ABSTRACT
Multiple sclerosis (MS) is an autoimmune neurodegenerative disease characterized by demyelination of the central nervous system (CNS). The exact cause of MS is still unknown; yet its incidence and prevalence rates are growing worldwide, making MS a significant public health challenge. The heterogeneous distribution of demyelination within and between MS patients translates in a complex and varied array of autonomic, motor, sensory and cognitive symptoms. Yet a unique aspect of MS is the highly prevalent (60–80%) temperature sensitivity of its sufferers, where neurological symptoms are temporarily exacerbated by environmental- or exercise-induced increases (or decreases) in body temperature. MS temperature sensitivity is primarily driven by temperature-dependent slowing or blocking of neural conduction within the CNS due to changes in internal (core) temperature; yet changes in skin temperature could also contribute to symptom exacerbation (e.g. during sunlight and warm ambient exposure). The impact of temperature sensitivity, and particularly of increases in core temperature, on autonomic (e.g. thermoregulatory/cardiovascular function) and motor symptoms (e.g. fatigue) is well described. However, less attention has been given to how increases (and decreases) in core and skin temperature affect sensory and cognitive symptoms. Furthermore, it remains uncertain whether changes in skin temperature alone could also trigger worsening of symptoms. Here we review the impact of temperature sensitivity on MS sensory and cognitive function and discuss additional factors (e.g. changes in skin temperature) that potentially contribute to temperature-induced worsening of symptoms in the absence of alteration in core temperature.
KEYWORDS: Multiple sclerosis, temperature sensitivity, demyelination, body temperature, skin, sensation, cognitive
Introduction
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system (CNS) that causes progressive disability in the majority of patients. Inflammatory demyelination, i.e. the partial or complete loss of the myelin sheath that envelops nerve fibres within the CNS, underlies the chronic neurodegeneration that characterizes MS and the plethora of clinical signs and symptoms that accompany the disease [1].
Variability in the location and degree of inflammatory demyelination within the CNS results in each patient experiencing a unique combination of symptoms. However, (temporary) symptom worsening following changes in body temperature (i.e. a combination of internal/core and external/skin temperatures), i.e. temperature sensitivity, is a common occurrence. As a result, MS patients experience significant challenges in maintaining appropriate physical activity levels and conducting normal working activities.
The impact of temperature sensitivity and particularly of increases in internal/core temperature, on autonomic (e.g. thermoregulatory/cardiovascular function) and motor symptoms (e.g. fatigue) is well described. However, less attention has been given to the mechanisms by which increases (and decreases) in core and skin temperature modulate sensory and cognitive symptoms in MS. Furthermore, it remains uncertain whether changes in skin temperature alone can trigger worsening of symptoms.
Here we review the impact of temperature sensitivity on MS sensory and cognitive function and discuss additional factors (e.g. changes in skin temperature) that potentially contribute to temperature-induced worsening of symptoms in the absence of alteration in core temperature.
Pathophysiology of MS
The prevailing hypothesis about the pathophysiology of MS contends that the myelin sheath of CNS axons is targeted, and ultimately destroyed, by a macrophage mediated, adaptive-immune cell driven autoimmune process. While the factors that initiate CNS autoimmunity are still unclear, the resulting inflammation and loss of myelin that characterize the acute MS lesion are known to occur through the operation of lymphocytes, macrophages, microglial cells and potentially astrocytes [2–7].
Myelin is necessary in facilitating fast conduction velocities of neural inputs within the CNS. Accordingly, inflammation of the CNS and the related lesions that occur in the white and grey matter across the CNS of MS patients, result in slowing and/or blocking of neural transmission within the CNS (Figure 1) [8,9].
Figure 1.
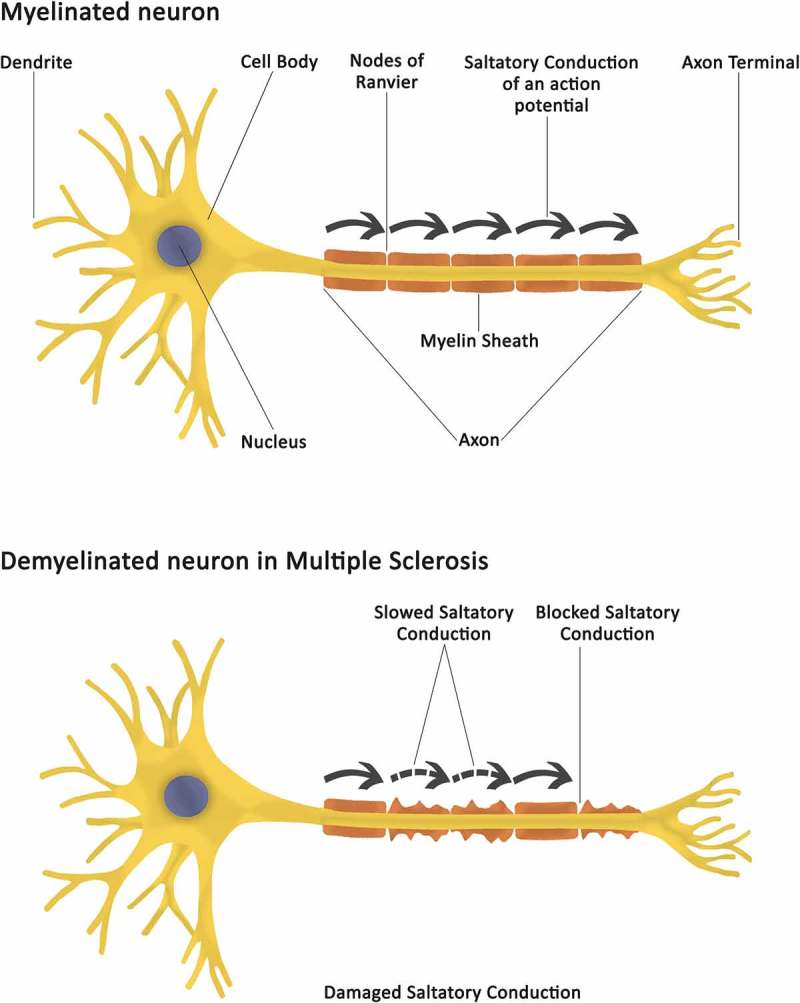
A schematic overview of a normally myelinated neuron and of a demyelinated neuron in MS. While in an unaffected myelinated neuron, saltatory conduction velocities of neural inputs are preserved along the axon, in a demyelinated neuron saltatory conduction between the Nodes of Ranvier is either slowed or blocked. Demyelination-induced conduction slowing/blocking, and related inability for neural signals to be appropriately exchanges between neurons, is at the root of MS-related signs and symptoms.
The appearance and location of new lesions in MS is unpredictable and vary within the CNS [10]. Brain lesions, as identified via magnetic resonance imaging (MRI), can occur at both cortical and subcortical sites. The periventricular region, cerebellum, brainstem, and optic pathways are frequently affected [11,12]. Hypothalamic lesions, which may be at the root of autonomic, endocrine and metabolic-related dysfunction observed in some patients with MS, are common [13]. While less prevalent than brain lesions, spinal lesions, particularly within the cervical cord, are also common [14]. Interestingly, re-myelination has been observed to occur in many MS lesions, yet this varies significantly between individuals [15] and is an inadequate or not a completely restorative process.
MS incidence and prevalence
Historically, clinical signs of MS were first observed in some patients in the early 19th century, when Robert Carswell and Jean Cruveilhier described the histopathology of the disease and the concept of neurodegeneration in the brain [16,17]. Later in the 19th century, Jean-Martin Charcot synthesized the clinical and neuropathology features of MS as a disease of the CNS. Thereafter, MS plaques and lesions were observed post mortem [18].
Signs and symptoms of MS may appear from childhood to late adulthood, with the average age for diagnosis being ~ 30 years old [12].
The estimated number of people with MS recorded in 2008 was reported to be about 2.1 million people worldwide. In 2013 this number had increased to 2.3 million [19]. Incidence rates vary widely, depending on geographic location and other factors, and fluctuate between 5 and 12 new cases per 100,000 people every year [20]. The disease has been shown to be more prevalent in females than in males [20]. Geographically, MS is generally reported to be less prevalent in areas close to the equator [21].. In relation to ethnic variability, Caucasians have a higher probability of being diagnosed with MS in comparison to other individuals [21].
MS can be classified into three types based on its clinical course: relapsing-remitting (RRMS) (85%) [18]; secondary-progressive (SPMS) [19]; primary-progressive (PPMS) [20–28] [Table 1]. RRMS is the most prevalent MS subtype and more common in females with younger disease onset [20]. SPMS is characterized by gradual deterioration of clinical symptoms that take place between or irrespective of relapses [24]. PPMS patients encounter disability without experiencing relapses or remissions. Despite a paucity of new lesions relative to other disease subtypes, persistently accelerated brain atrophy is typical [24].
