Abstract
As urban areas continue to grow, understanding how species respond and adapt to urban habitats is becoming increasingly important. Knowledge of the mechanisms behind observed phenotypic changes of urban-dwelling animals will enable us to better evaluate the impact of urbanization on current and future generations of wildlife and predict how animals respond to novel environments. Recently, urban ecology has emerged not only as a means of understanding organismal adaptation but also as a framework for exploring mechanisms mediating evolutionary phenomena. Here, we have identified four important research topics that will advance the field of urban ecology and shed light on the proximate and ultimate causes of the phenotypic differences commonly seen among species and populations that vary in their responses to urbanization. First, we address the ecological and socio-economic factors that characterize cities, how they might interact with each other, and how they affect urban species. Second, we ask which are the proximate mechanisms underlying the emergence over time of novel traits in urban organisms, focusing on developmental effects. Third, we emphasize the importance of understanding the ultimate causations that link phenotypic shifts to function. This question highlights the need to quantify the strength and direction of selection that urban individuals are exposed to, and whether the phenotypic shifts associated with life in the city are adaptive. Lastly, we stress the need to translate how individual-level responses scale up to population dynamics. Understanding the mechanistic underpinnings of variation among populations and species in their responses to urbanization will unravel species resilience to environmental perturbation, which will facilitate predictive models for sustainability and development of green cities that maintain or even increase urban biodiversity and wildlife health and wellbeing.
Introduction
A prevailing source of environmental change in the 21st century is rapid human population growth in urban areas. Today, 54% of the world’s human population lives in urban areas, and by 2050, this number is expected to increase to 66% (United Nations 2014). Urban land cover continually expands to accommodate this growth, leading to the destruction of natural habitat and reduced biodiversity as a result of local extinction processes (Kalnay and Cai 2003; While and Whitehead 2013). Responses to these changes vary considerably between and within species. Indeed, some species are unable to occupy urban habitats (urban avoiders) while others persist (urban adapters) and even thrive (urban exploiters; Blair 1996; Moller 2009; Sol et al. 2014; Sepp et al. 2018). Similarly, it has been suggested that within species, only individuals possessing certain traits may be able to colonize urban areas (Sol et al. 2013; Sprau and Dingemanse 2017). Recently, interest in urban ecology has grown exponentially, as evidenced by a proliferation of empirical studies, meta-analyses, and reviews (McIntyre 2000; Liker et al. 2008; Goddard et al. 2010; Audet et al. 2016; Marzluff 2017; Sepp et al. 2018; Tucker et al. 2018; Lapiedra 2018; Mulholland et al. 2018).
Present and past research in urban ecology has primarily focused on the following two questions: (1) do urban and rural populations differ in certain traits? and (2) do urban and rural areas differ in biodiversity and/or species abundance? The evidence accumulated so far points to globally widespread influences of urbanization on phenotypes (Alberti et al. 2017). Likewise, urbanization has been linked to profound and complex effects on biodiversity, which is often greatly reduced at intense levels of urban development, but can also flourish in sub-urban and peri-urban areas (Aronson Myla et al. 2017). As these two questions have already been widely investigated, we now need a deeper understanding of how and why patterns of phenotypic shift and biodiversity emerge in cities. To do so, we draw on Niko Tinbergen’s four questions of “survival value,” “ontogeny,” “evolution,” and “causation” (Tinbergen 1963). In the context of an integrative framework for urban ecology, we first need mechanistic studies that examine how novel phenotypic traits emerge in urban areas, focusing on ontogeny, developmental plasticity, and co-variation between different behavioral and physiological traits. We then need to identify whether phenotypic responses of urban populations are adaptive, what their function is, and to which urban-specific selective pressures they are subjected. The question of whether populations truly adapt to urban life (via genetic change and local adaptation) or only acclimate (via plasticity) is difficult to resolve (Isaksson 2015; Tucker et al. 2018). It is thus important to investigate proximate mechanisms, including genetic and epigenetic effects underlying the emergence of novel traits at the individual level (Sol et al. 2013), the modification of existing traits (Badyaev et al. 2008), or the filtering of individuals and species possessing specific traits from an original, non-urban population (Moller 2009; Banaszak-Cibicka and Żmihorski 2012; Sol et al. 2014). Individual responses can then be tied to population dynamics by quantifying how fitness of urban individuals scale up to influence the demography of populations. To date, few studies have successfully integrated all of these components (but see Badyaev et al. 2008).
Moreover, the field of urban ecology is hindered by the lack of a clear, standardized approach to quantifying urbanization. Cities are extremely complex environments that differ in ecological, structural, and socio-economic characteristics; such variation also exists within cities. This limits our ability to design comparative studies and interpret their results (Moller 2009; Ramalho and Hobbs 2012; Aronson et al. 2014; Sol et al. 2014; Sepp et al. 2018). Further, the majority of urban ecology studies to date have focused on only one or a few urban and rural areas (e.g., Partecke et al. 2005; Fokidis et al. 2009; Foltz et al. 2015). Given the potentially important effects of variation among cities, our ability to generalize from these focused studies is uncertain.
Outstanding questions
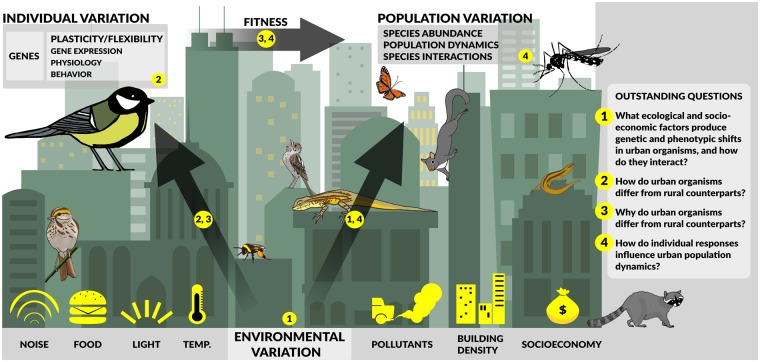
We have identified four pressing topics that will advance our knowledge of individual and population-level responses to urban environments. In the following sections, we give background to each question, outline gaps in knowledge, and suggest how these gaps can be addressed (Fig. 1).
Fig. 1.
Outstanding questions for urban ecology (1–4) in the context of environmental, individual, and population variation. Some representative common animals found in cities around the globe are pictured. Environmental variation can affect individual and population-level variation. Individual variation affects individual fitness which then can lead to changes at the population-level.
What ecological and socio-economic factors produce the observed genotypic and phenotypic shifts in urban animals, and how do they interact with each other?
Background
Cities are complex environments. There are large differences both within and between cities in age, climate, habitat structure, human population density, and socio-economic development. All these factors could contribute to the observed phenotypic and genotypic shifts observed in urban populations of wild species, and variation in those patterns. For example, wealthier cities in developed nations may have more urban parks and green spaces, whereas poorer cities may have fewer “managed” green spaces. The management of green space, as well as of urban waste, can have important consequences on the availability of food and nest sites for urban fauna. However, it is often unclear which factors are playing major roles. The reason has partly to do with sampling protocols, as few studies are explicitly designed to disentangle the effect of single factors. Nevertheless, global analyses of biodiversity datasets in urban areas have identified key ecological drivers of biodiversity loss due to urbanization, especially land cover (for instance the proportion of impervious surface) and the age of a city (Goddard et al. 2010; Aronson et al. 2014). In addition, socio-economic factors have also been shown to influence biodiversity (Hope et al. 2003; Kinzig et al. 2005; Aronson Myla et al. 2017). Conversely, studies that have thoroughly disentangled the effects of urban-specific factors on individual rather than on how different species respond are lacking, and this knowledge gap limits our understanding of whether specific city traits may influence adaptation to urban areas.
Gaps in knowledge
How do we define and quantify what urban is (and is not)?
