In contrast with previous findings [76], BRS increased to comparable amounts among untreated hyper- and normotensive subjects suggesting that cardioprotective mechanisms are preserved in the earlier stages of the disease [40]. Exposure to whole-body cryotherapy among hypertensive subjects supported this finding [69]. Finally, when examining cardiac electrical function cold-related ventricular repolarization was observed probably due to simultaneous co-activation of the parasympathetic and sympathetic nervous system, but unaffected by hypertension [40].
The use of antianginal medication in response to whole body cold exposure in hypertensive subjects has been reported in a few controlled studies. Interestingly, although the use of beta-blockers, combined alpha- and beta-blockers, as well as diuretics were effective in lowering the BP level, they did not affect the magnitude of the pressor response during whole-body cold exposure [63,65].
Also protective equipment and clothing may affect BP responses in cold. The use of a protective mask with a heating and humidifying properties mitigated the increase in BP especially among ageing hypertensive subjects (above 60 yrs.) [62]. An earlier study with healthy subjects showed similarly that proper head protection reduces cold-related increase in BP [77]. These findings are promising from the perspective of appropriate protection in cold environments of those with hypertension and possible other CVD's.
Cryotherapy is a very specific form of whole-body cold exposure and used in association with treating rheumatic disorders. It involves exposure of large skin areas to extreme cold for a brief period. Hypertension has also been examined in this context by a few studies. However, their study design does not allow distinguishing acute cold-related cardiovascular responses [68,69].
Local cold exposure
Only a few studies have examined how hypertension alters cardiovascular responses in response to local cooling. Smith et al. [66] showed that cooling of the forearm (clamped to 24 °C) among pre- or mildly hypertensive subjects did not affect the magnitude of vasoconstriction compared with controls. However, Rho/Rho-Kinase (ROCK) activity, which is thought to be secondary to endothelial dysfunction related vasoconstriction, increased among persons with hypertension. Also adrenergic-dependent vasoconstriction was upregulated suggesting an interaction between these pathways for maintaining basal vasomotor tone [66].
Another study applying facial cooling (trigeminal nerve stimulation) showed elevated cardiac workload among hypertensive subjects, as judged by augmented peripheral mean arterial pressure, sympathetic activity and altered central hemodynamics (e.g. increased augmentation pressure and AIx) [36]. HR did not change in response to face cooling among hypertensive persons as opposed to studies where it reduced among healthy persons [25,27,29–31,48,49]. However, a limitation of the study was that it did not include normotensive controls for comparison.
Cold pressor test
Compared to the previous cold stimuli eliciting thermoregulatory responses, the cold pressor test represents a generalized sympathoexcitatory stimulus. Similarly as healthy persons, also hypertensive subjects demonstrate an overall increase in mean arterial pressure, SBP, DBP MSNA and HR during the cold pressor test [40,57,60,71]. In addition, equally to whole-body cold exposure [61,76], a higher responsiveness to the cold pressor test has been detected among hypertensive compared with normotensive subjects [60].
Hypertension may alter central arterial responses detected as higher pulse wave velocity [75,78] compared with controls. The question of pulse pressure amplification and distensibility of brachial and carotid arteries were compared between borderline hypertensive and normotensive subjects in response to a cold pressure test [59]. The study showed that pulse pressure amplification tended to disappear in both groups because of earlier return of the carotid wave reflections and an increase in carotid pulse pressure. Arterial distensibility decreased, but only at the site of the carotid artery among hypertensive subject. This suggest that not only blood pressure level, but also intrinsic properties of arterial walls affect compliance in hypertension.
Jarvis et al. [40] examined the role of antihypertensive medication (renin inhibitors and diuretics) in response to the cold pressor test. Equally to the findings from studies employing whole-body cold exposure [63–65], antihypertensive medication lowered BP level, but not responsiveness to the cold pressor test in mild hypertension. The study did not allow a possibility to compare cardiovascular responses with normotensive subjects.
Family history of hypertension may predispose to later cardiovascular diseases and has shown higher reactivity to the cold pressor test. Two studies examining sympathetic reactivity (but with no special focus on cold-related responses) showed either a greater BP response [71] or no effect [57] among young women. Though the latter observed aggravated responses at the onset of isometric exercise among those with family history of hypertension.
In summary, hypertension results in aggravated [61,76] or comparable [38,42,43,63,65] increase in cardiovascular strain during whole-body cold exposure compared with controls. Local cooling suggest upregulation of pathways related to endothelial dysfunction of the microcirculation in essential hypertension. The variation in responses may partially depend on whether cooling of the head is involved or not, but also be related to differences in study populations (e.g. diseases progression, use of medication) and protocols. The higher cardiac workload observed in cold is primarily due to the increase in sympathetic activity and its effect on vasoconstriction and augmented BP [76]. The higher baseline BP together with its cold response among hypertensive subjects may more often involve an increased risk of cardiovascular events. For example, cold exposure affecting mainly the face increased SBP momentarily above 200 mmHg among untreated mildly hypertensive patients [38]. Antihypertensive medication lowers BP levels, but do not affect responsiveness to cold during whole body cooling [63–65] or the cold pressor test [40]. Appropriate protection in the cold may reduce the pressor response.
Coronary artery disease
Coronary artery disease, also known as ischemic heart disease, is a group of diseases that includes stable angina, unstable angina and myocardial infarction [79]. Stable CAD is characterized by episodes of reversible myocardial demand/supply mismatch related to ischemia and often inducible by exercise or other stressors [80]. CAD occurs when part of the smooth, elastic lining inside a coronary artery develops atherosclerosis. The artery's lining becomes hardened, stiffened, and swollen with calcium and fatty deposits, as well as abnormal inflammatory cells—to form a plaque. The limitation of blood flow to the heart causes ischemia, which may lead to myocardial infarction, cardiac muscle damage, tissue death and scarring. As cold exposure increases cardiovascular strain, this may pose an additional burden to an ischemic heart disease. Hence, it is well documented that the winter months are associated with worsening of angina symptoms [81] and a higher frequency of myocardial infarctions [4,8,82] and sudden cardiac deaths [6,83].
Cold pressor test and local cooling
There is a lack of evidence related to whole-body cold exposure-related cardiovascular responses of persons with CAD or angina (Table 1). All of the previous research have had a clinical focus and employed the cold pressor test [51,54,58,70] and only one utilized the cold face test [84]. Whether CAD aggravates cardiovascular responses related to the cold pressor test is controversial. Mudge et al. [55] showed comparable BP increase in response to the cold pressor test among persons with CAD and controls. Consistent with this finding Zeiher et al. [51] did not detect differences in hemodynamic responses in CAD patients with atherosclerosis compared with those having structurally normal arteries. Similarly, patients with CAD and those demonstrating only exertional chest pain, did not differ from each other in cardiovascular responses [84,85]. On the other hand, a combination of diagnosed CAD and chest pain demonstrated higher cardiovascular response towards the cold pressor test compared with those having only chest pain [58].
Despite the fact that the presence of CAD, or its severity, do not markedly impact the increase in cardiovascular strain in response to the cold pressor test, there are indications that myocardial oxygen supply is weakened [13]. Coronary artery stenosis results in a diminished flow-mediated vasodilatory response to cold. The endothelial dysfunction related to atherosclerosis attenuates endothelial-mediated vasodilation [51,86]. Earlier studies have shown that while normal coronary arteries dilate in response to the cold pressor test (as a result of coronary autoregulation), the diseased ones constrict [51,54]. Already Mudge et al. [55] demonstrated a higher coronary vascular resistance and reduced coronary blood flow in response to the cold pressor test. Persons with exertional angina showed increased coronary resistance in response to CPT especially in abnormally perfused left ventricular regions [87]. Even those with vasospastic angina (chest pain at rest, smooth muscle contractile disorder), demonstrated a blunted increase in coronary flow reserve during cold stimulation [88].
Reduced myocardial cold-related vasodilation prevents increasing coronary blood flow in response to the higher cardiac oxygen demand. This mismatch between oxygen demand and supply may lead to myocardial ischemia [13]. One indication of this are reports of angina in response to the cold pressor test [84]. Interestingly, although inhalation of cold air (−20 °C) resulted in angina in some CAD patients, this was not related to higher myocardial oxygen consumption or altered coronary blood flow [89]. In contrast, despite of reduced myocardial perfusion, not all experience angina or demonstrated ST-depression indicating ischemia in response to the cold pressor test [90]. Of note, transient myocardial perfusion defects may occur also in healthy persons [85] (Table 2). The observed ischemia in CAD patients may be related to altered myocardial functioning, as judged by delayed relaxation, impaired stiffness and reduced contractibility [70].
Table 2.
