Abstract
The endochondral ossification plays a critical role in vertebrate limb development and skeletal homeostasis, where limb mesenchyme cells form an intermediate cartilage scaffold that develops into growth plates and then replaced by bone. Although Indian hedgehog (Ihh) is known to control the hypertrophic differentiation process of chondrocytes, its role from the mesenchyme cells to the early stages of chondrogenesis is unclear. To define the function of Ihh in the mesenchymal cell's early stages of chondrogenesis, we specifically delete Ihh in Prx1-expressed mesenchyme cells at E9.5 using Prx1-Cre;Ihhfl/fl;Rosa26-ZsGreen1 mice. We found that deleting Ihh in the mesenchyme cells results in an early and quick ossification of the intermediate cartilage scaffold, causing the growth plate and phalange joint absence, short limbs, and dwarfishness. The green fluorescent protein (GFP)-positive cells derived from deleted Ihh mesenchyme cells overlap with von Kossa- and osteocalcin-positive staining area. These deleted Ihh/GFP-positive cells isolated from Prx1-Cre;Ihhfl/fl;Rosa26-ZsGreen1 newborn mice had osteogenic differentiation by showing a positive Alizarin red and von Kossa staining, as well as an enhanced Col1a1, osteocalcin, and Runx2 expression. Our findings demonstrate that deleting Ihh in mesenchyme cells during early limb development promotes intermediate cartilage scaffold ossification, which prevents growth plate formation that causes phalange joint absence, short limb, and dwarfish phenotype.
Keywords: : Indian hedgehog, mesenchymal cells, hypertrophic chondrocytes, synovial joint
Introduction
Endochondral ossification plays a critical role for vertebrate limb development, skeletal homeostasis, and fracture healing [1]. Endochondral bone development begins with mesenchymal condensation, then forms an intermediate cartilage scaffold that develops into growth plate, and finally replaced by bone. This developmental process is tightly regulated temporally and spatially by many factors, such as transcription, hormones, and cytokines [2].
The Hh homologous proteins in mammals are Sonic hedgehog, Indian hedgehog (Ihh), and Desert hedgehog, which all share the same signaling pathway [1]. Ihh, which is synthesized and expressed primarily in prehypertrophic chondrocytes of growth plate, has an essential function in cartilage development, endochondral bone formation [3], and synovial joint formation [4,5]. The main function of Ihh is to regulate chondrocyte hypertrophy and differentiation during endochondral ossification [6–8]. Ihh plays a critical role in prenatal and postnatal skeletal development and homeostasis. Interestingly, the mutation in Ihh causes brachydactyly type A1, which was the first recorded disorder of the autosomal dominant Mendelian trait in humans and characterized by shortened or absent middle phalanges in digits [9–11]. Understanding how an Ihh mutation causes the middle phalange absence is integral for comprehension, as well as its relevance to treatment of bone growth disturbances, such as dwarfish. The roles of Ihh in chondrogenesis and ossification are complex. Although this complexity has been demonstrated in numerous publications in the past two decades [6,10], the role of the Ihh pathway in bone formation is still under controversy. For example, Patched acts as a functional inhibitor of Hh signaling. The deleted Patched can address the role of enhanced Hh signaling. Ohba et al. [12] and Mak et al. [13] used the same genetic mouse models (delete Patched) and reported conflicting findings. Ohba et al. [12] showed that Patched1 haploinsufficiency in mature osteoblasts leads to more bone mass, whereas Mak et al. [13] showed that increased Hh signaling resulted in decreased bone mass. These controversial findings indicate that the process of bone development may involve a complex network because enhancing the Ihh downstream pathway may active other unknown signalings.
Although Ihh plays a critical role during limb growth in length via controlling growth plate chondrocyte hypertrophy and the endochondral ossification, its role in the mesenchyme cells to the earlier stages of chondrogenesis is unknown. Deleted Ihh from collagen type 2 alpha1-expressing (Col2a1) cells was perinatal lethal, limiting observations of postnatal osteoblast differentiation and bone formation [6,14]. Recently, we found that deleted Ihh in the mesenchyme cells accelerated abnormal mineralization. This is contradictory to the previous report in which deleted Ihh in Col2-expressed chondrocyte delays mineralization [14]. This indicates Ihh may have an opposite role in the mesenchyme cells during limb development and bone formation.