Defining what is urban and what is not is anything but trivial and perhaps only useful from a semantic point of view. Likewise, the term rural can also refer to villages, agricultural fields, wind farms, or energy extraction sites in forests. The important distinction for this field is to quantify the factors that are associated with urban or rural environments, and move away from categorical designations of these areas. A vast proportion of urban ecology studies defines urbanization only qualitatively, and refers to study sites as urban, sub-urban, peri-urban, or rural simply based on the researchers’ own knowledge of the study area. Other studies rely on satellite-based images to quantify land-cover types and categorize them as urban, for instance by using the proportion of impervious surface in a given area around a study site. Recent work has incorporated several landscape attributes in multi-variate analyses (for instance principal component analyses) to compute an integrated “urban score” to be used in subsequent models aimed at testing the effect of urbanization on biodiversity or individual-based variables (Giraudeau et al. 2014; Sprau et al. 2017). While such compound variables can have the benefit of defining the “citiness” of a particular sampling location, specific environmental variables may play a disproportionately stronger role than others in particular conditions. Depending on the research question of interest either method can have its own merit. Moreover, most definitions of urbanization have so far focused on cross-sectional samplings of environmental variables along urban gradients. Such a methodology fails to take into consideration the complex spatio-temporal dynamics of urban sprawl (Ramalho and Hobbs 2012). In a recent study by Salmón et al. (2018), they showed that by performing a spatio-temporal modeling on nitrogen oxide (NOx) pollution, the long- and medium-term pollution models (one-month and one-week) were highly associated with urbanization scores of the habitat. However, short-term variation in NOx (24-h) was not associated with the scoring of urbanization, or with various oxidative stress parameters measured in four species of songbirds. This finding may not be surprising given the importance of local weather conditions for daily pollution estimates, which do not affect other parameters of urbanization; however, approaches like this might provide a better understanding of whether a behavioral and physiological trait is an acute response to a specific and current stressor or a more long-term response to the urban environment. Although the urbanization score and NOx modeling revealed similar large-scale patterns on physiology, it also revealed that the fine-grained resolution in the NOx models gave better power to detect smaller effects on physiology compared to the more general urbanization scores (Salmón et al. 2018). Further, urbanization often proceeds non-linearly and at different speeds in different spatial directions. The result of this process is a mosaic of urban patches embedded into a matrix of remnant natural habitats. Each of these urban patches has its own history of urbanization. To recognize such complexity might explain large portions of variation in species abundance and diversity, and individual responses to the urban environment.
Can we model “city traits” in comparative global analyses?
The need to generalize the effects of urbanization has promoted studies at the global level. We advocate the need for such global studies, but we also think that we need to take into consideration within- and between-city differences in urbanization. Cities differ dramatically in the way they have been built in regard to the speed and pattern of urban sprawl, and this is especially evident when cities from different continents are compared (Ramalho and Hobbs 2012). Moreover, cities differ also in the matrix habitat they are built in, mostly depending on the geographic area they are located. For instance Phoenix, a global hotspot of urban ecology research, is a relatively isolated urban area surrounded by desert habitat. Conversely, New York City is located in a heavily urbanized metropolitan area surrounded by temperate habitat with mixed deciduous and evergreen vegetation. The variation in the matrix habitat could also generate important variation in findings and thus may at least partially explain why the same species can show different responses in different cities, or in different areas within the same city (Evans et al. 2009). If not accounted for, this variation may limit our ability to generalize from studies that focus on only one or a few cities. To take into account such variability and assign traits to different urban areas based on their ecological characteristics is likely to enhance the quality of global comparative analyses. Moreover, including in such analyses not only ecological but also socio-economic variables will likely improve the quality of the outcomes, as it is increasingly recognized that the type and history of urban development is crucial in determining its impact on biodiversity (Hope et al. 2003; Kinzig et al. 2005; Aronson Myla et al. 2017).
How do different urban-specific ecological factors interact to affect individual and species response?
Most studies aimed at testing the effects of urbanization on individual and species response have so far mostly focused on urbanization as a whole (see the section “Why do urban organisms differ from their non-urban counterparts?”). In the last couple of decades, urban-specific environmental factors such as anthropogenic light (Swaddle et al. 2015; Ouyang et al. 2018), noise (Slabbekoorn and Ripmeester 2008; Halfwerk and Slabbekoorn 2013), air pollution (Greaver et al. 2012; Salmon et al. 2018), toxicants (Järup 2003; Kobiela and Snell-Rood 2018), temperature (Arnfield 2003; Brans et al. 2017), or human presence (Chace and Walsh 2006; Corsini et al. 2017) have also been the focus of field studies and captive experiments. Recent studies have also tried to disentangle the relative contribution of some of these factors (Da Silva et al. 2014; Dominoni et al. 2014; Sprau et al. 2017). However, experiments are usually designed to separate the effects of these urban factors rather than explicitly testing their interactive effects (but see McMahon et al. 2017). Future studies should focus on the outcomes of such interactions and whether they produce synergistic (additive, multiplicative, etc.) or antagonistic effects. Recent studies have proposed integrated frameworks to address interactions between different stressors/stimuli that might be useful in the context of urban ecology as well (for instance, see Hale et al. 2013).
Significance and future prospects
To comprehensively understand how environmental variation influences individual, population, and species responses is a challenge that every ecologist is likely to face. To further add the complexity of the urban environment to this challenge can be daunting. However, it is a challenge that needs to be met to advance urban ecology research. To do so, we need to recognize and understand the complexity of the urban habitat, and specifically: (i) measure as many ecologically relevant variables as possible, including characteristics of the matrix around cities, (ii) measure socio-economic factors, (iii) consider the spatial and temporal variation of such variables between and within cities, (iv) consider the age and the history of development of a particular urban area, and, finally, (v) design studies aimed at testing the interaction between different urban-specific factors. We believe that integrated, precise measurements of urbanization will ensure that each individual analysis will be robust, and will also improve the quality of large-scale comparative analyses.
How do urban organisms differ from their rural counterparts? A multi-trait, integrative approach
Background
A growing body of evidence describes behavioral and physiological differences between urban and rural populations, and phenotypic shifts associated with urbanization have been globally demonstrated in plants and animals (see Alberti et al. [2017] for a review). However, both behavioral and physiological shifts due to urbanization are not always consistent within the same species measured in different cities (Evans et al. 2011; Ibáñez-Álamo et al. 2017), and even less between closely related species (Moller 2009; Sol et al. 2013; Alberti 2015; Ducatez et al. 2018). For instance, endocrine traits of birds show no general pattern of response to urbanization (Bonier 2012). Similarly, while levels of oxidative stress correlate overall positively with the degree of pollution, species-specific differences exist (Isaksson 2010; Salmon et al. 2018). We stress that while the lack of general patterns often reported might come partly from environmental differences among urban areas, it is also important to recognize the need to shift from the paradigm of investigating one phenotypic trait in one urban area to a more holistic understanding of how populations respond to urbanization by integrated measures of different traits at different levels of biological organization (e.g., gene, physiology, behavior), their co-variation, and the underlying mechanistic links between them. Moreover, although evidence points to phenotypic differences between urban and rural populations, we still do not understand how these differences arise. Specifically, these may come about via plastic (non-genetic) or evolutionary (genetic) responses. Thus far, evidence documenting either of these two processes is still relatively rare (Johnson and Munshi-South 2017). Furthermore, trait differences can arise as a result of founder effects, and insights from invasion ecology point to phenotype-dependent dispersal (Chapple et al. 2012). An additional level of complexity is given by potential non-random distributions of phenotypes in urban settings (Sprau and Dingemanse 2017). In this section, we want to highlight how these different processes can be unraveled through exploring underlying mechanisms for the observed patterns and through robust, integrative experimental designs.
Gaps in knowledge
Are multiple, correlated physiological and behavioral traits similarly affected by urbanization?