Exercise, cold and cardiovascular responses among persons with CVD obtained from controlled (n = 23) studies.
| Study | Patient group | Type of exercise | Type of cold exposure | Parameters | Results | Conclusion |
|---|---|---|---|---|---|---|
| WHOLE-BODY COLD EXPOSURE | ||||||
| Meyer et al. (2010) [94] | 13 men (mean 67 yrs.) ischemia during exercise, and ≥70% diameter stenosis of major artery, prior MI or reversible perfusion defect during exercise testing. | Maximal treadmill exercise tests | Cold air exposure (−20 °C) compared with +20 °C (t-shirt, pants) | ECG, HR, total exercise time, MET, RPP, HRR | Ischemic threshold was reached earlier (ca. 50 sec.) at −20 °C. No differences in other CV parameters or exercise performance. | Extreme cold lowered ischemic threshold even in the absence of, or without a history of angina. Cold did not affect exercise time, CV parameters (RPP), or result in arrhythmias. |
| Schmid et al. (2009) [108] | 22 males, 12 with CHF (59 yrs., EF 32%) and 10 with CAD (65 yrs., EF 52%). | Swimming (aqua therapy) | Cold water exposure (+22 °C) vs. +32 °C at rest and swimming (90 sec.) compared with 24-h Holter recording before immersion | RPP, arrhythmias (premature ventricular contractions, PVC), cardiac index, O2 | RPP increased at rest in cold water, but not during exercise. Cardiac index and VO2 increased to a similar extent when swimming in cold compared with warm water. PVCs increased in cold water in CHF but not in CAD patients. | Patients with compensated chronic heart failure tolerate water immersion and swimming in cold water well. However, increased PVCs might elevate the risk of high-grade ventricular arrhythmias. |
| Blanchet et al. (2003) [109] | 33 CHF patients (exercise limited dyspnoea, LVEF 26%, 3 females, 30 males, 60 ± 11 yrs.), and 12 healthy controls (4 females, 8 males, 52 ± 14 yrs.) | symptom-limited submaximal exercise, comparison of Cardevilol or Metoprolol treatment (beta-blockers) | Whole body exposure to cold air (−8 °C) and TN (+20 °C) | HR, SBP, NE, VO2, exercise performance, Lactate | CHF patients showed 21% decrease in exercise time in cold. Beta blockers increased exercise time and lowered peak exercise lactate levels at both temperatures. No difference in HR, SBP or RPP between temperatures. NE increased during exercise in cold only in CHF patients. | Patients with congestive heart failure showed decreased submaximal exercise time during moderate cold exposure. Beta-blocker therapy increased submaximal exercise time and attenuated the impact of cold exposure on functional capacity. |
| Juneau et al. (2002) [110] | 11 CHF patients (males, 61 ± 6 yrs., LVEF 25 ± 6%) | Symptom-limited maximal exercise testing, Lisinopril (ACE-inhibitor, three days prior to exercise tests) vs. placebo | Cold air (−8 °C) vs. TN (+20 °C) | HR, BP, RPP, exercise capacity | Higher RPP during exercise in the cold. Decreased exercise capacity in the cold with placebo group. Lisinopril attenuated the effect of cold on exercise. | Cold temperature increases cardiac demand in response to exercise and reduces maximal exercise capacity in patients with symptomatic heart failure. Acute treatment (three days) with Lisinopril attenuates the impact of cold on exercise capacity. |
| Marchant et al. (1994) [96] | 7 cold intolerant and 7 tolerant CAD subjects (57 ± 3 yrs.) | Symptom-limited exercise test (Bruce protocol) | Exposure to cold air (+6 °C + fan blowing 2 m/s on face and upper chest) vs. TN (+25 °C) | BP, HR, RPP, ECG, BRS, NE and E | Exercise in cold resulted in higher vascular resistance, SB, NE but not HR. SBP was higher in cold at onset of ischemia and angina. Unaltered RPP during exercise in the cold. Onset of ischemia and angina occurred earlier and BRS was impaired in cold-intolerant patients. | Higher BP during rest and exercise in cold increases myocardial oxygen demand. Cold-intolerant persons develop earlier onset of angina and ischemia in cold. They also have an impaired BRS and HR response that does not diminish due to increased BP. |
| Sheldahl et al. (1992) [18] | 16 men with CAD (asymptomatic, 63 ± 2 yrs.), 13 without CAD (61 ± 2 yrs.) and 12 young (40 ± 2 yrs.) | Self-paced snow shoveling (three methods) + treadmill exercise test | Cold ambient air exposure (ca. −1.5 °C) | ECG, BP VO2, left ventricular function | VO2 was lowest with CAD. Peak VO2 and HR ranged between 60–78%. LVEF and arrhythmias were comparable to treadmill exercise. | Selected energy expenditure varied in relation to individual peak VO2 and occurred at similar relative work intensities between CAD, controls and young persons. Arrhythmias and LVEF were similar to treadmill testing. |
| Peart et al. (1989) [98] | 11 men and 4 women (mean 49 yrs.) with stable CAD, no current medication (other than glyceryl trinitrates), reported cold sensitivity. | Symptom limited treadmill exercise test (Bruce protocol). | Cold air exposure (0 °C, cold air directed to face and trunk) compared with TN (+18–20 °C), treatment with either Nifedipine (calcium channel blocker) or Propranolol (beta blocker) for two weeks, repeat tests | ECG, BP, HR, RPP | Time to onset of angina, to 1 mm ST depression, and to peak exercise were shorter during exercise at 0 °C. RPP was similar between cold and TN, but was reached earlier with the different thresholds. Use of Nifedipine and Propranolol increased time to onset of angina, 1 mm ST-depression and time to peak exercise in cold and TN. Only Nifedipine enabled maintaining peak exercise time in cold. | Cold intolerance (symptom provoking) occurred in stable CAD patients during exercise testing at 0 °C. The reduction in the exercise time in cold persisted with Propranolol treatment, but to a lesser extent. Reduced exercise time in cold was not observed when treated with Nifedipine. |
| Juneau et al. (1989) [99] | 24 stable CAD patients (2 females, 22 males, mean 57 yrs.), reported cold sensitivity | Symptom limited treadmill exercise test (modified Bruce protocol). | Cold air exposure (−8 °C) compared with TN (+20 °C), placebo, Propranolol (beta blocker) or Diltiazem (calcium channel blocker) administration in either cold or TN. | HR, BP, ECG | Cardiovascular strain (elevated HR, SBP, DBP, RPP) was not different between exercise in cold vs. TN. Reported cold sensitive patients had lower ischemic threshold in the cold. Antianginal medication delayed the onset of ischemia both in cold and TN. Only Diltiazem prolonged exercise duration. | Cold does not worsen ischaemic threshold in most stable angina patients. Cold-sensitive patients showed earlier onset of ischemia. Both antianginal medications were effective for delaying ischemia to equal amounts in cold and TN. |
| Rosengren et al. (1988) [111] | 9 men with angina pectoris (mean 58 yrs.), ischemia during exercise (≥1 mm), cold susceptible | Symptom limited graded exercise testing (bicycle ergometer) | Whole body cold air exposure (mean −8 °C, variation from −2 °C to −13 °C), fan 3 m/s vs. TN (+22 °C) | HR, SBP, RPP, DBP, work capacity | Higher SBP, RPP, more pronounced ST-depression in 7/9 patients, higher RPP at the occurrence of ST-depression during exercise in cold. No effect on HR, or work capacity in cold. | Augmented cardiac work, secondary to increased BP, is related to cold-induced ST-depression among angina patients. |
| Areskog & Lassvik (1988) [81] | 17 male patients with angina pectoris, cold sensitive | Graded exercise test (bicycle) | Whole body cold air (−10 °C vs. +20 °C) and airway cooling (−10 °C, −35 °C vs. +20 °C) performed in a random order | HR, SBP, RPP | 10 subjects showed decreased maximal work load in cold exceeding 5% and explained by higher HR, SBP and RPP. Inhalation of −35 °C air resulted in comparable decrease in work capacity as exercise in −10 °C. | Cold susceptible persons with angina show a stronger sympathetic response during exercise in cold. A lower inhaled air temperature is required to produce the same response as with general skin cooling. |
| Brown & Oldridge (1985) [112] | 9 patients with history of cold-induced angina, 7 of 9 with previous MI, ischaemia during exercise | Symptom-limited maximal exercise test (bicycle) | Exposure to either cold air (−7.5 °C) vs. TN (+24 °C) and while breathing either cold (0.7 °C) vs. TN (+22.5 °C) air | HR, SBP, DBP, ventilation (VE), exercise capacity | Angina occurred sooner, and mean exercise time reduced in the cold, both while inspiring air of room temperature (−24%) and cold (−15%). Breathing cold air in the TN environment did not reduce exercise tolerance. SBP and RPP was higher during submaximal exercise in the cold. | Earlier onset of angina limits exercise performance in cold among angina patients. The effects are more related to the exposure of a cold environment rather than cold air inhalation. |
| Lassvik & Areskog (1980) [103] | 12 men with effort angina (mean 51 yrs.), cold sensitivity | Symptom-limited graded exercise test (bicycle ergometer) | Whole-body exposure to −10 °C vs. TN (+20 °C). Inhalation of cold air at various temperatures (−35 °C). Other test at either TN (+20 °C) or cold (−10 °C) climatic chamber and inhaling either TN or cold (−10 °C) air | ECG, HR, BP, exercise capacity, ST-depression | Inhalation of −35 °C decreased work capacity and caused higher HR, SBP and RPP in patients with effort angina, no changes with inhaling −10 °C in TN or cold environment. Exposure to cold while inhaling TN air decreased work capacity. | Skin cooling is more important in affecting cardiac work and working capacity than inhaling cold air in cold environment. However, inhaling very cold air decreases work capacity. Increased cardiac work reduces exercise capacity both during exercise or while inhaling cold air. |
| Backman et al. (1979) [104] | 26 patients with angina pectoris and coronary insufficiency (mean age 50 yrs., range 36–70) | Graded maximal exercise test (bicycle ergometer) | Exposure to cold air (−15 °C) vs. TN (+22 °C) while seated and in supine position | HR, BP, RPP, PE, exercise performance | Lowered maximal performance, higher perceived exertion, lower RPP in cold. Exercise in the supine position caused more pronounced ECG changes, reduced maximal performance and RPP compared to sitting. | Cold exposure leads to lowered work capacity, RPP and higher perceived exertion in cold. A supine body position in cold further lower the angina threshold and affect ECG, by increasing the central blood volume and the diastolic volume of the left heart and also myocardial oxygen consumption. |
