ABSTRACT
During macroautophagy, the phagophore-mediated formation of autophagosomes and their subsequent fusion with lysosomes requires extensive transformation of the endomembrane system. Membrane dynamics in eukaryotic cells is regulated by small GTPase proteins including Arfs and Rabs. The small GTPase proteins that regulate autophagic membrane traffic are mostly conserved in yeast and metazoans, but there are also several differences. In this mini-review, we compare the small GTPase network of yeast and metazoan cells that regulates autophagy, and point out the similarities and differences in these organisms.
KEYWORDS: Arf, autolysosome, autophagosome, autophagy, membrane fusion, membrane trafficking, Rab, Rag, TORC1
Introduction
Macroautophagy (hereafter autophagy) is a highly conserved catabolic pathway that eliminates unnecessary or damaged proteins and organelles through autophagosome-mediated lysosomal degradation. The released monomers can be used for energy metabolism or biosynthesis. Thus autophagy is not just a self-eating but it's a self-renewal process as well, which is indispensable for neuronal health, immunity and delayed aging.1
Autophagy starts with the emergence of a membrane cistern called phagophore from the preautophagosomal structure/phagophore assembly site (PAS). This structure has been described in yeast, and we use the same term for its functional metazoan equivalents in this review. The growing phagophore engulfs portions of the cytoplasm, and by sealing it forms an autophagosome, a double membrane transport vesicle. Then autophagosomes fuse with late endosomes and lysosomes (vacuole in yeast) to form degrading amphisomes and autolysosomes, respectively.1
Autophagy levels are strictly controlled by cellular metabolic status. Target of rapamycin kinase complex 1 (TORC1) is the central regulatory hub of metabolic and growth signaling pathways both in yeast and metazoan cells. Active TORC1 promotes protein synthesis and growth and downregulates autophagy, while its inactivation, for example by starvation, releases autophagy inhibition.1-5
Phagophore and autophagosome formation requires the coordinated action of Atg (Autophagy related) proteins that were discovered in budding yeast about 2 decades ago, followed by the identification of metazoan homologs.6 Most Atg proteins are present at the PAS, and form 5 evolutionary conserved complexes/functional units. The Atg1/unc-51-like kinase 1/2 (ULK1/2) complex is usually considered to sit on the top of the Atg hierarchy, and it is directly inhibited by TORC1 through the phosphorylation of multiple Atg1 complex subunits under nutrient rich circumstances. Concomitant with the activation of Atg1, the cellular endomembrane system undergoes a remarkable transformation, including the relocalization of Atg9, the only evolutionarily conserved transmembrane Atg protein, from Golgi and endosomes to the PAS.7 Numerous studies suggest that Atg9 transports membrane to phagophores in the form of small vesicles. The characteristic lipid and protein content of the phagophore membrane are set up by 2 additional components. The Vacuolar protein sorting 34 (Vps34) lipid kinase complex produces phosphatidyl-inositol 3-phosphate (PI3P), a lipid specific for autophagosomal and endosomal membranes. The protein conjugation system uses E1- and E2-like enzymes to conjugate the ubiquitin-like Atg12 to Atg5, which then bind to Atg16 to act as an E3-like complex that achieves the covalent attachment of ubiquitin-like Atg8/LC3 (light chain 3) family proteins to phosphatydil-ethanolamine. This lipid moiety anchors Atg8/LC3 into the phagophore and autophagosome membrane, making it an excellent marker protein for autophagic structures. Finally, PI3P effectors DFCP1 (double FYVE domain containing protein 1) and the complex of Atg18/WD repeat domain phosphoinositide-interacting protein (WIPI) with Atg2 also regulate the assembly of phagophores and autophagosomes.7