Research in urban ecology has typically focused on one or in very rare cases, a few traits (Badyaev et al. 2008; Sol et al. 2013). However, an organism’s interaction with the environment is both perceived at the neuroendocrine level as well as translated into behaviors (Adkins-Regan 2005). In particular, a specific behavior might originate from a single physiological pathway, or might be the result of a suite of physiological changes (Ouyang et al. 2016; Cohen et al. 2017). When studying covariation between physiology and behavior, we should distinguish between correlational and causal effects. For example, cause and effect can be demonstrated as physiological mechanisms that influence behavioral traits, whereas feedbacks can also occur in which physiology affects behavior, which then feeds back to affect physiology (Sih et al. 2015). Thus, to fully appreciate how behavioral shifts occur in urban populations, the potential physiological pathways underlying such shifts need to be measured in conjunction. Studying how behavior and physiology feedbacks change with urbanization gives insight into ecological processes such as niche expansion, dispersal, and social organization (Réale et al. 2007). Individual variation in behavioral and physiological traits affect key ecological and evolutionary processes: the pattern of phenotypic variation determines the outcome of natural selection and affects competition and the structure of ecological networks (Wolf and Weissing 2012; Wong and Candolin 2015).
In addition, recent research has highlighted that environmental change can alter the relationship between physiology and behavior. In normal conditions, physiological and behavioral responses might not be correlated with each other, but such relationships can arise when animals are exposed to environmental stressors (Killen et al. 2013). The opposite can also occur (Killen et al. 2013; Welbers et al. 2017; Hutton et al. 2018). Thus, to repeatedly measure the co-variation of physiological and behavioral traits in the same individual, for instance during different seasons or times of day, might shed light on specific ways that urbanization affect animals.
Another important aspect to consider is how urbanization affects behavioral traits that are normally correlated with each other. Indeed, recent studies suggest that organisms often exhibit behavioral syndromes, that is, suites of correlated behaviors across time and context (Sih et al. 2004). The existence of these syndromes indicates that there is a limit to the range of behavioral plasticity expressed by an individual, and thus highlights the need to track individuals across space and time to capture such plasticity. In summary, we believe that the study of correlated traits across situations and biological levels (e.g., physiology and behavior) would promote a deeper understanding of how urbanization affects phenotypes. In essence, when traits are correlated, e.g., different behaviors, or behaviors with physiological traits, they should be studied together longitudinally, rather than in isolated packages, as they have mostly been thus far (but see Charmantier et al. 2017).
What role do ontogeny, plasticity, and evolution play in generating the phenotypic variation associated with urbanization?
Plasticity is the first line of response when an individual is exposed to novel environments and stimuli, such as those that exist in urban areas, and it defines the potential of an organism to acclimate to these novel environmental conditions. Such plasticity can manifest itself during development or in adulthood. Unfortunately, studies on how ontogenetic (developmental) plasticity in urban organisms may promote phenotypic differences in adulthood are very rare. An exception is the work on house finches by Badyaev et al. (2008). Urban house finches in Tucson, AZ, possess larger and stronger beaks compared with their desert conspecifics (Badyaev et al. 2008), which confer a fitness advantage, e.g., higher juvenile survival, because they enable them to crack open and eat the larger seeds and nuts provided in garden feeders (Badyaev 2010). The researchers studied the developmental basis of this divergence in beak morphology in adulthood, demonstrating an earlier and accelerated tissue transformation in urban versus desert house finch embryos. Indeed, the mandibular primordia of the large-beaked urban finches express bone morphogenetic proteins earlier and at higher levels than those of the desert finches during embryonic development, leading to stronger beaks in adulthood (Badyaev et al. 2008). Studies like these can shed light onto the ontogenetic basis of phenotypic differences due to urbanization. Understanding the control system that underlies trait variation can help elucidate the evolution of reaction norms. For example, control systems can impose constraints if they cannot produce the optimal reaction norm, and can create addition pressures if the system is costly (Lessells 2008).
Moreover, quantifying the extent of reversible phenotypic plasticity in adulthood is also important, as it might underlie the capacity of individuals to respond to rapid environmental changes taking place during urban development. Such plasticity can be studied via translocation studies or testing how the same individuals respond to repeated, experimental exposures to urban challenges. We can also use laboratory studies to measure plasticity in a controlled setting. It is important to note that not all plasticity is adaptive. Anthropogenic environments may be ecological traps, such as the case of dark beetles that are killed in managed forests when they are attracted to forest fuel piles that are then milled (Hedin et al. 2008). Maladaptive behaviors are likely to occur when animals encounter very different conditions, e.g., urban environments, from those that shaped their traits under previous selection (Hale et al. 2016). Distinguishing between adaptive and maladaptive plasticity and their degree of flexibility will be important for understanding whether urban environments act as evolutionary traps or promote adaptive evolution (Robertson et al. 2013; Hale et al. 2016).
It is important to recognize that without robust experimental design, plastic, non-genetic responses can be easily confounded with genetic responses. For instance, while common-garden experiments are a common way to disentangle genetic versus environmental effects on behavior and physiology, they often lack control for the potential effect of parental and early-environmental influences on phenotypes (Partecke et al. 2005; Dominoni et al. 2013). Recently, Brans et al. (2017) used a multi-generational common-garden experiment with Daphnia to ask whether urban Daphnia have evolved higher heat-tolerance than rural water fleas. By breeding both the parental and F1 generation in a common environment, the authors limited the effect of any potential non-genetic influence on the results. They found higher heat tolerance in animals descended from individuals collected from urban ponds compared with descendants of individuals collected from rural ponds, partly mediated by smaller body size, suggesting adaptive thermal evolution in urban Daphnia. Similar studies will be instrumental in disentangling genetic and non-genetic responses to urbanization.
Furthermore, we also need to stress that the emergence of specific urban phenotypes might simply be a consequence of non-random distributions of phenotypes in urban settings, pre-selected from existing rural populations during the process of urbanization. In other words, urbanization might filter species, populations, and individuals on the basis of whether or not they possess traits that make them suitable to colonize and thrive in cities. The ideas of urban habitats being “filters” have been examined largely in community ecology (Croci et al. 2008; Maklakov et al. 2011; Banaszak-Cibicka and Żmihorski 2012), in which species have been identified as “winners or losers.” Much less attention has been devoted to the same process acting at the individual level within a population (but see Charmantier et al. 2017; Sprau and Dingemanse 2017), and we consider this as a ripe research field.
Significance and future prospects
To make strides in answering these questions, we need to not only measure phenotypic traits but we also need to measure these traits in conjunction, such that we can have information on (co)variation between and within individuals. To date, there are very few studies that have measured multiple behavioral traits (e.g., song, boldness, exploration) and multiple levels of causation (e.g., differences in physiology and morphology) within urban and rural populations and the few that have done so have been key in establishing trait covariance. To disentangle the role of parental, early developmental, environmental, or genetic effects in producing organisms that avoid or exploit urban environments, we need to design robust experiments, for instance cross-fostering or common-garden experiments (Partecke et al. 2006; Brans et al. 2017; Capilla-Lasheras et al. 2017; Kobiela and Snell-Rood 2018; Salmon et al. 2018). Lastly, with the growing amount of individual-based data collected, we should integrate between different levels of organization, e.g., genetic variation, epigenetic variation, gene expression, physiology, and behavior, to form of a holistic understanding of how new behaviors and life-histories emerge in urban environments.
Why do urban organisms differ from their non-urban counterparts?
