Assessment of cardiovascular (CV) risk forms the cornerstone of treatment of individuals requiring primary prevention of atherosclerotic CV disease (ASCVD). An estimate of the future risk of CV events is integral to clinical decision-making in these subjects as it permits tailoring therapy according to the likelihood of developing a vascular event in future. Traditionally, such an assessment is performed using population-based risk algorithms such as the Framingham risk score (FRS),1,2 the pooled cohort equation,3 QRISK2,4 etc. that return the probability of a person developing ASCVD event over next 10 years. Unfortunately, while such risk algorithms have proven accuracy for risk prediction at population level, their accuracy at individual-level is unsatisfactory, leading to treatment decisions that cannot be individualized. For example, a 20% 10-year ASCVD risk (considered high risk by most definitions) merely implies that 20 of 100 such individuals will develop an event over 10 years but cannot predict which 20.
Assessment of subclinical atherosclerosis has been proposed as a potential solution to this problem, which is inherent to clinical risk algorithms. It is based on the understanding that not everyone with CV risk factors develops atherosclerosis, but only those who develop atherosclerosis will eventually develop an ASCVD event. This implies that the presence of early evidence of atherosclerosis, and not merely that of clinical risk factors, should be the driver of clinical decision-making. In other words, this approach relies on tailoring therapy based on the actual presence of atherosclerosis rather than the ‘probability’ of developing atherosclerosis.
Several different measures of subclinical atherosclerosis have been developed over the years, including coronary artery calcium score (CACS), carotid intima-media thickness (CIMT), carotid plaques, brachial artery flow-mediated dilatation, ankle-brachial index (ABI), pulse wave velocity (PWV), etc. Pathological studies have validated their accuracy as surrogate markers of atherosclerosis,5, 6, 7 which has led to their extensive use in research studies looking at various aspects of the atherosclerotic process. This issue of Indian Heart Journal includes four such articles employing some of these tools as end-points,8, 9, 10, 11 which reiterates their enormous appeal for this purpose. However, this also raises a very pertinent question- while these tools seem to be excellent for research purposes, are they good enough for clinical use as well? Let us briefly review the evidence about their utility for clinical decision-making.
1. Coronary artery calcium score
In absence of the conditions causing dystrophic calcification, such as chronic kidney disease, hyperparathyroidism, etc., calcification in the coronary arteries occurs only in the presence of atherosclerosis. Thus, the presence of any amount of calcium in the coronary arteries can be considered as an evidence of ongoing atherosclerosis. Recent studies have also shown that unlike previously believed, coronary artery calcification is not a passive, age-related, end-stage event; rather, it is an active step in atherogenesis, occurring as a direct consequence of vascular inflammation.12
Coronary artery calcium (CAC) can be easily imaged and quantified using computed tomography (CT). Agatston method is the most commonly used method for CACS estimation and works by multiplying areas of calcium deposition (recognized by attenuation of >130 Hounsfield Units) with a density factor to yield CACS. Initially, electron-beam CT was used as the preferred modality for CACS estimation, but due to its lack of versatility for general CT imaging, it has now been largely superseded by multidetector CT.
Pathological studies have shown that CT-derived CACS correlates excellently with the total amount of calcium in the coronary arteries and also with the total atherosclerotic burden.5 However, as the calcification process is not dependent on the luminal encroachment by the plaque, the presence of calcium does not necessarily reflect the site of maximum stenosis.13
1.1. Predictive accuracy of CACS
Consistent with the excellent correlation between CACS and the total coronary atherosclerotic burden, numerous large-scale multicentric studies have shown that CACS is an excellent predictor of incident CV events14, 15, 16, 17, 18, 19 and this predictive accuracy is incremental to any of the conventional risk factors and risk algorithms.