Table 1.
MS subtypes, prevalence rates and characteristics.
| MS subtypes | Prevalence in MS population | Characteristics |
|---|---|---|
| Relapsing-Remitting (RRMS) | 85% | Unpredictable relapses, where new symptoms or the worsening of previous symptoms occur, followed by partial or complete recovery. The duration of the relapsing episodes can last from days to months. Incomplete recovery from relapse may result in residual, persistent disability. |
| Secondary Progressive (SPMS) | 90% of RRMS cases progress to SPMS | Gradual deterioration of clinical symptoms that take place between or irrespective of relapses. Most people with MS are initially diagnosed with RRMS, with a significant proportion transitioning to SPMS some 10–15 years later. MS patients with SPMS progress to a disability level that is manifested with relapse episodes of the MS symptoms. |
| Primary progressive (PPMS) | 10–15% | Encounter disability without experiencing relapses or remissions. |
Symptomatology of MS
Symptomatic axonal dysfunction occurs in eloquently distributed MS lesions, with individual clinical variations primarily reflecting the heterogeneity of lesion location, particularly in patients with early, relapsing disease. It is therefore common for patients to perceive their experience of MS and related symptomatology as “unique” when compared to other patients. Despite this variability, many MS patients experience an array of common symptoms [1,29].
Overall, these commonly shared symptoms can be categorized as motor, sensory, and cognitive. Autonomic nervous system dysfunction is also reported in MS patients and has been extensively reviewed elsewhere [1,30,31].
Motor symptoms
Motor deficits are frequently reported in MS (Figure 2). Spasticity (where disinhibited physiological reflexes in the spinal cord can produce involuntary, stiff and painful muscle movements in the extremities) occurs in 80% of the patients [32,33]. Similarly, 75% of the patients report walking difficulties and 50% accidental injuries due to frequent falls [32]. In turn, those symptoms result in many MS patients (i.e. ~ 50%) ultimately requiring walking aids or wheelchairs [34]. Other motor symptoms include: upper limb weakness, balance impairment, discoordination, difficulty swallowing, poor articulation, grip impairment, and tremor in the extremities [22,35–41].
Figure 2.
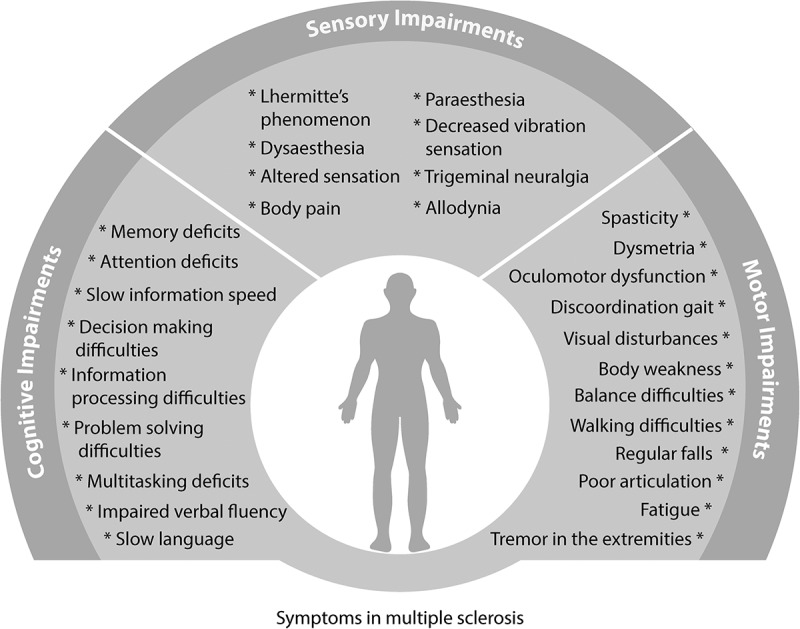
Summary of the cognitive, sensory and motor symptoms that have been recorded to occur during the course of MS. Cognitive impairments in MS affect executive functions, such as deficits in information processing, memory and language. Sensory symptoms constitute an uncomfortable subjective experience for the patient when stimuli touch their skin. Altered sensations are perceived, such as numbness, burning, pain and pins and needles sensation (allodynia, paraesthesia, dysesthesia, trigeminal neuralgia); electrical sensation that moves along the spinal cord (Lhermitte’s sign) and decreased vibration experience with the touch of a vibrating object on the skin. Motor symptoms include discoordination, tremor and ataxia in the limbs which subsequently result in regular falls and fatigue; visual impairments and articulation deficits.
Fatigue is a prevalent symptom in people with MS. 53 to 97% of MS patients report fatigue [34,42,43] and 69% report that this is the MS symptom that causes them the most distress [44]. Even though the experience of fatigue in MS is subjective and can occur in early disease patients, motor symptoms such as body weakness, incoordination, tremor in the extremities and exhaustion seem to play an essential role in its occurrence [45]. Based on the symptomatology, it is suggested that fatigue in MS is the outcome of excess axonal activity in the demyelinated CNS [46,47]. Notwithstanding, MS fatigue is a multifactorial phenomenon, and its subjective character in self-reported questionnaires is difficult to evaluate.
MS sufferers may also suffer from a cerebellar sign called dysmetria, frequently accompanied by ataxia, tremor and uncoordinated movements in the extremities. Lesions in the cerebellum play an essential part in the manifestation of symptoms, especially for limb coordination and motor performance [48].
Finally, eye movement disorders, such as internuclear ophthalmoplegia resulting from lesions in the medial longitudinal fasciculus in the brainstem [49,50] and nystagmus [49,51], are frequent in MS [34]. Poor visual acuity due to optic neuritis, and double vision due to brainstem lesions [52], also impact patients’ movement and balance [52].
It is important to note that while the majority of motor symptoms arise as a direct consequence of the disease, patients may also experience motor disturbances as side effects from medications such as corticosteroids (used to treat relapse symptoms) and anticholinergics (used to assist with urinary urgency and incontinence). These drug categories can cause fatigue, difficulty in breathing and eye disorders [53]; and are reported to affect pharyngeal sensations in patients that can lead to difficulties in swallowing when eating (dysphagia) [54]
Sensory symptoms
Sensory abnormalities and pain are observed in 80% of patients with MS, with significant consequences for quality of life (Figure 2) [55,56]. Like most symptoms, sensory deficits seem to correlate with the number and location of the lesions in the CNS [57–60].
Central or musculoskeletal pain is reported by 90% of patients during MS progression and is associated with plaques and lesions along the spinothalamic pathways [61]. Approximately 50% of MS patients experience pain and altered sensations such as tingling and numbness with higher incidence in their extremities, back and head (~ 65%) [61].
Allodynia and paraesthesia are also often reported sensory impairments in MS. Approximately 8% of MS patients experience mechanical allodynia, a painful sensation that comes with the light touch of a stimulus on the skin; ~ 35% of the MS patients experience cold allodynia when a cold stimulus touches the skin [61]. 40–80% of the patients claim that paraesthesia symptoms (tingling) affect their daily life, especially when they become persistent [56,62]. Furthermore, some people with MS may experience dysaesthesia and Lhermitte’s phenomenon as a result of spinal cord demyelination [63,64]. Dysaesthesia is manifested as an intense pain of pins and needles and burning sensation, usually present in the extremities [58,65]. Lhermitte’s sign is an electrical shock sensation occurring with neck flexion in 55–70% of patients with MS, often extending from the neck to the spine and in some patients involving the limbs [66,67].
Patients with MS can also experience trigeminal neuralgia, which manifests as sudden episodes of pain, burning sensation, itching in the areas covered by the trigeminal nerve on one side of the face [68,69]. This happens because of the damaged myelin around the trigeminal nerve nucleus and can result in reduced eating and speaking during these painful episodes [69]. Finally, vibration sensation is also affected in people with MS. Decreased vibration sensitivity was reported in the lower limbs in ~ 40% of the patients, while in the upper limbs this prevalence increases to ~ 60% [70].
Certain sensory symptoms can also arise secondary to the use of medications used in the management of MS [54]. For example, the disease modifying therapy interferon beta-1a may cause muscle ache, diffuse body pain, worsening spasticity and paraesthesiae that resolve with cessation of the drug [53].
Cognitive symptoms
Cognitive deficits are reported by 40–70% of MS sufferers, with a high incidence in the early stages of the disease (Figure 2) [71]. Cognitive impairments seem to be progressive in nature increasing with the level of disability in MS [72,73].
