Abstract
Background
The cost of conventional serological testing for toxoplasmosis discourages universal adoption of prenatal monthly screening programs to prevent congenital toxoplasmosis. Point-of-care (POC) technology may constitute a cost-effective approach.
Methods
We evaluated the diagnostic accuracy of 3 Toxoplasma POC tests against gold-standard testing performed at Palo Alto Medical Foundation Toxoplasma Serology Laboratory (PAMF-TSL). The POC tests included the following: Toxo IgG/IgM Rapid Test (Biopanda) and the OnSite Toxo IgG/IgM Combo-Rapid-test that detect IgG and IgM separately, and the Toxoplasma ICT-IgG-IgM-bk (LDBIO) that detects either or both immunoglobulin IgG/IgM in combination. Samples were selected from PAMF-TSL biobank (n = 210) and Centers for Disease Control and Prevention Toxoplasma 1998 Human Serum Panel (n = 100). Based on PAMF-TSL testing, Toxoplasma-infection status was classified in 4 categories: acute infections (n = 85), chronic infections (n = 85), false-positive Toxoplasma IgM (n = 60), and seronegative (n = 80). The POC testing was performed in duplicate following manufacturer’s instructions by investigators blinded to PAMF-TSL results. Sensitivity and specificity were calculated.
Results
A total of 1860 POC tests were performed. For detection of Toxoplasma IgG, sensitivity was 100% (170 of 170; 95% confidence interval [CI], 97.8%–100%) for all 3 POC kits; specificity was also comparable at 96.3% (77 of 80; 95% CI, 89.5%–98.9%), 97.5% (78 of 80; 95% CI, 91.3%–99.6%), and 98.8% (79 of 80; 95% CI, 93.2%–99.9%). However, sensitivity for detection of Toxoplasma IgM varied significantly across POC tests: Biopanda, 62.2% (51 of 82; 95% CI, 51.4%–71.9%); OnSite, 28% (23 of 82; 95% CI, 19.5%–38.6%); and LDBIO combined IgG/IgM, 100% (82 of 82; 95% CI, 95.5%–100%). Diagnostic accuracy was significantly higher for the LDBIO POC kit. The POC kits did not exhibit cross-reactivity for false-positive Toxoplasma-IgM sera.
Conclusions
The 3 evaluated POC kits revealed optimal sensitivity for Toxoplasma-IgG antibodies. The LDBIO-POC test exhibited 100% sensitivity for the combined detection of IgG/IgM in acute and chronic Toxoplasma infection. Biopanda and Onsite POC tests exhibited poor sensitivity for Toxoplasma-IgM detection.
Keywords: immunoglobulin G, immunoglobulin M, Toxoplasma gondii, toxoplasmosis, point-of-care testing
Toxoplasma gondii, the causative agent of toxoplasmosis, is an obligate intracellular protozoan of worldwide distribution that infects one third of the world’s human population [1]. When T gondii infection occurs in immunocompetent patients, it typically follows an asymptomatic and benign clinical course, whereas in immunocompromised hosts, toxoplasmosis can lead to life-threatening manifestations if diagnosed or treated late [2]. Of note, severe presentations of the acute infection in immunocompetent patients have been reported in association with atypical genotypes of the parasite [3, 4]. Primary T gondii infection that occurs during pregnancy or within 3 months before conception can result in congenital toxoplasmosis (CT) if not promptly diagnosed and treated [5]. Congenital toxoplasmosis can have devastating consequences for the fetus including stillbirth, neurological sequelae, and severe ocular disease [5]. The risk of CT and the severity of its clinical manifestations are influenced mainly by the gestational age at the time of maternal infection, T gondii strain, and antenatal anti-Toxoplasma treatment [6]. Maternal T gondii infections are usually asymptomatic; approximately half of T gondii-infected pregnant women who gave birth to infants with CT did not report any risk factors or any symptoms suggestive of infection [7]. Thus, timely diagnosis of T gondii infection during pregnancy relies largely on serological testing throughout gestation in the context of universal prenatal screening programs [8].
Maternal screening programs for CT have been widely adopted in France, Austria, Germany, and other European countries [9–11]. These programs consist of serial serological screening (eg, monthly in France, bimonthly in Austria, and every 2–3 months in Germany) of Toxoplasma-seronegative pregnant women coupled with prompt initiation of anti-toxo-plasma treatment upon maternal seroconversion [9–13]. Several observational studies over the past decade concluded that antenatal anti-Toxoplasma therapy reduces rates of mother-to-child transmission [9–11, 14] and mitigates the severity of clinical manifestations among congenitally infected infants [9, 11, 15–17]. Early initiation of treatment, within 3–4 weeks from maternal infection, is also critical [14, 16]. However, despite the fact that CT is a preventable and treatable condition, most pregnant women in the United States and worldwide are not routinely screened and treated accordingly for Toxoplasma infections [18]. In the United States, CT is usually diagnosed upon appearance of clinical and/or ultrasound findings in the fetus or at birth [5]. Consequently, reported cases of CT in the United States have higher rates of severe clinical manifestations at birth (ie, chorioretinitis, intracranial calcifications, and hydrocephalus) compared with their European counterparts [19–21].
Because approximately 5 to 8 serological tests would be performed in each Toxoplasma-seronegative woman, a major factor impeding uptake of monthly maternal screening programs worldwide is high cost of conventional serological testing, which is generally performed at commercial laboratories using automated platforms [22, 23]. Indeed, a decision-analytic economic model in the United States showed that universal monthly maternal screening can be cost saving in the United States, even with an incidence of acute maternal T gondii infection as low as 0.2 per 1000 pregnant women, if screening test costs ~$12.00 per test, a figure far below conventional laboratory-based testing cost [24]. Moreover, in Europe, and France in particular, where Toxoplasma-seroprevalence in the general population has declined (from 80% in 1960 to 36.7% in 2010) [25], numbers of seronegative pregnant women necessitating screening has increased, thereby endangering further financial viability of screening programs [25, 26]. Hence, alternative diagnostic tools are needed for toxoplasmosis diagnostics.