Before their fusion with late endosomes and lysosomes, nascent autophagosomes undergo a maturation process to become fusion competent. This fusion step is mediated by the Homotypic fusion and vacuole protein sorting (HOPS) tethering complex and a complex of appropriate SNARE proteins. While all 6 subunits of HOPS are found in yeast and metazoans, interestingly the SNAREs (Vam3, Vam7, Vti1 in yeast versus Syntaxin 17, Snap29 and Vamp7/8 in metazoans) are not conserved.8,9
The superfamily of Ras like proteins consists of several subfamilies of regulatory GTPases. Only two of these, Arfs (ADP ribosylation factor) and Rabs (Ras-related proteins in brain) are directly involved in membrane trafficking. Arfs and Rabs work similarly: both type of proteins are only active in GTP bound status and use a lipid anchor to associate with membranes. There is also functional divergence between these 2 subfamilies. The best studied binding partners of Arfs are coat proteins (such as Clathrin and Coatomer/COP), that is how they regulate vesicle budding. Arfs also have roles in vesicle positioning, controlling the microtubule network and the membrane lipid content.10 In contrast, Rabs interact with motor proteins or their adaptors and tethering factors, through which they can mediate not only vesicle movement but tethering and fusion as well.11
Autophagic degradation is controlled on different levels: 1. Signaling upstream of Atg proteins, 2. Autophagosome formation; 3. Fusion with endosomes and lysosomes, 4. Lysosomal degradation. For simplicity, we will discuss steps 3 and 4 together in later parts of this review. All of these steps depend on intracellular membrane dynamics, which is regulated mainly by Arf and Rab type small GTPases (with rare exceptions discussed below) (Fig. 1). Of course, the number of genes encoding small GTPases increased during the evolution of higher eukaryotes. There are ancestral Arfs and Rabs that have orthologues both in yeast and metazoans, but animal genomes also contain many paralogs with divergent cellular functions. In this mini-review, we summarize our current knowledge about the most critical small GTPases that control the autophagic pathway both in yeast and metazoans, and highlight the similarities and the most important differences between these organisms.
Figure 1.
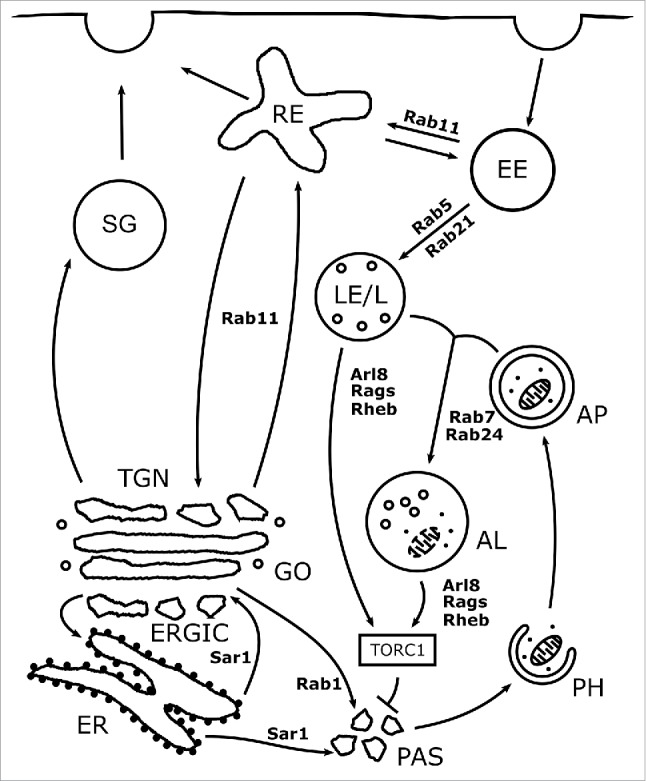
Small GTPases integrate autophagy into the membrane trafficking network. Key trafficking routes related to autophagy and their regulatory small GTPases in mammalian cells are depicted in this figure. Regulation of autophagy can be divided into 4 main steps. (1) Induction of autophagy. Autophagy is suppressed by TORC1, which is activated by Rheb during its transient recruitment to lysosomes by Rags. TORC1 activity is also regulated by Arl8 dependent lysosomal positioning. (2) Autophagosome formation. The Sar1 and Rab1 dependent early secretory and Rab11 dependent endosomal recycling pathways contribute to the delivery of membrane and Atg proteins to the PAS. (3) Autophagosome-lysosome fusion. The fusion of autophagosomes with late endosomes and lysosomes requires Rab7. The Rab21 dependent relocalization of Vamp7/8 from endosomes to lysosomes is also necessary for efficient fusion. (4) Autolysosomal degradation. The degradative capacity of autolysosomes depends on Rab5- and possibly Rab24-dependent biosynthetic transport. AL: Autolysosome, AP: Autophagosome, EE: Early endosome, GO: Golgi, LE/L: Late endosome/Lysosome, PH: Phagophore, RE: Recycling endosome, SG: Secretory granule.