| Lassvik & Areskog (1979) [105] | 11 patients with effort angina and history of cold intolerance (mean age 55 yrs., range 45–61) | Graded exercise test (bicycle ergometer) symptom limited, stopped with moderately severe angina, serial exercise tests | Exposure to +20 °C, +10 °C, 0 °C, −10 °C and −30 °C (few patients), random order | HR, BP, RPP, exercise performance | At rest SBP was progressively higher with decreasing temperature. Lesser absolute increase in BP during exercise in cold. No change in HR. Maximal workload decreased at −10 °C, maximal work capacity decreased with declining temperature. Decreased maximal capacity correlated with increase in RPP. | Decreased performance in cold may be explained by higher cardiac workload. Warm-up exercise, diminished the difference in SBP between a cold and TN environment with increasing work time. |
| Lassvik & Areskog (1979) [106] | 17 patients with angina pectoris (mean age 52 yrs. range 45–60 yrs.), cold susceptible, | Symptom-limited graded exercise test (bicycle ergometer) | Exposure to cold air (−10 °C) vs. TN (+20 °C) | HR, BP, RPP, ST-depression | Maximal work load decrease in cold. HR, SBP and RPP were higher during submaximal exercise in cold. No difference in HR, RPP and magnitude of ST-depression at maximal workloads. Work capacity decreased more in cold susceptible persons and correlated with higher HR and BP during exercise in cold. | Patients with angina pectoris showed intolerance to exercise in the cold that was related to higher HR and BP compared with exercise at TN. |
| Epstein et al. (1969) [107] | 6 males with CAD (age 37–60 yrs.) and 5 without CAD (2 females, 3 males, age 23–59 yrs.) | Exercise at sub anginal level (CAD patients, bicycle or treadmill), others exercised at levels that did not cause fatigue | Exposure to cold air (+15 °C) vs. TN (+25 °C) | HR, MAP,CO, SV, TPR, MCVP, LFMW | Lower temperature exercise resulted in higher SAP, TPR and left ventricular minute work. Exercise in cold did not alter HR, CO, SV. | Cold increases peripheral resistance during exercise. The rise in BP, by increasing myocardial oxygen demand, more likely provokes an attack of angina. |
| LOCAL COLD EXPOSURE | ||||||
| Dodds et al. (1995) [95] | 12 men with CAD (mean age 61 yrs.), four with previous MI, stable angina, (medication stopped 2 days prior to exercise tests) | Symptom-limited treadmill tests (Bruce-protocol) | Inhalation of either cold (−15 °C) or TN (+22 °C) air while exercising | HR, BP, RPP, ECG, Endothelin (ET-1) and Angiontensin-II (AT-II) | Cold air inhalation reduced time to angina and total exercise time. No effect on time for reaching 1 mm ST-depression. RPP was unaltered at the onset of angina, or occurrence of 1 mm ST-depression, but lower at peak exercise when breathing cold air. ET-decreased and AT-II levels increased with peak exercise but did not differ between exposures. | Cold air inhalation results in earlier onset of angina and reduced exercise capacity. Peripheral ET-1 and AT-II do not explain the mechanism of cold-induced angina. |
| Petersen et al. (1994) [97] | 10 men (mean age 59 yrs.) with CAD and MI within 6–12 mo, angina, ischemic ST-deviations during exercise | Symptom limited exercise test on bicycle ergometer | Inhalation of cold (−22 °C) or TN (+22 °C) air | A, NA, ANF, endothelin, renin, angiotensin II, MBF (scintigraphic imaging) | Myocardial ischemic score increased and endothelin levels during cold air inhalation exercise were higher compared with rest. No effect on HR, SBP, E and NE peak exercise response. | Inhalation of cold air during exercise was related to increased plasma endothelin levels and myocardial ischaemia. The ischemic response was not due to higher myocardial oxygen demand. |
| Hattenhauer & Neill (1975) [89] | 17 men with CAD (mean age 52 yrs, range 42–66 yrs.), 13 had previous MI, 11 cold susceptible | Atrial pacing (increase by 10 bpm/min) until angina occurred | Inhalation of cold (−20 °C) vs. TN (+20 °C) air | HR, LVEDP, SBP DBP, PO2, pH, MVO2, CBF, Lactate | Inhalation of cold air during rest increased HR, SBP, DBP, O2, PO2 and pH. Exercise increased SBP, DBP, and PO2 but no other parameters. | Angina pectoris induced by breathing cold air cannot be explained by a concurrent increase in myocardial work and oxygen consumption. |
| COLD PRESSOR TEST | ||||||
| Nesto et al. (1989) [100] | 16 men (mean 54 yrs.) with CAD, stable angina, ischemia during exercise | Graded maximal exercise test (Bruce protocol) | Cold pressor test during exercise compared with exercise only | HR, SBP, DBP, RPP, time to angina, time to ST-depression (1 mm) | Exercise with CPT increased SBP, DBP and RPP but did not change HR, total exercise time, time to angina or time to ST-depression. | Myocardial ischemia at a higher RPP during exercise and CPT could be due to competitive myocardial vasodilation as a result of exercise |
| Shea et al. (1987) [90] | 35 CAD patients (mean 39–74 yrs.) compared with healthy controls (n = 10) | Graded symptom-limited supine exercise | Cold pressor test (ice water for 3 min). CPT and exercise tests (conducted at TN) were separate. | HR, SBP, ST-depression, myocardial perfusion (positron emission tomography) | CPT resulted in abnormal myocardial perfusion, but only a few experienced angina or had ST-depression. In contrast, exercise resulted in reduced myocardial perfusion, reports of angina and ST-depression in most subjects. Peak SBP after CPT and exercise were similar. | Cold produces reduced myocardial perfusion that is asymptomatic and occur at lower HR compared with exercise. |
| de Servi et al. (1985) [101] | 11 (1 female, 10 males, mean age 53.5 ± 7 yrs.) CAD patients, ischaemia during exercise, cold susceptibility, medication ceased for the course of the study | Graded symptom limited exercise test | Exercise, followed by cold pressor test (ice water for 60 sec.) and another exercise test + cold pressor test | BP, HR, RPP, ST-depression, GCVF, ARCR | RPP increased during exercise and CPT and further when the two were combined. ARCR decreased during 1st exercise, increased (ns) during CPT, and decreased again during 2nd exercise + CPT bout. RPP increase during both exercise tests and CPT. Angina occurred at lower workload than the first exercise test. | Exercise reduced coronary resistance, but which increased during CPT. A combination of exercise and CPT reduces coronary resistance, but to a lesser extent in subjects with reduced exercise tolerance. |
| de Servi et al. (1980) [102] | 8 patients with CAD (2 females, 6 males, 52.3 yrs.) | Isometric exercise at 50% MVC for 2 ½ min | Cold pressor test (ice water for 1 min), followed by exercise at TN. Application of nifedipine (calcium channel blocker) and repeat CPT and exercise | HR, MAP, RPP, CBF (thermodilution technique) | CPT did not change CBF, but increased coronary resistance. Nifedipine did not alter CBF, but reduced coronary resistance during CPT. Increased HR and MAP was observed during CPT, but lower MAP and higher HR occurred after nifedipine treatment. Improved CBF and reduced resistance during isometric handgrip after nifedipine treatment. | Nifedipine was successful in reducing coronary vasoconstriction during CPT and increased CBF and reduced coronary vasoconstriction during isometric exercise. |
Abbreviations: ARCR = anterior region coronary resistance, BP = blood pressure, BRS = baroreflex sensitivity, CAD = coronary artery disease, CBF = coronary blood flow, CHF = chronic heart failure, CO = cardiac output, CPT = cold pressor test, CV = cardiovascular, DBP = diastolic blood pressure, E = epinephrine, ECG = electrocardiogram, GCVF = great cardiac vein flow, HR = heart rate, HRR = heart rate reserve, LVEF = left ventricular ejection fraction, LFMW = left ventricular minute work, LVEDP = left ventricular end diastolic pressure, MAP = mean arterial pressure, MBF = myocardial blood flow, MCVP = mean central venous pressure, MET = metabolic equivalent units, MVO2 = myocardial oxygen consumption, NE = norepinephrine, O2 = concentration of oxygen in blood (arterial, coronary-venous), PO2 = partial pressure of oxygen in blood (arterial and coronary-venous), PVC = premature ventricular contraction, SAP = systemic arterial pressure, SBP = systolic blood pressure, SV = stroke volume, TN = thermoneutral, TPR = total peripheral resistance, VE = ventilation, VO2 = oxygen consumption.
In summary, controlled studies assessing whole-body cooling and cardiovascular response in CAD patients at rest are lacking. Clinical research assessing reactivity to stress utilizing the cold-pressor test have shown either higher or comparable increase in cardiovascular strain among CAD patients compared with controls. Yet, many of the studies did not include healthy controls for comparison. CAD is often related to reduced myocardial blood flow in response to the cold pressor test. This may lead, but not always, to reports of angina related to myocardial ischemia.
Heart failure
Heart failure, often referred to as congestive (symptoms of congestion) or chronic (refers to long duration) heart failure (CHF) is a clinical syndrome in which there are characteristic signs and symptoms, such as oedema, breathlessness and fatigue, due to an underlying abnormality of cardiac function [91,92]. Heart failure is often a manifestation of CAD and includes having a previous MI. It occurs when the heart is unable to pump sufficiently to maintain blood flow to meet the body's needs. The end result is a fall in cardiac output or its inadequate increase during exercise. Under perfusion leads to baroreceptor activation, increased HR and BP with salt and water retention. Long-term progress of the disease includes enlargement of ventricles and atrium and pulmonary oedema. A heart failure patient has little physiological reserve to deal with increased cardiac workload that occurs while being cold exposed or during exercise. Cold-induced vasoconstriction is associated with an increased afterload, which a failing heart may not be able to compensate by increasing cardiac work [4].
To the best of my knowledge, there are no studies that have determined cold-related cardiovascular responses among heart failure patients. These would be important for understanding mechanisms behind the higher winter mortality [4]. Early classic studies suggest aggravated constrictor response reducing cutaneous blood flow at rest under thermoneutral conditions [93]. Only one recent study included heart failure patients in a trial studying the combined exposure to Finnish sauna followed by a head-out cold water-immersion [67]. This form of cold exposure produced comparable cardiovascular responses in heart failure patients compared with controls and did not provoke an excessive increase in adrenergic activity or arrhythmias. The results are to be interpreted with caution due to the small sample size and very short duration of the immersion.