MESA (Multi-Ethnic Study of Atherosclerosis) is a prospective multicenter study involving 6814 men and women of 45 to 84 years of age, of four different ethnicities. Overall, 500 (7.4%) incident ASCVD events were observed in the total study population over a median follow-up of 11.1 years. CACS strongly, and in a graded manner, predicted the 10-year risk of incident ASCVD, independent of standard risk factors.14 Importantly, the relationship between absolute CACS and the risk of ASCVD event was consistent across all different age, gender and ethnic subgroups. Overall, CACS = 0 was associated with 10-year event rates of 1.3% to 5.6%, while the event rates for those with CACS > 300 ranged from 13.1% to 25.6%. A doubling of the CACS at any level increased the probability of a coronary event by 25% in a 3.8-year follow-up period.20 Similar findings have been reported by several other population-based studies such as the HNR [Heinz Nixdorf Recall (Risk Factors, Evaluation of Coronary Calcium and Lifestyle)] study,15 Rotterdam study,16 Framingham Heart Study,17 etc. Furthermore, the excellent predictive accuracy of CACS has been demonstrated even in a relatively younger population in the CARDIA (Coronary Artery Risk Development in Young Adults) study.18 Even at a mean age of approximately 40.3 years, 10.2% individuals in this study had detectable CAC, with a mean score of 21.6. After adjusting for demographics, risk factors, and treatments, those with any CAC experienced a 5-fold increase in coronary heart disease (CHD) events and a 3-fold increase in CVD events, after a follow-up of 12.5 years. A CACS of >100 was associated with an absolute mortality rate of 22.4% over a follow-up of 12.5 years in this young population.
Recently, the MESA investigators have developed a risk score that incorporates CACS for prediction of CHD events. This risk score has been externally validated in the HNR and the Dallas Heart Study populations.21 A combined analysis of all three cohorts including 11,000 prospectively selected subjects with a 10-year follow-up, showed that addition of CACS to risk models significantly improved risk prediction compared to conventional risk factors.
1.2. The power of ‘zero’
An impressive finding from the studies evaluating predictive value of CACS is that the absence of any measurable CAC (i.e. CACS = 0) is associated with a very low risk of ASCVD events during follow-up.22, 23, 24, 25 A recent, comprehensive meta-analysis involving 13 studies examined the prognostic significance of CACS = 0. Overall, 29,312 individuals were found to have zero calcium score. The event rate in these individuals was just 0.47% over a mean follow-up of 50 months with a relative risk of 0.15 in comparison to CACS > 0, implying an 85% lower risk for individuals with zero calcium score.23 Furthermore, the absence of any measurable CAC has been demonstrated to have an overriding protective effect regardless of the underlying risk factor burden. In a large review of 44,052 patients referred for calcium scoring, individuals without any clinical risk factors but a CACS ≥ 400 experienced a significantly higher event rate (mortality rate 16.9 per 1000 person-years) than subjects with ≥3 risk factors but a CACS of 0 (mortality rate 2.7 per 1000 person-years).24 It has been suggested that among low- to intermediate-risk subjects, a CACS = 0 provides an almost 15-years ‘warranty’ (i.e. annual mortality rate <1%).25 Even among high-risk subjects, a CACS = 0 indicates relative safety from death, though the warranty period is shorter (roughly 5 years). However, it should be noted that the strong relationship between zero calcium score and very low CV event rate is not applicable to symptomatic individuals26 who are known to have soft coronary plaques without any calcium deposits.27,28 Similarly, the negative predictive value of zero CACS is also suboptimal in young individuals (<50 years of age) in whom it should be viewed with caution.