Regulation of autophagy by small GTPases
Control of autophagy induction: Regulators of TORC1 activity
In well fed conditions, active TORC1 localizes to late endosomal and vacuolar or the analogous lysosomal membranes in yeast and metazoan cells, respectively. The most important signal of nutrient rich status is the high intracellular amino acid (aa) concentration. TORC1 senses the aa level through Rag GTPases.2-4 Rags are exceptional, as their relationship to the Ras like GTPase superfamily is under debate.12 Mammalian cells express 4 Rag homologs, RagA, B, C and D, which form various heterodimeric complexes with each other. In its active form, a heterodimer always contains one GTP-bound RagA/B together with one GDP bound RagC/D subunit. This is an important difference between Rags and Ras like proteins, as the latter are only active in GTP bound form. Rags are recruited to the lysosomal membrane through binding the Ragulator complex. Ragulator serves as a RagA/B guanine nucleotide exchange factor (GEF) and activates the Rag heterodimer in an aa dependent manner. This Rag-Ragulator complex brings TORC1 to lysosomes, its site of activation.2,13 Low aa concentration causes the Rag complex to change its guanosine-bound status resulting in the inactivation of TORC1, which releases the Atg1 complex from TORC1 repression.2,4
Besides Rag mediated recruitment, TORC1 activity also depends on extracellular growth signals in metazoan cells, particularly the insulin/PI3K pathway. This signaling network activates Rheb (Ras homolog enriched in brain), a Ras related GTPase that associates with lysosomal membranes via its lipid anchor. Once the Rag complex brings TORC1 to lysosomes, it also interacts with Rheb, which enhances its kinase activity2 and thereby suppresses autophagy.
Yeast cells lack both insulin signaling and Rheb, but the Rag orthologues Gtr1 (GTP binding protein resemblance 1) and Gtr2 also associate with the vacuolar membrane and regulate TORC1 activity in an aa dependent manner.3
Another regulator of TORC1 activity is lysosome positioning. In mammalian cells, Arf-like 8 (Arl8) homologues are key determinants of the subcellular localization of TORC1 positive lysosomes. Constitutively active Arl8B promotes lysosomal transport toward the cell periphery, enhances TORC1 activity and downregulates autophagy, while RNAi silencing of this gene results in perinuclear distribution of lysosomes, decreased TORC1 activity and induction of autophagy.14 In addition to its indirect effect on autophagy induction through TORC1, hyperactive Arl8B also restricts the number of perinuclear lysosomes, hence inhibiting their fusion with autophagosomes.14
Delivery of membrane precursors and regulatory proteins to autophagosome formation sites
Even though plenty of regulators of autophagosome formation were identified, the membrane source(s) of the emerging phagophore are still under debate. Phagophore assembly clearly requires Atg9-positive membrane transport from various organelles including the Golgi and endosomes. There is growing evidence that phagophore formation overlaps with the secretory and endocytic recycling pathways, which deliver membranes and proteins from ER and endosomes toward Golgi and its cis and trans accessories: ERGIC (ER-to-Golgi Intermediate Compartment) and Trans Golgi Network (TGN). These organelles are all suggested to play a role in the generation of autophagic structures,15-17 and perturbation of their regulatory small GTPases also affects autophagosome formation. Additional studies showed that Atg proteins relocalize to PAS from other organelles upon autophagy induction.17-19 Relocalization of Atg proteins may be achieved by conventional membrane trafficking events regulated by small GTPases.
