Abstract
The lateral line system is a sensory system unique to fishes and amphibians. It is composed of distributed mechanosensory hair cell organs on the head and body (neuromasts), which are sensitive to pressure gradients and water movements. Over the last decade, we have pursued an interdisciplinary approach by combining behavioral, electrophysiology, and robotics experiments to study this fascinating sensory system. In behavioral and electrophysiology experiments, we have studied the larval lateral line system in the model genetic organism, zebrafish (Danio rerio). We found that the lateral line system, even in 5-day-old larvae, is involved in an array of behaviors that are critical to survival, and the deflection of a single neuromast can elicit a swimming response. In robotics experiments, we used a range of physical models with distributed pressure sensors to better understand the hydrodynamic environments from the local perspective of a fish or robot. So far, our efforts have focused on extracting control-related information for a range of application scenarios including characterizing unsteady flows such as Kármán vortex streets for station holding. We also used robot models to test biological hypotheses on how morphology and movement of fishes affect lateral line sensing. Overall, with this review we aim to increase the visibility and accessibility of this multi-disciplinary research approach.
Introduction
Fishes have evolved for over 500 million years in water and all (more than 30,000 known species) possess a distributed sensory system called the lateral line. This elaborate sensory system consists of neuromasts made of support cells and a cluster of hair cells whose apical cilia and stereovilli bundles are embedded in a dome-shaped, gelatinous cupula (Dijkgraaf 1963). The interaction between local fluid movements and the cupula causes deflections of the hair cells and the opening of mechanically gated ion channels allows cations to enter. Depolarization of the hair cells leads to glutamate release at the hair cell-afferent synapse and triggers action potentials in lateralis afferent neurons (Hudspeth and Corey 1977). There are two types of neuromasts: superficial and canal neuromasts. Superficial neuromasts project from the surface of the skin into the water and are sensitive to local flows near the body. In contrast, canal neuromasts are found in hollow, fluid-filled canals right below the surface and are sensitive to flows within the canal, governed by the pressure gradients across the canal pores, which are open to the environment (Kroese and Schellart 1992; Engelmann et al. 2000).
The refined ability to sense local flows and pressure gradients around the head and body enables fishes to respond to changes in their immediate surroundings. We think that the function of the lateral line system is two-fold. First, it is important for sensing the external environment. Previous studies have shown that the lateral line system plays a role in many critical behaviors such as rheotaxis (Oteiza et al. 2017), tracking hydrodynamic trails (Dehnhardt et al. 2001), localizing prey (Coombs and Conley 1997), and evading predators (Stewart et al. 2013). Second, it can provide sensory feedback through afferent and efferent pathways for controlling the movements of the body. There is compelling evidence that flow-relative control is key to enhance sensing and swimming performance as well as mediating respiratory locomotor coupling in fishes (Akanyeti et al. 2016).
Today’s research on the lateral line system is highly interdisciplinary, attracting talented scientists and engineers from different backgrounds. Recent advances in flow visualization (e.g., digital particle image velocimetry) and computational fluid dynamics methods have made it possible to study fluid-structure interactions at different scales (Akanyeti et al. 2011; Windsor 2014; Herzog et al. 2017). Molecular, anatomical, neurophysiological, and theoretical studies revealed important insights into the function and capabilities of individual neuromasts and the lateral line system as a whole (Hassan 1992; Ren and Mohseni 2012; Haehnel-Taguchi et al. 2014; Webb 2014; Lv et al. 2016). Organismal studies have taken advantage of pharmacological and laser treatments to ablate the lateral line system to interpret its function during relevant behaviors (Coombs and Conley 1997; Mirjany et al. 2011; Kohashi et al. 2012; Suli et al. 2012; Groneberg et al. 2015; Carrillo and McHenry 2016). 3D printed physical models and miniature pressure sensors allow us to systematically explore how morphology and movement of fishes affect lateral line sensing and swimming (Kruusmaa et al. 2014; Akanyeti et al. 2016, 2017). In addition, the accessibility and regenerative abilities of lateral line hair cells offer unique opportunities to study fundamental processes in hearing, development, and cell differentiation (Coffin et al. 2013).
Over a span of a decade, we (i.e., the authors) employed a combination of behavior, electrophysiology, and robotics approaches to better understand the function and organization of the lateral line system. Here, we summarize our own lateral line research and highlight the important findings. When appropriate, we also discuss relevant studies from other laboratories to put our research into context. However, we do not attempt to provide a comprehensive literature review of this diverse and fast-growing field; for this see Coombs et al. (2014).
Behavior experiments
The zebrafish larvae is a powerful vertebrate model organism in developmental, genetics, behavior, and neurophysiology research (Briggs 2002; Fero et al. 2010; Kalueff et al. 2013; Roberts et al. 2013; Wyatt et al. 2015). They have a tractable lateral line system (as depicted in Fig. 1) which allows us to investigate the hair cell function in vivo, which is advantageous to the field of auditory and vestibular research (Nicolson et al. 1998; Coffin et al. 2013).
Fig. 1.
(A) Schematic diagram of a zebrafish larva and its lateral line system at 7-day post fertilization (lateral view). Small circles represent the locations of the superficial neuromasts. At this age, the larvae possess only superficial neuromasts. Neuromasts are named according to their anatomical position: lateral branch (L), terminal lateral branch (Lt), secondary lateral branch (LII), dorsal branch (D), medial branch (MI), otic (O), mandibular (M), opercular (OP), supraorbital (SO), infraorbital (IO), and nasal (N) neuromasts. (B) Schematic diagram of a neuromast containing mechanosensory hair cells and support cells (lateral view). Each hair cell carries a kinocillium and a bundle of stereovilli at its apical surface, which are enclosed in a gelatinous cupula. The hair cells are innervated by peripheral projections of afferent neurons and two types of efferent neurons. Together, afferent and efferent neurons form the lateral line nerve.