1.3. CACS for guiding therapies
Although no randomized trial has been conducted so far to assess utility of CACS for guiding preventive therapies, several exploratory analyses have been performed which have provided valuable insights in to this aspect.29,30
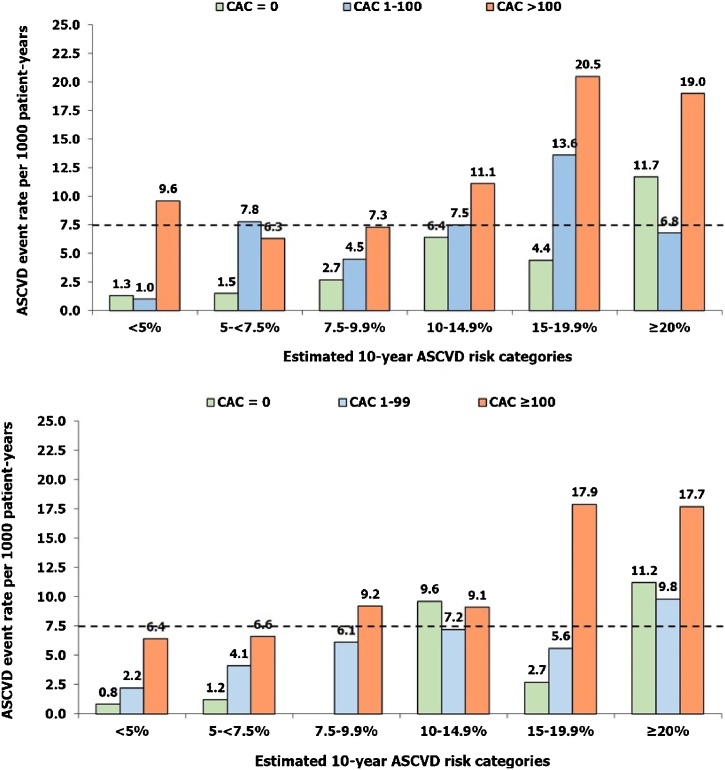
Nasir et al applied the 2013 American Heart Association/American College of Cardiology (AHA/ACC) guidelines for statin prescription to MESA participants.29 Among subjects with estimated 10-year ASCVD risk of 5–7.5% and 7.5–20%, a CACS = 0 was associated with 10-year event rates (∼1.5% and ∼4.5%, respectively) much below the threshold for statin prescription (>7.5%) (Fig. 1). Conversely, any CACS > 0 was associated with event rates higher than the accepted threshold for statin benefit in both these subgroups. Overall, the absence of CACS reclassified approximately one-half of ‘statin-eligible’ individuals as not eligible for statin therapy in this study. However, no incremental benefit of CACS was observed in individuals with estimated 10-year risk >20%. On the similar lines, Mahabadi et al applied the 2012 European Society of Cardiology (ESC) and the 2013 AHA/ACC guidelines for statin prescription to 3745 subjects included in the HNR study who had no CVD or lipid-lowering therapy at baseline.30 The study found that the two guidelines led to markedly different recommendations regarding statin therapy in this cohort. But regardless of the guideline applied, the addition of CACS (0 versus >100) significantly improved stratification of subjects into high versus low risk in both statin-eligible and statin-ineligible populations. In yet another analysis from the MESA, the event rates were correlated with CACS among patients who met inclusion criteria for the JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) study.31 Of these “statin eligible” subjects, 47% had CAC = 0, whereas 25% had CACS > 100. It was estimated that the 5-year number needed to treat to prevent 1 CV event was 124 for subjects with CACS = 0 but only 19 for those with CACS > 100. Collectively, these analyses strongly support the utility of CACS for guiding statin therapy in primary prevention setting, unless the patients are already at high-risk based on the presence of multiple CV risk factors.
Fig. 1.
Atherosclerotic cardiovascular disease (ASCVD) event rates observed in the study participants categorized according to the estimated 10-years ASCVD event rates (as estimated by the Pooled cohort equation) and the coronary artery calcium (CAC). Upper panel- Data from the MESA (Multi-Ethnic Study of Atherosclerosis) study (Nasir K, et al J Am Coll Cardiol. 2015;66:1657-68); Lower panel- HNR [Heinz Nixdorf Recall (Risk Factors, Evaluation of Coronary Calcium and Lifestyle)] study (Mahabadi AA, et al JACC Cardiovasc Imaging. 2017;10:143-153).
The dotted horizontal line represents estimated 10-year ASCVD risk of >7.5%, which is the threshold recommended for statin initiation by the 2013 American Heart Association/American College of Cardiology guidelines. In almost all the risk categories except >20% risk, the observed event rates among participants with CAC = 0 were much lower than this threshold.
CACS can also help in guiding aspirin use among patients without established CVD. The recent evidence suggests that aspirin is associated with harm when used in primary prevention setting32,33 and is currently not recommended for this purpose.34 However, aspirin therapy has been shown to be beneficial among individuals with significantly increased CACS (>100).35 Such high CACS essentially provides an evidence of underlying ASCVD and thus transforms this in to a “secondary prevention setting”, warranting prophylactic aspirin use.36
1.4. CACS progression
It has been shown that CACS increases at a rate of approximately 20–25% per year, with almost 20% subjects with initially zero CACS developing CACS > 0 within 4–5 years.37,38 The rate of progression is greater in men and in older individuals. It has been shown that any increase in CACS over time is associated with an increased risk of myocardial infarctions (MI) and all-cause mortality.39, 40, 41 The lowest risk is seen in those who have ‘double-zero’, i.e. CACS = 0 at two scans performed approximately 5-years apart.