ER is one of the most likely sources of autophagic membranes.15 ER-Golgi transport is mediated by vesicles enveloped by COP complexes. Sar1 (Secretion associated Ras-related GTPase 1) is an Arf family GTPase that binds to the COPII coat of the forming vesicles at ER exit sites, promote their budding, and helps coat disassembly before subsequent fusion with cis-Golgi.10,20 Consistent with the importance of the secretory pathway, overexpression of dominant negative Sar1 inhibited autophagosome formation and its knockdown decreased LC3 lipidation in mammalian cells.20 Sar1's function in autophagy is likely conserved, as yeast cells mutant for the Sar1 GEF Sec 12 (Secretory 12) are also defective in this process.21
Similarly to the early secretory pathway, intra-Golgi trafficking and transport from the recycling endosomes to TGN are also important for autophagosome formation. The best characterized regulatory GTPases of these pathways are Rab1 and Rab11. Rab1 is a Golgi resident GTPase that is important for both ER-to-Golgi and intra-Golgi trafficking.11 Rab1 orthologs colocalize with numerous Atg proteins including Atg9 and Atg8/LC3.18,20 Yeast studies showed that Atg9 promotes Yeast protein two 1 (Ypt1)/Rab1 localization to the PAS, and promotes Atg1 recruitment.22,23 Silencing of Ypt1 inhibits autophagosome formation, and leads to impaired vacuolar delivery of Atg8a: instead, it accumulates on abnormal cytoplasmic autophagic structures.24 Mammalian Rab1 may function similarly to yeast. Several studies showed that inhibition of Rab1 homologues or their GEF the TRAPPIII (Transport protein particle III) complex reduces the number of structures that are positive for WIPI2 or double FYVE containing protein 1 (DFCP1), two established markers of early autophagic structures.18,25 Additionally, Rab1b loss of function cells are defective in autophagosome formation.18,20 Based on these studies, Rab1 seems to be the best candidate among Rabs for mediating the fusion of membranaceous phagophore precursors at the PAS.
In mammalian cells, multiple studies showed that Rab11 positive recycling endosomes colocalize with ULK1, and they are necessary for delivering endocytosed Atg9 and Atg16L1 to the TGN and PAS.17,19 Additionally, overexpression of dominant negative Rab11a and b attenuate autophagosome formation.19 These results fit well with Drosophila and yeast data, which showed that inhibition of Drosophila Rab11 or its yeast orthologues Ypt31/32 leads to accumulation of immature early autophagic structures positive for Atg8.26,27 It thus seems likely that Rab11 and Rab1 mediated trafficking is key for proper autophagosome formation. In the course of the yeast secretory pathway, Rab1 is likely switched to Rab11.11 Interestingly, a recent study showed that during autophagy in mammalian cells, TGN-localized Rab11 and its effector TBC1D14 (Tre2/Bub2/Cdc16 1 domain 14) recruit TRAPPIII, which activates Rab1.18 This reversed Rab switch likely maintains the delivery of several Atg proteins toward the sites of autophagosome formation.
