ABSTRACT
Tumor-infiltrating lymphocytes (TILs) are predominantly present in breast cancer patients with estrogen receptor negative tumors, among whom increasing levels correlate with favorable outcomes. Nevertheless, currently available immune checkpoint inhibitors appear to benefit only a small number of women with breast cancer. Upregulation of additional immune checkpoint markers is one mechanism of resistance to current inhibitors that might be amenable to targeting with newer agents. T-cell Immunoglobulin and Mucin domain-containing molecule 3 (TIM-3) is an immune checkpoint receptor that is an emerging target for cancer immunotherapy. We investigated TIM-3 immunohistochemical expression in 3,992 breast cancer specimens assembled into tissue microarrays, linked to detailed outcome, clinico-pathological parameters and biomarkers including CD8, PD-1, PD-L1 and LAG-3. We scored and reported absolute counts for TIM-3+ intra-epithelial and stromal TILs (iTILs and sTILs), and find that breast cancer patients with TIM-3+ iTILs (≥ 1) represent a minority of cases (11%), with a predilection for basal-like breast cancers (among which 28% had TIM-3+ iTILs). TIM-3+ sTILs (≥ 2) represented 20% of cases and included more non-basal cases. The presence of TIM-3+ iTILs highly correlates with hematoxylin and eosin-stained stromal TILs and with other immune checkpoint markers (PD-1+ iTILs, LAG-3+ iTILs and PD-L1+ tumors). In prognostic analyses, early breast cancer patients with TIM-3+ iTILs have significantly improved breast cancer-specific survival whereas TIM-3+ sTILs did not reach statistical significance. In multivariate analyses, the presence of TIM-3+ iTILs is an independent favorable prognostic factor in the whole cohort as well as among ER negative patients. Our study supports TIM-3 as a target for breast cancer immunotherapy.
KEYWORDS: TIM-3, breast cancer, immunohistochemistry, tumor-infiltrating lymphocytes, LAG-3, PD-1, PD-L1, immune checkpoints
Introduction
The presence of small round dark mononuclear cells characteristic of tumor-infiltrating lymphocytes (TILs) on hematoxylin and eosin (H&E) – stained breast cancer specimens has garnered increased attention with the emergence of immune checkpoint inhibitors and has led to a re-examination of the role of the immune system in breast tumors. Accumulating evidence shows that the presence of an immune response in breast cancers correlates with estrogen receptor negative (ER-) subtypes (i.e. the HER2 and basal-like intrinsic subtypes) among whom there is an association with favorable outcomes 1–3. In contrast, the more common ER+ breast cancer subtypes rarely display such heightened immune responses, which when present are associated with unfavorable prognosis4-6.
Immune checkpoint inhibitors targeting cytotoxic T-Lymphocyte-associated antigen 4 (CTLA-4), programmed cell death-1 (PD-1) or its ligand (PDL-1) perform best in immunogenic cancers such as melanoma and non-small cell lung cancer7,8, but responses have recently been reported in triple negative/basal-like breast cancers9–11 (for reviews see refs. 12,13). However, even among such potentially immunogenic cancers, immune checkpoint inhibitors benefit only a relatively small number of patients7,12,14–18. As resistance may be due to the activation of alternative checkpoint pathways, additional immune checkpoints targets have become a subject of active research, including the T-cell Immunoglobulin and Mucin-domain- containing molecule 3 (TIM-3)19.
TIM-3 is an immune receptor discovered in 2002 that is expressed on a variety of immune cells including dendritic cells, macrophages, and T cells20-22. TIM-3 mediates its suppressive activity on immune cells via its ligands that include phosphatidylserine, CEACAM-1 and the widely expressed ligand galectin-9 23,24. TIM-3 is expressed on activated T cells and its signaling on cytotoxic T cells leads to an exhausted phenotype, characterized by a reduction in proliferation, decreased production of effector cytokines and apoptosis of effector T cells25. In addition, TIM-3+ TILs can co-express PD-1, with blockade of both receptors leading to a more pronounced tumor regression than either agent alone, at least in pre-clinical studies 26,27.
Multiple studies have now reported on the presence of TIM-3+ TILs in human tumors28-32. However, in breast cancer, TIM-3+ TILs have been evaluated by immunohistochemistry in a limited number of patients, with one recent study reporting positive associations with lymph node metastases33,34. The objective of our study is to evaluate the expression of TIM-3 on TILs in a large series of breast cancers powered for multivariate correlation with clinico-pathological parameters, survival, and other important immune biomarkers.
Results
Distribution of TIM-3+ iTILs in breast cancers
To define staining conditions and interpretation, we conducted an initial evaluation of TIM-3 staining and correlation with clinico-pathological parameters on a TMA consisting of 330 breast cancer patients (representing a training set). We observed 12% of breast cancer cases with TIM-3+ intratumoral tumor infiltrating lymphocytes (≥ 1 iTIL per 0.6 mm diameter core) whereas stromal TIM-3+ sTILs (≥ 1) were present in 48% of cases (Supplemental Table 1). We then proceeded with TIM-3 staining on a TMA comprising an independent cohort of 3,992 breast cancer cases, of which 3,148 cases were interpretable for TIM-3 immunohistochemistry staining (Supplemental Figure 1). The results were consistent with the training set as 11% of cases had ≥ 1 TIM-3+ iTILs and 40% of cases had ≥ 1 TIM-3+ sTILs (Supplemental Figure 2). TIM-3 expression on macrophages was only observed in 1% of cases and was not analyzed further.