Background
Urban environments are relatively novel and are characterized by several anthropogenic factors, e.g., increased anthropogenic food, light and noise levels, that make them unique. Hence, cities present wildlife with novel environmental conditions that are dramatically different from those under which they have evolved. Some of these conditions might exert strong selective pressures on urban organisms (Johnson and Munshi-South 2017). Consequently, cities can be seen as hubs of evolution in action. In fact, there has been a recent surge of interest in studying evolution in cities (see Johnson and Munshi-South [2017] for a review). Thus far, population genetic studies have been instrumental in this field. For example, Mueller et al. (2013) addressed the genetic nature of behavioral adaptation of blackbirds colonizing urban areas. They found evidence for consistent patterns of divergence between paired urban and rural birds at a microsatellite associated with the SERT gene. SERT has a number of hypothesized behavioral effects, including harm avoidance, which may be associated with tolerating the challenges of urban environments (Garroway and Sheldon 2013). Similarly, researchers have found that past history of urbanization of New York City is paralleled with changes in the genome and demographic history of the white-footed mouse (Harris et al. 2016). Using RAD sequencing, Perrier et al. (2018) found a small but significant effect of urbanization on genetic differentiation in European great tits. However, population genetic studies in urban ecology are still relatively rare and provide a very limited taxonomic sample. With molecular tools becoming cheaper and more accessible, much can be gained from investigating the consistency of the effects of urbanization on the spatial distribution of genetic diversity, the polygenic nature of gene–urbanization association, and potential signatures of selection in the genome of urbanized species (Bosse et al. 2017).
Despite population genetics being a useful tool to highlight evidence of genetic adaptation to urban life, it does not help us to fully understand why within a species urban organisms differ from their rural counterparts. In order to achieve this, we need to integrate genetic variation data with information on the strength of selection on a particular trait and its fitness value. For instance, several urban-specific environmental factors have been proposed to explain variation in life-histories associated with urbanization: food limitation, predation, anthropogenic pollution, etc. (Sepp et al. 2018). However, how these factors translate into selective pressures in urban environments is largely unknown. Moreover, how does individual fitness respond to spatio-temporal variation in such pressures along gradients of urbanization? And what traits are under selection? Quantifying selective pressures and obtaining long-term fitness data (in particular lifetime reproductive success) in urban environments remain daunting tasks, but key to understanding the evolution of urban-specialized traits.
Gaps in knowledge
Are behavioral/physiological changes in urban individuals adaptive?
We still do not have a clear understanding whether behavioral or physiological changes in urban individuals are adaptive or maladaptive. Repeatability, measured as the fraction of phenotypic variation that is due to differences among individuals relative to differences within an individual, can set the upper bound to heritable variation. However, for most traits we have no estimates of heritability or repeatability and no information if they change along urban gradients (Ouyang et al. 2011; Jenkins et al. 2014, Salmon et al. 2018). To fill these gaps, we would first need repeated measures of a trait within the same urban individuals. Then, we would need to measure the relationship between this trait and individual fitness. Last, we would need to assess how such a relationship might be affected by urban-specific environmental factors, to assess the strength of selection acting on the trait (see also next section). As an alternative perspective, top-down approaches using genomic data could be used to assess signatures of selection. For example, a large genomic dataset in European great tits suggests that beak shape and size evolved rapidly as a result of domestic garden feeders (Bosse et al. 2017; but see Perrier and Charmantier 2018).
What are the selective pressures that urban animals respond to?
Very few studies have quantified the selection pressures that urban animals are responding to. Selective pressures in urban environments, such as temporal variation in food, water, and predation, are often relaxed (Jokimäki et al. 2002; Marzluff 2017). Urban environments exhibit a range of changed ecological processes, e.g., increased primary productivity. To illustrate, although concrete surfaces in cities represent a net primary productivity of zero, city parks, gardens, and golf courses elevate local productivity relative to surrounding rural areas, with these green spaces lying close to the highest end of the productivity continuum (Kaye et al. 2005). These ecological processes should alter selective forces in cities, and might lead to the genetic differentiation of urban and wild populations. Alternatively or in conjunction to this, genetic changes associated with isolated wild populations due to habitat fragmentation may on the one hand result from increasingly urbanized landscapes (Shochat et al. 2006). On the other hand, continuous migration and gene flow, for instance in highly mobile species such as birds, might prevent the genetic differentiation of urban populations and dampen evolutionary responses.
As we highlighted above, to understand the evolutionary implications of urbanization it is imperative to obtain fitness data, preferably through experimental work in order to disentangle the fitness responses to different urban-specific environmental factors. In terms of reproductive success, food availability and quantity are often one of the characterized environmental traits, as least in avian systems (Schoech et al. 2009). Higher food availability is generally associated with increased reproductive success (Verboven et al. 2001) and earlier timing of reproduction (Schoech et al. 2009) in urban animals. However, urbanization may also be associated with a reduction in the quality of food, for instance via reduced availability of optimal diets, and this component is not always appreciated. Recently, a reduction of food quality was linked to reduced reproductive success in birds (Pollock et al. 2017) and higher disease susceptibility in coyotes (Murray et al. 2015). In terms of survival, there is no study that we are aware of that has characterized both how urban animals die and what selective pressures affect their survival, so it remains a clear research gap for urban evolutionary biology.
Do phylogenetically related species respond similarly to urbanization?
In a review of >800 avian species across five continents, Sol et al. (2014) found that most of the biodiversity loss can be attributed to a lack of appropriate adaptations for exploiting resources or avoiding risks associated with urbanized environments. Importantly, closely related species tended to respond to urbanization in the same way, e.g., avoider or exploiter, possibly sharing features that affect their tolerance to urban development (Lapiedra 2018). Moreover, recent work has also pointed to the reduction of avian phylogenetic uniqueness in urban habitats, which raises conservation concerns (McKinney 2006). These studies are a good start to answering this question and should be expanded to systems other than birds. In conjunction, a global network of researchers that work on the same system, e.g., house sparrows or Anolis lizards, may be useful to explore fundamental questions in different cities across the globe.
Significance and future prospects
These evolutionary and ultimate questions need data on life-time fitness that are often missing in field studies. In order to understand the forces of selection, we need basic information on the genetic variation of many of the behavioral or physiological traits that we are measuring. For example, we need to measure heritability and repeatability of key traits to quantify if these may constitute substrates of selection, and then measure selection coefficients. With selection coefficients, we can predict the rate of change in a trait over time and over different environmental conditions, to predict how likely a trait would be able to respond to current and future environmental change. However, it is important to note that phenotype–fitness relationships can be biased and generated by adaptive plastic responses to the environment (Bonier and Martin 2016). Moreover, we can use quantitative genomics to link genotype with phenotype. To measure evolutionary routes, we need to know which selective pressures promote trait divergence. Likely, this will include quantifying environmental factors and testing these factors using common-garden experiments in the laboratory. We can use phylogenetic relatedness to our advantage by comparing similar and dissimilar species pairs’ responses to environmental factors that characterize cities. Lastly, comparative phylogenetic models can predict how species may respond to urbanization especially for non-avian taxa, i.e., comparing phylogenetically similar species. In summary, greater attention should be paid to urban evolutionary aspects because the type and direction of physiological, behavior, and morphological changes can indicate how selective forces in urban environments differ from those in habitats less affected by humans.
How do individual-level responses influence population dynamics in urban areas?
Background
We mentioned in the “Introduction” section that research in urban ecology has so far primarily focused on questions related to how urbanization affects (i) population-level (urban vs. rural) phenotypic responses (Alberti et al. 2017) and (ii) biodiversity (Beninde et al. 2015; Aronson Myla et al. 2017). Despite the large number of studies that have investigated these questions, there is comparatively little knowledge about if and how the two aspects are linked. Urbanization is known to affect several demographic parameters, such as reproductive success and fertility, mortality, and longevity (although examples of longevity are rare; Chamberlain et al. 2009; Sepp et al. 2018). However, how these demographic effects translate into changes in population dynamics and ultimately into the likelihood of a species to increase or decrease in abundance over time is a somewhat neglected aspect of urban ecology. A mechanistic appreciation of the demographic processes that regulate urban populations is imperative if we want to understand how urbanization affects species abundance and biodiversity. Thus, we believe it should be a major focus of urban ecology research in the near future. Moreover, most studies so far have utilized a cross-sectional approach, comparing demographic traits and species abundance levels across gradients of urbanization or in paired urban/rural sites. While this approach is useful to identify patterns of changes in demography and biodiversity associated with urbanization, its utility for understanding the processes underlying these changes is limited. Alternative approaches, for instance longitudinal demographic analyses during different stages of urban development, or meta-population modelling, might be much more informative of such processes, drawing from the existing tools used in the fields of invasion biology and behavioral ecology.