Point-of-care (POC) rapid tests have improved access to efficient diagnostics across infectious diseases and global health, especially in low-income country settings [27, 28]. Point-of-care technology for antibody detection usually consists of immunochromatographic (ICT) membrane-based assays that can be performed without sophisticated laboratory infrastructure. Previous studies have shown optimal diagnostic performance for the Toxoplasma ICT IgG-IgM (LDBIO Diagnostics, Lyon, France) POC kit when compared with reference-serological methods in France [29, 30] and the United States [31]. However, direct comparisons of performance of various POC kits against reference-standard methods used in the United States, where T gondii strains are more diverse, have not been previously performed.
In this study, we sought to evaluate diagnostic accuracy for 3 Toxoplasma-IgG-IgM POC kits using a large number of sera from US patients previously tested by reference methods available at Palo Alto Medical Foundation, Toxoplasma Serology Laboratory ([PAMF-TSL] http://www.pamf.org/serology/), the reference laboratory for study and diagnosis of toxoplasmosis in the United States.
METHODS
Serological Samples
A total of 310 patient serum samples tested at PAMF-TSL were included: 210 were obtained from the PAMF-TSL biobank, and 100 from the Centers for Disease Control and Prevention (CDC) Toxoplasma 1998 Human Serum Panel (CDC-HSP) (Table 1). The PAMF-TSL samples were selected from archived specimens submitted between from February 27, 2013 to June 23, 2017. The CDC-HSP samples were selected from a biobank that contains known positive and negative sera available to researchers and laboratories in the United States. These samples are obtained by the CDC to help evaluate accuracy of commercial Toxoplasma antibody test kits (https://www.cdc.gov/dpdx/toxoplasmosis/index.html) and were submitted to investigators in a blinded fashion. All serum samples were previously tested at PAMF-TSL by gold-standard Toxoplasma-IgG method (Sabin-Feldman Dye test [DT]), double-sandwich immunoglobulin (Ig)M enzyme-linked immunosorbent assay, and additional confirmatory tests available only at PAMF-TSL.
Table 1.
Description and Source of Serum Samples
| Toxoplasma-Infection Status | Source | Total No. Samples | PAMF-TSL Criteriaa |
|---|---|---|---|
| Acute-Toxoplasma infection (n = 85) |
PAMF-TSL CDC-HSP |
50 35 |
IgG+/IgM+ Positive IgG Dye test plus positive IgM-ELISA |
| Chronic-Toxoplasma infection (n = 85) |
PAMF-TSL CDC-HSP |
50 35 |
IgG+/IgM− Positive IgG Dye test plus negative IgM-ELISA |
|
Toxoplasma seronegative (n = 80) |
PAMF-TSL CDC-HSP |
50 30 |
IgG−/IgM− Negative IgG Dye test plus negative IgM-ELISA |
| False-positive IgM (n = 60) |
PAMF-TSL | 60 | IgG−/IgM+ Sera from 33 patients who tested repeatedly positive by IgM-ELISA but failed to show IgG seroconversion (by Dye test) at follow-up testing (≥3 weeks from baseline) |
| Total samples (n = 310) |
PAMF-TSL CDC-HSP |
210 100 |
Toxoplasma-IgG+: 170/Toxoplasma-IgG−: 80 Toxoplasma-IgM+: 85/Toxoplasma-IgM−: 165 |
Abbreviations: CDC-HSP, Centers for Disease Control and Prevention Toxoplasma 1998 Human Serum Panel; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; IQR, interquartile range; PAMF-TSL, Palo Alto Medical Foundation, Toxoplasma Serology Laboratory.
aPAMF-TSL serological testing include IgG Dye test (positive ≥1:16 dilutions; negative <1:16 dilutions); IgM-ELISA (positive ≥ 2.0 units; indeterminate 1.7–1.9 units; and negative ≤1.6 units). All samples from patients with acute-Toxoplasma infection from PAMF-TSL had low IgG avidity (by VIDAS Toxo-IgG Avidity kit; bioMérieux, Marcy-l’Etoile, France); low avidity (cutoff <20%; median, 8.3%; IQR, 4.4%–13.3%; tested) and an acute pattern result when tested with the differential agglutination test (AC/HS test).
The PAMF-TSL samples (n = 210) were divided into 4 different groups: (1) acute-Toxoplasma infection (Toxoplasma-IgG+/IgM+ confirmed with additional testing [IgG-avidity, differential agglutination {AC/HS}, Toxoplasma-IgA, and Toxoplasma-IgE] indicative of a recently acquired infection) (n = 50); (2) chronic-Toxoplasma infection (Toxoplasma-IgG+/IgM−) (n = 50); (3) seronegative samples (Toxoplasma-IgG−/IgM−, never infected with T gondii) (n = 50); and (4) false-positive (FP) Toxoplasma-IgM (Toxoplasma-IgG−/IgM+) (n = 60) [23]. False-positive Toxoplasma-IgM serum samples were considered samples that tested repeatedly positive by IgM-enzyme-linked immunosorbent assay ([ELISA] at least in 2 separate test dates separated ≥3 weeks apart) but failed to show IgG seroconversion (when tested by DT) at follow-up testing at PAMF-TSL. In addition to IgM-ELISA and Toxoplasma-IgG DT, all of these samples were also tested with additional diagnostic tests, including differential agglutination (AC/HS), Toxoplasma-IgA, and Toxoplasma-IgE, and were all negative. Moreover, final interpretation of serological results from PAMF-TSL’s consulting physician (J.G.M.) stated that the serologic test results for these samples were compatible with FP Toxoplasma-IgM.
Of 100 CDC-HSP samples, 35 were established to have acute Toxoplasma infection, 35 to have chronic infection, and 30 uninfected. The POC testing results for CDC-HSP samples were returned to the CDC for data analysis and calculation of IgG and IgM diagnostic sensitivity and specificity for each POC kit.