As there were a large number of cases with TIM-3+ sTILs, a cut-off for dichotomization of ≥ 2 sTIL/0.6 mm core, a level reached in 20% of breast cancers, was selected based on analyses of Kaplan Meier curves of different TIM-3+ sTILs cutpoints (as described in Methods: Statistics). However, TIM-3+ iTILs were selected as the primary analysis parameter, to allow comparison with previously published immune biomarkers in this breast cancer cohort35,36.
The presence of TIM-3+ iTILs in breast cancer is associated with unfavorable clinico-pathological factors
Consistent with the results in the initial cohort of 330 patients, breast cancer cases with TIM-3+ iTILs in the validation cohort were significantly associated with younger age at presentation, higher grade, hormone receptor (ER/PR) negativity, and high Ki67 proliferation index [defined as ≥ 14%] (Table 1). In addition, the presence of TIM-3+ iTILs was much more common in the basal-like subtype relative to other subtypes (28% in basal-like vs 6% in luminal A). The results for TIM-3+ sTILs reflected similar associations with clinico-pathological parameters to TIM-3+ iTILs findings (Supplemental Table 2). However, more of the non-basal cases had TIM-3+ sTILs than was the case for iTILs.
Table 1.
TIM-3+ iTILs association with clinico-pathological parameters in breast cancer.
| Parameters | Negative N = 2816 |
TIM-3+ iTILs ≥ 1 N = 332 (11%) |
P-value* (χ2) |
|---|---|---|---|
| Age at diagnosis (years) | |||
| < 50 | 787 | 119 (13%) | 0.003 |
| ≥ 50 | 2029 | 213 (10%) | |
| Tumor size (cm) | |||
| ≤ 2 | 1467 | 163 (10%) | 0.331 |
| > 2 | 1334 | 166 (11%) | |
| Grade | |||
| 1&2 | 1286 | 96 (7%) | < 0.001 |
| 3 | 1414 | 219 (13%) | |
| Unknown | 116 | 12 | |
| Ki67 | |||
| Negative (< 14%) | 1469 | 100 (6%) | < 0.001 |
| Positive (≥ 14%) | 1085 | 209 (16%) | |
| Unknown | 262 | 23 | |
| ER | |||
| Negative | 716 | 159 (18%) | < 0.001 |
| Positive (> 1%) | 2091 | 172 (8%) | |
| Unknown | 9 | 1 | |
| Subtypes | |||
| Luminal A | 1209 | 73 (6%) | < 0.001 |
| Luminal B | 631 | 80 (11%) | |
| HER2E | 184 | 25 (12%) | |
| Basal-like | 205 | 81 (28%) | |
| Triple negative, non-basal | 182 | 40 (18%) | |
| Unknown | 240 | 17 |
* Chi-square test
TIM-3+ iTILs correlate with the presence of other immune checkpoint markers (LAG-3, PD-1, PD-L1) and overall H&E stils
Because this large cohort had been previously assessed for key immune biomarkers including PD-1, PD-L1 and LAG-3, we were able to analyze their correlations with TIM-3. In addition, we scored overall H&E stromal TILs to allow a parallel evaluation with immune checkpoint markers.
We found that breast tumors with TIM-3+ iTILs were highly significantly associated with the presence of additional immune checkpoint markers (Table 2). Indeed, nearly half of breast cancers that are positive for PD-L1 or PD-1+ iTILs or LAG-3+ iTILs were also infiltrated with TIM-3+ iTILs in the same 0.6 mm TMA core. However, only 3% (91/2736 interpretable cases) expressed all three immune checkpoint markers (TIM-3+/PD-1+/LAG-3+) (Table 2) when assessed by this method. We did not observe any particularly unique association pattern between the presence of TIM-3+ iTILs and any of the other individual immune checkpoint markers tested, suggesting that the TIM-3 checkpoint expression on TILs occur in tumors containing T cells positive for other exhausted markers. Furthermore, we found that all immune checkpoint markers correlated positively (p < 0.001) with H&E sTILs (Supplemental Figure 3). In this cohort, less than 1% of cases were categorized as lymphocyte-predominant breast cancer (LPBC, defined as ≥ 50% H&E sTILs).
Table 2.
Association of TIM-3+ iTILs with other immune biomarkers in breast cancer.