Gaps in knowledge
What is the impact of urbanization on components of fitness?
The need to obtain accurate demographic data is imperative to assess not only the impact of urbanization on fitness, but also the selective forces acting on urban populations (see the section “Why do urban organisms differ from their non-urban counterparts?”), and the consequences of such demographic changes for the persistence of wild species in urban areas. For instance, there is mounting evidence that passerine birds have reduced reproductive success in urban areas. This is partly due to the reduced investment in clutch size (Chamberlain et al. 2009; Sepp et al. 2018), but also to poor diet and health in early life which may reduce both pre- and post-fledging survival (Rodewald et al. 2013; Bailly et al. 2016; Smith et al. 2016; Capilla-Lasheras et al. 2017; Pollock et al. 2017; Salmón et al. 2017). However, most of these studies were limited to one or few years, whereas studies that have measured lifetime reproductive success (the most compelling fitness measure) in urban populations are non-existent. Similarly, there are very few examples of urban populations in which survival and especially longevity are measured accurately for most individuals (but see Sepp et al. 2018). A key challenge is therefore to move toward long-term monitoring of urban populations in order to obtain high quality data on individual reproduction and survival.
How are such changes in individual fitness linked to population dynamics in urban areas?
Very few studies have assessed population dynamics of species in urban areas (but see Riley et al. 2003; Harveson et al. 2007; Balogh et al. 2011). Such lack of knowledge limits our capacity to understand the drivers of change in population abundance associated with urbanization, as well as whether urban populations are sources or sinks. Likewise, evolutionary traps and range shifts are likely to interact as animals respond to rapid urbanization (Hale et al. 2016). If colonizers are more likely to encounter traps as they explore novel urban environments, what happens to these phenotypes and would they be removed from these environments? Perhaps the phenotypic traits that make an urban colonizer successful are not those that would persist in urban environments. Hence, it will be important to measure range shifts and dispersal strategies. In a recent study, Smith et al. (2016) have used a 3-year dataset on reproductive success and annual survival to build a stochastic demographic model and estimate population growth rate for spotted towhees (Pipilo maculatus) in four parks in Portland, OR, USA. Their model revealed that despite high levels of annual reproductive output, post-fledging survival can be very low. This pattern suggests that some urban populations might be sinks and must rely on immigration from source areas to be sustained. However, immigration as well as dispersal rates were not measured in this study. To obtain such estimates in urban areas where populations are likely to be distributed within a mosaic of small to large patches of remnant habitat can be daunting, but nevertheless essential for the understanding of population dynamics and the ecological connectivity of urban landscapes (LaPoint et al. 2015). Biotelemetry studies conducted in urban areas are increasing in number and scope (LaPoint et al. 2015), and we advocate more use of these tools to assess movements between sub-populations and thus inform metapopulation models with emigration and immigration rates. Alternatively, genetic information can be used to assess the direction and strength of gene flow and demographic history (Gaggiotti et al. 2009; Andreasen Alyson et al. 2012).
When are changes in individual fitness reflected in demographic changes during progressive urbanization?
An important aspect to consider when assessing the demographic consequences of urbanization, and thus its effects on biodiversity, is not only how and why, but also when during the different stages of urban development a species begins to show changes in demographic parameters that can lead to changes in abundance. It is important to also assess population density and land-use changes in areas surrounding cities as these areas can also develop at different time scales. Longitudinal analyses have been instrumental in elucidating the mechanisms underlying population dynamics in other study systems (Potts et al. 1980; Reed et al. 2013; Ewald et al. 2015; Haddad et al. 2015). However, as mentioned earlier, long-term ecological studies are rare in urban habitats. In this context, data obtained through citizen science projects can play a crucial role. Indeed, such data are intrinsically linked to the presence of humans and are therefore often collected within urban areas of different size and age (Bates et al. 2015; Bradsworth et al. 2017). Such data are increasingly used to assess long-term population trends and their underlying causes, and could be further exploited to understand early warning symptoms of demographic change linked to increasing urbanization.
Significance and future prospects
The widespread species loss associated with urbanization does not only happen during its first stages, but also during the complex process of urban sprawl, which creates a mosaic of different urban sub-habitats, from concrete-heavy business districts to greener suburban areas, that may or may not become unsuitable for certain species. Understanding when during this process species may cease to be able to cope with urban development is a research challenge that, if met, will provide us with unique knowledge about how urbanization affects biodiversity. We believe that meeting this challenge will require a mechanistic comprehension of this process that relies on long-term data on individual fitness, population growth, and habitat change.
Conclusions
There is a compelling need to expand and integrate different components of urban ecology to reach an integrative mechanistic understanding of how organisms respond to, cope with, and adapt to urbanization (Isaksson 2015; French et al. 2018). Urban sprawl has profound impacts on wild organisms, and the resulting disruption of physiology, behavior, and life history has major conservation implications (Knop et al. 2017; Ouyang et al. 2017; Kleist et al. 2018; Kernbach et al. 2018). In this context, there is a need to develop amelioration plans for species affected by urbanization, with the ultimate goal of designing environmentally sustainable cities with minimal ecological footprints. Despite the fact that the field of urban ecology is moving fast and attracting increasing scientific and public attention, we still lack a framework that can help us understand individual and population-level responses to urbanization.
The four questions we have raised here provide a framework and a pathway for an integrative understanding of urban ecology through a focus on mechanisms. We stress that we will need a combination of laboratory studies with controlled conditions, field studies that characterize fitness and the environment, and comparative and meta-analyses with global approaches for broad-scale patterns to form a holistic view of urban ecology. First, with clear definitions of the different factors that characterize a city, we can measure the socio-economic and ecological factors that influence the observable traits in animals, and their interactive effects. Second, we need to understand how urban animals differ from their rural counterparts through common garden and genomic studies that disentangle the genetic, epigenetic, and phenotypic contributions from development to adult phenotype. This will require us to not only focus on one trait but also on correlated traits. Next, to explore why individuals differ, we need to understand the evolutionary potential for adaptive change in traits of urban organisms. We will need to measure selection coefficients and use population genomic studies to explore global patterns. Lastly, to facilitate crosstalk between studies of individual-level phenotypic traits and biodiversity, we will need measures of lifetime fitness and links to population dynamics. With the expansion of the urban human population, having a concurrent increase in studies that address these knowledge gaps will help us build greener cities that maintain biodiversity and ecosystem function.
Acknowledgments
We wish to acknowledge the intellectual contributions of the participants in the Behavioral and Physiological Adaptations to Urban Environments workshop and symposium during the SICB 2018 meeting in San Francisco, CA. Lori Strong, Brett Burk, Ruedi Birenheide, and Richard Blob provided technical and administrative support.
Funding
This work was supported by the Society of Integrative and Comparative Biology (SICB); the National Science Foundation (NSF OIA-1738594 to J.Q.O.); the National Institutes of Health (NIH-P20 GM103650 to J.Q.O.); and the Swedish Research Council (VR; C0361301 to C.I.).