Exercise in the cold and cardiovascular responses in persons with cardiovascular diseases
The majority of the studies examining the combined effects of cold and exercise on cardiovascular responses among persons with cardiovascular diseases have studied CAD [18,81,89,90,94–112] and only a few heart failure [109,110]. To the best of my knowledge, none have included subjects with hypertension (Table 2). In contrast to studies conducted at rest, exercise-related research have either employed whole-body exposure to cold air [18,81,94,99,103,104,106,107,109,111,112] or water [108], cold air blown on face and upper chest [96,98], or while inhaled [89,95,97,103,112]. Only a few studies have included the cold pressor test stimulus performed in conjunction [100] or separately from exercise [90,101,102]. Depending of the mode of exposure the intensity of ‘cold’ has varied markedly. The majority of the exercise studies have employed the Bruce protocol, or its modification, which is a graded and symptom-limited test assessing maximal exercise capacity of cardiac patients [113]. Hence, the duration of exercise (and cold exposure itself) was often relatively short, on average 10–12 min. Only a few studies included submaximal exercise [18,107–109]. With the exception of one study [102], all others examined dynamic exercise (Table 2).
In the majority of the studies cardiovascular responses to varying cold exposures (cold, exercise, medication) were compared within the studied patient group. Only a few studies included healthy controls [18,90,107]. A portion of CAD patients report being cold susceptible and cardiovascular responses were compared between these groups [96]. The studied subjects were middle-aged (mean age 57 yrs.).
Coronary artery disease
Two previous reviews have creditably summarized the available evidence concerning with cold exposure, exercise and CAD [13,14].
Whole body cold exposure
A combination of cold exposure and exercise often results in increased cardiovascular strain compared with exercise alone. Several studies have shown elevated SBP [81,96,100,103,111,112], HR [81,103] or RPP [81,103,111,112] in response to symptom-limited graded exercise in a cold compared with thermoneutral environment. Though other studies have reported unaltered HR [94,96,99,105,107,111], SBP [99] and RPP [94,96,98,99,108]. The exact reasons for the deviating findings cannot be distinguished, but are likely due to variations in the study populations and designs. In studies that detected a higher RPP during exercise in the cold, this was probably more often driven through an increase in SBP, rather than HR [13,107].
Exercise is known to increase coronary blood flow, but this may occur to a lesser extent among patients with CAD [13]. Several studies have indicated that exercise in cold results in reaching the ischemic threshold earlier as indicated by an ST-depression of 1 ≥mm [94,96,98,99], while others have not [95,100]. Angina is considered to reflect ischemia and occurs sooner among CAD patients during exercise in the cold [98,112]. Though, ischemia may even occur without angina symptoms, or among those without a history of angina [94]. Furthermore, the onset of ischemia and angina may occur even earlier in cold-sensitive/intolerant CAD patients [96,99].
The possible mismatch between myocardial oxygen demand and supply [13,107] occurring during exercise in the cold among patients with CAD has been shown to lead to reduced exercise times in some [98,112], but not all [94,96] studies compared with thermoneutral conditions. Furthermore, maximal work capacity is often [103–106], but not always [111], reduced when comparing exercise in a cold environment with exercise alone. It is possible that especially cold-susceptible CAD patients demonstrate decreased maximal power while exercising in cold [81].
Applying antianginal medication has shown to be beneficial in patients with CAD in improving exercise capacity during exercise in the cold [98,99]. Both the use of calcium and beta blockers increased the time to onset of angina, the occurrence of ischaemia (ST-depression) and prolonged total exercise time [98]. However, it seemed that the use of calcium channel blockers were more effective for sustaining performance in the cold. In contrast, Juneau et al. [99] showed that beta and calcium channel blockers were equally effective in delaying the onset of exercise-induced ischemia.
Local cold exposure
Inhalation of cold air may be pronounced during exercise in association with oronasal breathing. Previous studies have detected reduced coronary blood flow among healthy persons while inhaling cold air [56] and further attenuated by aging [50].
The findings from patients with CAD are contradictory. Inhalation of cold air (−20 °C) and atrial pacing (used in parallel with exercise tests to assess myocardial function) resulted in reports of angina in a few of the patients. However, the occurrence of chest pain could not be explained by an increase in myocardial O2 consumption or reduction in coronary blood flow. Therefore, the authors suggest that inhalation of cold air could affect collaterals or coronary blood flow distribution, rather than large coronary artery or generalized arteriole constriction [89].
Inhalation of cold (−15 °C) compared with warm air (+22 °C) while exercising at room temperature has been shown to result in earlier onset of angina among stable angina patients. Furthermore, angina occurred sooner and mean total exercise time was shorter with cold air inhalation [95]. In addition, the total exercise time was reached at an earlier level of RPP. Similarly, decreased work capacity was also detected when inhaling very cold (−35 °C) compared with warm air (+20 °C) during a symptom-limited exercise test at neutral environmental temperature. At the same time, a higher cardiac workload (RPP) was observed with cold air inhalation and exercise [103].
Some of the studies have combined inhalation of cold air and exercise with concurrent cooling of the skin. Inhalation of moderately cold air (−10 °C) did not impair work capacity irrespective of environmental temperature [103]. In contrast, exposure to a cold environment (−10 °C) with inhalation of air at room temperature caused a significant decrease in exercise performance. Also Brown & Oldridge [112] documented decreased exercise capacity and earlier occurrence of angina among CAD patients both during exercise in a cold environment (−7.5 °C) and also when combined with inhaling cold (circa 0 °C) air. Both studies concluded that the effects on performance and appearance of angina are more related to the environment than inhaling cold air [103].
A few studies have examined mechanisms underlying reports of cold-induced angina or detected myocardial ischemia while inhaling cold air during exercise. One study employing graded exercise examined the role of vasoconstrictor peptides, endothelin or angiontensin as explanatory factors for cold-induced angina, but could not detect an effect of inhalation temperature [95]. In contrast, Petersen [97] demonstrated that inhaling cold (−22 °C) compared with warm (+22 °C) air during exercise was related to increased plasma endothelin levels and the detected myocardial ischaemia. Interestingly, the ischemic response was not related to a higher myocardial oxygen demand, as there were no differences in peak heart rate, SBP or catecholamines between inhaling cold or warm air.
Cold pressor test
In the examined studies cold pressor tests were applied either before or after [90,101,102], or in conjunction [100] with exercise. The simultaneous competing stimuli of the cold pressor test (vasoconstrictor stimulus) and exercise (stimulus for coronary vasodilation) were examined in a study where patients with CAD performed graded exercise in a thermoneutral environment with or without the cold stimulus [100]. The results showed higher SBP and DBP during submaximal workloads, as well as higher RPP throughout the exercise when compared with exercise without the cold pressor stimulus. Unexpectedly, the patients experienced angina and signs of ischemia at a higher RPP, from which the study suggest that coronary vasodilation occurring at exercise may outweigh the effects of the strong sympathetic stimulus of the cold pressor test. A somewhat similar finding from deServi et al. [101] showed that the higher coronary resistance during a cold pressor test does not persist during exercise in a neutral environment, when metabolic vasodilation occurs. However, the reduction in coronary resistance was smaller among CAD patients with reduced exercise tolerance. Of note, the study is limited by the interrelated repeated exercise and cold pressor tests. A calcium channel blocker (nifedipine) was successful in reducing coronary vasoconstriction and improving blood flow during isometric exercise combined with a cold pressor test [102]. However, the study did not assess simultaneous cardiovascular response of cold and exercise.
The aspect of myocardial blood supply in response to the separate effects of a cold pressor test and exercise have been investigated [90,101,102]. One study demonstrated that the cold pressor stimulus caused disturbances in the regional myocardial perfusion comparable to exercise in a neutral environment. Interestingly, the perfusion decrement related to the pressor test were not necessary accompanied by symptoms of angina [90]. Hence, it may be that the symptomatic patients or commonly accepted ECG manifestations of ischemia underestimate the true ischemic effects of a cold stimulus.
In summary, many of the previous studies among CAD patients demonstrate higher cardiac workload during exercise in the cold. Any contrasting findings have likely resulted from differences between patient groups, forms of cold exposure or employed exercise. In conjunction with the higher cardiac strain, myocardial oxygen supply may be weakened, as judged by reports of angina or detected ischemia. Performance during exercise in the cold may be impaired, as judged by lowered exercise times and peak levels. Antianginal medication has proven successful for CAD patients for improving performance during exercise in the cold.
Heart failure
Heart failure has in a few controlled exercise studies shown to be associated with reduced maximal and submaximal performance during cold exposure [109,110]. A study employing submaximal exercise in the cold showed a 21% reduction in exercise time in persons with heart failure compared with controls, no marked changes in RPP, but considerably increased release of norepinephrine. Furthermore, applying beta blocker therapy (Cardevilol, Metoprolol) attenuated the impact of cold on cardiovascular responses during exercise [109]. Exercise in cold water increased RPP, cardiac index, reduced peripheral vascular resistance and maximal exercise time by 17% in persons with heart failure. Remarkably, only three days use of a low-dose ACE inhibitor (Lisinopril) attenuated the effect of cold on exercise capacity [110].
Lastly, chronic heart failure patients have been shown to tolerate aqua therapy and swimming in moderately cold water (+22 °C) well, as judged by equally increased cardiac work and index, in both cold and warm water. However, CHF patients demonstrated higher amounts of premature ventricular contractions, probably as a result of the stimulation of autonomic nervous system and which could involve potential health risks [108].