| Immune biomarkers | TIM-3+ iTILs = 0 (n = 2816) |
TIM-3+ iTILs ≥ 1 (n = 332) |
P-value (χ2) |
|---|---|---|---|
| PD-L1* | |||
| Negative | 2374 | 215 (8%) | < 0.0001 |
| Positive (≥ 1%) | 133 | 97 (42%) | |
| PD-1+ iTILs* | |||
| Negative | 2388 | 198 (8%) | < 0.0001 |
| Positive (≥ 1) | 127 | 113 (47%) | |
| LAG-3+ iTILs* | |||
| Negative | 2344 | 169 (7%) | < 0.0001 |
| Positive (≥ 1) | 175 | 146 (45%) | |
| CD8+ iTILs* | |||
| Negative | 1881 | 111 (6%) | < 0.0001 |
| Positive (≥ 1) | 778 | 213 (22%) |
*Frequency in the whole cohort: PD-L1 ≥ 1% = 241/2918 (8.3%); PD-1+ iTILs ≥ 1 = 246/2908 (8.5%); LAG-3+ iTILs = 327/2921 (11%), from Burugu S et al., Annals of Oncology, 2017. CD8+ iTILs ≥ 1 = 1089/3403 (32%) from Liu S et al., Breast Cancer Research, 2012)
TIM-3+ iTILs are associated with good prognosis in early breast cancer
In univariate analyses, the presence of TIM-3+ iTILs in early breast tumors was associated with improved breast cancer-specific survival (BCSS) in the whole cohort (HR: 0.76, 95%CI 0.61–0.96, Log Rank p = 0.02) (Figure 1). When breast cancer subtypes were stratified in the analysis, only HER2+ and basal-like breast cancer patients with TIM-3+ iTILs displayed significantly improved BCSS (HER2+: HR: 0.27, 95%CI 0.10–0.72, Log Rank p = 0.005; Basal-like: HR: 0.48, 95%CI 0.29–0.78, Log Rank p = 0.003) (Figure 1). These results were similar using overall survival and relapse-free survival secondary endpoints (Supplemental Figure 4 for overall survival; Supplemental Figure 5 for relapse-free survival). In contrast, in exploratory analyses, the presence of TIM-3+ sTILs had a trend for favorable prognosis for BCSS and relapse-free survival and reached significance in the whole cohort for overall survival (Supplemental Figure 6 for BCSS; Supplemental Figure 7 for overall survival and Supplemental Figure 8 for relapse-free survival).
Figure 1.
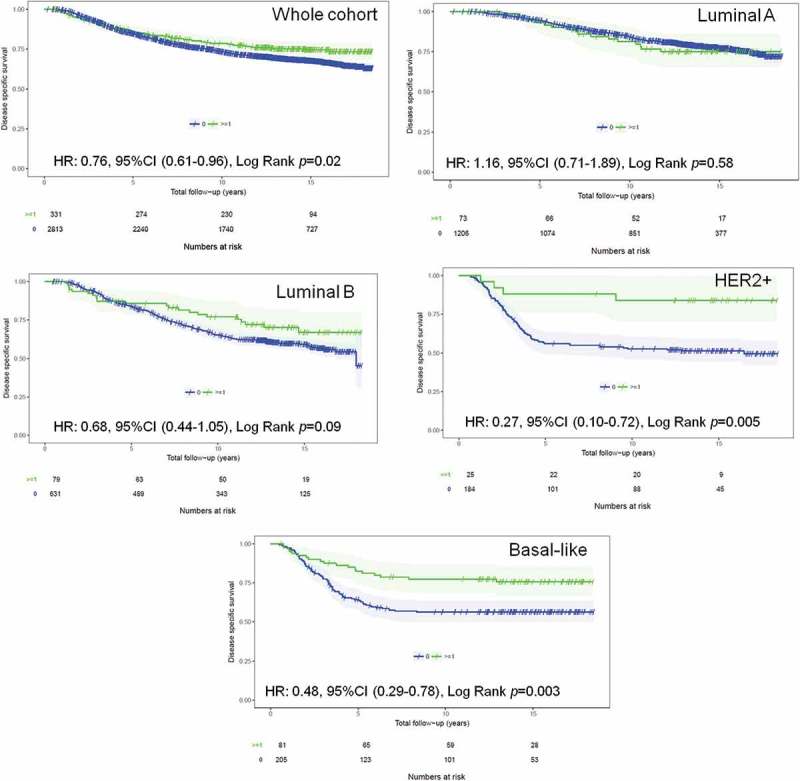
TIM-3+ iTILs association with BCSS in the whole (validation) cohort and by breast cancer subtype. Kaplan Meier curves of breast cancer-specific survival in breast cancer patients stratified by the presence or absence of TIM-3+ iTILs. KM curves in (A) the whole cohort, (B) Luminal A cases, (C) Luminal B, (D) HER2+ and (E) basal-like cases are shown with their corresponding numbers of patients, events and log rank p values. The number of patients still at risk at the end of each 5 years of follow-up is shown at the bottom of each panel.
In multivariate analyses that included H&E sTILs as a covariate, the presence of TIM-3+ iTILs remained a favorable prognostic factor in the whole cohort and ER- breast cancer patients (Table 3 – Whole cohort: HR: 0.64, 95%CI 0.48–0.85, p = 0.001; ER-: HR: 0.58, 95%CI 0.39–0.86, p = 0.004, Basal-like: HR: 0.58, 95%CI 0.32–1.03, p = 0.052). Similar findings were observed for TIM-3+ sTILs albeit not reaching significance for basal-like breast cancer patients (Supplemental Table 3). We also found that ER- breast cancer patients with tumors that were co-infiltrated with TIM-3+, PD-1+ and LAG-3+ TILs had a significant improved breast cancer specific survival, in univariate and multivariate analyses, relative to patients with a single positive, dual positive, or complete absence of these three immune checkpoint markers (Figure 2, Table 4).
Table 3.