It was shown that the lateral line system allows the larva to sense the strike of a predator especially in the absence of visual cues (McHenry et al. 2009; Stewart et al. 2013). To investigate whether the lateral line system plays role in evading suctions, we tested larval fish in a circular arena with a suction chamber located at the bottom (Olszewski et al. 2012). In control experiments with lateral line intact animals, the larvae consistently oriented away from the suction and elicited a burst swimming once they were dragged by the current. When the whole posterior lateral line was ablated using the aminoglycoside antibiotic neomycin, the ability of larvae to avoid the suction source was greatly diminished.
Larval rheotaxis in a flow chamber was demonstrated by Suli et al. (2012), which was also greatly diminished after the disruption of the lateral line system. The behavior of fish returned to normal after some time when hair cells were regenerated. The subject of rheotaxis in larval zebrafish was recently revisited by Oteiza et al. (2017), who combined behavior, neural imaging, and flow visualization approaches to provide a computational model on how the lateral line system mediates rheotaxis in larval zebrafish.
Electrophysiology experiments
To understand how the nervous system encodes and processes hydrodynamic stimuli, it is necessary to observe the neuronal activity of the lateral line system. Hair cells generate graded excitatory and inhibitory potentials in response to the deflection of their kinocilia (Corey and Hudspeth 1983; Ricci et al. 2013). Primary afferent neurons generate action potentials in response to the release of glutamate at the hair cell-afferent synapse. At rest, this results in a spontaneous base frequency of action potentials (Glowatzki and Fuchs 2002). Glutamate release increases when hair cells are excited and become depolarized, which is faithfully reflected in an increase in action potential frequency in the afferent neurons (Keen and Hudspeth 2006).
In the posterior lateral line, the afferent neurons form the lateral line nerve together with efferent fibers (Metcalfe et al. 1985). Extracellular recordings from the lateral line nerve in different fish species have shown that afferent activity can encode the frequency of water flow and that the lateral line system is stimulated in laminar and turbulent flows (Montgomery and Coombs 1992; Engelmann et al. 2000, 2003; Weeg and Bass 2002; Chagnaud et al. 2008). Also, efferent signals could be identified from extracellular recordings of the lateral line nerve and may increase or decrease their activity in response to hydrodynamic stimuli (Russell 1968; Flock and Russell 1973; Russell and Roberts 1974).
The cell bodies of the primary afferent neurons are in the lateral line ganglia, and to our knowledge, so far only afferent neurons of the posterior lateral line were the subject of electrophysiological investigations. Selective recordings from individual afferent neurons in the posterior lateral line ganglion have provided insight into the coding properties of the lateral line system. Afferent neuron activity has been recorded both intracellularly in whole cell patch configuration (Liao 2010; Liao and Haehnel 2012) and extracellularly in loose patch configuration (Obholzer et al. 2008; Trapani and Nicolson 2011; Haehnel et al. 2012; Levi et al. 2015; Lv et al. 2016).
With whole cell patch recordings, we were able to show that action potentials are generated in response to neuromast deflections caused by water jets (Liao 2010). Physiological properties of afferent neurons, such as excitability and spontaneous activity, vary in relation to their anatomical position and to which neuromasts they innervate (Liao and Haehnel 2012). The results indicated that smaller cells tended to innervate more rostral neuromasts and were more excitable, while larger cells innervated more caudal neuromasts and were less excitable. Next, we became interested in the question of how afferent neurons encode the mechanical deflections of the lateral line neuromasts. For controlled and precise mechanical deflections, we developed a stimulation device driven by a piezo actuator; this device was used to deflect the neuromasts over a range of frequencies and velocities (Haehnel-Taguchi et al. 2014; Levi et al. 2015). The main results from these studies are: (1) the stimulation frequencies up to 60 Hz were faithfully encoded by the afferent neurons and the firing of action potentials phase-locks to the stimulation frequency (Levi et al. 2015); (2) the activity of afferent neurons increased linearly between 0.1 and 1 µm/s of increasing deflection velocities (Haehnel-Taguchi et al. 2014); (3) the response of lateral line afferent neurons was phasic-tonic during the deflection phase, which suggests a tuning to a change of flow; (4) deflection of a single neuromast could elicit a locomotor response (as measured by fictive swimming recordings); (5) there was an increased probability of eliciting a locomotor response with increased lateral line activity; and (6) each afferent neuron was innervated up to three neighboring neuromasts, which was evident in the afferent recordings when multiple neuromasts were stimulated. This corroborated the anatomical findings showing afferent neurons contact hair cells of neighboring neuromasts with the same hair cell orientation (Faucherre et al. 2009).
Afferent recordings have become a useful tool to study the efficiency of ototoxic agents (Stewart et al. 2017). Further they can be employed to study the details of synapse function and transmitter release, by providing signals from the postsynaptic neurons (Obholzer et al. 2008; Lv et al. 2016). These approaches can be complemented by measurements of the presynaptic currents in hair cells using whole-cell patch clamp techniques (Ricci et al. 2013; Lv et al. 2016; Olt et al. 2016). These tools will be of great advantage in elucidating the details of the mechanotransduction process in hair cells, which to date is still not fully understood (Nicolson 2017).