Prx1 are specifically expressed at E9.5 in limb development [15]. To test the role of Ihh in the mesenchyme cells to the earlier stages of chondrogenesis, we have generated Prx1-Cre;Ihhfl/fl;Rosa26-ZsGreen1 mice to precisely delete Ihh in the mesenchyme cells. This unique and powerful genetic approach will generate mice lacking Ihh expression, specifically in mesenchyme. Prx1-Cre;Ihhfl/fl;Rosa26-ZsGreen1 mice can survive postnatal day 42 after deleting Ihh specifically in mesenchymal stem cells, providing an animal model in which we could observe the phenotype continuously and dynamically from prenatal development to postnatal bone formation. We can also track and isolate the deleted Ihh mesenchyme with enhanced green fluorescent protein (EGFP) [16]. Thus, we can determine the role of Ihh from the mesenchyme to the earlier stage of chondrogenesis and bone formation in vivo.
Materials and Methods
Generation of Prx1-Cre;Ihhfl/fl;Rosa26-ZsGreen1 mice
Prx1-Cre transgenic mice (Stock No.: 005584; The Jackson Laboratory, Bar Harbor, ME) were interbred with Ihhfl/fl mice (supplied by Beate Lanske) [14,15]. The Prx1-Cre;Ihhfl/+ mice were then interbred with B6.Cg-Gt (ROSA)26Sortm6(CAG-ZsGreen1)Hze (Stock No.: 007906; The Jackson Laboratory) [16] to obtain Prx1-Cre;Ihhfl/+;Rosa26-ZsGreen1 mice. The Prx1-Cre;Ihhfl/+;Rosa26-ZsGreen1 male mice were then crossed with female Ihhfl/fl mice to obtain Prx1-Cre;Ihhfl/fl;Rosa26-ZsGreen1 animals. These mice harbor a targeted mutation of the Gt (ROSA) 26Sor locus with loxP-flanked STOP cassette, preventing transcription of a CAG promoter-driven enhanced green fluorescent protein (ZsGreen1). When bred to mice that express Prx1-Cre recombinase, the resulting offspring will have the STOP cassette deleted in the limb's mesenchyme cells, resulting in an expression of ZsGreen1. Prx1-Cre;Ihhfl/fl;Rosa26-ZsGreen1 mice were born with the expected rate of Mendelian inheritance and showed dwarfish phenotype. The genetic loss is limited to limbs and calvarium, and thus, we can observe the phenotype continuously from prenatal to 6 weeks after birth or even longer in vivo [15]. A fostering system was also used for mutant pups as previously described [2,17]. Because the limbs of the mutant pups were significantly dwarfed at birth, we collected only the mutants and left them in a nest with one mother to foster as experimental group, the rest of mice were raised together by their mother as control. The Institutional Animal Care and Use Committee of Shanxi Medical University approved all experiments (approval no.: 2017003).
PCR analysis for genotyping
Genotyping of mice was performed by conventional PCR using the following specific primers: Cre allele; forward, 5′-CGCGGTCTGGCAGTAAAAACTATC-3′, reverse, 5′-CCCACCGTCAGTACGTGAGATATC-3′, Cre Internal control; forward, 5′-CTAGGCCACAGAATTGAAAGATCT-3′, reverse, 5′-GTAGGTGGAAATTCTAGCATCATCC-3′, Ihh allele; forward, 5′-AGCACCTTTTTTCTCGACTGCCTG-3′, reverse, 5′-TGTTAGGCCGAGAGGGATTTCGTG-3′, and wild-type ZsGreen; forward, 5′-AAG GGA GCT GCA GTG GAG TA-3′, reverse, 5′-CCG AAA ATC TGT GGG AAG TC-3′, mutant ZsGreen; forward, 5′-GGCATTAAA GCAGCGTATCC-3′, reverse, 5′-AACCAGAAGTGGCACCTG AC-3′.
Skeletal preparation
Skeletal preparations were performed as described [18]. Briefly, the bodies were fixed in 95% ethanol after removing skin, viscera, and adipose tissue. The skeletons were stained with 0.03% Alcian blue solution (Sigma-Aldrich, St. Louis, MO), dehydrated in 100% ethanol, and then incubated in 1% potassium hydroxide. Subsequently, counterstain bone in 0.01% Alizarin red solution (Sigma-Aldrich). Clear the samples by placing them in 1% potassium hydroxide and 20% glycerol, and finalize through gradient mix of glycerol and ethanol.