Regulation of autophagosome-lysosome fusion and lysosomal degradation
Completed autophagosomes must undergo maturation to gain competence for fusion with lysosomes. Proper autophagic degradation also requires the proper functioning of lysosomes. Endocytosis and autophagy converge and both utilize lysosomal degradation, and the biogenesis of endo- and autolysosomes use almost the same components of the membrane trafficking machinery. There are several similarities between autophagosomes and endosomes, including PI3P positivity or their HOPS dependent fusion with lysosomes.28-30
Rab5 and Rab7 are key regulatory GTPases of fusion events at early and late endosomal membranes, respectively. In the endocytic pathway, Rab5 acts upstream of Rab7, and the continuity between these factors is maintained by a Rab switch mechanism. This is performed by the Rab5 and PI3P dependent recruitment of the Mon1-Ccz1 complex, a Rab7 GEF that promotes early to late endosomal transition.31,32 In our recent study, we tested whether this Rab5-Rab7 hierarchy is also observed during autophagy in Drosophila fat cells. We found that Rab7 associates with autophagosomes and the Rab7-Mon1-Ccz1 module is important for autophagosome-lysosome fusion.33 These findings agree with earlier reports that Rab7, together with its activators (Mon1-Ccz1) and effectors (such as HOPS and the metazoan-specific PLEKHM1), are key factors for autophagosome-lysosome fusion in yeast and animal cells.34-36
As in course of endocytosis Rab5 acts upstream of Rab7, we would expect that loss of Rab5 also blocks autophagosome –lysosome fusion. Surprisingly, we found that this fusion event occurs normally in Rab5 null mutant fat cells of starved Drosophila. However, these autolysosomes were defective in the degradation of autophagic cargo, probably due to the perturbation of the endosomal and biosynthetic lysosomal transport pathways.33 Thus Rab7 acts independent of Rab5 in autophagosome-lysosome fusion. An earlier study showed that Rab5 loss of function HeLa and COS-7 cells were defective both in autophagosome formation and clearance of Huntingtin aggregates.37 Although we did not analyze Rab5's role in autophagosome formation, these findings suggest a more complex function of Rab5 during autophagy.
Interestingly, loss of one of the Rab5 homologues in yeast, Vps21, led to accumulation Atg8 positive structures.38 These structures did not fuse with the vacuole, but they were positive for FM4–64, a lipophilic dye widely used to stain endocytic and vacuolar membranes, but it normally does not label autophagosomes. Thus, the identity of these structures is somewhat ambiguous.38
A recent study suggested Rab21 as another regulator of autophagosome lysosome fusion. Unlike Rab7, Rab21 does not have a direct role in autophagosomal fusions. Instead, it is important for the lysosomal targeting of Vamp7 or Vamp8, subunits of the fusogenic SNARE complex in both Drosophila and mammalian cells.39
Several groups observed autophagy-dependent relocalization of mammalian Rab24 from ER and Golgi to autophagosomes and autolysosomes.40,41 Its exact role during autophagy is still under debate, but the accumulation of autolysosomes in Rab24 depleted cells under nutrient rich conditions suggests that it may promote autolysosomal maturation during basal autophagy.41
Role of small GTPases in the elimination of intracellular streptococcus and uncoupled mitochondria by non-canonical autophagy
Autophagy also contributes to the elimination of intracellular pathogens. The defense of non-phagocytic mammalian cells against Group A Streptococcus (GAS) highly depends on a non-canonical autophagic pathway. During this process, multiple phagophore-like LC3 positive structures emerge around GAS, and after their coalescence, these form a GAS-containing Autophagosome-like Vacuole (GcAV). Then GcAVs undergo homo- and heterotypic fusions with each other or lysosomes in order to eliminate GAS.42 Even though this unusual autophagic pathway also depends on Atg proteins as it begins with the formation of phagophore-like structures, the 2 processes show striking differences in how they utilize certain small GTPases.42,43
Remarkably, GAS clearance is also a Rab7 dependent process, but in this case Rab7 is important for the biogenesis of GcAVs. It cannot be ruled out that Rab7 functions in GcAV-lysosome fusion as well, but this speculation needs to be confirmed.42 Another GTPase that is involved in GcAV formation is Rab23. Depletion of this Rab results in fewer GcAVs and more intracellular GAS negative for LC3. Thus it seems likely that Rab23 is important for the selective targeting of GAS to autophagic structures.43 Finally, Rab9A was identified as a regulator of the late steps of GAS elimination, through its role in homo- and heterotypic fusion of GcAVs. This view is supported by findings that the number of GcAVs was increased but their size and colocalization with LAMP1 (Lysosome-associated membrane protein 1) was decreased in Rab9A knockdown cells.43
Recently, an interesting study showed that in mammalian cells treated with drugs that uncouple the mitochondrial electron transport chain, mitochondria are selectively captured by large LC3 positive phagophores that fuse in a Rab7 dependent manner.44 The similarities between this mitophagy pathway and GcAV formation raises the possibility that the Rab7-dependent anti-microbial, non-canonical autophagic pathway may has a general role in cellular physiology. While it is an attractive hypothesis that the cell uses the same mechanism for eliminating intracellular bacteria and their endosymbiotic descendants, further studies are necessary to confirm this.