Multivariate analyses of TIM-3+ iTILs in the whole cohort, among ER- and in basal-like patients for breast cancer-specific survival including H&E sTILs as a covariate.
| Whole cohort (# of events/n: 705/2379) | Hazard Ratio for BCSS (95% CI) | LRT P-value | |
|---|---|---|---|
| Age at diagnosis | |||
| (Reference group:> 50) | ≥ 50 | 1.06 (0.86–1.30) | 0.61 |
| Tumor grade | |||
| (Reference group: grade 1–2) | Grade 3 | 1.49 (1.26–1.77) | < 0.001 |
| Tumor size | |||
| (Reference group: ≤ 2cm) | > 2 | 1.63 (1.39–1.91) | < 0.001 |
| Lymphovascular invasion status | |||
| (Reference group: negative) | Positive | 1.33 (1.11–1.60) | 0.002 |
| Nodal status | |||
| (Reference group: negative) | Positive | 2.29 (1.86–2.82) | < 0.001 |
| Adjuvant systemic therapy | |||
| (Reference group: no AST) | TAM only | 0.73 (0.57–0.93) | 0.05 |
| Chemo only | 0.74 (0.56–0.99) | ||
| TAM+ chemo | 0.69 (0.49–0.97) | ||
| Breast cancer subtypes | |||
| (Reference group: Luminal A) | Luminal B/Ki67 | 1.81 (1.50–2.19) | < 0.001 |
| Luminal/HER2+ | 2.16 (1.64–2.84) | ||
| HER2+ | 2.54 (1.93–3.35) | ||
| Basal-like | 2.28 (1.74–2.99) | ||
| H&E sTILs | |||
| (10% increments) | 0.98 (0.98–0.99) | < 0.001 | |
| TIM-3+ iTILs | ≥ 1 | 0.64 (0.48–0.85) | 0.001 |
| (Reference group: 0) |
|
|
|
| Among ER-* (# of events/n: 255/705) |
Hazard Ratio for BCSS (95% CI) |
LRT P-value |
|
| Age at diagnosis | |||
| (Reference group:> 50) | ≥ 50 | 0.90 (0.66–1.23) | 0.50 |
| Tumor grade | |||
| (Reference group: grade 1–2) | Grade 3 | 1.91 (1.35–2.70) | < 0.001 |
| Tumor size | |||
| (Reference group: ≤ 2cm) | > 2 | 1.62 (1.23–2.12) | 0.001 |
| Lymphovascular invasion status | |||
| (Reference group: negative) | Positive | 1.32 (0.99–1.77) | 0.06 |
| Nodal status | |||
| (Reference group: negative) | Positive | 2.44 (1.76–3.38) | < 0.001 |
| Adjuvant systemic therapy | |||
| (Reference group: no AST) | TAM only | 0.89 (0.59–1.34) | 0.64 |
| Chemo only | 0.81 (0.55–1.19) | ||
| TAM+ chemo | 1.02 (0.59–1.75) | ||
| H&E sTILs | 0.98(0.97–0.99) | 0.002 | |
| (10% increments) | |||
| TIM-3+ iTILs | ≥ 1 | 0.58 (0.39–0.86) | 0.004 |
| (Reference group: 0) |
|
|
|
| Among basal-like (# of events/n: 94/263) |
Hazard Ratio for BCSS (95% CI) |
LRT P-value |
|
| Age at diagnosis | |||
| (Reference group:< 50) | ≥ 50 | 0.86 (0.50–1.46) | 0.57 |
| Tumor grade | |||
| (Reference group: grade 1–2) | Grade 3 | 1.39 (0.72–2.71) | 0.31 |
| Tumor size | |||
| (Reference group: ≤ 2cm) | > 2 | 1.40 (0.91–2.16) | 0.13 |
| Lymphovascular invasion status | |||
| (Reference group: negative) | Positive | 1.31 (0.82–2.10) | 0.26 |
| Nodal status | |||
| (Reference group: negative) | Positive | 2.01 (1.19–3.38) | 0.008 |
| Adjuvant systemic therapy | |||
| (Reference group: no AST) | TAM only | 1.65 (0.80–3.42) | 0.60 |
| Chemo only | 1.21 (0.65–2.25) | ||
| TAM+ chemo | 1.40 (0.47–4.19) | ||
| H&E sTILs | |||
| (10% increments) | 0.97 (0.95–0.99) | 0.002 | |
| TIM-3+ iTILs | |||
| (Reference group: 0) | ≥ 1 | 0.58 (0.32–1.03) | 0.052 |
* including HER2 positive and negative
Figure 2.