References
- Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Alberti M. 2015. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol Evol 30:114–26. [DOI] [PubMed] [Google Scholar]
- Alberti M, Correa C, Marzluff JM, Hendry AP, Palkovacs EP, Gotanda KM, Hunt VM, Apgar TM, Zhou Y.. 2017. Global urban signatures of phenotypic change in animal and plant populations. Proc Natl Acad Sci U S A 114:8951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen Alyson M, Stewart Kelley M, Longland William S, Beckmann Jon P, Forister Matthew L.. 2012. Identification of source-sink dynamics in mountain lions of the Great Basin. Mol Ecol 21:5689–701. [DOI] [PubMed] [Google Scholar]
- Arnfield AJ. 2003. Two decades of urban climate research: a review of turbulence, exchanges of energy and water, and the urban heat island. Int J Climatol 23:1–26. [Google Scholar]
- Aronson MFJ, La Sorte FA, Nilon CH, Katti M, Goddard MA, Lepczyk CA, Warren PS, Williams NSG, Cilliers S, Clarkson B, et al. 2014. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc Lond B Biol Sci 281:20133330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson Myla FJ, Lepczyk Christopher A, Evans Karl L, Goddard Mark A, Lerman Susannah B, MacIvor JS, Nilon Charles H, Vargo T.. 2017. Biodiversity in the city: key challenges for urban green space management. Front Ecol Environ 15:189–96. [Google Scholar]
- Audet J-N, Ducatez S, Lefebvre L.. 2016. The town bird and the country bird: problem solving and immunocompetence vary with urbanization. Behav Ecol 27:637–44. [Google Scholar]
- Badyaev AV. 2010. The beak of the other finch: coevolution of genetic covariance structure and developmental modularity during adaptive evolution. Philos Trans R Soc B Biol Sci 365:1111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev AV, Young RL, Oh KP, Addison C.. 2008. Evolution on a local scale: developmental, functional, and genetic bases of divergence in bill form and associated changes in song structure between adjacent habitats. Evolution 62:1951–64. [DOI] [PubMed] [Google Scholar]
- Bailly J, Scheifler R, Berthe S, Clément-Demange V-A, Leblond M, Pasteur B, Faivre B.. 2016. From eggs to fledging: negative impact of urban habitat on reproduction in two tit species. J Ornithol 157:377–92. [Google Scholar]
- Balogh AL, Ryder TB, Marra PP.. 2011. Population demography of gray catbirds in the suburban matrix: sources, sinks and domestic cats. J Ornithol 152:717–26. [Google Scholar]
- Banaszak-Cibicka W, Żmihorski M.. 2012. Wild bees along an urban gradient: winners and losers. J Insect Conserv 16:331–43. [Google Scholar]
- Bates AJ, Lakeman Fraser P, Robinson L, Tweddle JC, Sadler JP, West SE, Norman S, Batson M, Davies L.. 2015. The OPAL bugs count survey: exploring the effects of urbanisation and habitat characteristics using citizen science. Urban Ecosyst 18:1477–97. [Google Scholar]
- Beninde J, Veith M, Hochkirch A, Haddad N.. 2015. Biodiversity in cities needs space: a meta-analysis of factors determining intra-urban biodiversity variation. Ecol Lett 18:581–92. [DOI] [PubMed] [Google Scholar]
- Blair RB. 1996. Land use and avian species diversity along an urban gradient. Ecol Appl 6:506–19. [Google Scholar]
- Bonier F. 2012. Hormones in the city: endocrine ecology of urban birds. Horm Behav 61:763–72. [DOI] [PubMed] [Google Scholar]
- Bonier F, Martin PR.. 2016. How can we estimate natural selection on endocrine traits? Lessons from evolutionary biology. Proc R Soc B Biol Sci 283:20161887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse M, Spurgin LG, Laine VN, Cole EF, Firth JA, Gienapp P, Gosler AG, McMahon K, Poissant J, Verhagen I, et al. 2017. Recent natural selection causes adaptive evolution of an avian polygenic trait. Science 358:365–8. [DOI] [PubMed] [Google Scholar]
- Bradsworth N, White JG, Isaac B, Cooke R.. 2017. Species distribution models derived from citizen science data predict the fine scale movements of owls in an urbanizing landscape. Biol Conserv 213:27–35. [Google Scholar]
- Brans KI, Jansen M, Vanoverbeke J, Tüzün N, Stoks R, Meester LD.. 2017. The heat is on: genetic adaptation to urbanization mediated by thermal tolerance and body size. Glob Chang Biol 23:5218–27. [DOI] [PubMed] [Google Scholar]
- Capilla-Lasheras P, Dominoni DM, Babayan SA, O’Shaughnessy PJ, Mladenova M, Woodford L, Pollock CJ, Barr T, Baldini F, Helm B.. 2017. Elevated immune gene expression is associated with poor reproductive success of urban blue tits. Front Ecol Evol 5. [Google Scholar]
- Chace JF, Walsh JJ.. 2006. Urban effects on native avifauna: a review. Landsc Urban Plan 74:46–69. [Google Scholar]
- Chamberlain DE, Cannon AR, Toms MP, Leech DI, Hatchwell BJ, Gaston KJ.. 2009. Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151:1. [Google Scholar]
- Chapple DG, Simmonds SM, Wong BBM.. 2012. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol Evol 27:57–64. [DOI] [PubMed] [Google Scholar]
- Charmantier A, Demeyrier V, Lambrechts M, Perret S, Grégoire A.. 2017. Urbanization is associated with divergence in pace-of-life in great tits. Front Ecol Evol 5:53. [Google Scholar]
- Cohen AA, Isaksson C, Salguero-Gómez R.. 2017. Co-existence of multiple trade-off currencies shapes evolutionary outcomes. PLoS One 12:e0189124.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini M, Dubiec A, Marrot P, Szulkin M.. 2017. Humans and tits in the city: quantifying the effects of human presence on great tit and blue tit reproductive trait variation. Front Ecol Evol 5. [Google Scholar]
- Croci S, Butet A, Clergeau P.. 2008. Does urbanization filter birds on the basis of their biological traits. Condor 110:223–40. [Google Scholar]
- Da Silva A, Samplonius JM, Schlicht E, Valcu M, Kempenaers B.. 2014. Artificial night lighting rather than traffic noise affects the daily timing of dawn and dusk singing in common European songbirds. Behav Ecol 25:1037–47. [Google Scholar]
- Dominoni DM, Carmona-Wagner EO, Hofmann M, Kranstauber B, Partecke J.. 2014. Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J Anim Ecol 83:681–92. [DOI] [PubMed] [Google Scholar]
- Dominoni DM, Quetting M, Partecke J.. 2013. Artificial light at night advances avian reproductive physiology. Proc Biol Sci 280:20123017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatez S, Sayol F, Sol D, Lefebvre L. 2018. Are urban vertebrates city specialists, artificial habitat exploiters or environmental generalists? Integr Comp Biol (doi:10.1093/icb/icy101). [DOI] [PubMed]
- Evans KL, Chamberlain DE, Hatchwell BJ, Gregory RD, Gaston KJ.. 2011. What makes an urban bird? Glob Chang Biol 17:32–44. [Google Scholar]
- Evans KL, Gaston KJ, Sharp SP, McGowan A, Simeoni M, Hatchwell BJ.. 2009. Effects of urbanisation on disease prevalence and age structure in blackbird Turdus merula populations. Oikos 118:774–82. [Google Scholar]
- Ewald JA, Wheatley CJ, Aebischer NJ, Moreby SJ, Duffield SJ, Crick HQP, Morecroft MB.. 2015. Influences of extreme weather, climate and pesticide use on invertebrates in cereal fields over 42 years. Glob Chang Biol 21:3931–50. [DOI] [PubMed] [Google Scholar]
- Fokidis HB, Orchinik M, Deviche P.. 2009. Corticosterone and corticosteroid binding globulin in birds: relation to urbanization in a desert city. Gen Comp Endocrinol 160:259–70. [DOI] [PubMed] [Google Scholar]
- Foltz SL, Ross AE, Laing BT, Rock RP, Battle KE, Moore IT.. 2015. Get off my lawn: increased aggression in urban song sparrows is related to resource availability. Behav Ecol 26:1548–57. [Google Scholar]