Confounders in studies investigating the effects of cold and exercise on CVD
Controlled studies examining the single and combined effects of cold exposure and/or exercise are relatively few, but the interest is growing. Reaching conclusions based on the obtained evidence can be challenging due to several reasons. First of all, the type of cold exposure (intensity, duration, area of stimulation) varies remarkably between the different studies. The commonly employed cold pressor test which is clinically relevant for assessing cardiovascular diseases, does not reflect a true cold exposure. Also, the influence of seasonal variation on circulation and other cardiovascular risk factors should be taken into account. Studies examining exercise responses have likewise also used a variety of exercise protocols in terms of form (dynamic, isometric), intensity (submaximal, maximal, constant, graded) or duration. Especially, studies examining submaximal dynamic or isometric exercise are largely lacking. It is also likely that the characteristics of the participants have varied, with differences in age, anthropometry/body composition, gender, fitness etc. that are known to considerable affect thermal responses [20]. Ageing itself involves a continuum of functional, structural, cellular and molecular changes in the heart [114] and/or vasculature, which modify the observed cold-related cardiovascular responses. For example, ageing increase cold-related cardiac preload and afterload during cooling and contribute to increased myocardial oxygen demand [46]. It also affects cutaneous vascular responses, and where the adrenergically mediated vasodilator response may be lost [115]. Progression of arterial stiffness with ageing may also lead to augmented BP response towards cold [25,32,33,46]. Most of the previous controlled studies have included participants having CVD with either short disease progression, or causing mild to moderate disability. Hence, the susceptibility to cold may increase with more advanced disease states. However, in many cases ethical issues limit examining these population groups. Cardiovascular diseases are also often connected to other comorbid conditions, such as diabetes [116,117] which itself also affects thermal responses [118]. As the pathophysiology of neural and cardiovascular mechanisms are partially overlapping, it may be impossible to distinguish cold-related cardiovascular responses of an individual disease. Finally, a varying amount of medication used for cardiovascular diseases likely also affect cardiovascular and thermal response. As was shown antianginal medication is beneficial for both lowering baseline levels of BP in HTN, but also in improving performance while exercise in the cold among CAD patients. Differences between studies could also have been created due to varying washout times (e.g. from a few days to several weeks) when ceasing some of the medication prior to the experiments. Perhaps, for some of the controlled studies it is not possible, or may not even be necessary, to separate between disease groups or medication, if one wants to study susceptibility to thermal environments that has public health applicability.
Summing up: how does cold and exercise relate to higher CV morbidity and mortality
Acute responses
The mechanisms behind how cardiovascular diseases modify cold exposure and exercise-related cardiovascular responses can only be hypothesized (Figure 1). Cold exposure activates the sympathetic nervous system and the renin-angiotensin system which elevates BP both acutely and on long-term [74,119]. A higher BP itself, and its acute increase, are risk factors for adverse cardiovascular events, such as plaque ruptures and associated hemorrhages [83,120]. The acute BP increase may further be aggravated due to increased aortic stiffness due to healthy ageing [32], as well as in hypertension [73,121]. In addition, cold exposure involving both the whole-body and facial region may elicit a co-activation of both the sympathetic and parasympathetic nervous system (‘autonomic conflict’) and result in arrhythmias. These may, in theory, also occur with other stressors than submersion in cold water [122]. The higher cardiac workload associated with cold and reduced myocardial perfusion (e.g. due to stenosis) occurring with ischemic heart diseases may cause a mismatch between oxygen demand and supply and lead to either acute (plaque rupture and thrombus formation) or chronic ischemia (lumen narrowing of coronary artery) and consequently to MI or SCD [13,83]. Transient myocardial ischemia may occur due to cold-related coronary spams caused by altered endothelial function [123] and in the worst case lead to SCD [83]. The decreased skin blood flow as a result of cold-induced vasoconstriction together with increased diuresis leads to hemoconcentration and hyperviscocity [2]. Already mild cooling of the body surface for relatively short periods elicit hemoconcentration and increased levels of fibrinogen and cholesterol that could favor thrombus formation [28,124]. Also exercise in the cold elevates the coagulation potential [125]. This may together with cold-related increase in BP enhance the forces favoring vascular wall deformation, increase friction and shear stress on the aortic internal surface, and lead to ruptures or dissection [120].
It should be noted that, in addition to the acute cold-related effects and related health events, a lowering of environmental temperature often results in more pronounced lagged health effects compared with heat [2,126]. For example, the effect of cold on mortality occurs generally 2–3 days following the exposure, but effects are still observed within 10–25 lag days [2]. The effects of cold may be more indirect than heat and related to for example respiratory infections that may later result in hospitalizations [126].
Seasonal variation of factors affecting cold-related cardiovascular responses
In addition to the acute increase in BP also the cold season is associated with a higher BP [119,127]. A recent systematic review and meta-analysis [127] showed that lower ambient temperatures increase BP and especially persons with cardiovascular diseases are susceptible. In addition to acute responses, a cold season alters hemostatic factors and increases the coagulation potential, such as concentrations of t-PA, vWF, D-dimers [128] and fibrinogen [129]. These changes may explain at least a part of the higher CHD mortality [128].
In the end, also other factors influence the seasonal pattern of cardiovascular diseases and possibly contribute to the higher occurrence of adverse health events in the winter. Known risk factors for cardiovascular diseases are present at higher levels during the winter. These are for example a higher BP, BMI, waist circumference, HDL, LDL, triglycerides and glucose [130]. In addition, changes in vitamin D, certain hormones, air pollution, infections, diet and physical activity may further modify the risk of cardiovascular events [4].
Research gaps and conclusions
The separate effects of cold exposure and exercise on cardiovascular responses in healthy persons are well known. Less is known of how a cardiovascular disease alters these responses. This information enables understanding the pathophysiological responses that may contribute to the globally demonstrated higher cardiovascular morbidity and mortality of a specific climate.
With the available current information more studies with forms, intensity and duration of cold exposure that resembles situations that individuals may encounter in their habitual life are warranted. Furthermore, as regular exercise is important for everyone's health, its role can't be overemphasized for persons with a chronic diseases. Undisputed evidence indicate that health-enhancing exercise is an efficient form of secondary prevention, as it delays or prevent the progress of cardiovascular diseases [131] and reduces potential adverse health outcomes. Yet, as both cold exposure and exercise separately increase cardiovascular strain, information of their combined effects are needed. At present aerobic performance in the cold has not been comprehensively documented [12], and even less among persons with cardiovascular diseases. For example, symptoms of angina in the cold [13,81] may prevent individuals from practising regular exercise. Especially, information of how hypertension modifies cardiovascular responses during exercise in the cold are lacking. A further interest would also be to examine how isometric exercise in persons with cardiovascular diseases affect cardiovascular responses in cold [13]. This would help explaining why especially situations involving low temperatures and sudden or intense exercise (i.e. snow shoveling) may involve a health risk for persons with cardiovascular disease, such as an ischemic heart condition. The produced information would support individuals to practice regular year-round exercise outdoors. It would also be of value for health care experts when providing advice on appropriate protection for cold climates.
This line of research is particularly important as global populations are undergoing a major epidemiological transition in which cardiovascular diseases are estimated to increase in prevalence [114,132], have been recognized as being climate sensitive, and especially among the elderly population [3]. In addition, climate change is thought to bring about not only global warming, but possibly also a higher amount of extreme weather events [133] which may complicate preparedness of individuals. The expected higher share of non-optimal temperatures, and that of cold outweighing the health effects heat [9], provides further justification to pursue examining susceptible populations.