Conclusions
There are more Ras like proteins with roles in autophagic membrane traffic that we discussed above. The purpose of this mini-review is to give an overview of the ones that we consider as the most important regulators of this process (Fig. 1). We also focus on those GTPases that have a conserved function in autophagy in yeast and metazoans.
Small GTPase regulators can be divided into 3 categories. First, there are small GTPases including Rags, the Rab1-Rab11 axis and Rab7 that likely possess a conserved function in autophagy induction, autophagosome formation and fusion, respectively. The second category contains proteins whose function might have changed during evolution, such as Rab5. Finally, the metazoan innovations include Rheb, Rab21 and Rab24.
Despite extensive research and a recent Nobel prize, our knowledge of the mechanisms of the authophagy is far from complete. This is also true for small GTPases that control the various steps of this process. More than 20 Arf and 40 Rab subfamily proteins exist in mammalian cells, so further autophagy regulators will likely be identified among these.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all members of the Juhasz lab for stimulating discussions.
Funding
Work in the Juhasz lab is supported by the Hungarian Academy of Sciences (Momentum LP2014-2), the National Research, Development and Innovation Fund (K_16 119842), and by the State of Hungary (GINOP-2.3.2-15-2016-00006). ST is funded by the Premium Postdoctorate Research Program of the Hungarian Academy of Sciences (PPD-003/2016).
References
- [1].Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069-75; PMID:18305538; http://dx.doi.org/ 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008; 320:1496-501; PMID:18497260; http://dx.doi.org/ 10.1126/science.1157535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kira S, Tabata K, Shirahama-Noda K, Nozoe A, Yoshimori T, Noda T. Reciprocal conversion of Gtr1 and Gtr2 nucleotide-binding states by Npr2-Npr3 inactivates TORC1 and induces autophagy. Autophagy 2014; 10:1565-78; PMID:25046117; http://dx.doi.org/ 10.4161/auto.29397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 2008; 10:935-45; PMID:18604198; http://dx.doi.org/ 10.1038/ncb1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 2010; 22:132-9; PMID:20056399; http://dx.doi.org/ 10.1016/j.ceb.2009.12.004 [DOI] [PubMed] [Google Scholar]
- [6].Klionsky DJ, Cregg JM, Dunn WA Jr., Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, et al.. A unified nomenclature for yeast autophagy-related genes. Developmental cell 2003; 5:539-45; PMID:14536056 [DOI] [PubMed] [Google Scholar]
- [7].Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 2011; 27:107-32; PMID:21801009; DOI: 10.1146/annurev-cellbio-092910-154005 [DOI] [PubMed] [Google Scholar]
- [8].Takats S, Juhasz G. A genetic model with specifically impaired autophagosome-lysosome fusion. Autophagy 2013; 9:1251-2; PMID:23819962; http://dx.doi.org/ 10.4161/auto.25470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012; 151:1256-69; PMID:23217709; http://dx.doi.org/ 10.1016/j.cell.2012.11.001 [DOI] [PubMed] [Google Scholar]
- [10].Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol 2011; 12:362-75; PMID:21587297; http://dx.doi.org/ 10.1038/nrm3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem 2012; 81:637-59; PMID:22463690; http://dx.doi.org/ 10.1146/annurev-biochem-052810-093700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rojas AM, Fuentes G, Rausell A, Valencia A. The Ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol 2012; 196:189-201; PMID:22270915; http://dx.doi.org/ 10.1083/jcb.201103008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012; 150:1196-208; PMID:22980980; http://dx.doi.org/ 10.1016/j.cell.2012.