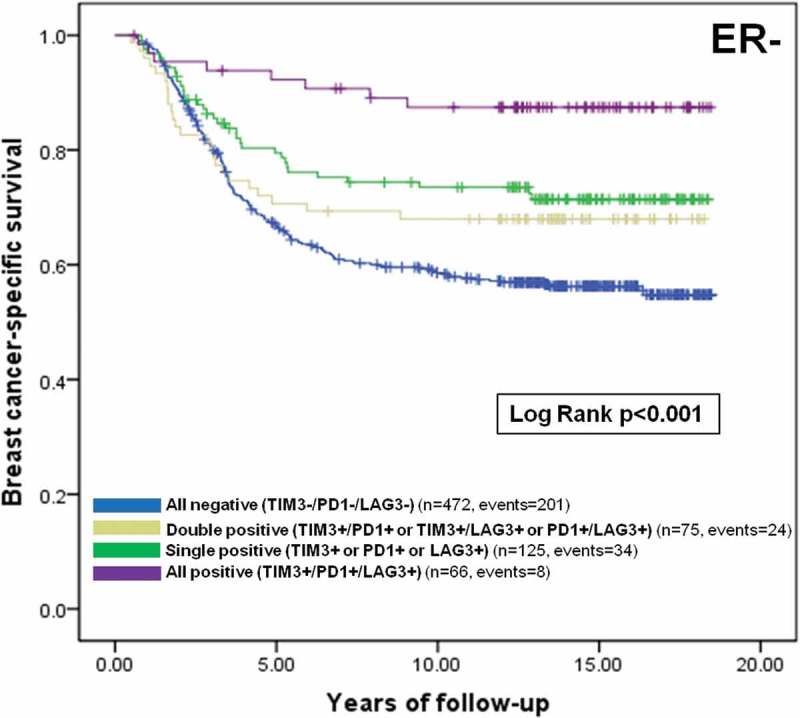
Prognostic value of TIM-3, PD-1 and LAG-3+ iTILs co-infiltration among ER- breast cancer patients. Kaplan Meier curve of breast cancer-specific survival among ER- breast cancer patients stratified by the presence or absence of one or more immune checkpoint markers is shown with corresponding number of patients, events and a log rank p value. Blue: All negative (TIM3-/PD1-/LAG3-), green: Single positive (TIM3+ or PD1+ or LAG3+), grey: Double positive (TIM3+/PD1+ or TIM3+/LAG3+ or PD1+/LAG3+), purple: All positive (TIM3+/PD1+/LAG3+).
Table 4.
Multivariate analyses of TIM-3/PD-1/LAG-3+ iTILs among ER- breast cancer patients for BCSS.
| Among ER- (# of events/n: 249/686) | Hazard Ratio for BCSS (95% CI) | P-value* | |
|---|---|---|---|
|
Age at diagnosis (Reference group:< 50) |
≥ 50 | 0.89 (0.64–1.24) | 0.49 |
|
Tumor grade (Reference group: grade 1–2) |
Grade 3 | 2.22 (1.56–3.16) | < 0.001 |
|
Tumor size (Reference group: ≤ 2cm) |
> 2 | 1.50 (1.14–1.98) | 0.004 |
|
Lymphovascular invasion status (Reference group: negative) |
Positive | 1.355 (0.998–1.840) | 0.052 |
|
Nodal status (Reference group: negative) |
Positive | 2.654 (1.887–3.731) | < 0.001 |
|
Adjuvant systemic therapy (Reference group: no AST) |
TAM only Chemo only TAM+ chemo |
0.851 (0.566–1.279) 0.739 (0.495–1.103) 1.193 (0.683–2.084) |
0.436 |
|
TIM-3/PD-1/LAG-3+ iTILs (Reference group: TIM3-/PD1-/LAG3-) |
TIM3-/PD1+/LAG3- TIM3-/PD1-/LAG3+ TIM3-/PD1+/LAG3+ TIM3+/PD1-/LAG3- TIM3+/PD1+/LAG3- TIM3+/PD1-/LAG3+ TIM3+/PD1+/LAG3+ |
0.499 (0.251–0.989) 0.498 (0.289–0.861) 0.586 (0.299–1.148) 0.615 (0.314–1.203) 0.348 (0.086–1.410) 0.959 (0.519–1.772) 0.165 (0.073–0.375) |
0.046 0.012 0.119 0.155 0.139 0.893 < 0.001 |
*Wald-test
Discussion
We report the first study of TIM-3 expression in a large (> 1000 case) series of early breast cancers. TIM-3 expression in this cohort was restricted to tumor-infiltrating lymphocytes and was present in about 12% of cases when 0.6 mm cores were evaluated for expression on intra-epithelial TILs, or 20% of cases when assessed on stromal TILs. The presence of TIM-3+ iTILs was associated with younger age, high grade and high Ki67 proliferation index and was enriched in the basal-like breast cancer subtype. Moreover, TIM-3+ iTILs highly correlated with co-infiltration of additional immune checkpoint markers PD-L1 (on carcinoma cells), PD-1 and LAG-3+ (on TILs). In prognostic analyses, early breast cancer patients with TIM-3+ iTILs had significantly improved survival for all assessed endpoints, as compared to patients whose tumors lacked TIM-3+ iTILs. In multivariate analyses, the prognostic effect was maintained in the whole cohort as well as among ER- and basal-like breast cancer patients.
Studies from our group and from others have been consistent in finding that the presence of immune checkpoint markers on intra-epithelial TILs in breast tumors is an uncommon event, and mostly restricted to ER- breast cancers33,36,37. However, TILs positive for immune checkpoint markers are able to discriminate breast cancer patients with favorable survival, consistent with an active anticancer immune microenvironment. Indeed, we found that breast tumors infiltrated with TIM-3+ iTILs highly correlate with tumors positive for other checkpoint markers (PD-1, PD-L1 and LAG-3). Results are consistent with other reported studies and imply that the expression of multiple different immune checkpoints can occur during tumor progression, reflecting an ongoing battle between cancer cells and the immune system38,39. In our cohort, coexpression of TIM-3 with PD-1 and LAG-3 is associated with a particularly favorable prognosis, perhaps reflecting an underlying robust immune recognition of the cancer cells that is difficult for the tumor to evade. Furthermore, other studies have reported TIM-3 expression on carcinoma cells to be associated with poor prognosis (for meta-analysis see ref. 40), which we did not observe in our large breast cancer cohort. These apparently conflicting results may be due to the different types of tumor and possible confounding by stage or other factors, as the smaller studies in other tumors were not powered for multivariate analyses.