The implications of increase in CACS among patients who are on statin therapy are less clear. Statin therapy leads to plaque pacification and calcification,42 leading to the formation of so-called “tomb of atherosclerosis”. Thus, increase in CACS among patients on statin therapy cannot be considered as a marker of increased ASCVD risk.
1.5. CACS for improving physician and patient behavior
By providing an objective evidence of ongoing coronary atherosclerosis, CACS can favorably influence patient- and physician-behavior towards greater adoption of risk reduction approaches. Gupta et al performed a meta-analysis of 6 studies involving 11,256 participants with a mean follow-up duration of 1.6–6.0 years.43 The meta-analysis found that the odds of aspirin initiation, lipid-lowering medication initiation and continuation, blood pressure–lowering medication initiation, and adoption of exercise and dietary change were significantly higher in individuals with non-zero CACS versus zero CACS. These findings remained significant even after adjustment for baseline patient characteristics and CV risk factors.
1.6. Clinical implications of CACS
The available evidence strongly suggests that given the imperfections inherent in conventional risk factors-based risk algorithms, CACS provides a robust alternate modality for more accurate risk prediction. The net reclassification ability of CACS is highest among intermediate risk individuals (10-year ASCVD risk 5–20%). In such individuals, the absence of CAC can be used to downgrade risk category and may also be used to avoid statin therapy (caution needed though). On the contrary, CACS >100 in these subjects would strongly argue in favor of aggressive risk factor modification and high-dose statin therapy. Notably, the use of CACS for CVD risk assessment does not increase downstream medical costs because any increase in further testing among those with high CACS is more than offset by lower resource utilization among those with normal CAC scans.44,45
CACS can be of particular value among Indians for a variety of reasons. Currently there is no validated ASCVD risk algorithm available for Indians, though previous studies have explored applicability of existing western algorithms for Indians.46, 47, 48 Moreover, as ASCVD in Indians tends to occur at a younger age than the western populations and since age is a strong determinant of 10-year ASCVD risk in all the currently available risk algorithms, the actual risk is often underestimated among Indians.49 CACS overcomes both these challenges. It has been shown that even though the absolute value of CACS varies with age, gender and ethnicity, its relationship with the future ASCVD risk does not change.14 Thus, it may be justifiable to directly extrapolate data regarding CACS to Indian population, though a formal validation is still warranted. At the same time, the logistic feasibility and safety of CACS estimation are also well-established. The modern CT scanners can perform CACS estimation with just 10–15 min of total room time, at about 1 mSv of radiation (almost equivalent to a mammogram), at a reasonable cost and without the need for contrast agents.12 The efficiency of CACS-based risk stratification can be further enhanced by limiting it to the subjects with multiple risk factors as shown in the CARDIA study.18 It was observed in the CARDIA study that among young individuals, the presence of conventional CV risk factors could help identify those individuals who were likely to have measurable CAC. Collectively, these evidences suggest that it may be reasonable to use CACS for CV risk stratification in Indian subjects (even as young as 30–35 years of age) who have risk factors for CVD but are at low 10-year risk of CV events as per the conventional algorithms. The presence of any CAC in such individuals should lead to aggressive risk factor modification with possibly statin therapy (certainly if CACS >100). If CACS is zero, then the scan may be repeated after a gap of 5 years to ensure that there is no progression to CACS > 0.