The efferent modulation of the lateral line system has been studied to an even lesser extent compared with its afferent pathways. Neuromasts receive input from cholinergic and dopaminergic efferent fibers (Metcalfe et al. 1985; Bricaud et al. 2001). The cholinergic efferent system that innervates the hair cells of the auditory, vestibular and, in fishes, the lateral line system is conserved among vertebrates (Köppl 2011). It had been suspected that the cholinergic system is involved in providing corollary discharge to inhibit the sensory components during locomotion (Feitl et al. 2010), which was confirmed in an electrophysiological study in tadpoles (Chagnaud et al. 2015). The function of the dopaminergic system which provides extensive projections to the neuromasts, the lateral line ganglia, and the medial octavolateralis nucleus are unknown to date (Haehnel-Taguchi et al. 2018). Recently it was shown that dopaminergic fibers may act on hair cells via paracrine dopamine release on D1 receptors (Toro et al. 2015). The dopaminergic efferent neurons, which originate from a nucleus in the ventral diencephalon, respond to mechanosensory stimuli in a dose dependent fashion, suggesting a possible feedback function (Reinig et al. 2017). Electro- and opto-physiological approaches promise to enhance our understanding of efferent modulation greatly in the future, not only for the lateral line system but also of hair cell systems in general, especially since the afferent and efferent structures in the mammalian auditory system are difficult to access. Overall, investigating the factors and behavioral states that may affect and modulate lateral line sensing will be an intriguing direction for future research.
Robotics experiments
Developing artificial lateral line systems
Inspired from the lateral line neuromasts and ciliated sensory cells in invertebrate systems (e.g., setae of crustaceans and filiform hairs of cricket’s cerci), a variety of approaches have been proposed to develop artificial flow sensors. Many of these approaches are based on micro-electro-mechanical systems (MEMS). While there are variations in design, fabrication, and readout methods, the main principles of MEMS sensing remain the same. A cantilever, projecting into the flow, is deformed due to the mechanical load applied by the moving fluid. These deformations change the electrical properties (e.g., capacitance and resistance) of the cantilever which are picked up by specialized circuits (Rizzi et al. 2014). For an extensive review on MEMS-based and other artificial hair cell designs, see Tao and Yu (2012) and Liu et al. (2016).
While good progress has been made in manufacturing and testing artificial hair cells in controlled laboratory conditions, they have yet to be employed in real-world applications. Current research efforts focus on understanding how size, geometry, and material properties of the cantilever affect the sensor performance, designing an antiparallel cantilever to achieve bi-directional flow sensitivity, developing an artificial cupula to increase the sensitivity, reliability, and robustness of the sensors (Kottapalli et al. 2017), building flexible sensor arrays (Jiang et al. 2017), and developing a multi-model artificial lateral line system (DeVries et al. 2015). In addition, there are efforts to adapt artificial lateral line systems for remote sensing in the field, for instance in rivers and water dams (Tuhtan et al. 2016), monitoring blood flow through the heart and increasing the aero/hydrodynamic efficiency of transportation vehicles.
Locomotion and sensing in unsteady flow regimes
To complement artificial hair cells, off-the-shelf miniature pressure sensors have been successfully employed to map the pressure distribution along robots and other physical models. So far in our research, we have mainly employed these pressure-based artificial lateral line systems. While much is known about the pressure-flow relationship in steady flows, our understanding has been limited to a few special cases in unsteady conditions (e.g., dipole fields are generated in still water by a vibrating sphere).
In an attempt to understand more complex flows, we chose to study Kármán vortex streets (KVS). In laboratory experiments, a KVS is generated by placing a cylinder in a steady flow. The flow moving past a cylinder creates vortices that shed alternately from each side of the cylinder. KVSs provide an opportunity to study a range of hydrodynamic conditions in a focused way because we can systematically change the frequency (i.e., vortex shedding frequency) and spacing (i.e., wake wavelength) of vortices by altering flow speed and cylinder size. In addition, a KVS includes four sub-hydrodynamic regions, each of which presents a unique set of sensing and control challenges. These regions are: suction region, where flow recirculates toward the cylinder; vortex formation region, where vortices are developed but still attached to the core; actual vortex street, where vortices are detached from the core and travel with constant velocity; and relatively steady flows on each side of the cylinder (Akanyeti et al. 2011).
Previous studies (e.g., Liao 2007; Przybilla et al. 2010) have shown that behind the cylinder, fish display a variety of unsteady behaviors including Kármán gaiting (slaloming between vortices), entraining in no flow zones, quasi-steady swimming, and getting in and out of KVSs (Fig. 2). Among these behaviors, we have studied Kármán gaiting the most. Over the years, we swam rainbow trout (Oncorhynchus mykiss) in steady flows and KVSs at varying flow speeds and cylinder diameters. The results from these experiments have been summarized in Liao and Akanyeti (2017). Briefly: (1) fish reduced muscle activity and consume less oxygen during Kármán gaiting than steady swimming (Liao 2004; Taguchi and Liao 2011); (2) depending on body size, there was a range of flow speeds and cylinder diameters that increase the probability of Kármán gaiting (Akanyeti and Liao 2013a); (3) fish were typically one–two body lengths downstream from the cylinder while Kármán gaiting (in this region, vortices are strong, and the average flow speed is significantly lower than the incoming flow speed); (4) Kármán gait kinematics were closely related to flow parameters, and could be modeled with a traveling wave equation (Akanyeti and Liao 2013b); (5) fish also Kármán gait behind tandem cylinders (i.e., two cylinders placed one after another) when downstream spacing between the cylinders was appropriate (Stewart et al. 2016); and (6) both vision and lateral line system played active roles during Kármán gaiting (Liao 2006).
Fig. 2.