Evidence indicates that executive functions (attention, memory and information processing) are impaired in MS, with patients experiencing challenges to the performance of activities of daily living as a result [74,75]. Cognitive dysfunction in MS manifests itself in deficits in working memory (i.e. short term memory) [76], verbal (use of language) and nonverbal memory (e.g. visual content, auditory information) [77–79], (visuo-) spatial memory (i.e. the ability to navigate in one’s environment based on location of objects and oneself) [74,80], in recall and recognition (i.e. retrieve and identify information from past memories) [77,78] and in the ability to retain old memories [81,82]. Furthermore, deficits in attention [79,80], information processing, decision making and problem solving [81,83,84] especially during multitasking activities [85], seem to be frequent. Finally, language and verbal fluency are often impaired in MS [75] and these defects manifest in slow speech capacity and slow information processing speed [74].
Cognitive impairments in MS are extensively associated with lesions and atrophy in the brain [86,87] and especially with neocortical grey matter loss [88] and with white matter lesions in the corpus callosum that connects the two cerebral hemispheres together [89]. White matter lesions associated with cognitive impairment in MS are found in cerebellar peduncle, corona radiate, optic radiation, superior longitudinal fasciculus, anterior limb of internal capsule and cingulate [90]. Decreased grey matter has been shown for bilateral caudate, left insula and right temporal lobe and was related to cognitive deterioration [89].
It should be acknowledged that certain aspects of the cognitive symptomatology might be also secondary to sleep disorders associated with MS [91]. Abnormal sleep patterns in MS are common (prevalence: ~ 60% of patients) [91] and can be triggered by sleeping problems such as sleep apnea, chronic insomnia disorders and restless leg syndrome [92]. As well as contributing to fatigue in MS, sleep disturbances appear to negatively affect cognitive functions such concentration, memory, attention and information processing [91,92].
Finally, MS medications may also affect patients’ cognitive abilities and thus consideration should be given to potential disease-independent changes in cognitive performance when administering neuropsychological testing to this patient population. For example, anticholinergic drugs may cause cognitive deterioration in attention, processing speed, and verbal memory [93].
Temperature sensitivity in MS
A unique aspect of MS symptomatology is the highly prevalent temperature sensitivity of its sufferers, where neurological symptoms are temporarily exacerbated by environmental- or exercise-induced increases (or decreases) in body (core and skin) temperature.
Heat sensitivity or Uhthoff’s phenomenon occurs in 60–80% of MS patients [1], where increases in core body temperature as little as ~ 0.5°C can trigger temporary symptoms worsening. This phenomenon is generally triggered by exposure to warm environment, hot baths, or exercise and lasts until core temperature returns to baseline values (36.5 to 37°C) [94,95]. However, decreases in body (core and skin) temperature resulting from cold baths or exposure to cold ambient temperatures can also trigger a worsening of clinical symptoms [96].
Temperature sensitivity episodes are also known as pseudo-exacerbations or pseudo-relapses. While the increase in symptoms may be similar in nature to those experienced during an exacerbation or relapse, the worsening of symptoms is not associated with the disease’s active progression and is temporary in nature, with symptoms subsiding upon restoration of core temperature. MS patients therefore experience significant challenges in maintaining appropriate physical activity levels [97] and conducting normal working activities. Early retirement due to heat intolerance and fatigue is highly prevalent amongst MS patients [98]. Despite being a frequent complaint in patients with MS, our understanding of how increases or decreases in body (core and skin) temperature contribute to transient worsening of MS symptoms is incomplete.
Mechanisms of heat sensitivity in MS
The manifestation of temperature-induced pseudo-exacerbations in MS seems to be primarily driven by a transient slowing or blocking of neural conduction within CNS nerve fibres, due to core temperature-induced changes in the excitability of demyelinated axons [1,99]. The degree of demyelination and the direction (i.e. increases vs. decreases) and magnitude of changes in body temperature both contribute to symptoms worsening. As a result, MS patients might experience symptoms worsening due to heat or cold sensitivity.
The effects of increases in core temperature on conduction block have been investigated in demyelinated nerve fibres using animal models [57]. For example, Rasminsky (1973) [100] investigated the activities between internodes in a demyelinated rat ventral root nerve fibre which would fire slower action potentials across the axon in comparison to a myelinated nerve fibre. Conduction within the demyelinated nerve fibre was blocked when fibre temperature was raised to and beyond 36.5°C. On the contrary conduction was (partially) re-established when fibre’s temperature was lowered below 36.5°C. In highlighting conduction slowing in demyelinated fibres exposed to increased temperatures, this study provided insights on the potential roots of the role of changes in core temperature in the symptoms worsening observed in MS patients experiencing heat sensitivity.
Mechanistically, it would appear that demyelination reduces the axon safety factors, such that less current is available to excite nodes of Ranvier and to propagate action potentials effectively along the axon (Figure 1) [1]. Furthermore, there is evidence that segmental axonal demyelination in MS unmasks an increased density of membrane potassium channels with a high predilection for current leak via potassium efflux with new sodium channels also being inserted within the axonal membrane as an ion channel adaptation to demyelination [101]. Interestingly, the newly incorporated sodium channels exhibit an altered high sensitivity to temperature-induced pore closure, where elevations in temperature of as little as 0.2–0.5°C are sufficient to compromise action potential depolarization [101]. The picture that emerges is that a combination of changes in the intrinsic excitability of demyelinated fibers and in the temperature sensitivity of the ion channels that contribute to their resting membrane potentials, result in the conduction slowing and/or block that is typical of Uhthoff’s phenomena.
Such a low temperature threshold (0.2–0.5°C) for compromising action potential depolarization within the CNS in MS has been mechanistically confirmed in humans, where recent evidence from MS patients with internuclear ophthalmoparesis (i.e. saccadic eye disorder) exposed to passive whole-body heating indicated that ocular symptoms worsening could occur with increases in core temperature as little as of 0.2°C, and that symptoms worsening could be mitigated by restoring core temperature to baseline values via active body cooling [102].
Despite the evidence above, the exact pathophysiology of temperature-dependent conduction slowing/blocking in demyelinated axons in MS remains uncertain [103] and consequently, there is still no available pharmacological intervention available that can ameliorate the impact of temperature sensitivity on MS patients’ quality of life [104].
Heat-induced worsening of sensory and cognitive MS symptoms
The majority of experimental studies that have assessed the impact of increases in body (skin and core) temperature on MS symptoms worsening have primarily focused on autonomic symptoms, with less attention being given to sensory and cognitive symptoms.
A common approach to the investigation of heat-induced symptoms worsening has been based on observations and subjective evaluations of symptoms development during hot baths, exercise, exposures to warm ambient temperatures and during seasonal changes. In this respect, observations of symptoms worsening during passive heating through hot baths were often used in previous decades for the identification of clinical signs in MS [105].
With regards to evaluation of sensory symptoms worsening, a recent study attempted to characterise how controlled changes in body temperature could affect the skin’ sensitivity to thermal stimuli, i.e. thermosensory function. In this study, quantification of afferent thermosensory function in RRMS was performed before and after exercise-induced increases in patients’ body (core and skin) temperature (mean body temperature increase: ~ 0.4 oC). It was found that while the perception of warm stimuli (34°C and 38°C) remained relatively similar between pre and post exercise, the perception of cold stimuli (26°C and 22°C) decreased in the MS group compared to age-matched heathy controls. This reduced cold thermosensitivity was attributed to impaired afferent neural functions within demyelinated pathways sub serving cold sensing [106].
As a result of the limited mechanistic evidence on how controlled changes in body temperature lead to worsening of sensory symptoms, our knowledge on this area mainly relies on patients self-reporting symptoms via surveys and questionnaires during warm ambient exposures.
In one such a survey, patients with MS with heat sensitivity reported that they favour staying in a room with a temperature of ~ 20°C. Temperatures higher than the aforementioned were indeed associated with higher incidence of experiencing weakness in legs, balance deficits, pain, fatigue, spasms, paraesthesia, concentration difficulties and bladder complications [107].
Similarly to what reported for sensory symptoms, the majority of the evidence on available the impact of heat on cognitive function in MS arises from patients’ self-reports and observational studies evaluating the impact of outdoor weather on MS symptoms.
In one such cognitive study involving a cohort of MS patients performing a letter cognitive task, functional magnetic resonance imaging (fMRI) was used to show that cognitive deterioration in sustained attention and overall thinking was more pronounced as outdoor temperatures increased (i.e. from ~ −5°C to ~ 26°C) [108]. Interestingly, imaging data showed that despite warmer outdoor temperatures induced a decline in cognitive performance, some brain regions (i.e. frontal gyrus, dorsolateral, prefrontal and parietal lobe areas) appeared to be more active in the MS than in the age-gender matched healthy control group. It was suggested that reorganizational brain processes might have taken place in the MS group to compensate for the damage caused by demyelination [108]. Importantly, and irrespective of any potential re-organization, these results indicated that environmental temperature could indeed play a role in cognitive symptoms worsening in MS. However, it should be noted that how that may occur and what physiological mechanisms might be underlying this were not tested, leaving any potential explanation speculative.