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, et al.. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol 2011; 13:453-60; PMID:21394080; http://dx.doi.org/ 10.1038/ncb2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 2008; 182:685-701; PMID:18725538; http://dx.doi.org/ 10.1083/jcb.200803137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ge L, Zhang M, Schekman R. Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment. eLife 2014; 3:e04135; PMID:25432021; http://dx.doi.org/ 10.7554/eLife.04135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell 2013; 154:1285-99; PMID:24034251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lamb CA, Nuhlen S, Judith D, Frith D, Snijders AP, Behrends C, Tooze SA. TBC1D14 regulates autophagy via the TRAPP complex and ATG9 traffic. EMBO J 2016; 35:281-301; PMID:26711178; http://dx.doi.org/ 10.15252/embj.201592695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Longatti A, Lamb CA, Razi M, Yoshimura S, Barr FA, Tooze SA. TBC1D14 regulates autophagosome formation via Rab11- and ULK1-positive recycling endosomes. J Cell Biol 2012; 197:659-75; PMID:22613832; http://dx.doi.org/ 10.1083/jcb.201111079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zoppino FC, Militello RD, Slavin I, Alvarez C, Colombo MI. Autophagosome formation depends on the small GTPase Rab1 and functional ER exit sites. Traffic 2010; 11:1246-61; PMID:20545908; http://dx.doi.org/ 10.1111/j.1600-0854.2010.01086.x [DOI] [PubMed] [Google Scholar]
- [21].Ishihara N, Hamasaki M, Yokota S, Suzuki K, Kamada Y, Kihara A, Yoshimori T, Noda T, Ohsumi Y. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol Biol Cell 2001; 12:3690-702; PMID:11694599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kakuta S, Yamamoto H, Negishi L, Kondo-Kakuta C, Hayashi N, Ohsumi Y. Atg9 vesicles recruit vesicle-tethering proteins Trs85 and Ypt1 to the autophagosome formation site. J Biol Chem 2012; 287:44261-9; PMID:23129774; http://dx.doi.org/ 10.1074/jbc.M112.411454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang J, Menon S, Yamasaki A, Chou HT, Walz T, Jiang Y, Ferro-Novick S. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proc Natl Acad Sci U S A 2013; 110:9800-5; PMID:23716696; http://dx.doi.org/ 10.1073/pnas.1302337110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lynch-Day MA, Bhandari D, Menon S, Huang J, Cai H, Bartholomew CR, Brumell JH, Ferro-Novick S, Klionsky DJ. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci U S A 2010; 107:7811-6; PMID:20375281; http://dx.doi.org/ 10.1073/pnas.1000063107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, et al.. alpha-Synuclein impairs macroautophagy: implications for Parkinson's disease. J Cell Biol 2010; 190:1023-37; PMID:20855506; http://dx.doi.org/ 10.1083/jcb.201003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Szatmari Z, Kis V, Lippai M, Hegedus K, Farago T, Lorincz P, Juhász G, Sass M. Rab11 facilitates cross-talk between autophagy and endosomal pathway through regulation of Hook localization. Mol Biol Cell 2014; 25:522-31; PMID:24356450; http://dx.doi.org/ 10.1091/mbc.E13-10-0574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Geng J, Nair U, Yasumura-Yorimitsu K, Klionsky DJ. Post-Golgi Sec proteins are required for autophagy in Saccharomyces cerevisiae. Mol Bio Cell 2010; 21:2257-69; PMID:20444978; http://dx.doi.org/ 10.1091/mbc.E09-11-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 2008; 19:5360-72; PMID:18843052; http://dx.doi.org/ 10.1091/mbc.E08-01-0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T, Mizushima N. The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol Biol Cell 2014; 25:1327-37; PMID:24554770; http://dx.doi.org/ 10.1091/mbc.E13-08-0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Takats S, Pircs K, Nagy P, Varga A, Karpati M, Hegedus K, Kramer H, Kovács AL, Sass M, Juhász G. Interaction of the HOPS complex with Syntaxin 17 mediates autophagosome clearance in Drosophila. Mol Biol Cell 2014; 25:1338-54; PMID:24554766; http://dx.doi.org/ 10.1091/mbc.E13-08-0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell 2010; 141:497-508; PMID:20434987 [DOI] [PubMed] [Google Scholar]