- French SS, Webb AC, Hudson SB, Virgin EE. 2018. Town and country reptiles: A review of reptilian responses to urbanization. Integr Comp Biol (doi:10.1093/icb/icy052). [DOI] [PubMed]
- Gaggiotti OE, Bekkevold D, Jørgensen HBH, Foll M, Carvalho GR, Andre C, Ruzzante DE.. 2009. Disentangling the effects of evolutionary, demographic, and environmental factors influencing genetic structure of natural populations: Atlantic herring as a case study. Evolution 63:2939–51. [DOI] [PubMed] [Google Scholar]
- Garroway CJ, Sheldon BC.. 2013. Urban behavioural adaptation. Mol Ecol 22:3430–2. [DOI] [PubMed] [Google Scholar]
- Giraudeau M, Mousel M, Earl S, McGraw K.. 2014. Parasites in the city: degree of urbanization predicts poxvirus and coccidian infections in house finches (Haemorhous mexicanus). PLoS One 9:e86747.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard MA, Dougill AJ, Benton TG.. 2010. Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25:90–8. [DOI] [PubMed] [Google Scholar]
- Greaver TL, Sullivan TJ, Herrick JD, Barber MC, Baron JS, Cosby BJ, Deerhake ME, Dennis RL, Dubois J-JB, Goodale CL, et al. 2012. Ecological effects of nitrogen and sulfur air pollution in the US: what do we know? Front Ecol Environ 10:365–72. [Google Scholar]
- Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, et al. 2015. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:e1500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JD, Davies G, Fairbrass AJ, Matthews TJ, Rogers CDF, Sadler JP.. 2013. Mapping lightscapes: spatial patterning of artificial lighting in an urban landscape. PLoS One 8:e61460.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale R, Morrongiello JR, Swearer SE.. 2016. Evolutionary traps and range shifts in a rapidly changing world. Biol Lett 12:20160003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfwerk W, Slabbekoorn H.. 2013. The impact of anthropogenic noise on avian communication and fitness In: Gil D, Brumm H, editors. Avian urban ecology: behavioral and physiological adaptations. Oxford, UK: Oxford University Press; p. 84–97. [Google Scholar]
- Harris SE, Xue AT, Alvarado-Serrano D, Boehm JT, Joseph T, Hickerson MJ, Munshi-South J.. 2016. Urbanization shapes the demographic history of a native rodent (the white-footed mouse, Peromyscus leucopus) in New York City. Biol Lett 12:pii: 20150983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harveson PM, Lopez RR, Collier BA, Silvy NJ.. 2007. Impacts of urbanization on Florida Key deer behavior and population dynamics. Biol Conserv 134:321–31. [Google Scholar]
- Hedin J, Isacsson G, Jonsell M, Komonen A.. 2008. Forest fuel piles as ecological traps for saproxylic beetles in oak. Scand J Forest Res 23:348–57. [Google Scholar]
- Hope D, Gries C, Zhu W, Fagan WF, Redman CL, Grimm NB, Nelson AL, Martin C, Kinzig A.. 2003. Socioeconomics drive urban plant diversity. Proc Natl Acad Sci U S A 100:8788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton P, Wright CD, DeNardo DF, McGraw KJ. 2018. No effect of human presence at night on disease, body mass, or metabolism in rural and urban house finches (Haemorhous mexicanus). Integr Comp Biol (doi:10.1093/icb/icy093). [DOI] [PubMed]
- Ibáñez-Álamo JD, Rubio E, Bitrus Zira K.. 2017. The degree of urbanization of a species affects how intensively it is studied: a global perspective. Front Ecol Evol 5:41. [Google Scholar]
- Isaksson C. 2010. Pollution and its impact on wild animals: a meta-analysis on oxidative stress. EcoHealth 7:342–50. [DOI] [PubMed] [Google Scholar]
- Isaksson C. 2015. Urbanization, oxidative stress and inflammation: a question of evolving, acclimatizing or coping with urban environmental stress. Funct Ecol 29:913–23. [Google Scholar]
- Järup L. 2003. Hazards of heavy metal contamination. Br Med Bull 68:167–82. [DOI] [PubMed] [Google Scholar]
- Jenkins BR, Vitousek MN, Hubbard JK, Safran RJ.. 2014. An experimental analysis of the heritability of variation in glucocorticoid concentrations in a wild avian population. Proc R Soc B Biol Sci 281:20141302.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MTJ, Munshi-South J.. 2017. Evolution of life in urban environments. Science 358:eaam8327.. [DOI] [PubMed] [Google Scholar]
- Jokimäki J, Clergeau P, Kaisanlahti-Jokimäki M-L.. 2002. Winter bird communities in urban habitats: a comparative study between central and northern Europe. J Biogeogr 29:69–79. [Google Scholar]
- Kalnay E, Cai M.. 2003. Impact of urbanization and land-use change on climate. Nature 423:528–31. [DOI] [PubMed] [Google Scholar]
- Kaye JP, McCulley RL, Burke IC.. 2005. Carbon fluxes, nitrogen cycling, and soil microbial communities in adjacent urban, native and agricultural ecosystems. Glob Chang Biol 11:575–87. [Google Scholar]
- Kernbach ME, Hall RJ, Burkett-Cadena ND, Unnasch TR, Martin LB. 2018. Dim light at night: physiological effects and ecological consequences for infectious disease. Integr Comp Biol (doi:10.1093/icb/icy080). [DOI] [PubMed]
- Killen SS, Marras S, Metcalfe NB, McKenzie DJ, Domenici P.. 2013. Environmental stressors alter relationships between physiology and behaviour. Trends Ecol Evol 28:651–8. [DOI] [PubMed] [Google Scholar]
- Kinzig AP, Warren P, Martin C, Hope D, Katti M.. 2005. The effects of human socioeconomic status and cultural characteristics on urban patterns of biodiversity. Ecol Soc 10:23. [Google Scholar]
- Kleist NJ, Guralnick RP, Cruz A, Lowry CA, Francis CD.. 2018. Chronic anthropogenic noise disrupts glucocorticoid signaling and has multiple effects on fitness in an avian community. Proc Natl Acad Sci U S A 115:E648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop E, Zoller L, Ryser R, Gerpe C, Hörler M, Fontaine C.. 2017. Artificial light at night as a new threat to pollination. Nature 548:206–9. [DOI] [PubMed] [Google Scholar]
- Kobiela ME, Snell-Rood EC. 2018. Nickel exposure has complex transgenerational effects in a butterfly. Integr Comp Biol (doi:10.1093/icb/icy096). [DOI] [PubMed]
- Lapiedra O. 2018. Urban behavioral ecology: lessons from Anolis lizards. Integr Comp Biol (doi:10.1093/icb/icy109). [DOI] [PubMed]
- LaPoint S, Balkenhol N, Hale J, Sadler J, Ree R, Evans K.. 2015. Ecological connectivity research in urban areas. Funct Ecol 29:868–78. [Google Scholar]
- Lessells CM. 2008. Neuroendocrine control of life histories: what do we need to know to understand the evolution of phenotypic plasticity? Philos Trans R Soc B Biol Sci 363:1589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liker A, Papp Z, Bókony V, Lendvai ÁZ.. 2008. Lean birds in the city: body size and condition of house sparrows along the urbanization gradient. J Anim Ecol 77:789–95. [DOI] [PubMed] [Google Scholar]
- Maklakov AA, Immler S, Gonzalez-Voyer A, Rönn J, Kolm N.. 2011. Brains and the city: big-brained passerine birds succeed in urban environments. Biol Lett 7:730–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff JM. 2017. A decadal review of urban ornithology and a prospectus for the future. Ibis 159:1–13. [Google Scholar]
- McIntyre NE. 2000. Ecology of urban arthropods: a review and a call to action. Ann Entomol Soc Am 93:825–35. [Google Scholar]
- McKinney ML. 2006. Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–60. [Google Scholar]
- McMahon TA, Rohr JR, Bernal XE.. 2017. Light and noise pollution interact to disrupt interspecific interactions. Ecology 98:1290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AP. 2009. Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159:849–58. [DOI] [PubMed] [Google Scholar]