2. Carotid ultrasound imaging
Carotid ultrasound imaging for assessment of CIMT and carotid plaques has been another commonly used modality for assessment of subclinical atherosclerosis. Several studies have shown that increased CIMT is significantly associated with an increased risk of vascular events.50, 51, 52, 53, 54, 55 A number of studies have been performed among Indians subjects also, even describing normal reference ranges.56, 57, 58, 59 However, the incremental value of CIMT over conventional CV risk factors has been rather inconsistent. This inconsistency can be attributed to the differences in the CIMT methodology used in various studies- e.g. carotid segments [common carotid artery (CCA), bulb and/or internal carotid artery (ICA)] included in CIMT measurement, near wall or far wall, single versus multiple measurements, imaging in only single plane or from multiple angles, mean value or maximum value, plaques included or not included, etc.60 Of these different factors, the carotid segment used for imaging seems to be the most relevant for determining predictive accuracy of CIMT. The current guidelines recommend measurement of CIMT at the far wall of the CCA owing to its ease of measurement and greater reproducibility.61 However, it is now well understood that CCA-IMT alone correlates mainly with the stroke risk but is not a good predictor of CHD events or overall CVD risk. A meta-analysis that included major clinical studies with CIMT assessment of single or multiple carotid segments showed that for every 0.1-mm increase in CIMT, the future risk of MI increased by 10% to 15%.62 However, another meta-analysis that evaluated only CCA-IMT in 45,828 patients from 14 population-based studies showed that the addition of CIMT to conventional risk factors could reclassify only 0.8% subjects in the overall cohort and 3.6% among those at intermediate risk.63
The major reason for these differences is that unlike CCA, carotid bulb and ICA have greater predilection for development of atherosclerotic changes and atherosclerotic plaques. For these reasons, ICA and bulb IMT correlate better with ASCVD risk factors, overall atherosclerotic burden and the future risk of ASCVD events.64,65 Several studies have also shown consistently that plaques have greater predictive accuracy for ASCVD risk (esp. CHD events) than CIMT and the greater accuracy of ICA and bulb CIMT is likely to reflect the inclusion of plaques in the measurement of CIMT of these segments.60 Unfortunately, measurement of bulb and ICA IMT is technically more challenging and less reproducible than CCA-IMT.
As mentioned above, carotid plaques have greater predictive accuracy for ASCVD risk than CIMT.65 The presence of lumen encroaching but hemodynamically non-obstructive plaques is associated with a high risk of CV events that is comparable to the risk seen with CACS > 100.66 The more recent studies have also shown that in comparison to qualitative assessment of plaque (as present or absent), quantitative assessment in the form of total plaque area and volume is even more sensitive and accurate for prediction of ASCVD risk.60,67 Thus, comprehensive assessment of carotid plaque burden may emerge as a useful tool for ASCVD risk prediction. Compared with CACS, carotid ultrasound has the advantages of being less expensive, more widely available and radiation-free. However, further improvements in ultrasound technology to allow automated, reproducible quantitative assessment of plaque burden and standardization of assessment protocol are required before it can be used more routinely. In the meantime, routine use of CIMT assessment is not recommended by the current guidelines (class III recommendation).34 Nonetheless, when available, the presence of CCA-IMT >75% percentile for age, gender and ethnic group or the presence of a carotid plaque should lead to aggressive risk factor modification in an individual who is otherwise at a low risk for CV events.
3. Other modalities
A number of studies have also employed brachial artery flow-mediated vasodilatation as a measure of endothelial function and a marker of subclinical atherosclerosis.68, 69, 70 Although conceptually sound, the technique suffers from inherent susceptibility of endothelial function to physiological stimuli and technological challenges in measuring brachial artery diameter reproducibly.
Aortic PWV is a measure of arterial stiffness and has been shown to predict CV risk in many studies.71, 72, 73, 74 However, as arterial stiffness is closely linked with blood pressure, the utility of arterial stiffness assessment is confined mainly to evaluation and management of hypertensive subjects.75
Finally, ABI is a non-invasive tool to detect hemodynamically significant occlusive disease in the lower limb arteries. ABI < 0.9 is a reliable marker of peripheral artery disease and is present in 12–27% of the asymptomatic individuals above 55 years of age.76,77 However, its potential to reclassify patients into different ASCVD risk categories remains controversial.34,78
4. Conclusions
An ideal tool for subclinical atherosclerosis assessment that has excellent predictive accuracy, good reclassification ability, is reproducible, not time-consuming and is relatively safe has been elusive so far. However, the current evidence regarding CACS suggests that it could be a close contender. Robust data now exists to support the utility of CACS for guiding preventive therapy among individuals who are candidates for primary prevention of ASCVD, esp. those at intermediate ASCVD risk. CACS may be particularly useful among Indians for whom no prospectively validated clinical risk algorithm currently exists. However, the routine use of other atherosclerosis imaging modalities for ASCVD risk prediction is not recommended at present; though, with further refinements in ultrasound imaging, quantitative carotid plaque imaging may emerge as a potential tool for this purpose.