Histology
After the animals were euthanized, hind limbs were harvested and immersed in 10% formalin for 24 h. The paraffin sections were prepared through normal procedures. Safranin-O staining was performed using 0.5% Safranin-O solution and counterstaining of 0.2% fast green to assess glycosaminoglycan production. Von Kossa staining was performed using 4% silver nitrate solution with counterstaining of 1% fast red solution to evaluate the mineralization of bone [2].
Immunohistochemistry
For immunohistochemistry, 6-μm-thick sections were collected on positively charged glass slides and dried on a hot plate. Then, sections were deparaffinized with xylene and rehydrated in a descending series of ethanol concentrations. Endogenous peroxidase was blocked by treating the sections with 3% hydrogen peroxide in methanol (Sigma-Aldrich) for 30 min. Antigen was retrieved with 5 mg/mL of hyaluronidase in phosphate-buffered saline for 20 min at 37°C. The sections then were incubated with primary antibodies against osteocalcin (M173; Takara Bio, Otsu, Japan; 1:1000) at 4°C overnight. Thereafter, the sections were treated sequentially with biotinylated secondary antibody and SP conjugate (Zymed Laboratories/Invitrogen), and then developed in DAB chromogen (Zymed Laboratories/Invitrogen, Frederick, MD). For tartrate-resistant acid phosphatase (TRAP) staining, a commercial TRAP Kit (Sigma-Aldrich) was used according to the manufacturer's protocol. Photography was taken with a Nikon E800 microscope (Nikon, Melville, NY) [19].
RNA in situ hybridization
RNA in situ hybridization for Collagen X messenger RNA (mRNA) was performed using the RNAscope® 2.0 paraffin-embedded (FFPE) Reagent Kit (Advanced Cell Diagnostics, Inc., Hayward, CA) according to the manufacturer's instructions. A detailed description of the RNAscope 2.0 Assay has been published previously [20].
Radiographical analysis and microcomputed tomography
Plain radiographs were taken using a Faxitron X-ray apparatus (Faxitron, Tucson, AZ). Mice were anesthetized by intraperitoneal injections of ketamine hydrochloride (100 mg/kg body weight) and imaged by microcomputed tomography (micro-CT) at P5, P10, P21, and P42 (vivaCT 80; SCANCO MEDICAL, Bassersdorf, Switzerland) with a source voltage of 70 keV, current of 114 μA, and 15.6 μm isotropic resolution, following recommended guidelines from Bouxsein et al. [21]. The trabecular region was isolated from the cortical region in each two-dimensional image by manual contouring analysis. The complete bone marrow cavity of the tibia was evaluated, thereby avoiding sampling errors incurred by random deviations of a single section. The resulting gray-scale images were segmented using a low-pass filter to remove noise and a fixed threshold was used (220) to extract the mineralized bone phase. After three-dimensional (3D) reconstruction, bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and connectivity density were calculated using built-in software.
Primary murine chondrocyte isolation and culture
Murine costal chondrocytes were isolated from the ventral parts of the rib cages of 6-day-old mice and cultured in F-12 media with 10% fetal bovine serum (FBS) (Thermo Fisher Scientifc, Inc., Waltham, MA) as previously described [22,23]. Briefly, the pieces of murine rib cartilage were subjected to enzymatic treatment with 3% collagenase D. Undigested bony tissues were discarded by filtration, and then the chondrocytes were seeded on tissue culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ) at a density of 1 × 105 cells/cm2 in Ham's F-12 medium with 10% FBS and cultured at 37°C in a thermal incubator under 5% carbon dioxide. Throughout the experimental culture, the medium was refreshed every other day.
Isolate and identify deleted Ihh/GFP-positive cells by flow cytometry and fluorescent microscopy
The flow cytometry process was done on a flow cytometer FC500 (Beckman Coulter, Indianapolis, IN) machine to investigate the percentage of chondrocyte-expressed detectable EGFP compared with cells from Ihhfl/fl mice. To detect EGFP, 6-μm-thick frozen sections of P5 were incubated for 15 min with 10 μg/mL of Hoechst nuclear dye (Pierce, Rockford, IL) while protected from light. The GFP-positive cells isolated from day 6 rib cage were cultured 5 days and fixed in 10% formalin for 20 min and stained with DAPI (Invitrogen, Frederick, MD). Photography was performed with a Nikon E800 fluorescence microscope (Nikon).