Strengths of our study include the use of a large cohort of early breast cancer patients, treated consistently according to provincial guidelines, linked to detailed long-term outcome data and assessed using a training and validation approach to biomarker interpretation. Some limitations include, first, the necessity in such a large series to rely on TMA cores, representing a 0.28 mm2 surface area sampling of a tumor for assessment of the tumor immune microenvironment. Second, infiltration of TILs bearing multiple immune biomarkers could only be inferred from single stains and therefore does not directly identify co-expression on the same lymphocyte. Third, breast cancer patients in the cohort received what would now be considered older treatments (predating trastuzumab, taxanes and aromatase inhibitors) which may affect extrapolation of some of the observed prognostic and predictive associations to more contemporary treatment regimens.
Accumulating evidence suggests resistance to anti- CTLA-4 or anti-PD-1/PD-L1 inhibitors can occur in otherwise immunogenic cancers through compensatory upregulation of additional immune checkpoints39,41. TIM-3 has recently emerged as a target for cancer immunotherapy following pre-clinical studies suggesting its non-redundant functions in comparison to the better-characterized checkpoint markers PD-1/PD-L1, and efficacious treatment synergy when TIM-3 is targeted in combination with anti-PD1/PDL1 antibodies26,27,42,43. Although ER- breast cancer, in particular basal and triple negative breast cancer, is considered the most immunogenic subtype, reports from immune checkpoint inhibitor clinical trials are not as encouraging. Early reports suggest metastatic breast cancer patients may benefit most from PD-1/PD-L1 blockade monotherapy in the first-line setting, or in combination with chemotherapy agents for second or third-line therapy with an objective response rate ranging from 10%-40% 9-11 (for review, see ref. 12). The findings from our study imply that TIM-3 inhibitors could potentially help to treat PD-1 refractory or metastatic tumors. Currently, four early phase clinical trials testing the efficacy of anti-TIM-3 in combination with anti-PD-1/PDL1 in advanced tumors have opened [NCT03066648, NCT02608268, NCT02817633, and NCT03099109]. Our data support that this appears to be a relevant combinatorial strategy to assess in breast cancer, particularly in patients with non-BRCA mutated basal-like tumors, an aggressive subtype for which targeted therapies are not currently available.
Methods
Study cohorts
A training set consisting of 330 breast cancer patients was used to finalize biomarker staining and interpretation conditions for an initial analysis of TIM-3. These patients were diagnosed with invasive breast cancer at the University of British Columbia hospitals between 1989 and 2002 and have been previously described44. A detailed description of the validation set consisting of 3,992 breast cancer patients has been previously published45,46. In brief, newly diagnosed invasive breast cancers from centres across the province of British Columbia performing breast cancer excision surgery, referred to the British Columbia Cancer Agency (BCCA) between 1986 and 1992 and for which both blocks from a central estrogen receptor testing laboratory and detailed clinico-pathologic, treatment and outcome data collected by the BCCA Breast Cancer Outcomes Unit were available were assembled into 17 single core tissue microarray blocks. None of these patients (training and validation cohorts) received neoadjuvant treatment. Table 1 summarizes the basic clinico-pathological parameters of the study populations. The median follow-up for both cohorts is 13 years. The Clinical Research Ethics Board of the University of British Columbia and the British Columbia Cancer Agency Breast Cancer Outcomes Unit approved the access to the samples and corresponding de-identified outcome data.
Immunohistochemistry and scoring
Tissue microarrays (TMAs) were built from formalin-fixed paraffin-embedded primary excision specimens from patients in the training and validation cohorts and represented as 0.6mm cores across 3 blocks for the training cohort and 17 blocks for the validation cohort. These TMAs have been previously stained and scored for multiple biomarkers including ER, PR, HER2, Ki67, EGFR, CK5/6, CD8, LAG-3, PD-1 and PD-L136. Breast cancer intrinsic subtypes were previously determined from both cohorts by immunohistochemistry (IHC) benchmarked against a gene expression gold standard (the PAM50 intrinsic subtype classifier) 47 . Briefly, ER+ (≥ 1%) or PR+ (≥ 1%), HER2- (including IHC 2+ cases that were HER2- by fluorescence in situ hybridization) and low (< 14%) Ki67 were defined as Luminal A; hormone receptor positive cases which were also either HER2+ or had high Ki67 were defined as Luminal B; HER2+/ER-/PR- cases were defined as HER2E, and triple negative cases that were positive for EGFR+ or CK5/6+ were defined as basal-like.
Overall stromal TILs were scored on H&E-scanned images of the TMA cores using the assessment recommendations of the International TILs Working Group48, whereby stromal TILs are scored as the percentage of intertumoral stromal surface area (i.e. excluding areas occupied by carcinoma cells) containing mononuclear lymphocytic infiltrates.