In a more mechanistic attempt to evaluate cognitive symptoms worsening during heat exposure, Hämäläinen et al. [95] recently assessed potential cognitive deterioration in MS patients and (age-gender matched) healthy controls performing cognitive tasks during a 25-min sauna exposure (ambient temperature increasing from ~ 40°C to ~ 55°C, with < 5% relative humidity). As a result of the sauna exposure, MS patients’ core (gastrointestinal) temperature increased by ~ 0.5°C (as compared to a ~ 0.2 increase in the control group) and these showed deteriorated performance in terms of sustained attention, arithmetic calculations, ability to focus, information processing speed and flexibility and reaction times in comparison to baseline measurements [95].
The sensory and cognitive studies above seem to provide support to the classic view that increases in core temperature of as little as ~ 0.5°C that would drive heat sensitivity in MS and that such mechanisms could impact both sensory and cognitive symptoms (Figure 3). However, it is important to note that very little evidence is available as to whether an increase in core temperature is a necessary prerequisite for symptoms worsening to take place, or whether changes in skin temperature alone (or in combination with rises in core temperature) can also contribute to symptoms worsening. This question is particularly relevant, as anecdotal evidence from patients seem to point to the fact that even brief warm ambient exposures as well as direct sunlight can trigger symptoms worsening, despite the latter being unlikely to drive rises in core temperature. Furthermore, increased exposure to warmer outdoor temperatures (likely to induce more frequent rises in skin temperature) seem to be associated with higher mortality in MS (at least in the USA), a fact that highlights the importance of gathering a better understanding of the underlying mechanisms that lead to heat sensitivity [109].
Figure 3.
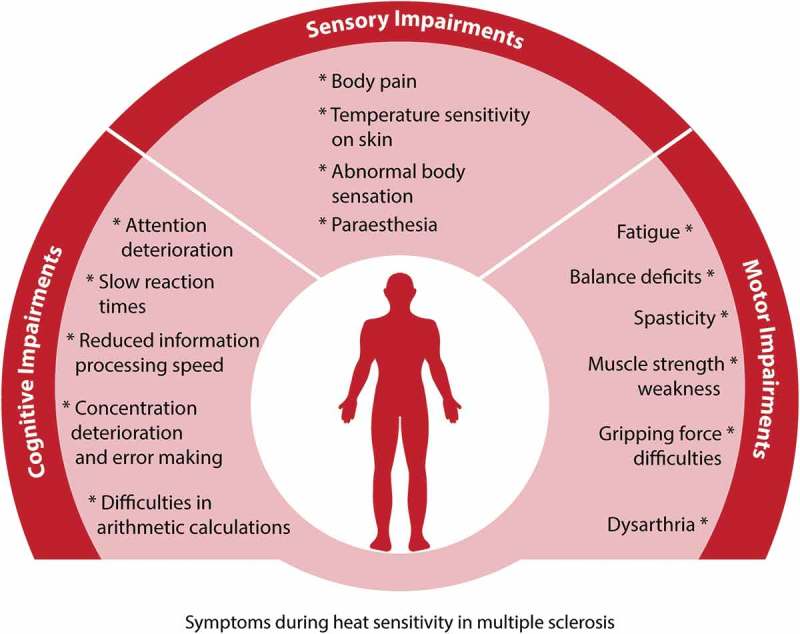
Summary of symptoms exacerbated by increases in body temperature in MS. Rises in body temperature induce: 1) cognitive deficits in attention, concentration and information processing; 2) sensory deficits such as altered sensations, body pain, and decreases in temperature sensitivity of the skin; 3) motor deficits including spasticity, muscle weakness, balance impairments, fatigue and speech difficulties (dysarthria). All the above symptoms have been observed and/or quantified during increases in body temperature in people with Multiple Sclerosis. These symptoms are pseudo-exacerbations of ongoing MS symptoms and thus, they subside as soon as body temperature is restored to its previous normothermic levels.
A reason why it is reasonable to hypothesise that increases in skin temperature could also contribute to heat sensitivity is that in the majority of studies assessing the impact of heat sensitivity in MS, it is common occurrence that rises in core temperature are also accompanied by proportional increases in skin temperature. This is an almost unavoidable occurrence with some of the heating procedures used, especially when external heat (e.g. warm air, hot water) is used to raise internal temperature (as opposed to endogenous heat produced via exercise).
For example, in a recent study people with MS were exposed to moderate heating (passive heating) (40°C, 30% RH) in an environmental chamber for 60 min which increased their skin temperature ~ 3.8°C without changes in their core (intestinal) temperature (37.4°C). In that experiment, postural sway was found to be increased and posture stability deteriorated after short term heat exposure in comparison to an age- and gender-matched healthy control group [110]. The motor symptoms that appeared with increased ambient temperatures were associated with increases in skin, but not core, temperature in MS. The above claim challenges the existing dogma that heat sensitivity only occurs in MS with increases in core temperature.
Recent observations performed with MS patients undergoing passive heat stress accomplished via a water-perfused suit, indicated that increases in patients’ core temperature (gastrointestinal tract) (~ 0.6°C) resulted in fatigue perception and impairments in hand motor function in comparison to age- and gender- matched healthy group [111]. However, MS patients’ skin temperature was also raised during the passive heating protocol (~ 3.5°C), making the independent contribution of core versus skin temperature rises to the development of such heat-induced pseudo-exacerbations difficult to isolate.
Interestingly, further evidence on the role of increases in skin temperature in MS heat sensitivity might arise from a hot water immersion study evaluating autonomic and motor function in MS. In this study, MS patients reported general body weakness (especially in the extremities), blurring in vision, tachycardia, loss of leg strength and dysarthria (difficulty in speaking), as a result of both whole-body immersion (15–34 min) in hot water (~ 40°C-42°C), as well as during upper or lower limb immersion (17–50 min) in hot water (~ 41°C-45°C) [112]. As patients’ oral temperature (i.e. a surrogate for core temperature) was observed to increase (range: 0.1°C −1.8°C) during whole-body immersion only, the fact that both conditions induced a worsening of autonomic and motor performance would point to the potential role that an increase in skin temperature only (i.e. during the upper/lower limb immersion) could play in triggering heat sensitivity [112]. The age- and gender- matched healthy control group did not exhibit any symptoms or signs, such, as weakness, during testing.
It is important to note that the above study focused more on observations than on quantifications of heat-induced symptoms. Furthermore, the onset of symptoms in relation to changes in core or skin temperature through different time points during the experiment was not assessed, thus the independent contribution of rises in skin versus core temperature in the development of heat sensitivity in MS requires further mechanistic evaluation.
Overall, it appears from the analysis above that despite sensory and cognitive symptoms representing a significant burden to MS patients’ quality of life, knowledge on how these symptoms are impacted by heat sensitivity remains fragmentary. Furthermore, mechanistic evidence about the independent and combined role of increases in internal (core) versus external (skin) body temperatures remain ambiguous and require further investigation to better elucidate how heat sensitivity is triggered and develops. Finally, the potential neuropsychological priming (i.e. placebo effect) produced by a patient’s assumption that heat (or cold) exposure might negatively affect their own health should not be underestimated. Heightened levels and anxiety in MS patients undergoing environmental heat (or cold) exposure could also partly contribute to symptom worsening.
Mechanisms of cold sensitivity in MS
While heat sensitivity seems to be the primary patient complaint in the context of temperature sensitivity, cold sensitivity is also reported by MS sufferers (Figure 4). Twenty percent of people with MS experience deterioration of their symptoms during winter and cold ambient temperatures, while 5% of the patients report cold sensitivity during cold baths [96]. While the incidence rates of cold sensitivity in MS are generally smaller (~ 15%) than that of heat sensitivity [113,114], cold-induced pseudo-exacerbations still play a major role in determining patients’ quality of life.
Figure 4.