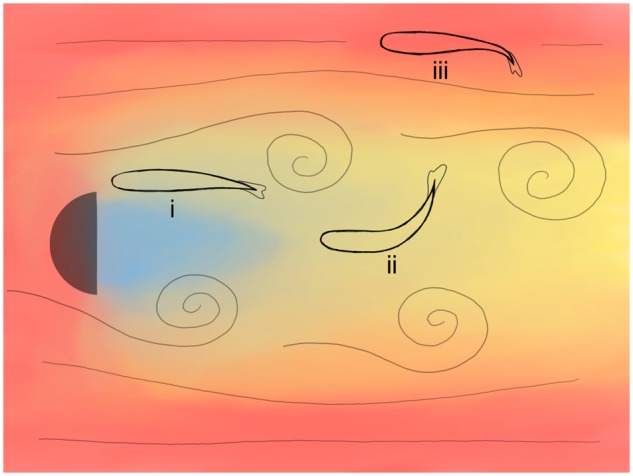
Schematic diagram of a Kármán vortex street and a rainbow trout (dorsal view) while entraining behind a D-shaped cylinder (i), Kármán gaiting inside the vortex street (ii), and steady swimming outside the vortex street (iii). Blue and red colors indicate regions with low and high flow velocity, respectively.
Pressure measurements in KVS
To better understand KVSs in a frame of reference relevant to a swimming fish or a robot, we designed a boat-shaped sensor platform consisting of an array of 10 pressure sensors on each side (Venturelli et al. 2012). This platform was employed in a steady flow and a KVS to measure local pressures in relation to the orientation and lateral position of the platform. After analyzing the pressure data, we found that: (1) compared with the steady flow, the average turbulence intensity was 30% higher in the KVS, and at least 50% of the sensors were simultaneously detecting the vortex shedding frequency as the dominant frequency; (2) in both flow regimes, there was a direct correlation between the orientation of the platform and the pressure difference across sides, which increased with turning angle; (3) in the KVS, there was also a direct correlation between the lateral displacement of the platform and the pressure difference across sides, where again, the pressure difference increased with lateral displacement; and (4) in addition, we were able to track and estimate the speed of shed vortices by calculating cross-correlations among the neighboring sensors on each side. Our results support the hypothesis proposed by Chagnaud et al. (2008) that fish use a similar method to track moving objects and estimate flow velocity.
Next, a 3D printed fusiform platform (inspired from fish head), consisting of 16 pressure sensors (Chambers et al. 2014), was used. Compared with 2D horizontal arrays presented in Venturelli et al. (2012), in this study, the sensors were distributed in 3D. We also extended our investigation to nine KVSs, which were generated by all possible combinations between three cylinders and three flow speeds. This allowed evaluating the individual effect of flow parameters on sensing. For instance, some KVSs have distinct vortex shedding frequencies but similar wake wavelengths, whereas others have distinct wavelengths but similar frequencies. Another distinction in these experiments was that the sensor platform was mobile. The head was first moved to and away from the cylinder. It was then moved laterally across the wake from one side of the cylinder to the other. In both scenarios, the platform was moving slowly to minimize any alterations that it may induce on the flow. The main results are: (1) the flow parameters derived from pressure measurements provided sufficient information to differentiate each KVS by estimating its cylinder size and flow speed; (2) when moving laterally, the relative position of the head with respect to the KVS’s centerline was estimated using the measures of turbulence intensity and the pressure difference between the tip and side sensors; and (3) when moving toward the cylinder, we could successfully identify the suction zone, vortex formation region, and actual vortex street using the measures of turbulence intensity, strength of the vortex shedding frequency, and pressure difference. In addition, the movement increased the dynamic sensing range of the platform. This is because when the sensor platform was static, it could only take pressure measurements at one location and a reference signal was required to analyze these measurements. In contrast, this reference signal was not required while moving. The platform was taking snapshots of the environment at different locations. By analyzing pressure variations over time, the orientation and position of the platform was recovered successfully. Overall, our results suggest that 3D pressure measurements provide useful information for detection, characterization, and localization of a source that produces an unsteady wake.
How does morphology and movement of the robot affect pressure sensing?
Most of our theoretical understanding on how a sensor platform affects its surrounding fluid comes from steady forward locomotion, e.g., a gliding fish or robot (Dubois et al. 1974; Akanyeti et al. 2013; Windsor 2014). Self-generated pressures during unsteady locomotion (i.e., the velocity of the platform varies over time) are far less understood. Undulatory movements during steady swimming, startle response (e.g., C-start), burst and coast swimming, and Kármán gaiting are all examples of unsteady locomotion in fishes. To begin to elucidate the relationship between self-generated pressures and unsteady locomotion, we first focused on forward accelerations and decelerations along the swimming direction. In Akanyeti et al. (2013), the 3D fusiform head was moved forward and backward using a periodic, sine wave trajectory in still water and in steady flows at varying flow speeds up to one body length per second. The head was driven with an external, computer-controlled actuator. This experimental range allowed us to investigate self-generated pressures at varying swimming velocity and acceleration magnitudes. The main results are: (1) at low swimming velocities, the self-generated pressures matched closely with the acceleration profile of the head, whereas at high swimming velocities, the self-generated pressures matched closely with the velocity profile; (2) distributed pressure sensing can be used to estimate the swimming velocity of the head and sensory feedback on swimming velocity can be used to correct for cruising speed or to estimate the traveling distance; and (3) the magnitude of self-generated pressures varied depending on the position along the head, where they were highest on the front and increased exponentially with swimming velocity (up to 2 mmHg).