Quantitative real-time PCR analysis
Total RNA was extracted from these GFP-positive cells by using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) and reversely transcribed into complementary DNA by using the PrimeScript™ RT-PCR Kit (Takara, Dalian, China). Real-time PCR was conducted utilizing SYBR Premix Ex TaqTM (Takara) according to the manufacturer's instructions. The 18S mRNA was served as an internal reference to normalize the levels of gene expression for all samples. The specific primers used for gene expression analysis are listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/scd).
The cycle threshold value for target gene was measured and calculated by computer software IQ50 (Bio-Rad Laboratories, Hercules, CA). Relative mRNA level was calculated as x = 2−ΔΔCt, in which ΔΔCt = ΔCt E – ΔCt C, ΔCt E = Ctexp − Ct18S, and ΔCt C = CtC − Ct18S [24]. Each sample was analyzed in triplicate.
Statistical analysis
Each measure was performed in triplicate. Data were obtained from at least three independent experiments and presented as mean ± standard deviation. Student's t-test was used to compare the difference between the deleted Ihh and its littermate control. P value <0.05 was considered significant.
Results
Prx1-Cre;Ihhfl/fl;Rosa26−ZsGreen1 mice exhibit severe limb abnormalities
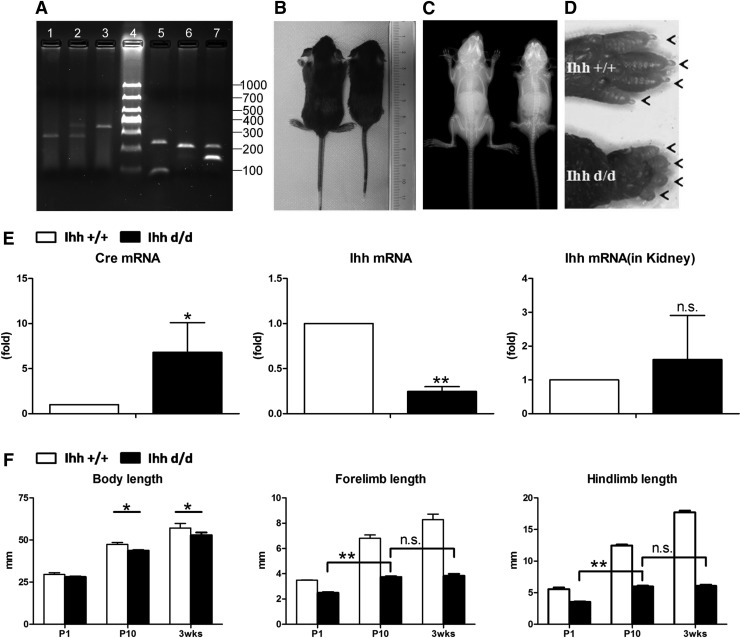
PCR was used for genotyping to confirm disruption of both Ihh alleles and the presence of Cre, ZsGreen1 (Fig. 1A). As expected, mutant mice displayed a severe phenotype that restricted their limbs, while their general body size and digit number were not affected (Fig. 1B–D). Gene expression analysis using RNA isolated from costal chondrocytes of Ihh +/+ and Ihh d/d mice at postnatal day 6 (P6) confirmed strong Cre expression and a statistically significant deletion of Ihh (90%) in mutants. In contrast, Ihh expression in kidney tissues of Ihhfl/fl and mutant mice was comparable (Fig. 1E). The movement of mutant mice was limited due to its short limb phenotype, which resulted in feeding difficulties and subsequent malnutrition. A fostering system was used for mutant pups to maintain sufficiently old mice for this study [2,17]. Mutant mice continued to exhibit short limbs at 3 weeks of age, but their body length was only slightly reduced compared with the control group. The limb length data confirmed a severe shortening in mutants, and no further limb growth was observed between P10 and 3 weeks (Fig. 1F). Our observations are consistent with previous findings where Prx1Cre recombinase-mediated deletions of a floxed Ihh gene expressed in mesenchymal cells during adulthood can be highly specific and remain highly efficient for months after induction [2,17].
FIG. 1.