- Mueller JC, Partecke J, Hatchwell BJ, Gaston KJ, Evans KL.. 2013. Candidate gene polymorphisms for behavioural adaptations during urbanization in blackbirds. Mol Ecol 22:3629–37. [DOI] [PubMed] [Google Scholar]
- Mulholland TI, Ferraro DM, Boland KC, Ivey KN, Le M-L, LaRiccia CA, Vigianelli JM, Francis CD. 2018. Effects of experimental anthropogenic noise exposure on the reproductive success of secondary cavity nesting birds. Integr Comp Biol (doi:10.1093/icb/icy079). [DOI] [PubMed]
- Ouyang JQ, Davies S, Dominoni D.. 2018. Hormonally mediated effects of artificial light at night on behavior and fitness: linking endocrine mechanisms with function. J Exp Biol 221:jeb156893.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang JQ, de Jong M, van Grunsven RHA, Matson KD, Haussmann MF, Meerlo P, Visser ME, Spoelstra K.. 2017. Restless roosts: light pollution affects behavior, sleep, and physiology in a free-living songbird. Glob Chang Biol 23:4987–94. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Hau M, Bonier F.. 2011. Within seasons and among years: when are corticosterone levels repeatable? Horm Behav 60:559–64. [DOI] [PubMed] [Google Scholar]
- Ouyang JQ, Lendvai ÁZ, Moore IT, Bonier F, Haussmann MF.. 2016. Do hormones, telomere lengths, and oxidative stress form an integrated phenotype? A case study in free-living tree swallows. Integr Comp Biol 56:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partecke J, Gwinner E, Bensch S.. 2006. Is urbanisation of European blackbirds (Turdus merula) associated with genetic differentiation? J Ornithol 147:549–52. [Google Scholar]
- Partecke J, Van’t Hof T, Gwinner E.. 2005. Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J Avian Biol 36:295–305. [Google Scholar]
- Perrier C, Charmantier A.. 2018. On the timing of bill length evolution in British great tits: a comment on Bosse et al. 2017. bioRxiv ( 10.1101/269175). [DOI]
- Perrier C, Lozano del Campo A, Szulkin M, Demeyrier V, Gregoire A, Charmantier A.. 2018. Great tits and the city: distribution of genomic diversity and gene–environment associations along an urbanization gradient. Evol Appl 11:593–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CJ, Capilla-Lasheras P, McGill RAR, Helm B, Dominoni DM.. 2017. Integrated behavioural and stable isotope data reveal altered diet linked to low breeding success in urban-dwelling blue tits (Cyanistes caeruleus). Sci Rep 7:5014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GR, Coulson JC, Deans IR.. 1980. Population dynamics and breeding success of the Shag, Phalacrocorax aristotelis, on the Farne Islands, Northumberland. J Anim Ecol 49:465–84. [Google Scholar]
- Ramalho CE, Hobbs RJ.. 2012. Time for a change: dynamic urban ecology. Trends Ecol Evol 27:179–88. [DOI] [PubMed] [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ.. 2007. Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. [DOI] [PubMed] [Google Scholar]
- Reed TE, Jenouvrier S, Visser ME.. 2013. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J Anim Ecol 82:131–44. [DOI] [PubMed] [Google Scholar]
- Riley SPD, Sauvajot RM, Fuller TK, York EC, Kamradt DA, Bromley C, Wayne RK.. 2003. Effects of urbanization and habitat fragmentation on bobcats and coyotes in Southern California. Conserv Biol 17:566–76. [Google Scholar]
- Robertson BA, Rehage JS, Sih A.. 2013. Ecological novelty and the emergence of evolutionary traps. Trends Ecol Evol 28:552–60. [DOI] [PubMed] [Google Scholar]
- Rodewald AD, Kearns LJ, Shustack DP.. 2013. Consequences of urbanizing landscapes to reproductive performance of birds in remnant forests. Biol Conserv 160:32–9. [Google Scholar]
- Salmón P, Nilsson JF, Watson H, Bensch S, Isaksson C.. 2017. Selective disappearance of great tits with short telomeres in urban areas. Proc R Soc B Biol Sci 284:20171349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon P, Stroh E, Herrera-Duenas A, von Post M, Isaksson C.. 2018. Oxidative stress in birds along a NOx and urbanisation gradient: an interspecific approach. Sci Total Environ 622–623:635–43. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Bridge ES, Boughton RK, Wilcoxen TE.. 2009. Environment, glucocorticoids, and the timing of reproduction. Gen Comp Endocrinol 163:201–7. [DOI] [PubMed] [Google Scholar]
- Sepp T, McGraw KJ, Kaasik A, Giraudeau M.. 2018. A review of urban impacts on avian life-history evolution: does city living lead to slower pace of life? Glob Chang Biol 24:1452–69. [DOI] [PubMed] [Google Scholar]
- Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D.. 2006. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–91. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson JC.. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–8. [DOI] [PubMed] [Google Scholar]
- Sih A, Mathot KJ, Moirón M, Montiglio P-O, Wolf M, Dingemanse NJ.. 2015. Animal personality and state-behaviour feedbacks: a review and guide for empiricists. Trends Ecol Evol 30:50–60. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Ripmeester EAP.. 2008. Birdsong and anthropogenic noise: implications and applications for conservation. Mol Ecol 17:72–83. [DOI] [PubMed] [Google Scholar]
- Smith SB, McKay JE, Richardson JK, Shipley AA, Murphy MT.. 2016. Demography of a ground nesting bird in an urban system: are populations self-sustaining? Urban Ecosyst 19:577–98. [Google Scholar]
- Sol D, González-Lagos C, Moreira D, Maspons J, Lapiedra O.. 2014. Urbanisation tolerance and the loss of avian diversity. Ecol Lett 17:942–50. [DOI] [PubMed] [Google Scholar]
- Sol D, Lapiedra O, González-Lagos C.. 2013. Behavioural adjustments for a life in the city. Anim Behav 85:1101–12. [Google Scholar]
- Sprau P, Dingemanse NJ.. 2017. An approach to distinguish between plasticity and non-random distributions of behavioral types along urban gradients in a wild passerine bird. Front Ecol Evol ( 10.3389/fevo.2017.00092). [DOI] [Google Scholar]
- Sprau P, Mouchet A, Dingemanse NJ.. 2017. Multidimensional environmental predictors of variation in avian forest and city life histories. Behav Ecol 28:59–68. [Google Scholar]
- Swaddle JP, Francis CD, Barber JR, Cooper CB, Kyba CCM, Dominoni DM, Shannon G, Aschehoug E, Goodwin SE, Kawahara AY, et al. 2015. A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol Evol 30:550–60. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. 1963. On aims and methods of ethology. Z Tierpsychol 20:410–33. [Google Scholar]
- Tucker MA, Böhning-Gaese K, Fagan WF, Fryxell JM, Van Moorter B, Alberts SC, Ali AH, Allen AM, Attias N, Avgar T, et al. 2018. Moving in the Anthropocene: global reductions in terrestrial mammalian movements. Science 359:466–9. [DOI] [PubMed] [Google Scholar]
- Verboven N, Tinbergen JM, Verhulst S.. 2001. Food, reproductive success and multiple breeding in the great tit Parus major. Ardea 89:387–406. [Google Scholar]
- United Nations. 2014. World urbanization prospects. Department of Economic and Social Affairs, Population Division.
- Welbers AAMH, van Dis NE, Kolvoort AM, Ouyang J, Visser ME, Spoelstra K, Dominoni DM.. 2017. Artificial light at night reduces daily energy expenditure in breeding great tits (Parus major). Front Ecol Evol 5. [Google Scholar]
- While A, Whitehead M.. 2013. Cities, urbanisation and climate change. Urban Stud 50:1325–31. [Google Scholar]
- Wolf M, Weissing FJ.. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 27:452–61. [DOI] [PubMed] [Google Scholar]
- Wong BBM, Candolin U.. 2015. Behavioral responses to changing environments. Behav Ecol 26:665–73. [Google Scholar]