Point-of-Care Kits
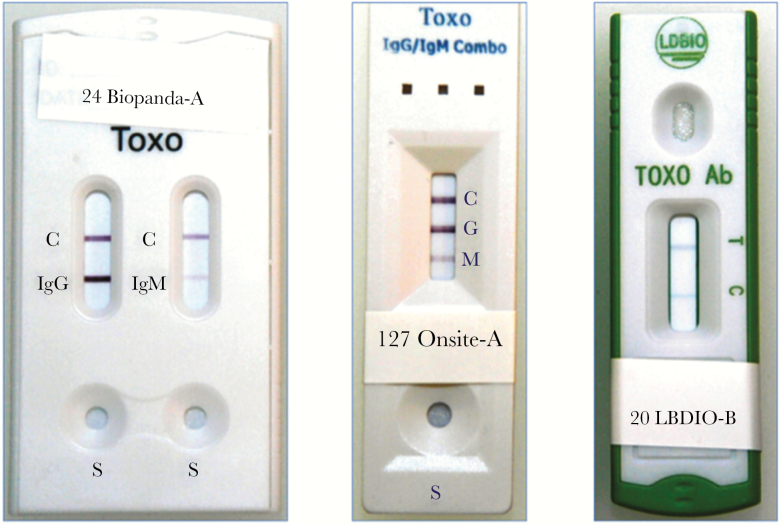
Three POC kits were included: (1) Toxo IgG/IgM Rapid Test (Biopanda Reagents, Belfast, UK) (Biopanda POC test); (2) OnSite Toxo IgG/IgM Combo Rapid Test (CTK-Biotech, San Diego, CA) (OnSite POC test); and (3) Toxoplasma ICT IgG-IgM (LDBIO Diagnostics, Lyon, France) (LDBIO POC test). All 3 POC kits are based on a lateral-flow immunochromatography method that uses a nitrocellulose membrane strip containing a test (T) and control (C) band. For the Biopanda POC and OnSite POC tests, their cassette arrangement allows detection and differentiation of Toxoplasma-IgG and Toxoplasma-IgM antibodies in 2 different testing bands (IgG and IgM). For the LDBIO POC kit, test band (T band) simultaneously detects Toxoplasma-IgG and Toxoplasma-IgM not allowing their differentiation (Figure 1). The POC testing and interpretation of results were performed (1) following manufacturer’s instructions and (2) in duplicates by 2 independent investigators (L.N.B. and C.A.G.). Final readings were ascertained by these (L.N.B. and C.A.G.) and 2 additional investigators (J.G.M. and D.G.C.-I.). All investigators performing and reading results (L.N.B., C.A.G., J.G.M., and D.G.C.-I.) were blinded to both PAMF-TSL’s and CDC’s results when POC tests were performed.
Figure 1.
Toxoplasma point-of-care (POC) kits for detection of Toxoplasma immunoglobulin IgG and IgM antibodies. (Left) Biopanda POC test Toxo IgG/IgM Rapid Test (Biopanda Reagents, Belfast, UK) cassette showing 2 separate strips for Toxoplasma gondii IgG and IgM detection, each one with a control band (C) (sample no. 24: positive IgG, positive IgM). (Center) OnSite POC test Toxo IgG/IgM Combo Rapid test (CTK Biotech, San Diego, CA) cassette showing 1 single testing strip with 3 separate testing bands for control (C), T gondii IgG (G) and IgM (M) antibodies (from top to bottom) (sample no. 127: positive IgG, positive IgM). (Right) LDBIO POC test Toxoplasma ICT IgG and IgM (LDBIO Diagnostics, Lyon, France) cassette showing 1 testing strip with a testing band for simultaneous detection of T gondii IgG/IgM antibodies (T) and a control band (C) (sample no. 20: positive combined IgG/IgM).
Serological Testing at Palo Alto Medical Foundation Toxoplasma Serology Laboratory
Toxoplasma-IgG testing was performed using Sabin-Feldman IgG DT, the gold-standard method for Toxoplasma-IgG detection in the United States. The DT is a very sensitive and specific test in which live tachyzoites are lysed in the presence of complement and T gondii-specific IgG derived from patients’ samples. Toxoplasma-IgM testing was performed using a laboratory-developed, double-sandwich IgM-ELISA that uses sonicated T gondii antigen prepared from live tachyzoites (www.pamf.org/serology/clinicianguide.html). When indicated (eg, sera with positive Toxoplasma-IgM test results), additional tests such as Toxoplasma-IgG avidity, differential agglutination (AC/HS), Toxoplasma-IgA, and Toxoplasma-IgE, were performed by PAMF-TSL [23]. According to PAMF-TSL results, sera from PAMF-TSL biobank and CDC were classified in 4 groups, described above under “Serological Samples”: (1) acute Toxoplasma infection; (2) chronic Toxoplasma infection; (3) seronegative samples uninfected with T gondii; and (4) FP Toxoplasma-IgM.
Statistical Analysis
Sensitivity, specificity, positive predicted values (PPVs), and negative predicted values (NPVs) were calculated against PAMF-TSL IgG and IgM test results, respectively. Diagnostic accuracy was calculated as proportion of correctly classified tests (true positive [TP]+true negative [TN]) among all tests performed (TP+TN+FP+false negative [FN]) [32], as suggested by the Standards for Reporting of Diagnostic Accuracy (STARD) statement [33]. Continuous variables were compared using Student t test or Wilcoxon signed-rank test. All tests for significance were 2-sided, and P values ≤.05 were considered significant. Statistical analysis was performed using Prism 7.0 software (GraphPad, La Jolla, CA).
RESULTS
A total of 1860 POC tests (310 samples × 3 POC kits × 2 [duplicate testing of each POC kit]) were performed.