Funding Statement
The production of the present review was supported through funding from the Finnish Ministry of Education and Culture (Cardiovascular responses to year-round health-enhancing exercise in persons with coronary artery disease).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Song X, Wang S, Hu Y, et al. Impact of ambient temperature on morbidity and mortality: an overview of reviews. Sci Total Environ. 2017;586:241–254. doi: 10.1016/j.scitotenv.2017.01.212. [DOI] [PubMed] [Google Scholar]
- [2].Liu C, Yavar Z, Sun Q. Cardiovascular response to thermoregulatory challenges. Am J Physiol Heart Circ Physiol. 2015;309(11):1793. doi: 10.1152/ajpheart.00199.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bunker A, Wildenhain J, Vandenbergh A, et al. Effects of air temperature on climate-sensitive mortality and morbidity outcomes in the elderly; a systematic review and meta-analysis of epidemiological evidence. EBioMedicine. 2016;6:258–268. doi: 10.1016/j.ebiom.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fares A. Winter cardiovascular diseases phenomenon. N Am J Med Sci. 2013;5(4):266–279. doi: 10.4103/1947-2714.110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ryti NRI, Guo Y, Jaakkola JJK. Global association of cold spells and adverse health effects: a systematic review and meta-analysis. Environ Health Perspect. 2016;124(1):12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ryti NRI, Mäkikyrö EMS, Antikainen H, et al. Risk of sudden cardiac death in relation to season-specific cold spells: a case-crossover study in Finland. BMJ Open. 2017;7(11):017398. doi: 10.1136/bmjopen-2017-017398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ikäheimo TM, Lehtinen T, Antikainen R, et al. Cold-related cardiorespiratory symptoms among subjects with and without hypertension: the National FINRISK Study 2002. Eur J Public Health. 2014;24(2):237–243. doi: 10.1093/eurpub/ckt078. [DOI] [PubMed] [Google Scholar]
- [8].Nagarajan V, Fonarow GC, Ju C, et al. Seasonal and circadian variations of acute myocardial infarction: findings from the get with the guidelines-coronary artery disease (GWTG-CAD) program. Am Heart J. 2017;189:85–93. doi: 10.1016/j.ahj.2017.04.002. [DOI] [PubMed] [Google Scholar]
- [9].Gasparrini A, Guo Y, Hashizume M, et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015;386(9991):369–375. doi: 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang J, Yin P, Zhou M, et al. Cardiovascular mortality risk attributable to ambient temperature in China. Heart. 2015;101(24):1966–1972. doi: 10.1136/heartjnl-2015-308062. [DOI] [PubMed] [Google Scholar]
- [11].Berko J, Ingram DD, Saha S, et al. Deaths attributed to heat, cold, and other weather events in the United States, 2006–2010. Natl Health Stat Report. 2014;30(76):1–15. [PubMed] [Google Scholar]
- [12].Castellani JW, Tipton MJ. Cold stress effects on exposure tolerance and exercise performance. Compr Physiol. 2015;6(1):443–469. doi: 10.1002/cphy.c140081. [DOI] [PubMed] [Google Scholar]
- [13].Manou-Stathopoulou V, Goodwin CD, Patterson T, et al. The effects of cold and exercise on the cardiovascular system. Heart. 2015;101(10):808–820. doi: 10.1136/heartjnl-2014-306276. [DOI] [PubMed] [Google Scholar]
- [14].Emmett JD. A review of heart rate and blood pressure responses in the cold in healthy subjects and coronary artery disease patients. J Cardiopulm Rehabil. 1995;15(1):19–24. doi: 10.1097/00008483-199501000-00003. [DOI] [PubMed] [Google Scholar]
- [15].Janardhanan R, Henry Z, Hur DJ, et al. The snow-shoveler's ST elevation myocardial infarction. Am J Cardiol. 2010;106(4):596–600. doi: 10.1016/j.amjcard.2010.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Franklin BA, Bonzheim K, Gordon S, et al. Snow shoveling: a trigger for acute myocardial infarction and sudden coronary death. Am J Cardiol. 1996;77(10):855–858. doi: 10.1016/S0002-9149(97)89181-3. [DOI] [PubMed] [Google Scholar]
- [17].Franklin BA, Hogan P, Bonzheim K, et al. Cardiac demands of heavy snow shoveling. JAMA. 1995;273(11):880–882. doi: 10.1001/jama.1995.03520350062030. [DOI] [PubMed] [Google Scholar]
- [18].Sheldahl LM, Wilke NA, Dougherty SM, et al. Effect of age and coronary artery disease on response to snow shoveling. J Am Coll Cardiol. 1992;20(5):1111–1117. doi: 10.1016/0735-1097(92)90366-U. [DOI] [PubMed] [Google Scholar]
- [19].Klug G, Schenk S, Dörler J, et al. Occurrence of acute myocardial infarction in winter tourists: data from a retrospective questionnaire. Clin Res Cardiol. 2011;100(8):669–674. doi: 10.1007/s00392-011-0294-3. [DOI] [PubMed] [Google Scholar]
- [20].Castellani JW, Young AJ. Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton Neurosci. 2016;196:63–74. doi: 10.1016/j.autneu.2016.02.009. [DOI] [PubMed] [Google Scholar]
- [21].Johnson JM, Minson CT, Kellogg DL. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol. 2014;4(1):33–89. doi: 10.1002/cphy.c130015. [DOI] [PubMed] [Google Scholar]
- [22].Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol. 2010;109(4):1221–1228. doi: 10.1152/japplphysiol.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Greaney JL, Kenney WL, Alexander LM. Sympathetic regulation during thermal stress in human aging and disease. Auton Neurosci. 2016;196:81–90. doi: 10.1016/j.autneu.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wilson TE, Sauder CL, Kearney ML, et al. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol. 2007;103(4):1257–1262. doi: 10.1152/japplphysiol.00401.2007. [DOI] [PubMed] [Google Scholar]
- [25].Kingma BRM, Frijns AJH, Saris WHM, et al. Increased systolic blood pressure after mild cold and rewarming: relation to cold-induced thermogenesis and age. Acta Physiol (Oxf). 2011;203(4):419–427. doi: 10.1111/j.1748-1716.2011.02336.x. [DOI] [PubMed] [Google Scholar]
- [26].Mäkinen TM, Mäntysaari M, Pääkkönen T, et al. Autonomic nervous function during whole-body cold exposure before and after cold acclimation. Aviat Space Environ Med. 2008;79(9):875–882. doi: 10.3357/ASEM.2235.2008. [DOI] [PubMed] [Google Scholar]
- [27].Korhonen I. Blood pressure and heart rate responses in men exposed to arm and leg cold pressor tests and whole-body cold exposure. Int J Circumpolar Health. 2006;65(2):178–184. doi: 10.3402/ijch.v65i2.18090. [DOI] [PubMed] [Google Scholar]
- [28].Keatinge WR, Coleshaw SR, Cotter F, et al. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. Br Med J (Clin Res Ed). 1984;289(6456):1405–1408. doi: 10.1136/bmj.289.6456.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202–207. doi: 10.1007/s10286-006-0332-9. [DOI] [PubMed] [Google Scholar]
- [30].Reyners AK, Tio RA, Vlutters FG, et al. Re-evaluation of the cold face test in humans. Eur J Appl Physiol. 2000;82(5–6):487–492. doi: 10.1007/s004210000217. [DOI] [PubMed] [Google Scholar]
- [31].Stemper B, Hilz MJ, Rauhut U, et al. Evaluation of cold face test bradycardia by means of spectral analysis. Clin Auton Res. 2002;12(2):78–83. doi: 10.1007/s102860200024. [DOI] [PubMed] [Google Scholar]
- [32].Hess KL, Wilson TE, Sauder CL, et al. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol. 2009;107(4):1076–1082. doi: 10.1152/japplphysiol.00605.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Greaney JL, Stanhewicz AE, Kenney WL, et al. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J Appl Physiol. 2014;117(6):648–657. doi: 10.1152/japplphysiol.00516.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Muller MD, Gao Z, Drew RC, et al. Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J Appl Physiol. 2011;111(6):1694–1702. doi: 10.1152/japplphysiol.00909.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Edwards DG, Roy MS, Prasad RY. Wave reflection augments central systolic and pulse pressures during facial cooling. Am J Physiol Heart Circ Physiol. 2008;294(6):2535. doi: 10.1152/ajpheart.01369.2007. [DOI] [PubMed] [Google Scholar]
- [36].Prodel E, Barbosa TC, Mansur DE, et al. Effects of face cooling on pulse waveform and sympathetic activity in hypertensive subjects. Clin Auton Res. 2017;27(1):45–49. doi: 10.1007/s10286-016-0391-5. [DOI] [PubMed] [Google Scholar]
- [37].Edwards DG, Gauthier AL, Hayman MA, et al. Acute effects of cold exposure on central aortic wave reflection. J Appl Physiol. 2006;100(4):1210–1214. doi: 10.1152/japplphysiol.01154.2005. [DOI] [PubMed] [Google Scholar]
- [38].Hintsala H, Kandelberg A, Herzig K, et al. Central aortic blood pressure of hypertensive men during short-term cold exposure. Am J Hypertens. 2014;27(5):656–664. doi: 10.1093/ajh/hpt136. [DOI] [PubMed] [Google Scholar]
- [39].Wilson TE, Crandall CG. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev. 2011;39(1):12–17. doi: 10.1097/JES.0b013e318201eed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jarvis SS, Okada Y, Levine BD, et al. Central integration and neural control of blood pressure during the cold pressor test: a comparison between hydrochlorothiazide and aliskiren. Physiol Rep. 2015;3(9). doi: 10.14814/phy2.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Siegrist PT, Gaemperli O, Koepfli P, et al. Repeatability of cold pressor test-induced flow increase assessed with H(2)(15)O and PET. J Nucl Med. 2006;47(9):1420–1426. [PubMed] [Google Scholar]
- [42].Hintsala HE, Kiviniemi AM, Tulppo MP, et al. Hypertension does not alter the increase in cardiac baroreflex sensitivity caused by Moderate Cold Exposure. Front Physiol. 2016;7:204. doi: 10.3389/fphys.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hintsala H, Kenttä TV, Tulppo M, et al. Cardiac repolarization and autonomic regulation during short-term cold exposure in hypertensive men: an experimental study. PLoS One. 2014;9(7):e99973. doi: 10.1371/journal.pone.0099973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sanchez-Gonzalez MA, Figueroa A. Cold exposure attenuates post exercise cardiovagal reactivation and sympathetic withdrawal. Auton Neurosci. 2013;176(1–2):95–97. doi: 10.1016/j.autneu.2013.02.002. [DOI] [PubMed] [Google Scholar]