During undulatory swimming, fish go through simultaneous heave and yaw movements. To better understand the self-pressures during these movements, we performed experiments using a 3D printed fish head (Akanyeti et al. 2016). The head was based on a CAD model obtained from scanned images of a trout. It consisted of four pressure sensors arranged from snout to operculum along a horizontal line. Similar to previous experiments, the head was controlled using an external actuator. Self-generated pressures were evaluated as a function of swimming velocity, oscillation frequency, and the phase difference between heave and yaw movements. We found that: (1) self-pressures were mostly correlated with four kinematics variables: forward, lateral, and angular velocity and lateral acceleration; (2) they increased exponentially with swimming velocity and oscillation frequency; and (3) they also varied depending on the phase difference between heave and yaw movements. These results had some interesting implications for biological research in lateral line sensing. It has been long presumed that the ability of the lateral line system to detect external stimuli is hindered by the self-generated stimuli of swimming, and that fish have several ways to deal with this problem. First, they can use an efferent system to change the sensitivity of hair cells (Feitl et al. 2010) or filter out self-generated noise (Montgomery and Bodznick 1994). Second, they can minimize head and body motions, as when gliding or remaining stationary. In Akanyeti et al. (2016), we show that there is another way: fish can automatically reduce self-generated pressures up to 50% by adjusting the timing (i.e., phase difference) between heave and yaw movements. With this, they also attain heightened sensitivity around the anterior region of the head where the majority of the encounters related to feeding are initiated.
Designing a pressure-relative controller for underwater robots
We discussed various methods to extract meaningful information from hydrodynamic environments using pressure-based artificial lateral line systems. We are ultimately interested in designing pressure-relative controllers that increase the autonomy and efficiency of underwater robots. In Salumae et al. (2012), we implemented a Braitenberg controller which successfully turns the fish robot into the incoming flow by comparing the pressures across the head (inspired from the rheotaxis behavior observed in fish). This work was later extended to station holding in steady flows and KVSs (Salumae and Kruusmaa 2013), path-following (Jung et al. 2013), and localization (Muhammad et al. 2017). In Jezov et al. (2012), another controller was developed to synchronize the tail movements of the robot with incoming vortices in order to increase its propulsive efficiency.
Challenges and future directions
Flow and pressure-relative controllers have a tremendous potential to increase the awareness and movement efficiency of underwater robots. We believe that we have barely tapped this potential. As artificial lateral line sensors are becoming more available, new control algorithms are needed to reconcile pressure and flow measurements. So far, the self-generated pressures have been studied mostly around the head and only in one or two behaviors. It is desirable to extend these studies to whole body and possibly to other ecologically relevant behaviors. Once we know more about self-generated stimuli, we can better extrapolate contextual cues from local flow and pressure measurements. Another fruitful direction is to use robot surrogates to systematically explore the individual contributions of multiple design parameters that may be relevant for fish locomotion and sensing. We are highly in favor of interdisciplinary approaches to address biological questions. However, we emphasize that the experimental results obtained with robot surrogates can be suggestive at best. Biological organisms are extremely complex and there are often key differences between biomimetic robots and their biological counterparts which may affect the assertions made. Mimicking biology is tricky due to the limitations of current technologies, hence creative approaches are needed to navigate around these limitations.
Summary
In this review, we discuss some of our contributions to the lateral line research and its applications to underwater robotics. So far, our research efforts have fallen into three main areas: (1) behavior experiments to better understand the function and relevance of the lateral line system in fishes; (2) electrophysiology experiments to better understand how the peripheral nervous system encodes and processes lateral line information; and (3) robotics experiments to study what information is available to lateral line sensing and to develop new controllers to increase the autonomy of underwater robots. We summarized our key findings for each experimental area in separate sections. We have also outlined some of the key challenges lying ahead and discuss possible avenues for future work. The remarkable locomotion and sensing capabilities of fishes continue to inspire biologists and engineers. On the one hand, multi-disciplinary approaches are needed to decipher the biomechanical, neural, and hydrodynamic mechanisms of how aquatic animals interact with their surroundings. On the other hand, biological principles are becoming more and more adopted by robotics researchers. As underwater robots are progressively getting smaller, we are looking for new technologies to increase their autonomy, maneuverability, and efficiency. The ultimate goal is to develop adaptive, evolvable, and sustainable robots so that they can survive in our ever-changing world.
Author contributions
M.H.-T., J.C.L., and O.A. contributed equally in reviewing the literature. M.H.-T. and O.A. wrote the paper.
Acknowledgments
We thank Allison Sophie Zwarycz for drawing Figure 2, and proof reading the manuscript.
Funding
The majority of robotics experiments was carried out under the FILOSE project, supported by the European Union (FP7-ICT-2007-3). The rest of the work was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft HA 6481/1-1 (to M.H.-T.), National Institute on Deafness and Other Communication Disorders Grant RO1-DC-010809 and National Science Foundation Grant IOS 1257150 (to J.C.L.), and Research Coordination Network Travel Grant DBI-RCN 1062052 (to O.A. and J.C.L.). The manuscript is part of a symposium, Sensory Feedback and Animal Locomotion: Perspectives from Biology and Biorobotics, which was held at the 2018 annual meeting of Society for Integrative and Comparative Biology in San Francisco, California. Support for O.A.’s participation in this symposium was provided by Photron (https://photron.com), the Company of Biologists, the Society for Comparative and Integrative Biology (Divisions of Comparative Biomechanics, Vertebrate Morphology, Animal Behavior, and NNSB), the Air Force Office of Scientific Research (FA9550-16-1-0165), and the National Science Foundation (IOS-1747859).