Diagnostic Accuracy of Point-of-Care Testing for Toxoplasma-Immunoglobulin G
Sensitivity calculations for Toxoplasma-IgG detection were done for 170 sera (85 from PAMF-TSL biobank and 85 from CDC-HSP). Sensitivity was 100% for all 3 POC kits (95% confidence interval [CI], 97.8%–100%; 170 of 170). Specificity for Toxoplasma-IgG detection was comparable among all 3 POC kits: 96.3% (95% CI, 89.5%–98.9%; 77 of 80), 97.5% (95% CI, 91.3%–99.6%; 78 of 80), and 98.8% (95% CI, 93.2%–99.9%; 79 of 80), for Biopanda, OnSite, and LDBIO POC tests, respectively (Tables 2–4). Diagnostic accuracy for IgG detection was 98.8% (247 of 250), 99.2% (248 of 250), and 99.6% (249 of 250) for Biopanda, OnSite, and LDBIO POC tests, respectively.
Table 2.
Results of Point-of-Care Testing for Detection of Toxoplasma IgG and/or IgM Antibodies
| POC Kit | Toxoplasma Antibodies | IgG+ (Positives/Reference Testing at PAMF-TSL) (%) |
IgG−/IgM− (Positives/Reference Testing at PAMF-TSL) (%) |
Toxoplasma Antibodies | IgM+a (Positives/Reference Testing at PAMF-TSL) (%) |
IgM−a (Positives/Reference Testing at PAMF-TSL) (%) |
|---|---|---|---|---|---|---|
| Biopanda | IgG | 170 of 170 (100) |
3 of 80 (3.8) |
IgM | 51 of 82b (62.2) |
19 of 165 (11.5) |
| OnSite | IgG | 170 of 170 (100) |
2 of 80 (2.5) |
IgM | 23 of 82b (28) |
4 of 165 (2.4) |
| LDBIOc | IgG/IgM Combined | 170 of 170 (100) |
1 of 80 (1.3) |
IgG/IgM Combined | 82 of 82b (100) |
NA |
Abbreviations: CDC, Centers for Disease Control and Prevention; CDC-HSP, CDC Toxoplasma 1998 Human Serum Panel; Ig, immunoglobulin; NA, not applicable; PAMF-TSL, Palo Alto Medical Foundation, Toxoplasma Serology Laboratory; POC, point of care.
aAll IgM positive sera were also IgG positive by reference testing. The IgM negative sera were either IgG+IgM− sera (n = 85) or IgG−IgM− sera (n = 80).
bThree of 85 samples (from the CDC-HSP 1998) were excluded from the calculation of analytical sensitivity for IgM that were provided to us by the CDC report, because PAMF-TSL IgM-ELISA results were <2.0 units (cutoff for positive).
cLDBIO POC test detects in combination Toxoplasma IgG/IgM.
Table 4.
Diagnostic Accuracy of Three POC Kits for Toxoplasma IgG and IgM Detection
| POC Kit | Toxoplasma Antibodies | Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) | NPVb (%) (95% CI) |
PPVb (%) (95% CI) |
|---|---|---|---|---|---|
| Biopanda | IgG | 100 (97.8–100) |
96.3 (89.5–98.9) | 100 (95.3–100) | 98.3 (95.0–99.5) |
| IgM | 62.2 (51.4–71.9) |
88.5 (82.7–92.5) |
82.5 (76.2–87.4) | 72.9 (61.5–81.9) |
|
| OnSite | IgG | 100 (97.8–100) | 97.5 (91.3–99.6) |
100 (95.3–100) | 98.8 (95.8–99.8) |
| IgM | 28.0 (19.5–38.6) |
97.6 (93.9–99.1) |
73.2 (66.9–78.6) |
85.2 (67.5–94.1) |
|
| LDBIOa | IgG/IgM Combined | 100 (97.8–100) |
98.8 (93.2–99.9) |
100 (95.4–100) | 99.4 (96.8–99.9) |
Abbreviations: CI, confidence interval; Ig, immunoglobulin; POC, point of care; NPV, negative predictive value; PPV, positive predictive value.
aAs the LDBIO-POC test reports Toxoplasma IgG and IgM antibodies combined in a single testing band (T band), specificity for LDBIO-POC test was calculated over 80 seronegative sera (Toxoplasma-IgG−/IgM−)
bThe above PPV and NPV for the 3 POC tests are representative of the performance of these tests in a group of samples in which 33% were Toxoplasma IgM+ and 68% were Toxoplasma IgG+. In pragmatic clinical settings, the prevalence of Toxoplasma IgM+ individuals is significantly lower (usually <1% [<10 of 1000]).
Detailed serological results for Toxoplasma-IgG and Toxoplasma-IgM titers were available only for sera from PAMF-TSL biobank. For the 100 Toxoplasma-IgG+ patients from PAMF-TSL biobank, median IgG DT titer was 1:512 (range, 1:32 to 1:32000). The IgG titers from patients with acute Toxoplasma infection were significantly higher compared with patients with chronic Toxoplasma infection (median 8000 [interquartile range {IQR}, 2048–8000] vs 192 [IQR, 64–512], respectively; P < .0001). Of note, all 3 POC kits showed optimal sensitivity for IgG detection across all tiers of IgG DT titers. Diagnostic performance for all POC kits according to infection status is shown in Table 3.
Table 3.