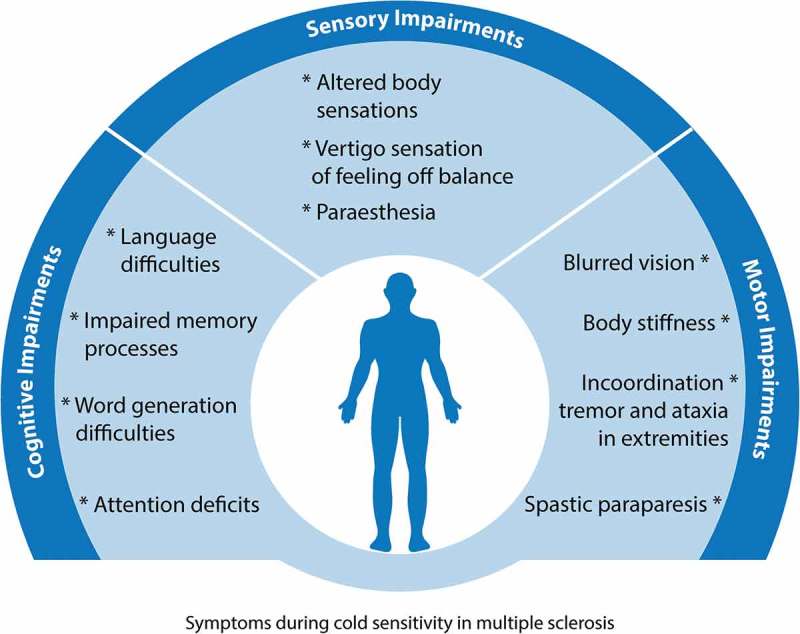
Summary of symptoms exacerbated by decreases in body temperature in MS. Drops in body temperature induce: 1) cognitive deficits in memory, language and attention; 2) altered sensations, such as tingling, numbness, burning sensation over the body (paraesthesia), and vertigo sensation that results in body imbalance; 3) motor deficits including body stiffness, tremor in the extremities, and visual difficulties. These cold-induced pseudo-exacerbations of symptoms subside as soon as the patient’s body temperature recovers to its normothermic levels.
The primary driver of cold sensitivity in MS seems to be associated with the presence of demyelinating lesions within the hypothalamus (i.e. the main CNS area controlling body temperature), which result in thermoregulatory dysfunction in the form of blunted autonomic responses (i.e. vasoconstriction, shivering) to cold stress [115].
The presence of hypothalamic lesions often leads to episodes of hypothermia in MS patients [116]. In some extreme cases, core (rectal) temperatures as low as of 29°C have been recorded in MS patients presenting demyelinating plaques in the midbrain, pons, medulla, and hypothalamus, which eventually led to mortality in the patients [117].
It is important to note that while the presence of hypothalamic lesions could affect thermoregulatory function and potentially drive cold sensitivity in MS, pseudo-exacerbations due to decreases in body temperature per se could still be involved in a worsening of symptoms during cold exposures. Yet there is limited evidence as to whether and how decreases in body temperature might affect salutatory conduction within CNS demyelinated fibres and whether this is analogous to what known with regards to heat sensitivity.
Cold-induced worsening of sensory and cognitive MS symptoms
Cold induced pseudo-exacerbations in MS have been observed and they have been subjectively evaluated during weather changes, passive body cooling and cold baths. Yet in light of the relatively lower prevalence of cold sensitivity amongst the MS population, most of our knowledge on this topic arises from case studies of patients presenting abnormally low core temperatures. These case studies can provide insights on the worsening of motor, sensory and cognitive symptoms as a result of decreases in body temperature; yet systematic investigations of the mechanisms behind cold sensitivity in MS are warranted.
Amongst those case studies, some stand out for the extent of the decreases in core temperature that is observed in some patients, especially when this is compared to the range of normal core temperatures observed in resting healthy individuals (i.e. ~ 36.7–37°C).
For example, in a MS patient admitted to a medical unit with a few days history of withdrawal and lethargy that eventually lapsed into coma, core (rectal) temperature was observed to have reached 29°C; following gentle rewarming over 3 days the patient was able to recover, although testing after rewarming showed improvements in cognitive function lagged behind the re-establishment of normothermia [117]. Interestingly, the same patient presented further hypothermic episodes within the following year, suggesting that recurrent thermoregulatory dysfunctions in this patient could have been associated with the presence of lesions to hypothalamic thermoregulatory centres.
Further case studies reported that exposure to cold stimuli or to cold weather induced or worsened paraesthesia symptoms and vision, and also induced ataxia and vertigo, in MS patients [118]. In one of such patients, a worsening of symptoms such as numbness and sensation impairments in lower extremities, increased stiffness and walking difficulties and mild spastic paraparesis started to arise with the beginning of the winter season. The same patient was then observed to present worse motor and sensory symptoms during psychophysical tests in colder (19°C) than warmer (25°C) ambient room temperatures, as she presented lower core (oral) temperatures (36.4°C-36.6°C) under colder conditions. Interestingly, the patient’s symptoms improved with body warming that resulted in subsequent increases in core temperature [118].
Another case study showed that exposure to cold weather worsened symptoms and triggered difficulties in walking, stiff gait and feeling of falling [118]. When the same patient was exposed to cold ambient temperatures (~ 2°C) in an environmental chamber, this showed difficulties in walking, with slow and rigid gait, tremor and uncoordinated upper extremity movements, which were further exacerbated and eventually led to spasticity when ambient temperature was further lowered [119].
In a study where MS patients wore a cooling jacket perfusing 10°C water for 2h, an associated 1°C drop in these patients’ core temperature (tympanic temperature) resulted in slowing down patients’ cognitive processes during neuropsychological testing (i.e. attention in auditory stimuli, language in word generation, immediate recall and delayed recall for verbal and visual memory) but not in the healthy controls (no age-gender matched group) [120]. Electrophysiological recordings of event-related potentials with the presentation of auditory stimuli showed slowed acoustic processing time and deficits in attention processing peak and latencies. The cognitive impairments were related to slow conduction velocities in the parts of the brainstem that are responsible for auditory evaluation of stimuli [120], a fact which suggested a core temperature-dependent mechanisms in the context of cold-induced slowing of neural transmission.
Altogether, the case studies reviewed above indicate that decreases in core temperature could impair sensory and cognitive functions in MS. However, mechanistic evidence on how pseudo-exacerbations driven by cold exposures could affect symptoms in MS patients who might not present hypothalamic lesions is lacking. Our understanding of the underlying physiology of cold sensitivity in MS remains therefore fragmentary.
Finally, it is important to note that while decreases in body temperature per se might have negative effects in some MS patients, body cooling can on the contrary be beneficial to dampen the impact of rises in body temperature in heat sensitive patients.
MS heat-sensitive patients are indeed advised to take advantage of body cooling that can decrease their core temperature, especially when it is known that a heat load might be experienced (e.g. before doing exercise or being exposed to warm ambients).
There is broad evidence on the beneficial effect of pre-cooling and per-cooling (i.e. cooling during heat exposure) in heat-sensitive patients [121–124]. For example, cold water immersion (~ 21°C-27°C) and related decreases in core temperature (oral temperature) (i.e. from ~ 36.7°C to ~ 35.5°C) have been shown to improve vision impairment, back pain, difficulties in swallowing, spasticity, sensation impairment in lower extremities [125]. Similarly, forearm, hand or whole body cooling with cold air exposure in MS have been shown to reduce tremor, spasticity symptoms, back pain, and improve vision, speech, and exercise performance [113,125,126].
Finally, a simple method such as cold water ingestion, can also improve exercise tolerance in the heat in heat-sensitive MS patients, without any significant change in their core temperature (rectal temperature) [127].
In the context of the evidence above, it therefore remains to be established how different MS patients with different presentations of temperature sensitivity (i.e. heat vs. cold sensitivity) might benefit from different approaches to the manipulation of their body temperature within ranges that are optimal for their physiological and cognitive function.
Conclusions and future directions
MS is a demyelinating disease of the CNS that induces significant disability due to a variety of motor, sensory and cognitive symptoms. A unique aspect of MS is the highly prevalent temperature sensitivity of its sufferers, where neurological symptoms are temporarily exacerbated by environmental- or exercise-induced increases (or decreases) in body temperature.
Current evidence suggests that conduction slowing within the CNS due to rises in core temperature could trigger exacerbation of sensory and cognitive symptoms, as observed in heat sensitive patients; yet only few mechanistic studies are available to confirm such hypothesis. As temperature-induced pseudo-exacerbations in MS have been reported to occur also without any changes in patients’ core temperature (e.g. during partial body heating or sunlight exposure), questions arise as to whether additional factors, such as changes in skin temperature, could be involved in the triggering of MS heat-related pseudo-exacerbations.
Limited knowledge is available on the impact of cold in MS pseudo-exacerbation and related sensory and cognitive symptoms, with the majority of evidence arising from case studies of hypothermic patients presenting hypothalamic lesions.
Future research will therefore have to: 1) better elucidate the physiological mechanisms and potential triggers of temperature sensitivity; 2) better quantify the extent to which sensory and cognitive functions are impacted by rises or drop in body temperature; 3) better determine the impact of cold on symptom worsening in patients who might not present hypothalamic lesions.
The fundamental knowledge arising from the research envisaged above will be useful in guiding the design of therapeutic interventions, and of more appropriate working and leisure environments, that will ultimately help lifting the burden posed by temperature sensitivity on MS patient’s quality of life.