References
- Akanyeti O, Chambers LD, Ježov J, Brown J, Venturelli R, Kruusmaa M, Megill WM, Fiorini P.. 2013. Self-motion effects on hydrodynamic pressure sensing: part I. Forward-backward motion. Bioinspir Biomim 8:026001.. [DOI] [PubMed] [Google Scholar]
- Akanyeti O, Liao JC.. 2013a. The effect of flow speed and body size on Kármán gait kinematics in rainbow trout. J Exp Biol 216:3442.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanyeti O, Liao JC.. 2013b. A kinematic model of Kármán gaiting in rainbow trout. J Exp Biol 216:4666.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanyeti O, Putney J, Yanagitsuru YR, Lauder GV, Stewart WJ, Liao JC.. 2017. Accelerating fishes increase propulsive efficiency by modulating vortex ring geometry. Proc Natl Acad Sci U S A 114:13828.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanyeti O, Thornycroft PJM, Lauder GV, Yanagitsuru YR, Peterson AN, Liao JC.. 2016. Fish optimize sensing and respiration during undulatory swimming. Nat Commun 7:11044.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanyeti O, Venturelli R, Visentin F, Chambers L, Megill WM, Fiorini P.. 2011. What information do Kármán streets offer to flow sensing? Bioinspir Biomim 6:036001.. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Chaar V, Dambly-Chaudier C, Ghysen A.. 2001. Early efferent innervation of the zebrafish lateral line. J Comp Neurol 434:253–61. [DOI] [PubMed] [Google Scholar]
- Briggs JP. 2002. The zebrafish: a new model organism for integrative physiology. Am J Physiol Integr Comp Physiol 282:R3–9. [DOI] [PubMed] [Google Scholar]
- Carrillo A, McHenry MJ.. 2016. Zebrafish learn to forage in the dark. J Exp Biol 219:582–9. [DOI] [PubMed] [Google Scholar]
- Chagnaud BP, Banchi R, Simmers J, Straka H.. 2015. Spinal corollary discharge modulates motion sensing during vertebrate locomotion. Nat Commun 6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnaud BP, Brücker C, Hofmann MH, Bleckmann H.. 2008. Measuring flow velocity and flow direction by spatial and temporal analysis of flow fluctuations. J Neurosci 28:4479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers LD, Akanyeti O, Venturelli R, Ježov J, Brown J, Kruusmaa M, Fiorini P, Megill WM.. 2014. A fish perspective: detecting flow features while moving using an artificial lateral line in steady and unsteady flow. J R Soc Interface 11:20140467.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Brignull H, Raible DW, Rubel EW.. 2013. Hearing loss, protection, and regeneration in the larval zebrafish lateral line. New York (NY): Springer; p. 313–47. [Google Scholar]
- Coombs S, Bleckmann H, Fay RR, Popper AN, (eds.). 2014. The lateral line system. In: Springer handbook of auditory research. New York (NY: ): Springer. [Google Scholar]
- Coombs S, Conley RA.. 1997. Dipole source localization by mottled sculpin. I. Approach strategies. J Comp Physiol A 180:387–99. [DOI] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ.. 1983. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci 3:962–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehnhardt G, Mauck B, Hanke W, Bleckmann H.. 2001. Hydrodynamic trail-following in harbor seals (Phoca vitulina). Science 293:102–4. [DOI] [PubMed] [Google Scholar]
- DeVries L, Lagor FD, Lei H, Tan X, Paley DA.. 2015. Distributed flow estimation and closed-loop control of an underwater vehicle with a multi-modal artificial lateral line. Bioinspir Biomim 10:025002.. [DOI] [PubMed] [Google Scholar]
- Dijkgraaf S. 1963. The functioning and significance of the lateral-line organs. Biol Rev 38:51–105. [DOI] [PubMed] [Google Scholar]
- Dubois AB, Cavagna GA, Fox RS.. 1974. Pressure distribution on the body surface of swimming fish. J Exp Biol 60:581–91. [DOI] [PubMed] [Google Scholar]
- Engelmann J, Hanke W, Mogdans J, Bleckmann H.. 2000. Hydrodynamic stimuli and the fish lateral line. Nature 408:51–2. [DOI] [PubMed] [Google Scholar]
- Engelmann J, Kröther S, Bleckmann H, Mogdans J.. 2003. Effects of running water on lateral line responses to moving objects. Brain Behav Evol 61:195–212. [DOI] [PubMed] [Google Scholar]
- Faucherre A, Pujol-Martí J, Kawakami K, López-Schier H.. 2009. Afferent neurons of the zebrafish lateral line are strict selectors of hair-cell orientation. PLoS One 4:e4477.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitl KE, Ngo V, McHenry MJ.. 2010. Are fish less responsive to a flow stimulus when swimming? J Exp Biol 213:3131–7. [DOI] [PubMed] [Google Scholar]
- Fero K, Yokogawa T, Burgess HA.. 2010. Zebrafish Models in Neurobehavioral Research (Neuromethods). Totowa (NJ: ): Humana Press. [Google Scholar]
- Flock A, Russell I.. 1973. Efferent nerve fibres: postsynaptic action on hair cells. Nat New Biol 243:89–91. [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA.. 2002. Transmitter release at the hair cell ribbon synapse. Nat Neurosci 5:147–54. [DOI] [PubMed] [Google Scholar]
- Groneberg AH, Herget U, Ryu S, De Marco RJ.. 2015. Positive taxis and sustained responsiveness to water motions in larval zebrafish. Front Neural Circuits 9:9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehnel-Taguchi M, Akanyeti O, Liao JC.. 2014. Afferent and motor neuron activity in response to single neuromast stimulation in the posterior lateral line of larval zebrafish. J Neurophysiol 112:1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehnel-Taguchi M, Fernandes AM, Böhler M, Schmitt I, Tittel L, Driever W.. 2018. Projections of the diencephalospinal dopaminergic system to peripheral sense organs in larval zebrafish (Danio rerio). Front Neuroanat 12:20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehnel M, Taguchi M, Liao JC.. 2012. Heterogeneity and dynamics of lateral line afferent innervation during development in zebrafish (Danio rerio). J Comp Neurol 520:1376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan ES. 1992. Mathematical description of the stimuli to the lateral line system of fish derived from a three-dimensional flow field analysis—I. The cases of moving in open water and of gliding towards a plane surface. Biol Cybern 66:443–52. [Google Scholar]
- Herzog H, Klein B, Ziegler A.. 2017. Form and function of the teleost lateral line revealed using three-dimensional imaging and computational fluid dynamics. J R Soc Interface 14:20160898.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ, Corey DP.. 1977. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A 74:2407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezov J, Akanyeti O, Chambers LD, Kruusmaa M.. 2012. Sensing oscillations in unsteady flow for better robotic swimming efficiency. 2012 IEEE International Conference on Systems, Man, and Cybernetics (SMC), IEEE. p. 91–6.