TIM-3 immunohistochemistry was conducted with anti-TIM-3 rabbit monoclonal antibody clone D5D5R from Cell Signaling (Cat# 45208) as employed in other publications 21,33,49,50, here using a Ventana Ultra automated stainer (Ventana Medical Systems) in concordance with manufacturer’s protocol. In brief, slides underwent antigen retrieval with Standard Cell Conditioning 1 reagent (Ventana Medical Systems) followed by 60 minutes of primary antibody incubation (applied at 1:50 dilution) with no heat, and visualized using a chromoMap DAB detection kit (Ventana Medical Systems). Membranous staining in tonsil tissue served as a positive control in each staining run. TIM-3+ lymphocytes scores were reported as absolute counts per TMA core for intra-epithelial or stromal locations. TIM-3+ intra-epithelial lymphocytes (TIM-3+ iTILs) were defined as TIM-3+ lymphocytes located within carcinoma nests whereas TIM-3+ stromal lymphocytes (TIM-3+ sTILs) were those not in direct contact with the carcinoma nest.
Statistics
IBM SPSS software (version 24.0) and R (version 3.3.2) were used to conduct all the statistical analyses.TIM-3+ iTILs scores were dichotomized ≥ 1 (as positive) vs. 0 (as negative). In addition, TIM-3 expression on other immune cells (non-lymphocytes) was assessed, but as only 1% of cases were positive on the training set this staining pattern was not further analyzed.
For prognostic analyses, the primary end-point, breast cancer-specific survival, was defined as the time from date of diagnosis to date of death attributed to breast cancer. Patients were censored at death from another cause or if alive at end of follow-up. Relapse-free survival and overall survival were secondary end-points. Relapse-free survival was defined as time from date of diagnosis to date of any type of breast cancer relapse (local, regional, distant, or contralateral) and overall survival as time from date of diagnosis to date of death, irrespective of the cause of death. Correlation with survival was conducted using Kaplan-Meier curves, log-rank test and Cox regression models. Proportional hazard assumptions were assessed by visual examinations of Kaplan-Meier plots. The effect size was adjusted in multivariate Cox regression models by taking into account significant clinicopathological parameters (age, tumor grade, tumor size, lymphovascular invasion and nodal status).
Clinico-pathological and prognostic associations for TIM-3+ iTILs were analyzed first on the training cohort (n = 330) and further tested on the validation cohort (n = 3,992) in a pre-specified formal written statistical plan, presented at the British Columbia Cancer Agency Breast Cancer Outcomes Unit. Furthermore, half of the validation cohort served for a training and a validation approach specifically for correlations and combinatorial analyses among immune biomarkers (TIM-3, PD-L1, PD-1, LAG-3, CD8) due to the low number of positive cases observed in the training set. In addition, 40% of cases in the validation cohort were considered TIM-3+ sTIL positive based on a ≥ 1 positive TIL cut-point. A cut-point of ≥ 2 positive TILs for TIM-3+ sTILs, representing 20% of cases, was selected following testing of various cut-points (≥ 1, ≥ 2) based on the distribution on half of the validation cohort set in prognostic analyses. In these cases, a pre-specified written statistical plan for validation on the other half of the set was presented prior to statistical analyses. Prognostic analyses of co-infiltrated immune checkpoint markers were nevertheless considered exploratory. All statistical tests performed were two-sided at α = 0.05.
Supplementary Material
Funding Statement
This work was supported by the Canadian Cancer Society under grant #705463 awarded to TON. SB is a recipient of a studentship from the Fonds de recherche du Québec-Santé;Fonds de la recherche en sante du Quebec (FRSQ) [31806];
Disclosure
Torsten O. Nielsen reports a proprietary interest in the Prosigna test for breast cancer intrinsic subtype, not part of the current study. All other co-authors have declared no conflicts of interest.
References
- 1.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et al Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 3.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, et al Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 4.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, et al Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. The Lancet Oncology. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 5.Krishnamurti U, Wetherilt CS, Yang J, Peng L, Li X.. Tumor-infiltrating lymphocytes are significantly associated with better overall survival and disease-free survival in triple-negative but not estrogen receptor-positive breast cancers. Human Pathology. 2017;64:7–12. doi: 10.1016/j.humpath.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Heindl A, Sestak I, Naidoo K, Cuzick J, Dowsett M, Yuan Y. Relevance of Spatial Heterogeneity of Immune Infiltration for Predicting Risk of Recurrence After Endocrine Therapy of ER+ Breast Cancer. Journal of the National Cancer Institute. 2018;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin J, Chiarion-Sileni V, Gonzalez R, Jj Grob, Cl Cowey, Cd Lao, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in Untreated Melanoma. The New England Journal of Medicine. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 9.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 Study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, Boccia R, Lippman ME, Somer R, Smakal M, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Research and Treatment. 2018;167:671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McArthur HL, Diab A, Page DB, Yuan J, Solomon SB, Sacchini V, Comstock C, Durack JC, Maybody M, Sung J, Ginsberg A, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2016;22:5729–5737. doi: 10.1158/1078-0432.CCR-16-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emens LA. Breast cancer immunotherapy: facts and hopes. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2018;24:511–520. doi: 10.1158/1078-0432.CCR-16-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwa MJ, Adams S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer, Wiley; 2018. [DOI] [PubMed] [Google Scholar]