Conflict of interest
No conflict of interest to declare for any of the authors
References
- 1.Wilson P.W., D’Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 2.D’Agostino R.B., Sr, Vasan R.S., Pencina M.J. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 3.Goff D.C., Jr, Lloyd-Jones D.M., Bennett G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2013 doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hippisley-Cox J., Coupland C., Vinogradova Y. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of qrisk2. BMJ. 2008;336:1475–1482. doi: 10.1136/bmj.39609.449676.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rumberger J.A., Simons D.B., Fitzpatrick L.A., Sheedy P.F., Schwartz R.S. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 6.Pignoli P., Tremoli E., Poli A., Oreste P., Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 7.Persson J., Formgren J., Israelsson B., Berglund G. Ultrasound-determined intima-media thickness and atherosclerosis. Direct and indirect validation. Arterioscler Thromb. 1994;14:261–264. doi: 10.1161/01.atv.14.2.261. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann T., Sengupta S., Bhrushundi M.P., Kulkarni H., Sengupta P.P., Fergus I. HIV related stigma, perceived social support and risk of premature atherosclerosis in South Asians. Indian Heart J. 2018;70:630–636. doi: 10.1016/j.ihj.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saedi S., Ghadrdoost B., Pouraliakbar H., Zahedmehr A., Jebelli A. The association between increased carotid intima–media thickness and SYNTAX score in coronary artery disease: a single center study. Indian Heart J. 2018;70:627–629. doi: 10.1016/j.ihj.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sancheti S., Shah P., Phalgune D.S. Correlation of endothelial dysfunction measured by flow-mediated vasodilatation to severity of coronary artery disease. Indian Heart J. 2018;70:622–626. doi: 10.1016/j.ihj.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma K.H., Sharma N., Shah K., Patil S. Impact of coronary artery disease on augmentation index as measured by estimated central blood pressure: a case control study in asian indians. Indian Heart J. 2018;70:615–621. doi: 10.1016/j.ihj.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenland P., Blaha M.J., Budoff M.J., Erbel R., Watson K.E. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangiorgi G., Rumberger J.A., Severson A. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 14.Budoff M.J., Young R., Burke G. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ascvd) events: the multi-ethnic study of atherosclerosis (mesa) Eur Heart J. 2018;39:2401–2408. doi: 10.1093/eurheartj/ehy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erbel R., Mohlenkamp S., Moebus S. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf recall study. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 16.Vliegenthart R., Oudkerk M., Hofman A. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 17.Ferencik M., Pencina K.M., Liu T. Coronary artery calcium distribution is an independent predictor of incident major coronary heart disease events: results from the Framingham heart study. Circ Cardiovasc Imaging. 2017:10. doi: 10.1161/CIRCIMAGING.117.006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr J.J., Jacobs D.R., Jr, Terry J.G. Association of coronary artery calcium in adults aged 32–46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–399. doi: 10.1001/jamacardio.2016.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson J.E., Allison M.A., Carr J.J. Calcium/vitamin d supplementation and coronary artery calcification in the women’s health initiative. Menopause. 2010;17:683–691. doi: 10.1097/gme.0b013e3181d683b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detrano R., Guerci A.D., Carr J.J. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 21.McClelland R.L., Jorgensen N.W., Budoff M. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the mesa (multi-ethnic study of atherosclerosis) with validation in the hnr (heinz nixdorf recall) study and the dhs (dallas heart study) J Am Coll Cardiol. 2015;66:1643–1653. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaha M.J., Cainzos-Achirica M., Greenland P. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the multi-ethnic study of atherosclerosis (mesa) Circulation. 2016;133:849–858. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarwar A., Shaw L.J., Shapiro M.D. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Nasir K., Rubin J., Blaha M.J. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5:467–473. doi: 10.1161/CIRCIMAGING.111.964528. [DOI] [PubMed] [Google Scholar]
- 25.Valenti V., Ó Hartaigh B., Heo R. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900–909. doi: 10.1016/j.jcmg.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaha M.J., Blumenthal R.S., Budoff M.J., Nasir K. Understanding the utility of zero coronary calcium as a prognostic test: a bayesian approach. Circ Cardiovasc Qual Outcomes. 2011;4:253–256. doi: 10.1161/CIRCOUTCOMES.110.958496. [DOI] [PubMed] [Google Scholar]