Results of Point-of-Care Testing for Detection of Toxoplasma IgG/IgM per Toxoplasma Infection Status
| POC Kit |
Toxoplasma Antibodies | POC Result | Toxoplasma Infection Status | ||
|---|---|---|---|---|---|
| Acute Infection (IgG+/IgM+) n = 85a (%) |
Chronic Infection (IgG+/IgM−) n = 85 (%) |
Seronegative (IgG−/IgM−) n = 80 (%) |
|||
| Biopanda |
IgG | Positive | 85 (100) | 85 (100) | 3 (3.8) |
| Negative | 0 (0) | 0 (0) | 77 (96.3) | ||
| IgM | Positive | 51 (62.2) | 16 (18.9) | 3 (3.8) | |
| Negative | 31 (37.8) | 69 (81.1) | 77 (96.2) | ||
| OnSite |
IgG | Positive | 85 (100) | 85 (100) | 2 (2.5) |
| Negative | 0 (0) | 0 (0) | 78 (97.5) | ||
| IgM | Positive | 23 (28.0) | 3 (3.5) | 1 (1.3) | |
| Negative | 59 (72.0) | 82 (96.5) | 79 (98.7) | ||
| LDBIO |
IgG/IgM Combined |
Positive | 85 (100) | 85 (100) | 1 (1.3) |
| Negative | 0 (0) | 0 (0) | 79 (98.7) | ||
Abbreviations: CDC, Centers for Disease Control and Prevention; CDC-HSP, CDC Toxoplasma 1998 Human Serum Panel; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin; PAMF-TSL, Palo Alto Medical Foundation, Toxoplasma Serology Laboratory; POC, point of care.
a Three of 85 samples (from the CDC-HSP 1998) were excluded from the calculation of analytical sensitivity for IgM that were provided to us by the CDC report, because PAMF-TSL IgM-ELISA results were <2.0 units (cutoff for positive).
Diagnostic Accuracy of Point-of-Care Testing for Toxoplasma-Immunoglobulin M
Sensitivity calculations for detection of Toxoplasma-IgM was performed for 82 (instead of 85) Toxoplasma-IgM-positive serum samples because 3 of 35 positive Toxoplasma-IgM samples from the CDC-HSP serum panel were dilutions of 3 true Toxoplasma-IgM-positives specimens, and their corresponding PAMF-TSL IgM-ELISA results were <2.0 (0.5, 0.8, and 1.6 units). Therefore, these 3 samples were excluded from sensitivity calculation in results reported to us by the CDC (Tables 2 and 3).
Sensitivity for Toxoplasma-IgM varied significantly across POC kits: Biopanda POC test 62.2% (95% CI, 51.4%–71.9%; 51 of 82), OnSite-POC test 28% (95% CI, 19.5%–38.6%; 23 of 82), and LDBIO-POC test for combined IgG/IgM detection 100% (95% CI, 95.5%–100%; 82 of 82) (P < .0001 for all comparisons). Specificity for Toxoplasma-IgM was significantly lower for Biopanda compared with OnSite and LDBIO POC tests: 88.5% (95% CI, 82.7%–92.5%; 146 of 165), 97.6% (95% CI, 93.9–99.1%; 161 of 165), and 98.8% (95% CI, 93.2%–99.9%; 79 of 80), respectively (P = .0012 for Biopanda vs OnSite POC tests; P = .005 for Biopanda vs LDBIO POC tests) (Table 4). Diagnostic accuracy for IgM detection was significantly lower for Biopanda and OnSite POC tests in comparison with LDBIO POC test: 79.8% (197 of 247), 74.5% (184 of 247), and 99.4% (161 of 162), respectively (P < .0001 for both Biopanda vs LDBIO POC test and OnSite vs LDBIO POC test).
Cross-Reactivity of Point-of-Care Kits With Nonspecific Immunoglobulin M
All 3 POC kits showed negative results for IgM (0 of 60) when tested against FP Toxoplasma-IgM samples (nonspecific IgM) (Table 5). In 3 of 60 (5%) samples, the Biopanda POC kit tested falsely positive for IgG. Of note, IgM-ELISA titers from patients with nonspecific IgM were significantly lower compared with patients with true acute Toxoplasma infection (median 3.3 [IQR, 2.7–4.4] vs 6.8 [IQR, 5.0–8.4], respectively; P < .0001).
Table 5.
Results of POC Testing Against Selected Sera With False-Positive IgM Results for Toxoplasma IgM
| POC Kit | Toxoplasma Antibodies | Positive/Total (%) |
|---|---|---|
| Biopanda | IgG | 3 of 60 (5%) |
| IgM | 0 of 60 (0%) | |
| OnSite | IgG | 0 of 60 (0%) |
| IgM | 0 of 60 (0%) | |
| LDBIO | IgG/IgM combined | 0 of 60 (0%) |
Abbreviations: Ig, immunoglobulin; POC, point-of-care.
Interobserver and Intertest Agreement
There were instances when Biopanda and OnSite POC cassettes displayed poorly visualized testing bands (ie, very faint colored bands-ambiguous bands) (Supplementary Material, Figure S1). Ambiguous bands were seen in 44 of 620 (7.1%) of Biopanda POC tests (5 for IgG and 39 for IgM bands); 13 of 39 (33%) of ambiguous IgM bands were seen in both POC duplicate tests for a given sample. For OnSite, ambiguous bands were seen in 28 of 620 (4.5%) POC tests (2 for IgG and 26 for IgM bands); 19 of 26 (73%) of ambiguous IgM bands were seen in both POC duplicate tests. However, because Biopanda and OnSite POC tests manufacturer’s instructions indicate that any shade of color on testing bands should be interpreted as positive, all ambiguous-bands for these 2 POC tests were re-evaluated by 2 other investigators (J.G.M., D.G.C.-I.) blinded also to both PAMF-TSL results, and their corresponding duplicate POC test and results were classified accordingly. There were no ambiguous bands for LDBIO POC tests. Lastly, per manufacturer instructions, all 1860 POC test results were considered valid because all of them exhibited the presence of the control (C) band.