- [32].Nordmann M, Cabrera M, Perz A, Brocker C, Ostrowicz C, Engelbrecht-Vandre S, Ungermann C. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol 2010; 20:1654-9; PMID:20797862; http://dx.doi.org/ 10.1016/j.cub.2010.08.002 [DOI] [PubMed] [Google Scholar]
- [33].Hegedus K, Takats S, Boda A, Jipa A, Nagy P, Varga K, Kovács AL, Juhász G. The Ccz1-Mon1-Rab7 module and Rab5 control distinct steps of autophagy. Mol Biol Cell 2016; 27:3132-42; PMID:27559127; http://dx.doi.org/ 10.1091/mbc.E16-03-0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McEwan DG, Popovic D, Gubas A, Terawaki S, Suzuki H, Stadel D, Coxon FP, Miranda de Stegmann D, Bhogaraju S, Maddi K, et al.. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol Cell 2015; 57:39-54; PMID:25498145; http://dx.doi.org/ 10.1016/j.molcel.2014.11.006 [DOI] [PubMed] [Google Scholar]
- [35].Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol 1999; 147:435-46; PMID:10525546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang CW, Stromhaug PE, Shima J, Klionsky DJ. The Ccz1-Mon1 protein complex is required for the late step of multiple vacuole delivery pathways. J Biol Chem 2002; 277:47917-27; PMID:12364329; http://dx.doi.org/ 10.1074/jbc.M208191200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ravikumar B, Imarisio S, Sarkar S, O'Kane CJ, Rubinsztein DC. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci 2008; 121:1649-60; PMID:18430781; http://dx.doi.org/ 10.1242/jcs.025726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen Y, Zhou F, Zou S, Yu S, Li S, Li D, Song J, Li H, He Z, Hu B, et al.. A Vps21 endocytic module regulates autophagy. Mol Biol Cell 2014; 25:3166-77; PMID:25143401; http://dx.doi.org/ 10.1091/mbc.E14-04-0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jean S, Cox S, Nassari S, Kiger AA. Starvation-induced MTMR13 and RAB21 activity regulates VAMP8 to promote autophagosome-lysosome fusion. EMBO Rep 2015; 16:297-311; PMID:25648148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Munafo DB, Colombo MI. Induction of autophagy causes dramatic changes in the subcellular distribution of GFP-Rab24. Traffic 2002; 3:472-82; PMID:12047555 [DOI] [PubMed] [Google Scholar]
- [41].Yla-Anttila P, Mikkonen E, Happonen KE, Holland P, Ueno T, Simonsen A, Eskelinen EL. RAB24 facilitates clearance of autophagic compartments during basal conditions. Autophagy 2015; 11:1833-48; PMID:26325487; http://dx.doi.org/ 10.1080/15548627.2015.1086522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yamaguchi H, Nakagawa I, Yamamoto A, Amano A, Noda T, Yoshimori T. An initial step of GAS-containing autophagosome-like vacuoles formation requires Rab7. PLoS Pathog 2009; 5:e1000670; PMID:19956673; http://dx.doi.org/ 10.1371/journal.ppat.1000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nozawa T, Aikawa C, Goda A, Maruyama F, Hamada S, Nakagawa I. The small GTPases Rab9A and Rab23 function at distinct steps in autophagy during Group A Streptococcus infection. Cell Microbiol 2012; 14:1149-65; PMID:22452336; http://dx.doi.org/ 10.1111/j.1462-5822.2012.01792.x [DOI] [PubMed] [Google Scholar]
- [44].Yamano K, Fogel AI, Wang C, van der Bliek AM, Youle RJ. Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. eLife 2014; 3:e01612; PMID:24569479; http://dx.doi.org/ 10.7554/eLife.01612 [DOI] [PMC free article] [PubMed] [Google Scholar]