- 27.Knez A., Becker A., Leber A. Relation of coronary calcium scores by electron beam tomography to obstructive disease in 2,115 symptomatic patients. Am J Cardiol. 2004;93:1150–1152. doi: 10.1016/j.amjcard.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 28.Chang S.M., Nabi F., Xu J. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol. 2009;54:1872–1882. doi: 10.1016/j.jacc.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 29.Nasir K., Bittencourt M.S., Blaha M.J. Implications of coronary artery calcium testing among statin candidates according to American college of cardiology/American heart association cholesterol management guidelines: Mesa (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2015;66:1657–1668. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 30.Mahabadi A.A., Mohlenkamp S., Lehmann N. Cac score improves coronary and cv risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. JACC Cardiovasc Imaging. 2017;10:143–153. doi: 10.1016/j.jcmg.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Blaha M.J., Budoff M.J., DeFilippis A.P. Associations between c-reactive protein, coronary artery calcium, and cardiovascular events: implications for the jupiter population from mesa, a population-based cohort study. Lancet. 2011;378:684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Group A.S.C. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018 doi: 10.1056/NEJMoa1804988. [DOI] [PubMed] [Google Scholar]
- 33.McNeil J.J., Nelson M.R., Woods R.L. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018 doi: 10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piepoli M.F., Hoes A.W., Agewall S. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miedema M.D., Duprez D.A., Misialek J.R. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–460. doi: 10.1161/CIRCOUTCOMES.113.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hecht H., Blaha M.J., Berman D.S. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr. 2017;11:157–168. doi: 10.1016/j.jcct.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Kronmal R.A., McClelland R.L., Detrano R. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the multi-ethnic study of atherosclerosis (mesa) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 38.Erbel R., Lehmann N., Churzidse S. Progression of coronary artery calcification seems to be inevitable, but predictable—results of the Heinz Nixdorf recall (hnr) study. Eur Heart J. 2014;35:2960–2971. doi: 10.1093/eurheartj/ehu288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budoff M.J., Hokanson J.E., Nasir K. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Budoff M.J., Young R., Lopez V.A. Progression of coronary calcium and incident coronary heart disease events: Mesa (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehmann N., Erbel R., Mahabadi A.A. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the hnr study (Heinz Nixdorf recall) Circulation. 2018;137:665–679. doi: 10.1161/CIRCULATIONAHA.116.027034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puri R., Nicholls S.J., Shao M. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 43.Gupta A., Lau E., Varshney R. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacological and lifestyle preventive therapies: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2017;10:833–842. doi: 10.1016/j.jcmg.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rozanski A., Gransar H., Shaw L.J. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the eisner (early identification of subclinical atherosclerosis by noninvasive imaging research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong J.C., Blankstein R., Shaw L.J. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA cholesterol management guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10:938–952. doi: 10.1016/j.jcmg.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Bansal M., Kasliwal R.R., Trehan N. Comparative accuracy of different risk scores in assessing cardiovascular risk in Indians: a study in patients with first myocardial infarction. Indian Heart J. 2014;66:580–586. doi: 10.1016/j.ihj.2014.10.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bansal M., Kasliwal R.R., Trehan N. Relationship between different cardiovascular risk scores and measures of subclinical atherosclerosis in an Indian population. Indian Heart J. 2015;67:332–340. doi: 10.1016/j.ihj.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garg N., Muduli S.K., Kapoor A. Comparison of different cardiovascular risk score calculators for cardiovascular risk prediction and guideline recommended statin uses. Indian Heart J. 2017;69:458–463. doi: 10.1016/j.ihj.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bansal M., Shrivastava S., Mehrotra R., Agarwal V., Kasliwal R.R. Low framingham risk score despite high prevalence of metabolic syndrome in asymptomatic north-Indian population. J Assoc Physicians India. 2009;57:17–22. [PubMed] [Google Scholar]