DISCUSSION
We report the diagnostic performance of 3 POC tests for detection of Toxoplasma IgG and IgM antibodies using a large number of sera from US patients tested by PAMF-TSL reference gold-standard IgG and IgM tests. There are 2 major conclusions from our study. First, all POC tests (Biopanda, OnSite, and LDBIO) demonstrated optimal analytical sensitivity and specificity for Toxoplasma-IgG testing irrespectively of Toxoplasma-infection status (acute vs chronic infection) and IgG titers. Second, except for LDBIO POC test that detects IgG-IgM in one testing band and therefore showed 100% diagnostic sensitivity for acute Toxoplasma infection, Biopanda and OnSite POC tests showed poor diagnostic sensitivity for Toxoplasma-IgM detection (62.2% and 28%, respectively). Notably, no POC tests showed cross-reactivity with sera from patients with FP Toxoplasma-IgM results. Hence, our results support use of the LDBIO-POC platform as a simple, low-cost, and rapid diagnostic tool capable of efficiently detecting Toxoplasma-IgG and/or Toxoplasma-IgM antibodies. Our study also supports the LDBIO-POC test as an initial serological screening test in programs for universal Toxoplasma prenatal testing.
The underperforming diagnostic accuracy for Toxoplasma-IgM detection noted for the 2 POC tests that were designed to detect IgG and IgM separately deserves further discussion. There are some technical differences between these 3 POC tests. The Biopanda and OnSite POC tests use recombinant T gondii antigens for binding to specific anti-Toxoplasma antibodies (IgG and IgM, respectively), whereas the LDBIO POC test uses as antigen whole-cell lysates of tachyzoites from the T gondii RH Sabin Type I strain. Additional mechanistic differences on lateral flow assays design may also account for the higher diagnostic performance observed with the LDBIO POC kit (see Supplementary Material). Biopanda and OnSite offer in their POC cassettes detection of Toxoplasma-IgM antibodies in a distinct testing band (M band, Figure 1). False-negative results for IgM may lead to delay confirmatory testing, missing the opportunity for timely initiation of anti-Toxoplasma therapy in seroconverting pregnant women. In addition, the Biopanda and the OnSite POC tests exhibited the presence of ambiguous bands, defined as poorly visualized testing bands with very faint color, which were seen in 7.1% (44 of 620) and 4.5% (28 of 620) of the tests performed by Biopanda and OnSite, respectively. The above, added to an excessive number of FP results (11.5% for Biopanda IgM), represents a significant drawback that, in clinical practice, may lead to confusion and unnecessary confirmatory testing at expenses of increasing testing cost and patient’s anxiety. The failure of the Biopanda (62.2% sensitivity) and OnSite POC tests (28% sensitivity) to optimally detect Toxoplasma IgM represents a serious handicap for the implementation of such POC tests in universal screening programs during pregnancy.
The LDBIO Toxoplasma POC kit showed robust performance in our study, with 100% sensitivity for the combined detection of IgG and IgM detection and low rate of FP IgG/IgM POC test results (1.3%, 1 of 80 in IgG/IgM seronegative sera). Of note, the LDBIO reports Toxoplasma-IgG and Toxoplasma-IgM antibodies combined in a single testing band (T band); therefore, independent calculation of IgM sensitivity and specificity for this POC test, without taking into account the presence of IgG, was not possible (Tables 2 to 3). The diagnostic accuracy for LDBIO has been documented in prior studies using various reference standard methods for Toxoplasma-IgG and IgM detection [29–31]. To date, at least ~2000 sera and whole blood samples from France, United States, and Morocco have been tested (including the 310 sera from this study). Chapey et al [29] evaluated the LDBIO POC test in 400 sera from France and demonstrated diagnostic sensitivity and specificity of 97% and 96%, respectively, for the combined detection of Toxoplasma IgG-IgM when compared with the fully automated chemiluminescence Architect test (Abbott North, Chicago, IL). Our team, led by McLeod et al demonstrated 100% sensitivity and specificity for the LDBIO POC test when tested against 180 sera from United States patients, including seropositive individuals with diverse T gondii serotypes previously tested at PAMF-TSL [31]. Mahinc et al tested 1002 sera from France and reported 100% analytical sensitivity in comparison with Toxoplasma-IgG II Western blot analysis and Platelia Toxo-IgM (Bio-Rad) for IgG and IgM detection, respectively. In Mahinc et al’s [30] study, the LDBIO POC kit showed reliable performance even at low IgG titers, outperforming the Architect IgG test in its gray zone of reporting. More recently, the McLeod-Lykins team tested LDBIO POC test’s suitability using fingerstick whole blood in 244 whole blood samples from 205 consenting individuals from the National Chicago-Based, Congenital Toxoplasmosis Collaborative Study (n = 208 samples) and Morocco (n = 39 samples), including 101 seropositive sera, demonstrating 100% sensitivity and specificity when compared with automated commercial laboratory testing for IgG and IgM or PAMF-TSL testing [34]. In light of accumulated evidence for LDBIO POC test’s accuracy in comparison with reference-standard methods in the United States and France, the LDBIO POC kit can be deemed as an optimal low-cost technology for population-based serological screening and/or universal monthly prenatal screening. This POC test has been shown to perform well independently of implicated T gondii strain and across a wide range of Toxoplasma-IgG antibody titers [31]. If cost of LDBIO POC kit can be maintained at <$5.00, this assay may become an attractive option for first-line testing across maternal screening programs.
Our study has some limitations. Despite large sample size and inclusion of sera from acute and chronic Toxoplasma infection and FP IgM results, we did not include sera from patients with early T gondii infection (early seroconversion) to assess for IgM diagnostic accuracy in the absence of Toxoplasma IgG. These sera are scarce given lack of routine prenatal screening for Toxoplasma infection in the United States, impeding access to patients with documented seroconversion at early stages of infection. Therefore, in our study, IgM titers from patients with acute Toxoplasma infection were remarkably high (IgM-ELISA titers; median 6.8 [IQR, 5.0–8.4]; cutoff ≥2.0 units). As a result, POC analytical performance for IgM detection at lower IgM titers remains unknown, and we encourage investigators to address this issue in future prospective studies. Reassuringly, Mahinc et al [30] tested sera from 17 individuals with acute seroconversion that were only IgM positive and IgG negative and provided proof of concept that the LDBIO POC test can detect early Toxoplasma IgM (16 of 17 positive).