- 14.Bellmunt J, De Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al. Pembrolizumab as second-line therapy for advanced Urothelial Carcinoma. The New England Journal of Medicine. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman- Censits J, Perez-Gracia JL, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. The Lancet Oncology. 2017;18:1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 17.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. The New England Journal of Medicine. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 19.Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Seminars in cancer biology. Elsevier.2017. [DOI] [PubMed] [Google Scholar]
- 20.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 21.De Mingo Pulido A, Gardner A, Hiebler S, Soliman H, Rugo HS, Krummel MF, Coussens LM, Ruffell B. TIM-3 regulates cd103(+) dendritic cell function and response to chemotherapy in breast cancer. Cancer Cell. 2018;33:60–74 e6. doi: 10.1016/j.ccell.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut. 2015;64:1593–1604. doi: 10.1136/gutjnl-2014-307671. [DOI] [PubMed] [Google Scholar]
- 23.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nature Immunology. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 24.Sabatos-Peyton CA, Nevin J, Brock A, Venable JD, Tan DJ, Kassam N, Xu F, Taraszka J, Wesemann L, Pertel T et al. Blockade of Tim-3 binding to phosphatidylserine and CEACAM1 is a shared feature of anti-Tim-3 antibodies that have functional efficacy. Oncoimmunology. 2018;7:e1385690. doi: 10.1080/2162402X.2017.1385690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunological Reviews. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. The Journal of Experimental Medicine. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of Experimental Medicine. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granier C, Dariane C, Combe P, Verkarre V, Urien S, Badoual C, Roussel H, Mandavit M, Ravel P, Sibony M, et al. Tim-3 expression on tumor-infiltrating pd-1(+)cd8(+) t cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Research. 2017;77:1075–1082. doi: 10.1158/0008-5472.CAN-16-0274. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 30.Japp AS, Kursunel MA, Meier S, Malzer JN, Li X, Rahman NA, Jekabsons W, Krause H, Magheli A, Klopf C, et al. Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunology, Immunotherapy: CII. 2015;64:1487–1494. doi: 10.1007/s00262-015-1752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Meng Q, Bartek J Jr., Poiret T, Persson O, Rane L, Rangelova E, Illies C, Peredo IH, Luo X, et al. Tumor-infiltrating lymphocytes (TILs) from patients with glioma. Oncoimmunology. 2017;6:e1252894. doi: 10.1080/2162402X.2016.1252894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 2017;6:e1261779. doi: 10.1080/2162402X.2016.1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solinas C, Garaud S, De Silva P, Boisson A, Van Den Eynden G, De Wind A, Risso P, Rodrigues Vitoria J, Richard F, Migliori E, et al. Immune checkpoint molecules on tumor-infiltrating lymphocytes and their association with tertiary lymphoid structures in human breast cancer. Frontiers in Immunology. 2017;8:1412. doi: 10.3389/fimmu.2017.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Xiang R, Wu B, Li J, Luo G. T-cell immunoglobulin mucin-3 expression in invasive ductal breast carcinoma: clinicopathological correlations and association with tumor infiltration by cytotoxic lymphocytes. Molecular and Clinical Oncology. 2017;7:557–563. doi: 10.3892/mco.2017.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Research: BCR. 2012;14:R48. doi: 10.1186/bcr3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burugu S, Gao D, Leung S, Chia SK, Nielsen TO. LAG-3+ tumor infiltrating lymphocytes in breast cancer: clinical correlates and association with PD-1/PD-L1+ tumors. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2017;28:2977–2984. doi: 10.1093/annonc/mdx557. [DOI] [PubMed] [Google Scholar]
- 37.Bottai G, Raschioni C, Losurdo A, Di Tommaso L, Tinterri C, Torrisi R, Reis-Filho JS, Roncalli M, Sotiriou C, Santoro A, et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Research: BCR. 2016;18:121. doi: 10.1186/s13058-016-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 39.Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nature Medicine. 2017;23:551–555. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Cai P, Liang T, Wang L, Hu L. TIM-3 is a potential prognostic marker for patients with solid tumors: A systematic review and meta-analysis. Oncotarget. 2017;8:31705–31713. doi: 10.18632/oncotarget.15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang RY, Francois A, McGray AR, Miliotto A, Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology. 2017;6:e1249561. doi: 10.1080/2162402X.2016.1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngiow SF, Von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Research. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 44.Bortnik S, Choutka C, Horlings HM, Leung S, Baker JH, Lebovitz C, Dragowska WH, Go NE, Bally MB, Minchinton AI, et al. Identification of breast cancer cell subtypes sensitive to ATG4B inhibition. Oncotarget. 2016;7:66970–66988. doi: 10.18632/oncotarget.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, Nielsen TO. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Research: BCR. 2014;16:432. doi: 10.1186/s13058-014-0492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernar PS, Parker JS et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. Journal of the National Cancer Institute. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international tils working group 2014. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen TC, Chen CH, Wang CP, Lin PH, Yang TL, Lou PJ, Ko JY, Wu CT, Chang YL. The immunologic advantage of recurrent nasopharyngeal carcinoma from the viewpoint of Galectin-9/Tim-3-related changes in the tumour microenvironment. Scientific Reports. 2017;7:10349. doi: 10.1038/s41598-017-10386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu JF, Ma SR, Mao L, Bu LL, Yu GT, Li YC, Huang CF, Deng WW, Kulkarni AB, Zhang WF. T-cell immunoglobulin mucin 3 blockade drives an antitumor immune response in head and neck cancer. Molecular Oncology. 2017;11:235–247. doi: 10.1002/1878-0261.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