- 50.Chambless L.E., Heiss G., Folsom A.R. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the atherosclerosis risk in communities (aric) study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 51.O’Leary D.H., Polak J.F., Kronmal R.A., Manolio T.A., Burke G.L., Wolfson S.K., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 52.Chambless L.E., Folsom A.R., Clegg L.X. Carotid wall thickness is predictive of incident clinical stroke: the atherosclerosis risk in communities (aric) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 53.Lorenz M.W., von Kegler S., Steinmetz H., Markus H.S., Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the carotid atherosclerosis progression study (caps) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 54.van der Meer I.M., Bots M.L., Hofman A. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam study. Circulation. 2004;109:1089–1094. doi: 10.1161/01.CIR.0000120708.59903.1B. [DOI] [PubMed] [Google Scholar]
- 55.Yeboah J., McClelland R.L., Polonsky T.S. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansa G., Bhargava K., Bansal M., Tandon S., Kasliwal R.R. Carotid intima-media thickness and coronary artery disease: an Indian perspective. Asian Cardiovasc Thorac Ann. 2003;11:217–221. doi: 10.1177/021849230301100308. [DOI] [PubMed] [Google Scholar]
- 57.Kasliwal R.R., Bansal M., Gupta H., Agrawal S. Association of carotid intima-media thickness with left main coronary artery disease. Indian Heart J. 2007;59:50–55. [PubMed] [Google Scholar]
- 58.Jadhav U.M., Kadam N.N. Association of microalbuminuria with carotid intima-media thickness and coronary artery disease—a cross-sectional study in western india. J Assoc Phys India. 2002;50:1124–1129. [PubMed] [Google Scholar]
- 59.Kasliwal R.R., Bansal M., Desai N. A study to derive distribution of carotid intima media thickness and to determine its correlation with cardiovascular risk factors in asymptomatic nationwide Indian population (score-india) Indian Heart J. 2016;68:821–827. doi: 10.1016/j.ihj.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naqvi T.Z., Lee M.S. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–1038. doi: 10.1016/j.jcmg.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 61.Stein J.H., Korcarz C.E., Hurst R.T. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American society of echocardiography carotid intima-media thickness task force endorsed by the society for vascular medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Lorenz M.W., Markus H.S., Bots M.L., Rosvall M., Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 63.Den Ruijter H.M., Peters S.A., Anderson T.J. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 64.Ebrahim S., Papacosta O., Whincup P. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British regional heart study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 65.Inaba Y., Chen J.A., Bergmann S.R. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 66.Belcaro G., Nicolaides A.N., Ramaswami G. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the cafes-cave study(1)) Atherosclerosis. 2001;156:379–387. doi: 10.1016/s0021-9150(00)00665-1. [DOI] [PubMed] [Google Scholar]
- 67.Mortensen M.B., Fuster V., Muntendam P. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the bioimage study. J Am Coll Cardiol. 2016;68:881–891. doi: 10.1016/j.jacc.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 68.Gokce N., Keaney J.F., Jr, Hunter L.M., Watkins M.T., Menzoian J.O., Vita J.A. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 69.Bhargava K., Hansa G., Bansal M., Tandon S., Kasliwal R.R. Endothelium-dependent brachial artery flow mediated vasodilatation in patients with diabetes mellitus with and without coronary artery disease. J Assoc Physicians India. 2003;51:355–358. [PubMed] [Google Scholar]
- 70.Patti G., Pasceri V., Melfi R. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–75. doi: 10.1161/01.CIR.0000151308.06673.D2. [DOI] [PubMed] [Google Scholar]
- 71.Boutouyrie P., Tropeano A.I., Asmar R. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 72.Laurent S., Boutouyrie P., Asmar R. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 73.Mattace-Raso F.U., van der Cammen T.J., Hofman A. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 74.Willum-Hansen T., Staessen J.A., Torp-Pedersen C. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 75.Williams B., Mancia G., Spiering W. 2018 ESC/esh guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 76.Hiatt W.R. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 77.McDermott M.M., Greenland P., Liu K. The ankle brachial index is associated with leg function and physical activity: the walking and leg circulation study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 78.Ankle Brachial Index C., Fowkes F.G., Murray G.D. Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]