Acknowledgments
We thank Anna Vlachaki and Salman Asghar for assisting in the creation of the figures.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Davis SL, Wilson TE, White AT, et al. Thermoregulation in multiple sclerosis. J Appl Physiol. 2010;109(5):1531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barnett MH, Henderson APD, Prineas JW.. The macrophage in MS: just a scavenger after all? Pathology and pathogenesis of the acute MS lesion. Mult Scler. 2006;12(December2005):121–32. [DOI] [PubMed] [Google Scholar]
- [3].Duffy SS, Lees JG, The M-TG. Contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int. 2014;2014:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fletcher JM, Lalor SJ, Sweeney CM, et al. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lagumersindez-Denis N, Wrzos C, Mack M, et al. Differential contribution of immune effector mechanisms to cortical demyelination in multiple sclerosis. Acta Neuropathol. 2017;134(1):15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Singh AK, Novakova L, Axelsson M, et al. High interferon-γ uniquely in Vδ1 T cells correlates with markers of inflammation and axonal damage in early multiple sclerosis. Front Immunol. 2017;8(MAR). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Van Der Valk P, De Groot CJA. Staging of multiple sclerosis (MS) lesions: pathology of the time frame of MS. Neuropathol Appl Neurobiol. 2000;26(1):2–10. [DOI] [PubMed] [Google Scholar]
- [8].Lucchinettil CF, Bruckz W, Rodriguez ’ M, et al. Distinct patterns of multiple sclerosis pathology indicates heterogeneity in pathogenesis. Brain Pathol. 1996;6:259–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Riccitelli G, Rocca MA, Pagani E, et al. Mapping regional grey and white matter atrophy in relapsing-remitting multiple sclerosis. Mult Scler. 2012;18(7):1027–037. [DOI] [PubMed] [Google Scholar]
- [10].Muzio L, Martino G, Furlan R. Multifaceted aspects of inflammation in multiple sclerosis: the role of microglia. J Neuroimmunol. 2007;191(1–2):39–44. [DOI] [PubMed] [Google Scholar]
- [11].Lagumersindez-Denis N, Wrzos C, Mack M, et al. Differential contribution of immune effector mechanisms to cortical demyelination in multiple sclerosis. Acta Neuropathol. 2017;134(1):15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shivane AG, Chakrabarty A. Multiple sclerosis and demyelination. Curr Diagnostic Pathol. 2007;13(3):193–202. [Google Scholar]
- [13].Huitinga I, Cja DG, Van Der Valk P, et al. Hypothalamic lesions in multiple sclerosis. J Neuropathol Exp Neurol. 2001;60(12):1208–218. [DOI] [PubMed] [Google Scholar]
- [14].Hua LH, Donlon SL, Sobhanian MJ, et al. Thoracic spinal cord lesions are influenced by the degree of cervical spine involvement in multiple sclerosis. Spinal Cord. 2015;53(7):520–25. [DOI] [PubMed] [Google Scholar]
- [15].Strijbis EMM, Kooi EJ, Van Der Valk P, et al. Cortical remyelination is heterogeneous in multiple sclerosis. J Neuropathol Exp Neurol. 2017;76(5):390–401. [DOI] [PubMed] [Google Scholar]
- [16].The CA. 150th anniversary of the first depiction of the lesions of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1988;51(10):1249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Murray TJ. The history of multiple sclerosis: the changing frame of the disease over the centuries. J Neurol Sci. 2009;277((SUPPL):1. [DOI] [PubMed] [Google Scholar]
- [18].Kumar DR, Aslinia F, Yale SH, et al. Jean-martin charcot: the father of neurology. Clin Med Res. 2011;9(1):46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Browne P, Chandraratna D, Angood C, et al. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022–024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pugliatti M, Rosati G, Carton H, et al. The epidemiology of multiple sclerosis in Europe. Eur J Neurol. 2006;13(7):700–22. [DOI] [PubMed] [Google Scholar]
- [21].Kurtzke JF. Multiple sclerosis in time and space–geographic clues to cause. J Neurovirol. 2000;6(Suppl 2):S134–40. [PubMed] [Google Scholar]
- [22].Goldenberg MM. Multiple sclerosis review. P.T. 2012;37(3):175–84. [PMC free article] [PubMed] [Google Scholar]
- [23].Vukusic S, Confavreux C. Primary and secondary progressive multiple sclerosis. J Neurol Sci. 2003;206(2):153–55. [DOI] [PubMed] [Google Scholar]
- [24].Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–12. [DOI] [PubMed] [Google Scholar]
- [25].Olek MM. Multiple sclerosis etiology, diagnosis, and new treatment strategies. Springer;2007. [Google Scholar]
- [26].Huang WJ, Chen WW, Zhang X. Multiple sclerosis: pathology, diagnosis and treatments (review). Exp Ther Med. 2017;13:3163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].O’Loughlin E, Hourihan S, Chataway J, et al. The experience of transitioning from relapsing remitting to secondary progressive multiple sclerosis: views of patients and health professionals. Disabil Rehabil. 2017; 39(18):1821–828. [DOI] [PubMed] [Google Scholar]
- [28].Oreja-Guevara C. Overview of magnetic resonance imaging for management of relapsing-remitting multiple sclerosis in everyday practice. Eur J Neurol. 2015;22(Suppl 2):22–27. [DOI] [PubMed] [Google Scholar]
- [29].Simon C. Multiple Sclerosis. InnovAiT. 2009;2(4):529–31. [Google Scholar]
- [30].C-A H, Jörg J. Autonomic dysfunction in multiple sclerosis. J Neurol. 2006;253(S1):3–9. [DOI] [PubMed] [Google Scholar]
- [31].Acevedo A, Nava C, Arriada N, et al. Cardiovascular dysfunction in multiple sclerosis. Acta Neurol Scand. 2000;101(2):85–88. [DOI] [PubMed] [Google Scholar]
- [32].Lee Y, Chen K, Ren Y, et al. Robot-guided ankle sensorimotor rehabilitation of patients with multiple sclerosis. Mult Scler Relat Disord. 2017;11:65–70. [DOI] [PubMed] [Google Scholar]
- [33].Patejdl R, Zettl UK. Spasticity in multiple sclerosis: contribution of inflammation, autoimmune mediated neuronal damage and therapeutic interventions. Autoimmun Rev. 2017;16:925–36. [DOI] [PubMed] [Google Scholar]
- [34].Gallien P, Robineau S. Sensory-motor and genito-sphincter dysfunctions in multiple sclerosis. Biomed Pharmacother. 1999;53:380–85. [DOI] [PubMed] [Google Scholar]
- [35].Dujmovic I, Radovanovic S, Martinovic V, et al. Gait pattern in patients with different multiple sclerosis phenotypes. Mult Scler Relat Disord. 2017;13:13–20. [DOI] [PubMed] [Google Scholar]
- [36].Gandolfi M, Munari D, Geroin C, et al. Sensory integration balance training in patients with multiple sclerosis: A randomized, controlled trial. Mult Scler. 2015;21(11):1453–462. [DOI] [PubMed] [Google Scholar]
- [37].Hebert JR, Manago MM. Reliability and validity of the computerized dynamic posturography sensory organization test in people with multiple sclerosis. Int J MS Care. 2017;19(3):151–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hunt CM, Widener G, Allen DD. Variability in postural control with and without balance-based torso- weighting in people with multiple sclerosis and healthy controls. Phys Ther. 2014;94(10):1489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Newsome SD, Wang JI, Kang JY, et al. Quantitative measures detect sensory and motor impairments in multiple sclerosis. J Neurol Sci. 2011;305(1–2):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Prosperini L, Fortuna D, Giannì C, et al. The diagnostic accuracy of static posturography in predicting accidental falls in people with multiple sclerosis. Neurorehabil Neural Repair. 2013;27(1):45–52. [DOI] [PubMed] [Google Scholar]
- [41].Thoumie P, Mevellec E. Relation between walking speed and muscle strength is affected by somatosensory loss in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;73(3):313–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lerdal A, Celius EG, Moum T. Fatigue and its association with sociodemographic variables among multiple sclerosis patients. Mult Scler J. 2003;9(5):509–14. [DOI] [PubMed] [Google Scholar]
- [43].Van Kessel K, Moss-Morris R. Understanding multiple sclerosis fatigue: A synthesis of biological and psychological factors. J Psychosom Res. 2006;61(5):583–85. [DOI] [PubMed] [Google Scholar]