Because our study evaluated the diagnostic accuracy of POC kits compared with reference laboratory methods for Toxoplasma serology testing, the estimated PPVs and NPVs were representative of a group of tested samples with 68% IgG and 33% IgM seropositivity. Positive predicted values and NPVs vary according to the prevalence of the disease or condition in a determined population, and thus the PPVs and NPVs for a pragmatic cohorts with different IgG and IgM seropositivity rates would be very different; eg, for a population with 1% IgM seropositivity, a POC test with sensitivity and specificity for IgM detection of 62.2% and 88.5%, respectively, would have a PPV of only 5.2%, and a NPV of 99.6% and a POC test with 28% and 97.6% sensitivity and specificity, respectively, would have a PPV of 10.5% and NPV of 99.26%.
Our study was a proof-of-concept study and the first to directly compare 3 commercial POC tests for Toxoplasma diagnostics. As such, our results have notable implications for obstetricians, pediatricians, neonatologists, infectious diseases specialists, and others including policy makers in public health. As a neglected disease, CT represents a significant burden to healthcare systems around the world, with 190000 infants per annum affected worldwide carrying 1.2 million disability-adjusted life years [35–37]. Integration of POC platforms into Toxoplasma diagnostics holds enormous promise given its critical elements such as rapid turnaround time, simplicity, low cost, and completion of testing during same clinical encounter [38]. However, careful evaluation of POC test accuracy and diagnostic performance is a pivotal step before any consideration for clinical use. The World Health Organization has proposed the “ASSURED” criteria for the ideal POC assay: Affordable, Sensitive, Specific, User-friendly, Rapid/robust, Equipment-free and Deliverable to users [28]. In this frame, our study represents the initial stage to move forward POC diagnostics in toxoplasmosis. Given their low complexity and minimal risk for incorrect results, POC kits with robust analytical performance such as the LDBIO POC test, which has also been shown to have excellent diagnostic accuracy when tested with whole blood collected directly from a fingerstick (via a capillary tube) [34], can receive Clinical Laboratory Improvement Amendments waivers, enabling them to be used in POC settings and thereby contribute to mitigate costs associated with serological testing in maternal screening programs [24]. Despite enthusiasm, several questions regarding the application of POC diagnostics in clinical grounds remain to be addressed, and future studies are needed to prospectively evaluate effectiveness of POC test-based prenatal screening strategies, including cost effectiveness in individual country settings, impact on clinical outcomes, and their integration to clinical workflows in perinatal care [39]. Work is in progress for the evaluation of POC test-based prenatal screening integration in the clinical workflow in prenatal care (Thrasher Research Fund award no. 13798).
Positive POC test results indicating acquisition of an acute Toxoplasma infection during gestation (eg, seroconversion from IgG−/IgM− serostatus to an IgM+ or IgG+ or combined IgG+/IgM+ status between 2 consecutive screenings) would always require confirmatory testing with conventional laboratory-based methods to confirm the diagnosis and guide clinical management. Moreover, positive POC test results at the first prenatal care visit (either IgG+/IgM− or positive for the combined IgG/IgM detection) also require confirmatory testing, to confirm that these reflect chronic infection, acquired before gestation. Pregnant women found to be chronically infected early in gestation would not need additional monthly screening for the rest of their pregnancy because they are not at risk of transmitting the infection to their fetus, unless severely immunocompromised and not on prophylactic therapy. Despite the need for confirmatory testing for positive results, POC test-based prenatal screening programs are still cost saving because they significantly limit the number of women who will need such confirmatory testing (even in settings with seropositivity rates among pregnant women as high as 50%, a POC test-based screening program can cut the need for laboratory-based screening by half).
CONCLUSIONS
In conclusion, we demonstrated that all 3 POC tests (Biopanda, OnSite, and LDBIO POC tests) showed optimal diagnostic sensitivity and specificity for Toxoplasma-IgG detection across different Toxoplasma-infection states. However, the LDBIO POC test showed 100% sensitivity for the combined detection of IgG/IgM in acute and chronic Toxoplasma infection, whereas Biopanda and OnSite POC tests showed poor diagnostic sensitivity for Toxoplasma IgM. An accurate, low-cost, and reliable POC diagnostic test for Toxoplasma infection can benefit patients and mitigate the cost of serological testing in universal prenatal screening programs for Toxoplasma infections.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was funded by the E.W, AI Thrasher Foundation (number 13796; to Y. M., D. G. C.-I., J. G. M., and R. M.) and the Mann Cornwell, Rooney, Morel, Taub, and Engel families.