- Jiang Y, Ma Z, Fu J, Zhang D.. 2017. Development of a flexible artificial lateral line canal system for hydrodynamic pressure detection. Sensors 17:1220.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung DS, Pott PP, Salumae T, Kruusmaa M.. 2013. Flow-aided path following of an underwater robot. 2013 IEEE International Conference on Robotics and Automation, IEEE. p. 4602–7.
- Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, et al. ; Zebrafish Neuroscience Research Consortium. 2013. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10:70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen EC, Hudspeth AJ.. 2006. Transfer characteristics of the hair cell’s afferent synapse. Proc Natl Acad Sci U S A 103:5537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi T, Nakata N, Oda Y.. 2012. Effective sensory modality activating an escape triggering neuron switches during early development in zebrafish. J Neurosci 32:5810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C. 2011. Evolution of the octavolateral efferent system In: Ryugo DK, Fay RR, Popper AN, editors. Auditory and vestibular efferents. Springer handbook of auditory research. New York (NY: ): Springer; p. 217–59. [Google Scholar]
- Kottapalli A, Bora M, Kanhere E, Asadnia M, Miao J, Triantafyllou M.. 2017. Cupula-inspired hyaluronic acid-based hydrogel encapsulation to form biomimetic MEMS flow sensors. Sensors 17:1728.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese AB, Schellart NA.. 1992. Velocity- and acceleration-sensitive units in the trunk lateral line of the trout. J Neurophysiol 68:2212–21. [DOI] [PubMed] [Google Scholar]
- Kruusmaa M, Fiorini P, Megill W, de Vittorio M, Akanyeti O, Visentin F, Chambers L, El Daou H, Fiazza M-C, Jezov J, et al. 2014. FILOSE for svenning: a flow sensing bioinspired robot. IEEE Robot Autom Mag 21:51–62. [Google Scholar]
- Levi R, Akanyeti O, Ballo A, Liao JC.. 2015. Frequency response properties of primary afferent neurons in the posterior lateral line system of larval zebrafish. J Neurophysiol 113:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JC. 2004. Neuromuscular control of trout swimming in a vortex street: implications for energy economy during the Karman gait. J Exp Biol 207:3495–506. [DOI] [PubMed] [Google Scholar]
- Liao JC. 2006. The role of the lateral line and vision on body kinematics and hydrodynamic preference of rainbow trout in turbulent flow. J Exp Biol 209:4077–90. [DOI] [PubMed] [Google Scholar]
- Liao JC. 2007. A review of fish swimming mechanics and behaviour in altered flows. Philos Trans R Soc B Biol Sci 362:1973–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JC. 2010. Organization and physiology of posterior lateral line afferent neurons in larval zebrafish. Biol Lett 6:402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JC, Akanyeti O.. 2017. Fish swimming in a Kármán vortex street: kinematics, sensory biology and energetics. Mar Technol Soc J 51:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JC, Haehnel M.. 2012. Physiology of afferent neurons in larval zebrafish provides a functional framework for lateral line somatotopy. J Neurophysiol 107:2615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Wang A, Wang X, Liu P.. 2016. A review of artificial lateral line in sensor fabrication and bionic applications for robot fish. Appl Bionics Biomech 2016:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C, Stewart WJ, Akanyeti O, Frederick C, Zhu J, Santos-Sacchi J, Sheets L, Liao JC, Zenisek D.. 2016. Synaptic ribbons require ribeye for electron density, proper synaptic localization, and recruitment of calcium channels. Cell Rep 15:2784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry MJ, Feitl KE, Strother JA, Van Trump WJ.. 2009. Larval zebrafish rapidly sense the water flow of a predator’s strike. Biol Lett 5:477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe WK, Kimmel CB, Schabtach E.. 1985. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol 233:377–89. [DOI] [PubMed] [Google Scholar]
- Mirjany M, Preuss T, Faber DS.. 2011. Role of the lateral line mechanosensory system in directionality of goldfish auditory evoked escape response. J Exp Biol 214:3358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Coombs S.. 1992. Physiological characterization of lateral line function in the Antarctic fish Trematomus bernacchii. Brain Behav Evol 40:209–16. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, Bodznick D.. 1994. An adaptive filter that cancels self-induced noise in the electrosensory and lateral line mechanosensory systems of fish. Neurosci Lett 174:145–8. [DOI] [PubMed] [Google Scholar]
- Muhammad N, Toming G, Tuhtan JA, Musall M, Kruusmaa M.. 2017. Underwater map-based localization using flow features. Auton Robots 41:417–36. [Google Scholar]
- Nicolson T. 2017. The genetics of hair-cell function in zebrafish. J Neurogenet 31:102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson T, Rüsch A, Friedrich RW, Granato M, Ruppersberg JP, Nüsslein-Volhard C.. 1998. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron 20:271–83. [DOI] [PubMed] [Google Scholar]
- Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, Busch-Nentwich E, Seiler C, Sidi S, Söllner C, Duncan RN, et al. 2008. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci 28:2110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski J, Haehnel M, Taguchi M, Liao JC.. 2012. Zebrafish larvae exhibit rheotaxis and can escape a continuous suction source using their lateral line. PLoS One 7:e36661.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olt J, Allen CE, Marcotti W.. 2016. In vivo physiological recording from the lateral line of juvenile zebrafish. J Physiol 594:5427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteiza P, Odstrcil I, Lauder G, Portugues R, Engert F.. 2017. A novel mechanism for mechanosensory-based rheotaxis in larval zebrafish. Nature 547:445–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybilla A, Kunze S, Rudert A, Bleckmann H, Brucker C.. 2010. Entraining in trout: a behavioural and hydrodynamic analysis. J Exp Biol 213:2976–86. [DOI] [PubMed] [Google Scholar]
- Reinig S, Driever W, Arrenberg AB.. 2017. The descending diencephalic dopamine system is tuned to sensory stimuli. Curr Biol 27:318–33. [DOI] [PubMed] [Google Scholar]
- Ren Z, Mohseni K.. 2012. A model of the lateral line of fish for vortex sensing. Bioinspir Biomim 7:036016.. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Bai J-P, Song L, Lv C, Zenisek D, Santos-Sacchi J.. 2013. Patch-clamp recordings from lateral line neuromast hair cells of the living zebrafish. J Neurosci 33:3131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi F, Qualtieri A, Chambers LD, Epifani G, Megill WM, De Vittorio M.. 2014. Stress-driven artificial hair cell for flow sensing In: Bleckmann H, Mogdans J, Coombs SL, editors. Flow sensing in air and water berlin. Heidelberg: Springer; p. 499–519. [Google Scholar]
- Roberts AC, Bill BR, Glanzman DL.. 2013. Learning and memory in zebrafish larvae. Front Neural Circuits 7:126.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ. 1968. Influence of efferent fibres on a receptor. Nature 219:177–8. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Roberts BL.. 1974. Active reduction of lateral-line sensitivity in swimming dogfish. J Comp Physiol A 94:7–15. [Google Scholar]
- Salumae T, Kruusmaa M.. 2013. Flow-relative control of an underwater robot. Proc R Soc A Math Phys Eng Sci 469:20120671. [Google Scholar]
- Salumae T, Rano I, Akanyeti O, Kruusmaa M.. 2012. Against the flow: a Braitenberg controller for a fish robot. 2012 IEEE International Conference on Robotics and Automation, IEEE. p. 4210–5.
- Stewart WJ, Cardenas GS, McHenry MJ.. 2013. Zebrafish larvae evade predators by sensing water flow. J Exp Biol 216:388–98. [DOI] [PubMed] [Google Scholar]
- Stewart WJ, Johansen JL, Liao JC.. 2017. A non-toxic dose of cobalt chloride blocks hair cells of the zebrafish lateral line. Hear Res 350:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WJ, Tian F, Akanyeti O, Walker CJ, Liao JC.. 2016. Refuging rainbow trout selectively exploit flows behind tandem cylinders. J Exp Biol 219:2182.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suli A, Watson GM, Rubel EW, Raible DW.. 2012. Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells. PLoS One 7:e29727.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi M, Liao JC.. 2011. Rainbow trout consume less oxygen in turbulence: the energetics of swimming behaviors at different speeds. J Exp Biol 214:1428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Yu X.. 2012. Hair flow sensors: from bio-inspiration to bio-mimicking—a review. Smart Mater Struct 21. [Google Scholar]
- Toro C, Trapani JG, Pacentine I, Maeda R, Sheets L, Mo W, Nicolson T.. 2015. Dopamine modulates the activity of sensory hair cells. J Neurosci 35:16494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JG, Nicolson T.. 2011. Mechanism of spontaneous activity in afferent neurons of the zebrafish lateral-line organ. J Neurosci 31:1614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuhtan JA, Fuentes-Pérez JF, Strokina N, Toming G, Musall M, Noack M, Kämäräinen JK, Kruusmaa M.. 2016. Design and application of a fish-shaped lateral line probe for flow measurement. Rev Sci Instrum 87:045110.. [DOI] [PubMed] [Google Scholar]
- Venturelli R, Akanyeti O, Visentin F, Ježov J, Chambers LD, Toming G, Brown J, Kruusmaa M, Megill WM, Fiorini P.. 2012. Hydrodynamic pressure sensing with an artificial lateral line in steady and unsteady flows. Bioinspir Biomim 7:036004.. [DOI] [PubMed] [Google Scholar]
- Webb JF. 2014. Lateral line morphology and development and implications for the ontogeny of flow sensing in fishes In: Bleckmann H, Mogdans J, Coombs SL, editors. Flow sensing in air and water. Berlin (Germany: ): Springer; p. 247–70. [Google Scholar]
- Weeg MS, Bass AH.. 2002. Frequency response properties of lateral line superficial neuromasts in a vocal fish, with evidence for acoustic sensitivity. J Neurophysiol 88:1252–62. [DOI] [PubMed] [Google Scholar]
- Windsor SP. 2014. Hydrodynamic imaging by blind Mexican cavefish In: Bleckmann H, Mogdans J, Coombs SL, editors. Flow sensing in air and water: behavioral, neural and engineering principles of operation. Berlin (Germany: ): Springer; p. 103–25. [Google Scholar]
- Wyatt C, Bartoszek EM, Yaksi E.. 2015. Methods for studying the zebrafish brain: past, present and future. Eur J Neurosci 42:1746–63. [DOI] [PubMed] [Google Scholar]