- [45].Cui J, Durand S, Levine BD, et al. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol Heart Circ Physiol. 2005;289(6):2429. doi: 10.1152/ajpheart.00383.2005. [DOI] [PubMed] [Google Scholar]
- [46].Wilson TE, Gao Z, Hess KL, et al. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):1627. doi: 10.1152/ajpregu.00099.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yamazaki F, Sone R. Modulation of arterial baroreflex control of heart rate by skin cooling and heating in humans. J Appl Physiol. 2000;88(2):393–400. [DOI] [PubMed] [Google Scholar]
- [48].Heindl S, Struck J, Wellhöner P, et al. Effect of facial cooling and cold air inhalation on sympathetic nerve activity in men. Respir Physiol Neurobiol. 2004;142(1):69–80. doi: 10.1016/j.resp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- [49].Hilz MJ, Stemper B, Sauer P, et al. Cold face test demonstrates parasympathetic cardiac dysfunction in familial dysautonomia. Am J Physiol. 1999;276(6 Pt 2):1833. [DOI] [PubMed] [Google Scholar]
- [50].Muller MD, Gao Z, Mast JL, et al. Aging attenuates the coronary blood flow response to cold air breathing and isometric handgrip in healthy humans. Am J Physiol Heart Circ Physiol. 2012;302(8):1737. doi: 10.1152/ajpheart.01195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zeiher AM, Drexler H, Wollschlaeger H, et al. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol. 1989;14(5):1181–1190. doi: 10.1016/0735-1097(89)90414-2. [DOI] [PubMed] [Google Scholar]
- [52].Fairbairn TA, Motwani M, Mather AN, et al. Cardiac MR imaging to measure myocardial blood flow response to the cold pressor test in healthy smokers and nonsmokers. Radiology. 2014;270(1):82–90. doi: 10.1148/radiol.13122345. [DOI] [PubMed] [Google Scholar]
- [53].Ritter CO, Kowalski M, Weng AM, et al. Quantitative myocardial perfusion imaging with a MR cold pressor test. Magn Reson Med. 2012;67(1):246–250. doi: 10.1002/mrm.22941. [DOI] [PubMed] [Google Scholar]
- [54].Nabel EG, Ganz P, Gordon JB, et al. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77(1):43–52. doi: 10.1161/01.CIR.77.1.43. [DOI] [PubMed] [Google Scholar]
- [55].Mudge GH, Grossman W, Mills RM, et al. Reflex increase in coronary vascular resistance in patients with ischemic heart disease. N Engl J Med. 1976;295(24):1333–1337. doi: 10.1056/NEJM197612092952401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Muller MD, Gao Z, McQuillan PM, et al. Coronary responses to cold air inhalation following afferent and efferent blockade. Am J Physiol Heart Circ Physiol. 2014;307(2):228. doi: 10.1152/ajpheart.00174.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Matthews EL, Greaney JL, Wenner MM. Rapid onset pressor response to exercise in young women with a family history of hypertension. Exp Physiol. 2017;102(9):1092–1099. doi: 10.1113/EP086466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sevre K, Rostrup M. Blood pressure and heart rate responses to cold pressor test in patients admitted to hospital due to chest pain. Blood Press. 1999;8(2):110–113. doi: 10.1080/080370599438284. [DOI] [PubMed] [Google Scholar]
- [59].Lafleche AB, Pannier BM, Laloux B, et al. Arterial response during cold pressor test in borderline hypertension. Am J Physiol. 1998;275(2 Pt 2):409. [DOI] [PubMed] [Google Scholar]
- [60].Benetos A, Safar ME. Response to the cold pressor test in normotensive and hypertensive patients. Am J Hypertens. 1991;4(7 Pt 1):627–629. doi: 10.1093/ajh/4.7.627. [DOI] [PubMed] [Google Scholar]
- [61].Greaney JL, Kenney WL, Alexander LM. Neurovascular mechanisms underlying augmented cold-induced reflex cutaneous vasoconstriction in human hypertension. J Physiol (Lond). 2017;595(5):1687–1698. doi: 10.1113/JP273487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Seifert J, McNair M, Declercq P, et al. A heat and moisture mask attenuates cardiovascular stress during cold air exposure. Ther Adv Cardiovasc Dis. 2013;7(3):123–129. doi: 10.1177/1753944713481512. [DOI] [PubMed] [Google Scholar]
- [63].Komulainen S, Rintamäki H, Virokannas H, et al. Blood pressure responses to whole-body cold exposure: effect of metoprolol. J Hum Hypertens. 2004;18(12):905–906. doi: 10.1038/sj.jhh.1001758. [DOI] [PubMed] [Google Scholar]
- [64].Komulainen S, Oja T, Rintamäki H, et al. Blood pressure and thermal responses to whole body cold exposure in mildly hypertensive subjects. J Therm Biol. 2004;29(7):851–856. doi: 10.1016/j.jtherbio.2004.08.065. [DOI] [Google Scholar]
- [65].Komulainen S, Tähtinen T, Rintamäki H, et al. Blood pressure responses to whole-body cold exposure: effect of carvedilol. Eur J Clin Pharmacol. 2000;56(9–10):637–642. doi: 10.1007/s002280000208. [DOI] [PubMed] [Google Scholar]
- [66].Smith CJ, Santhanam L, Alexander LM. Rho-Kinase activity and cutaneous vasoconstriction is upregulated in essential hypertensive humans. Microvasc Res. 2013;87:58–64. doi: 10.1016/j.mvr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Radtke T, Poerschke D, Wilhelm M, et al. Acute effects of Finnish sauna and cold-water immersion on haemodynamic variables and autonomic nervous system activity in patients with heart failure. Eur J Prev Cardiol. 2016;23(6):593–601. doi: 10.1177/2047487315594506. [DOI] [PubMed] [Google Scholar]
- [68].Missmann M, Himsl M, Mur E, et al. Impact of Whole Body Cryotherapy at −110 °C on Subjects with Arterial Hypertension. Arch Immunol Ther Exp (Warsz). 2016;64(1):75–82. doi: 10.1007/s00005-015-0363-9. [DOI] [PubMed] [Google Scholar]
- [69].Zalewski P, Buszko K, Zawadka-Kunikowska M, et al. Cardiovascular and autonomic responses to whole-body cryostimulation in essential hypertension. Cryobiology. 2014;69(2):249–255. doi: 10.1016/j.cryobiol.2014.07.014. [DOI] [PubMed] [Google Scholar]
- [70].Kudaiberdieva G, Timuralp B, Ata N, et al. Cold exposure and left ventricular diastolic performance in coronary artery disease. Angiology. 2003;54(2):187–193. doi: 10.1177/000331970305400208. [DOI] [PubMed] [Google Scholar]
- [71].Greaney JL, Matthews EL, Wenner MM. Sympathetic reactivity in young women with a family history of hypertension. Am J Physiol Heart Circ Physiol. 2015;308(8):816. doi: 10.1152/ajpheart.00867.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. 2017;389(10064):37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kaplan NM, Vitor RG. Kaplan's clinical hypertension. 11th ed Philadelphia: Wolters Kluwer Health; 2015. [Google Scholar]
- [74].Sun Z. Cardiovascular responses to cold exposure. Front Biosci (Elite Ed). 2010;2:495–503. doi: 10.2741/e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Safar ME. Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol. 2017. doi: 10.1038/nrcardio.2017.155. [DOI] [PubMed] [Google Scholar]
- [76].Greaney JL, Kenney WL, Alexander LM. Sympathetic function during whole-body cooling is altered in hypertensive adults. J Appl Physiol. 2017. doi: 10.1152/japplphysiol.00613.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Li Y, Alshaer H, Fernie G. Blood pressure and thermal responses to repeated whole body cold exposure: effect of winter clothing. Eur J Appl Physiol. 2009;107(6):673–685. doi: 10.1007/s00421-009-1176-5. [DOI] [PubMed] [Google Scholar]
- [78].Girerd X, Chanudet X, Larroque P, et al. Early arterial modifications in young patients with borderline hypertension. J Hypertens Suppl. 1989;7(1):45. doi: 10.1097/00004872-198902001-00014. [DOI] [PubMed] [Google Scholar]
- [79].Willerson J, Maseri A, Armstrong P. Coronary heart disease syndromes: pathophysiology and clinical recognition. In: Cardiovascular medicine. 3rd ed London: Springer London; 2007. p. 667–698. [Google Scholar]
- [80].Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- [81].Areskog NH, Lassvik C. Angina pectoris in the cold. Arctic Med Res. 1988;47 Suppl 1:269–271. [PubMed] [Google Scholar]
- [82].Bhaskaran K, Hajat S, Haines A, et al. Effects of ambient temperature on the incidence of myocardial infarction. Heart. 2009;95(21):1760–1769. doi: 10.1136/hrt.2009.175000. [DOI] [PubMed] [Google Scholar]
- [83].Ryti NRI, Mäkikyrö EMS, Antikainen H, et al. Cold spells and ischaemic sudden cardiac death: effect modification by prior diagnosis of ischaemic heart disease and cardioprotective medication. Sci Rep. 2017;7:41060. doi: 10.1038/srep41060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Neill WA, Duncan DA, Kloster F, et al. Response of coronary circulation to cutaneous cold. Am J Med. 1974;56(4):471–476. doi: 10.1016/0002-9343(74)90478-1. [DOI] [PubMed] [Google Scholar]
- [85].Rodger JC, Railton R, Parekh P, et al. Effect of cold stimulation on myocardial perfusion. An investigation using thallium-201 scintigraphy. Br Heart J. 1984;52(1):57–62. doi: 10.1136/hrt.52.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zeiher AM, Drexler H, Wollschläger H, et al. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84(5):1984–1992. doi: 10.1161/01.CIR.84.5.1984. [DOI] [PubMed] [Google Scholar]
- [87].Feldman RL, Whittle JL, Marx JD, et al. Regional coronary hemodynamic responses to cold stimulation in patients without variant angina. Am J Cardiol. 1982;49(4):665–673. doi: 10.1016/0002-9149(82)91944-0. [DOI] [PubMed] [Google Scholar]
- [88].Park SM, Shim WJ, Ahn JC, et al. Changes of coronary blood flow in vasospastic angina under cold stimulation by transthoracic Doppler echocardiography. J Korean Med Sci. 2005;20(2):204–208. doi: 10.3346/jkms.2005.20.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hattenhaur M, Neill WA. The effect of cold air inhalation on again pectoris and myocardial oxygen supply. Circulation. 1975;51(6):1053–1058. doi: 10.1161/01.CIR.51.6.1053. [DOI] [PubMed] [Google Scholar]
- [90].Shea MJ, Deanfield JE, deLandsheere CM, et al. Asymptomatic myocardial ischemia following cold provocation. Am Heart J. 1987;114(3):469–476. doi: 10.1016/0002-8703(87)90740-X. [DOI] [PubMed] [Google Scholar]