- [44].Fisk JD, Pontefract A, Ritvo PG, et al. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21(1):9–14. [PubMed] [Google Scholar]
- [45].Mills RJ, Young CA. A medical definition of fatigue in multiple sclerosis. QJM. 2008;101(1):49–60. [DOI] [PubMed] [Google Scholar]
- [46].Ayache SS, Chalah MA. Fatigue in multiple sclerosis – insights into evaluation and management. Clin Neurophysiol. 2017;47(2):139–71. [DOI] [PubMed] [Google Scholar]
- [47].Biberacher V, Schmidt P, Selter RC, et al. Fatigue in multiple sclerosis: associations with clinical, MRI and CSF parameters. Mult Scler J. 2018;24(8):1115–125. [DOI] [PubMed] [Google Scholar]
- [48].Elzamarany E, Afifi L, El-Fayoumy NM, et al. Motor cortex rTMS improves dexterity in relapsing-remitting and secondary progressive multiple sclerosis. Acta Neurol Belg. 2016;116(2):145–50. [DOI] [PubMed] [Google Scholar]
- [49].Prasad S, Galetta SL. Eye movement abnormalities in multiple sclerosis. Neurol Clin. 2010;28:641–55. [DOI] [PubMed] [Google Scholar]
- [50].Hickman SJ, Raoof N, McLean RJ, et al. Vision and multiple sclerosis. Mult Scler Relat Disord. 2014;3:3–16. [DOI] [PubMed] [Google Scholar]
- [51].Iyer PM, Fagan AJ, Meaney JF, et al. Horizontal nystagmus and multiple sclerosis using 3-Tesla magnetic resonance imaging. Ir J Med Sci. 2016;185(4):881–86. [DOI] [PubMed] [Google Scholar]
- [52].Swingler RJ, Compston DAS. The morbidity of multiple sclerosis. QJM. 1992;83(1):325–37. [PubMed] [Google Scholar]
- [53].Jongen PJ, Stavrakaki I, Voet B, et al. Patient-reported adverse effects of high-dose intravenous methylprednisolone treatment: a prospective web-based multi-center study in multiple sclerosis patients with a relapse. J Neurol. 2016;263(8):1641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cockburn N, Pateman K, Taing MW, et al. Managing the oral side-effects of medications used to treat multiple sclerosis. Aust Dent J. 2017;62(3):331–36. [DOI] [PubMed] [Google Scholar]
- [55].Heijenbrok MW, Anema JR, Faes TJ, et al. Quantitative measurement of vibratory sense and temperature sense in patients with multiple sclerosis. Electromyogr Clin Neurophysiol. 1992;32(0301–150X(Print)):385–88. [PubMed] [Google Scholar]
- [56].Sanders EACM, Arts RJHM. Paraesthesiae in multiple sclerosis. J Neurol Sci. 1986;74(2–3):297–305. [DOI] [PubMed] [Google Scholar]
- [57].Davis FA, Jacobson S. Altered thermal sensitivity in injured and demyelinated nerve. J Neurol Neurosurg Psychiatry. 1971;34(5):551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Indaco A, Iachetta C, Nappi C, et al. Chronic and acute pain syndromes in patients with multiple sclerosis. Acta Neurol (Napoli). 1994;16(3):97–102. [PubMed] [Google Scholar]
- [59].Blanco Y, Compta Y, Graus F, et al. Midbrain lesions and paroxysmal dysarthria in multiple sclerosis. Mult Scler. 2008;14(5):694–97. [DOI] [PubMed] [Google Scholar]
- [60].Costantino A, Black SE, Carr T, et al. Dorsal midbrain syndrome in multiple sclerosis with magnetic resonance imaging correlation. Can J Neurol Sci. 1986;13(1):62–5. [DOI] [PubMed] [Google Scholar]
- [61].Afshari D, Moradian N, Khalili M, et al. Evaluation of pulsing magnetic field effects on paresthesia in multiple sclerosis patients, a randomized, double-blind, parallel-group clinical trial. Clin Neurol Neurosurg. 2016;149:171–74. [DOI] [PubMed] [Google Scholar]
- [62].Svendsen KB, Jensen TS, Hansen HJ, et al. Sensory function and quality of life in patients with multiple sclerosis and pain. Pain. 2005;114(3):473–81. [DOI] [PubMed] [Google Scholar]
- [63].Beckmann Y, Özakbaş S, Bülbül NG, et al. Reassessment of Lhermitte’s sign in multiple sclerosis. Acta Neurol Belg. 2015;115(4):605–08. [DOI] [PubMed] [Google Scholar]
- [64].Stenager E, Knudsen L, Jensen K. Acute and chronic pain syndromes in multiple sclerosis. A 5-year follow-up study. Ital J Neurol Sci. 1995;16(8):629–32. [DOI] [PubMed] [Google Scholar]
- [65].Okada S, Kinoshita M, Fujioka T, et al. Two cases of multiple sclerosis with painful tonic seizures and dysesthesia ameliorated by the administration of mexiletine. Jpn J Med. 1991;30(4):373–75. [DOI] [PubMed] [Google Scholar]
- [66].Etemadifar M, Mehrbod N, Dehghani L, et al. Prevalence of Lhermitte’s sign in multiple sclerosis versus neuromyelitis optica. Iran J Neurol. 2014;13(1):50–51. [PMC free article] [PubMed] [Google Scholar]
- [67].Rae-Grant AD, Eckert NJ, Bartz S, et al. Sensory symptoms of multiple sclerosis: a hidden reservoir of morbidity. Mult Scler. 1999;5:179–83. [DOI] [PubMed] [Google Scholar]
- [68].Hooge JP, Redekop WK. Trigeminal neuralgia in multiple sclerosis. Neurology. 1995;45(7):1294–296. [DOI] [PubMed] [Google Scholar]
- [69].Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(Pt 12):2347–360. [DOI] [PubMed] [Google Scholar]
- [70].Jamali A, Sadeghi-Demneh E, Fereshtenajad N, et al. Somatosensory impairment and its association with balance limitation in people with multiple sclerosis. Gait Posture. 2017;57:224–29. [DOI] [PubMed] [Google Scholar]
- [71].Inglese M, Adhya S, Johnson G, et al. Perfusion magnetic resonance imaging correlates of neuropsychological impairment in multiple sclerosis. J Cereb Blood Flow Metab. 2008;28(1):164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Keser Z, Hasan KM, Mwangi B, et al. Diffusion tensor imaging-defined sulcal enlargement is related to cognitive impairment in multiple sclerosis. J Neuroimaging. 2017;27:3. [DOI] [PubMed] [Google Scholar]
- [73].Rocca MA, De Meo E, Filippi M. Functional MRI in investigating cognitive impairment in multiple sclerosis. Acta Neurol Scand. 2016;134:39–46. [DOI] [PubMed] [Google Scholar]
- [74].Jønsson A, Andresen J, Storr L, et al. Cognitive impairment in newly diagnosed multiple sclerosis patients: A 4-year follow-up study. J Neurol Sci. 2006;245(1–2):77–85. [DOI] [PubMed] [Google Scholar]
- [75].Sepulcre J, Vanotti S, Hernández R, et al. Cognitive impairment in patients with multiple sclerosis using the Brief Repeatable Battery-Neuropsychology test. Mult Scler. 2006;12(2):187–95. [DOI] [PubMed] [Google Scholar]
- [76].Foong J, Rozewicz L, Quaghebeur G, et al. Executive function in multiple sclerosis. The role of frontal lobe pathology brain. 1997;120(1):15–26. [DOI] [PubMed] [Google Scholar]
- [77].Einarsson U, Gottberg K, Von Koch L, et al. Cognitive and motor function in people with multiple sclerosis in Stockholm County. Mult Scler. 2006;12(3):340–53. [DOI] [PubMed] [Google Scholar]
- [78].Randolph JJ, Wishart HA, Saykin AJ, et al. FLAIR lesion volume in multiple sclerosis: relation to processing speed and verbal memory. J Int Neuropsychol Soc. 2005;11:2. [DOI] [PubMed] [Google Scholar]
- [79].Schulz D, Kopp B, Kunkel A, et al. Cognition in the early stage of multiple sclerosis. J Neurol. 2006;253(8):1002–010. [DOI] [PubMed] [Google Scholar]
- [80].Scherer P, Penner IK, Rohr A, et al. The Faces Symbol Test, a newly developed screening instrument to assess cognitive decline related to multiple sclerosis: first results of the Berlin Multi-Centre FST Validation Study. Mult Scler J. 2007;13(3):402–11. [DOI] [PubMed] [Google Scholar]
- [81].Diamond BJ, Johnson SK, Kaufman M, et al. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189–99. [DOI] [PubMed] [Google Scholar]
- [82].Griffiths SY, Yamamoto A, Boudreau VG, et al. Memory interference in multiple sclerosis. J Int Neuropsychol Soc. 2005;11:6. [DOI] [PubMed] [Google Scholar]
- [83].Azcárraga-Guirola E, Rodríguez-Agudelo Y, Velázquez-Cardoso J, et al. Electrophysiological correlates of decision making impairment in multiple sclerosis. Eur J Neurosci. 2017;45(2):321–29. [DOI] [PubMed] [Google Scholar]
- [84].Beatty WW, Monson N. Problem solving by patients with multiple sclerosis comparison of performance on the Wisconsin and California Card Sorting Tests. J Int Neuropsychol Soc. 1996;2(2):134–40. [DOI] [PubMed] [Google Scholar]
- [85].Morse CL, Schultheis MT, McKeever JD, et al. Multitasking in multiple sclerosis: can it inform vocational functioning? Arch Phys Med Rehabil. 2013;94(12):2509–514. [DOI] [PubMed] [Google Scholar]