Potential conflicts of interest. R. M. and J. G. M. performed a literature review for Sanofi-Pasteur during the time this manuscript was prepared; no compensation was received for this review. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363:1965–76. [DOI] [PubMed] [Google Scholar]
- 2. Montoya JG, Gomez CA. Toxoplasmosis after solid organ transplantation. In: Per Ljungman DS, MichaelBoeckh Transplant Infections. 4th ed Switzerland: Springer, 2016. [Google Scholar]
- 3. Demar M, Ajzenberg D, Maubon D, et al. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin Infect Dis 2007; 45:e88–95. [DOI] [PubMed] [Google Scholar]
- 4. de-la-Torre A, Sauer A, Pfaff AW, et al. Severe South American ocular toxoplasmosis is associated with decreased Ifn-gamma/Il-17a and increased Il-6/Il-13 intraocular levels. PLoS Negl Trop Dis 2013; 7(11):e2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maldonado YA, Read JS. AAP Committee on infectious diseases. Diagnosis, Treatment, and Prevention of Congenital Toxoplasmosis in the United States. Pediatrics. 2017;139:e20163860. [DOI] [PubMed] [Google Scholar]
- 6. Pomares C, Montoya JG. Laboratory diagnosis of congenital toxoplasmosis. J Clin Microbiol 2016; 54:2448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyer K, Hill D, Mui E, et al. Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin Infect Dis 2011; 53:1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones JL, Dargelas V, Roberts J, et al. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis 2009; 49:878–84. [DOI] [PubMed] [Google Scholar]
- 9. Wallon M, Peyron F, Cornu C, et al. Congenital Toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin Infect Dis 2013; 56:1223–31. [DOI] [PubMed] [Google Scholar]
- 10. Prusa AR, Kasper DC, Pollak A, et al. The Austrian Toxoplasmosis register, 1992–2008. Clin Infect Dis 2015; 60:e4–10. [DOI] [PubMed] [Google Scholar]
- 11. Hotop A, Hlobil H, Gross U. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2012; 54:1545–52. [DOI] [PubMed] [Google Scholar]
- 12. De Paschale M, Agrappi C, Manco MT, et al. Implementation of screening for Toxoplasma gondii infection in pregnancy. J Clin Med Res 2010; 2:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Logar J, Petrovec M, Novak-Antolic Z, et al. Prevention of congenital toxoplasmosis in Slovenia by serological screening of pregnant women. Scand J Infect Dis 2002; 34:201–4. [DOI] [PubMed] [Google Scholar]
- 14. Thiébaut R, Leproust S, Chêne G, Gilbert R. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet 2007; 369:115–22. [DOI] [PubMed] [Google Scholar]
- 15. Cortina-Borja M, Tan HK, Wallon M, et al. Prenatal treatment for serious neurological sequelae of congenital toxoplasmosis: an observational prospective cohort study. PLoS Med 2010; 7:e1000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kieffer F, Wallon M, Garcia P, et al. Risk factors for retinochoroiditis during the first 2 years of life in infants with treated congenital toxoplasmosis. Pediatr Infect Dis J 2008; 27:27–32. [DOI] [PubMed] [Google Scholar]
- 17. Gras L, Wallon M, Pollak A, et al. Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: a cohort study in 13 European centres. Acta Paediatr 2005; 94:1721–31. [DOI] [PubMed] [Google Scholar]
- 18. Peyron F, Mc Leod R, Ajzenberg D, et al. Congenital toxoplasmosis in France and the United States: one parasite, two diverging approaches. PLoS Negl Trop Dis 2017; 11:e0005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McLeod R, Boyer K, Karrison T, et al. Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis 2006; 42:1383–94. [DOI] [PubMed] [Google Scholar]
- 20. Olariu TR, Remington JS, McLeod R, et al. Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J 2011; 30:1056–61. [DOI] [PubMed] [Google Scholar]
- 21. Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. N Engl J Med 1994; 330:1858–63. [DOI] [PubMed] [Google Scholar]
- 22. Wallon M, Peyron F. Congenital toxoplasmosis: a plea for a neglected disease. Pathogens 2018; 7:E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis 2002; 185Suppl 1:S73–82. [DOI] [PubMed] [Google Scholar]
- 24. Stillwaggon E, Carrier CS, Sautter M, McLeod R. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Negl Trop Dis 2011; 5:e1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nogareda F, Le Strat Y, Villena I, et al. Incidence and prevalence of Toxoplasma gondii infection in women in France, 1980–2020: model-based estimation. Epidemiol Infect 2014; 142:1661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prusa AR, Kasper DC, Sawers L, et al. Congenital toxoplasmosis in Austria: prenatal screening for prevention is cost-saving. PLoS Negl Trop Dis 2017; 11:e0005648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drancourt M, Michel-Lepage A, Boyer S, Raoult D. The point-of-care laboratory in clinical microbiology. Clin Microbiol Rev 2016; 29:429–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peeling RW, Mabey D. Point-of-care tests for diagnosing infections in the developing world. Clin Microbiol Infect 2010; 16:1062–9. [DOI] [PubMed] [Google Scholar]
- 29. Chapey E, Wallon M, Peyron F. Evaluation of the LDBIO point of care test for the combined detection of toxoplasmic IgG and IgM. Clin Chim Acta 2017; 464:200–1. [DOI] [PubMed] [Google Scholar]
- 30. Mahinc C, Flori P, Delaunay E, et al. Evaluation of a new immunochromatography technology test (LDBio Diagnostics) to detect Toxoplasma IgG and IgM: comparison with the routine architect technique. J Clin Microbiol 2017; 55:3395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Begeman IJ, Lykins J, Zhou Y, et al. Point-of-care testing for Toxoplasma gondii IgG/IgM using Toxoplasma ICT IgG-IgM test with sera from the United States and implications for developing countries. PLoS Negl Trop Dis 2017; 11:e0005670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC 2009; 19:203–11. [PMC free article] [PubMed] [Google Scholar]
- 33. Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003; 138:W1–12. [DOI] [PubMed] [Google Scholar]
- 34. Lykins J, Li X, Levigne P, Zhou Y, El Bissati K, Clouser F, et al. Rapid, inexpensive, fingerstick, whole-blood, sensitive, specific, point-of-care test for anti-Toxoplasma antibodies. PLoS Negl Trop Dis 2018; 12(8): e0006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torgerson PR, Mastroiacovo P. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ 2013; 91:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Havelaar AH, Kemmeren JM, Kortbeek LM. Disease burden of congenital toxoplasmosis. Clin Infect Dis 2007; 44:1467–74. [DOI] [PubMed] [Google Scholar]
- 37. Lykins J, Wang K, Wheeler K, et al. Understanding toxoplasmosis in the United States through “large data” analyses. Clin Infect Dis 2016; 63:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller MB, Bahk K, Carroll KC, Klugman K, Ledeboer N.. Changing Diagnostic Paradigms for Microbiology: Report on an American Academy of Microbiology Colloquium held in Washington, DC, from 17 to 18 October 2016. Washington, DC: American Society for Microbiology; 2017. [PubMed] [Google Scholar]
- 39. Pai NP, Vadnais C, Denkinger C, et al. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low-and middle-income countries. PLoS Med 2012; 9:e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.