- [91].Pearse SG, Cowie MR. Heart failure: classification and pathophysiology. Medicine. 2014;42(10):556–561. doi: 10.1016/j.mpmed.2014.07.012. [DOI] [Google Scholar]
- [92].McMurray JJV, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- [93].Zelis R, Mason DT, Braunwald E. A comparison of the effects of vasodilator stimuli on peripheral resistance vessels in normal subjects and in patients with congestive heart failure. J Clin Invest. 1968;47(4):960–970. doi: 10.1172/JCI105788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Meyer P, Guiraud T, Curnier D, et al. Exposure to extreme cold lowers the ischemic threshold in coronary artery disease patients. Can J Cardiol. 2010;26(2):50. doi: 10.1016/S0828-282X(10)70007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Dodds PA, Bellamy CM, Muirhead RA, et al. Vasoconstrictor peptides and cold intolerance in patients with stable angina pectoris. Br Heart J. 1995;73(1):25–31. doi: 10.1136/hrt.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Marchant B, Donaldson G, Mridha K, et al. Mechanisms of cold intolerance in patients with angina. J Am Coll Cardiol. 1994;23(3):630–636. doi: 10.1016/0735-1097(94)90747-1. [DOI] [PubMed] [Google Scholar]
- [97].Pétersen CL, Hansen A, Frandsen E, et al. Endothelin release and enhanced regional myocardial ischemia induced by cold-air inhalation in patients with stable angina. Am Heart J. 1994;128(3):511–516. doi: 10.1016/0002-8703(94)90624-6. [DOI] [PubMed] [Google Scholar]
- [98].Peart I, Bullock RE, Albers C, et al. Cold intolerance in patients with angina pectoris: effect of nifedipine and propranolol. Br Heart J. 1989;61(6):521–528. doi: 10.1136/hrt.61.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Juneau M, Johnstone M, Dempsey E, et al. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation. 1989;79(5):1015–1020. doi: 10.1161/01.CIR.79.5.1015. [DOI] [PubMed] [Google Scholar]
- [100].Nesto RW, Lamas GA, Barry J. Paradoxical elevation of threshold to angina pectoris by cold pressor test in men with significant coronary artery disease. Am J Cardiol. 1989;63(11):656–659. doi: 10.1016/0002-9149(89)90246-4. [DOI] [PubMed] [Google Scholar]
- [101].de Servi S, Mussini A, Angoli L, et al. Effects of cold stimulation on coronary haemodynamics during exercise in patients with coronary artery disease. Eur Heart J. 1985;6(3):239–246. doi: 10.1093/oxfordjournals.eurheartj.a061847. [DOI] [PubMed] [Google Scholar]
- [102].de Servi S, Mussini A, Specchia G, et al. Effects of nifedipine on coronary blood flow and coronary resistance during cold pressor test and isometric exercise in patients with coronary artery disease. Eur Heart J. 1980;1(Suppl B):43–47. doi: 10.1093/eurheartj/1.suppl_2.43. [DOI] [PubMed] [Google Scholar]
- [103].Lassvik C, Areskog NH. Angina pectoris during inhalation of cold air. Reactions to exercise. Br Heart J. 1980;43(6):661–667. doi: 10.1136/hrt.43.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Backman C, Holm S, Linderholm H. Reaction to cold of patients with coronary insufficiency. Ups J Med Sci. 1979;84(2):181–187. doi: 10.3109/03009737909179154. [DOI] [PubMed] [Google Scholar]
- [105].Lassvik C, Areskog NH. Effects of various environmental temperatures on effort angina. Ups J Med Sci. 1979;84(2):173–180. doi: 10.3109/03009737909179153. [DOI] [PubMed] [Google Scholar]
- [106].Lassvik CT, Areskog NH. Angina in cold environment. Reactions to exercise. Br Heart J. 1979;42(4):396–401. doi: 10.1136/hrt.42.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Epstein SE, Stampfer M, Beiser GD, et al. Effects of a reduction in environmental temperature on the circulatory response to exercise in man. Implications concerning angina pectoris. N Engl J Med. 1969;280(1):7–11. doi: 10.1056/NEJM196901022800102. [DOI] [PubMed] [Google Scholar]
- [108].Schmid J, Morger C, Noveanu M, et al. Haemodynamic and arrhythmic effects of moderately cold (22 degrees C) water immersion and swimming in patients with stable coronary artery disease and heart failure. Eur J Heart Fail. 2009;11(9):903–909. doi: 10.1093/eurjhf/hfp114. [DOI] [PubMed] [Google Scholar]
- [109].Blanchet M, Ducharme A, Racine N, et al. Effects of cold exposure on submaximal exercise performance and adrenergic activation in patients with congestive heart failure and the effects of beta-adrenergic blockade (carvedilol or metoprolol). Am J Cardiol. 2003;92(5):548–553. doi: 10.1016/S0002-9149(03)00723-9. [DOI] [PubMed] [Google Scholar]
- [110].Juneau M, Larivée L, White M. Cold temperature impairs maximal exercise performance in patients with heart failure: attenuation by acute ACE inhibitor therapy. Can J Cardiol. 2002;18(9):981–986. [PubMed] [Google Scholar]
- [111].Rosengren A, Wennerblom B, Bjurö T, et al. Effects of cold on ST amplitudes and blood pressure during exercise in angina pectoris. Eur Heart J. 1988;9(10):1074–1080. doi: 10.1093/oxfordjournals.eurheartj.a062402. [DOI] [PubMed] [Google Scholar]
- [112].Brown CF, Oldridge NB. Exercise-induced angina in the cold. Med Sci Sports Exerc. 1985;17(5):607–612. doi: 10.1249/00005768-198510000-00015. [DOI] [PubMed] [Google Scholar]
- [113].Bruce RA, Blackmon JR, Jones JW, et al. Exercising testing in adult normal subjects and cardiac patients. Pediatrics. 1963;32:756. [PubMed] [Google Scholar]
- [114].Steenman M, Lande G. Cardiac aging and heart disease in humans. Biophys Rev. 2017;9(2):131–137. doi: 10.1007/s12551-017-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Monahan KD, Feehan RP, Sinoway LI, et al. Contribution of sympathetic activation to coronary vasodilatation during the cold pressor test in healthy men: effect of ageing. J Physiol (Lond). 2013;591(11):2937–2947. doi: 10.1113/jphysiol.2013.251298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Naito R, Miyauchi K. Coronary artery disease and type 2 diabetes mellitus. Int Heart J. 2017;58(4):475–480. doi: 10.1536/ihj.17-191. [DOI] [PubMed] [Google Scholar]
- [117].Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380(9841):601–610. doi: 10.1016/S0140-6736(12)60987-8. [DOI] [PubMed] [Google Scholar]
- [118].Kenny GP, Sigal RJ, McGinn R. Body temperature regulation in diabetes. Temperature (Austin). 2016;3(1):119–145. doi: 10.1080/23328940.2015.1131506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Barnett AG, Sans S, Salomaa V, et al. The effect of temperature on systolic blood pressure. Blood Press Monit. 2007;12(3):195–203. doi: 10.1097/MBP.0b013e3280b083f4. [DOI] [PubMed] [Google Scholar]
- [120].Vitale J, Manfredini R, Gallerani M, et al. Chronobiology of acute aortic rupture or dissection: a systematic review and a meta-analysis of the literature. Chronobiol Int. 2015;32(3):385–394. doi: 10.3109/07420528.2014.983604. [DOI] [PubMed] [Google Scholar]
- [121].Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension. 2010;55(1):9–14. doi: 10.1161/HYPERTENSIONAHA.107.090464. [DOI] [PubMed] [Google Scholar]
- [122].Shattock MJ, Tipton MJ. ‘Autonomic conflict’: a different way to die during cold water immersion? J Physiol. 2012;590(14):3219–3230. doi: 10.1113/jphysiol.2012.229864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Raizner AE, Chahine RA, Ishimori T, et al. Provocation of coronary artery spasm by the cold pressor test. Hemodynamic, arteriographic and quantitative angiographic observations. Circulation. 1980;62(5):925–932. doi: 10.1161/01.CIR.62.5.925. [DOI] [PubMed] [Google Scholar]
- [124].Neild PJ, Syndercombe-Court D, Keatinge WR, et al. Cold-induced increases in erythrocyte count, plasma cholesterol and plasma fibrinogen of elderly people without a comparable rise in protein C or factor X. Clin Sci (London, England: 1979). 1994;86(1):43–48. doi: 10.1042/cs0860043. [DOI] [PubMed] [Google Scholar]
- [125].Nagelkirk PR, Hogan KB, Hoare JM. Ambient temperature affects thrombotic potential at rest and following exercise. Thromb Res. 2012;130(2):248–252. doi: 10.1016/j.thromres.2011.10.015. [DOI] [PubMed] [Google Scholar]
- [126].Anderson BG, Bell ML. Weather-related mortality: how heat, cold, and heat waves affect mortality in the United States. Epidemiology. 2009;20(2):205–213. doi: 10.1097/EDE.0b013e318190ee08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wang Q, Li C, Guo Y, et al. Environmental ambient temperature and blood pressure in adults: a systematic review and meta-analysis. Sci Total Environ. 2017;575:276–286. doi: 10.1016/j.scitotenv.2016.10.019. [DOI] [PubMed] [Google Scholar]
- [128].Ghebre MA, Wannamethee SG, Rumley A, et al. Prospective study of seasonal patterns in hemostatic factors in older men and their relation to excess winter coronary heart disease deaths. J Thromb Haemost. 2012;10(3):352–358. doi: 10.1111/j.1538-7836.2012.04617.x. [DOI] [PubMed] [Google Scholar]
- [129].Stout RW, Crawford V. Seasonal variations in fibrinogen concentrations among elderly people. Lancet. 1991;338(8758):9–13. doi: 10.1016/0140-6736(91)90004-9. [DOI] [PubMed] [Google Scholar]
- [130].Marti-Soler H, Gubelmann C, Aeschbacher S, et al. Seasonality of cardiovascular risk factors: an analysis including over 230 000 participants in 15 countries. Heart. 2014;100(19):1517–1523. doi: 10.1136/heartjnl-2014-305623. [DOI] [PubMed] [Google Scholar]
- [131].Corrà U, Piepoli MF, Carré F, et al. Secondary prevention through cardiac rehabilitation: physical activity counselling and exercise training: key components of the position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur Heart J. 2010;31(16):1967–1974. doi: 10.1093/eurheartj/ehq236. [DOI] [PubMed] [Google Scholar]
- [132].Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015;201(Suppl 1):1. doi: 10.1016/S0167-5273(15)31026-3. [DOI] [PubMed] [Google Scholar]
- [133].Schneider A, Rückerl R, Breitner S, et al. Thermal control, weather, and aging. Curr Environ Health Rep. 2017;4(1):21–29. doi: 10.1007/s40572-017-0129-0. [DOI] [PubMed] [Google Scholar]