- [86].Hynčicová E, Vyhnálek M, Kalina A, et al. Cognitive impairment and structural brain changes in patients with clinically isolated syndrome at high risk for multiple sclerosis. J Neurol. 2017;264(3):482–93. [DOI] [PubMed] [Google Scholar]
- [87].Sbardella E, Petsas N, Tona F, et al. Assessing the correlation between grey and white matter damage with motor and cognitive impairment in multiple sclerosis patients. PLoS One. 2013;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Amato MP, Portaccio E, Goretti B, et al. Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol. 2007;64(8):1157. [DOI] [PubMed] [Google Scholar]
- [89].Rossi F, Giorgio A, Battaglini M, et al. Relevance of brain lesion location to cognition in relapsing multiple sclerosis. PLoS One. 2012;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhang X, Zhang F, Huang D, et al. Contribution of gray and white matter abnormalities to cognitive impairment in multiple sclerosis. Int J Mol Sci. 2016;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hughes AJ, Dunn KM, Chaffee T. Sleep disturbance and cognitive dysfunction in multiple sclerosis: a systematic review. Curr Neurol Neurosci Rep. 2018;18. [DOI] [PubMed] [Google Scholar]
- [92].Braley TJ, Boudreau EA. Sleep disorders in multiple sclerosis. Curr Neurol Neurosci Rep. 2016;16:5. [DOI] [PubMed] [Google Scholar]
- [93].Cruce R, Vosoughi R, Freedman MS. Cognitive impact of anticholinergic medication in MS: adding insult to injury? Mult Scler Relat Disord. 2012;1(4):156–61. [DOI] [PubMed] [Google Scholar]
- [94].Romberg A, Ikonen A, Ruutiainen J, et al. The effects of heat stress on physical functioning in persons with multiple sclerosis. J Neurol Sci. 2012;319(1–2):42–46. [DOI] [PubMed] [Google Scholar]
- [95].Hämäläinen P, Ikonen A, Romberg A, et al. The effects of heat stress on cognition in persons with multiple sclerosis. Mult Scler J. 2012;18(4):489–97. [DOI] [PubMed] [Google Scholar]
- [96].Simmons RD, Ponsonby A-L, Iaf VDM, et al. What affects your MS? Responses to an anonymous, Internet-based epidemiological survey. Mult Scler. 2004;10(2):202–11. [DOI] [PubMed] [Google Scholar]
- [97].White LJ, Dressendorfer RH. Exercise and multiple sclerosis. Sports Medicine. 2004;34:1077–100. [DOI] [PubMed] [Google Scholar]
- [98].Economic PA. Impact of Multiple Sclerosis in 2010 Australian MS longitudinal Study. Australia: Multiple Sclerosis Research Australia; 2011. [Google Scholar]
- [99].Quandt FN, Davis FA. Action potential refractory period in axonal demyelination: a computer simulation. Biol Cybern. 1992;67(6):545–52. [DOI] [PubMed] [Google Scholar]
- [100].The RM. Effects of temperature on conduction in demyelinated single nerve fibers. Arch Neurol. 1973;28(5):287–92. [DOI] [PubMed] [Google Scholar]
- [101].Frohman TC, Davis SL, Beh S, et al. Uhthoff’s phenomena in MS—clinical features and pathophysiology. Nat Rev Neurol. 2013;9(9):535–40. [DOI] [PubMed] [Google Scholar]
- [102].Frohman TC, Davis SL, Frohman EM. Modeling the mechanisms of Uhthoff’s phenomenon in MS patients with internuclear ophthalmoparesis. Ann N Y Acad Sci. 2011;1233(1):313–19. [DOI] [PubMed] [Google Scholar]
- [103].Kiernan MC. Some do not like it hot. J Physiol. 2017;11:3251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kanagaratnam M, Pendleton C, Souza DA, et al. Diuretic-sensitive electroneutral Na + movement and temperature effects on central axons. J Physiol. 2017;595(11):3471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Berger JR, Sheremata W. The hot bath test and multiple sclerosis. JAMA. 1982;247:979. [PubMed] [Google Scholar]
- [106].Filingeri D, Chaseling G, Hoang P, et al. Afferent thermosensory function in relapsing-remitting Multiple Sclerosis following exercise-induced increases in body temperature. Exp Physiol. 2017;102(8):887–93. [DOI] [PubMed] [Google Scholar]
- [107].Flensner G, Ek A-C, Söderhamn O, et al. Sensitivity to heat in MS patients: a factor strongly influencing symptomology–an explorative survey. BMC Neurol. 2011;11(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Leavitt VM, Wylie G, Chiaravalloti N, et al. Warmer outdoor temperature is associated with task-related increased BOLD activation in patients with multiple sclerosis. Brain Imaging Behav. 2014;8(1):128–32. [DOI] [PubMed] [Google Scholar]
- [109].Sun H. Temperature dependence of multiple sclerosis mortality rates in the United States. Mult Scler. 2017;23(14):1839–846. [DOI] [PubMed] [Google Scholar]
- [110].Poh PYS, Adams AN, Huang M, et al. Increased postural sway in persons with multiple sclerosis during short-term exposure to warm ambient temperatures. Gait Posture. 2017;53:230–35. [DOI] [PubMed] [Google Scholar]
- [111].White AT, VanHaitsma TA, Vener J, et al. Effect of passive whole body heating on central conduction and cortical excitability in multiple sclerosis patients and healthy controls. J Appl Physiol. 2013;114(12):1697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gutherie TC. Visual and motor changes in patients with multiple sclerosis. A result of induced changes in environmental temperature. AMA Arch NeurPsych. 1951;65(4):437–51. [DOI] [PubMed] [Google Scholar]
- [113].Grahn DA, Murray JV, Hc H. Cooling via one hand improves physical performance in heat-sensitive individuals with Multiple Sclerosis: A preliminary study. BMC Neurol. 2008;8(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Syndulko K, Woldanski A, Rw B, et al. Preliminary evaluation of lowering tympanic temperature for the symptomatic treatment of multiple sclerosis. J Neurol Rehabil. 1995;9(4):205–15. [Google Scholar]
- [115].Sullivan F, Hutchinson M, Bahandeka S, et al. Chronic hypothermia in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1987;50(6):813–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Linker RA, Mohr A, Cepek L, et al. Core hypothermia in multiple sclerosis: case report with magnetic resonance imaging localization of a thalamic lesion. Mult Scler. 2006;12(1):112–15. [DOI] [PubMed] [Google Scholar]
- [117].White KD, Scoones DJ, Newman PK. Hypothermia in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996;61(4):369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Honan WP, Heron JR, Foster DH, et al. Paradoxical effects of temperature in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1987;50(9):1160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Geller M. Appearance of signs and symptoms of multiple sclerosis in response to cold. Mt Sinai J Med. 1974;41(1):127–30. [PubMed] [Google Scholar]
- [120].Geisler MW, Gaudino EA, Squires NK, et al. Cooling and multiple sclerosis: cognitive and sensory effects. J Neurol Rehabil. 1996;10:17–22. [Google Scholar]
- [121].White T, Wilson TE, Davis SL, et al. Effect of precooling on physical performance in multiple sclerosis. Mult Scler. 2000;6(3):176–80. [DOI] [PubMed] [Google Scholar]
- [122].Ku YT, Montgomery LD, Wenzel KC, et al. Physiologic and thermal responses of male and female patients with multiple sclerosis to head and neck cooling. Am J Phys Med Rehabil. 1999;78(5):447–56. [DOI] [PubMed] [Google Scholar]
- [123].Reynolds LF, Short CA, Westwood DA, et al. Head Pre-Cooling Improves Symptoms of Heat-Sensitive Multiple Sclerosis Patients. Can J Neurol Sci. 2011;38(1):106–11. [DOI] [PubMed] [Google Scholar]
- [124].Coyle PK, Krupp LB, Doscher C, et al. Immunological Effects of Cooling in Multiple Sclerosis. Neurorehabil Neural Repair. 1996;10(1):9–15. [Google Scholar]
- [125].Watson CW. Effect of lowering of body temperature on the symptoms and signs of multiple sclerosis. N Engl J Med. 1959;261:1253–259. [DOI] [PubMed] [Google Scholar]
- [126].Feys P. Effects of peripheral cooling on intention tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76(3):373–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Chaseling GK, Filingeri D, Barnett M, et al. Cold-water ingestion improves exercise tolerance of heat-sensitive people with MS. Med Sci Sports Exerc. 2018;50(4):643–648:1. [DOI] [PubMed] [Google Scholar]