Deleted Ihh in mesenchymal cells causes limb abnormalities. (A) Prx1-Cre;Ihhfl/fl;Rosa26−ZsGreen1 mouse genotyping by PCR. Lane 1: WT Ihhfl/fl (382 bp); lane 2: heterozygous Ihhfl/fl (382 and 484 bp); lane 3: homozygous Ihhfl/fl (484 bp); lane 4: 50-bp DNA marker; lane 5: transgene (100 bp) and internal control (324 bp); lane 6: WT ZsGreen1 (297 bp); and lane 7: heterozygous ZsGreen1 (297 and 199 bp). (B, C) Phenotype of Prx1-Cre;Ihhfl/fl;Rosa26−ZsGreen1 mice and the control littermates at P21. (D) Mutant has small size, a short limb, but no digit number change. Arrow represents digit. (E) Cre and Ihh expression. A significantly reduced Ihh expression confirms the efficient and specific ablation of Ihh in the limbs. **P < 0.01, *P < 0.05 (vs. Ihhfl/fl); n = 4 for the chondrocytes RNA; n = 4 for kidney RNA. (F) Measurements of body length, forelimb length, and hind limb length. No growth is observed in mutant limbs between P10 and P21. **P < 0.01, *P < 0.05. Body length; n = 5. Forelimb length; n = 5. Hind limb length; n = 5. Ihh, Indian hedgehog; mRNA, messenger RNA; n.s., not significantly different; WT, wild type.
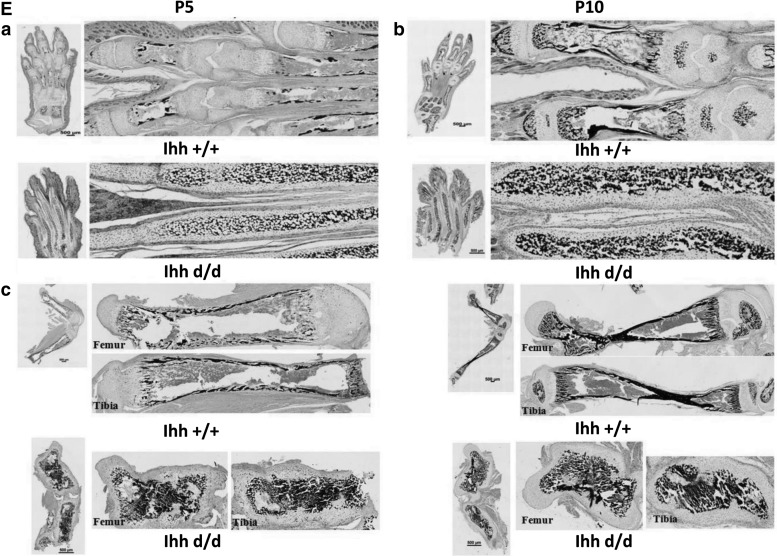
Accelerated pathological matrix calcification, no growth plate and phalange joint formation in Ihh-deficient mice
Whole skeletal staining with Alizarin red/Alcian blue (days 5 and 10 after birth) showcased that accelerating bone formation led to no digital joint formation (Fig. 2A), which is consistent with the X-ray results (Fig. 2B). The body of the sternum and xiphoid process showed an abnormal calcification and early fusion (Fig. 2C). Safranin-O staining confirmed that mutant bones were shorter with hypertrophic conversions, whereas control mice exhibited normal chondrocyte differentiation and bone formation (Fig. 2D). Control mice group displayed normal von Kossa staining patterns in limbs. In comparison, von Kossa staining detected abundant, but severely disorganized, mineralization in the center of mutant bones (Fig. 2E).
FIG. 2.
Deletion of Ihh in mesenchyme accelerates abnormal bone formation and no digital joint, no growth plate, and no second ossification center formation in vivo. (A) Whole skeletal staining with Alizarin red/Alcian blue (P5 and P10) showed that abnormal bone formation led to no joint formation due to accelerating bone formation (red color: bone). (B) X-ray (P5 and P10) further confirmed no joint formation. WT mice (Ihh +/+), homozygotes (Ihh d/d). (C) Whole-body staining with Alizarin red/Alcian blue showed an abnormal bone formation (P5) in the sternum and early fusion of the xiphoid process. Arrow indicates enlarged view of the dotted area on the left. (D) No digital joints, no growth plate, and no second ossification center formation in the limbs of P5 and P10 Prx1-Cre;Ihhfl/fl;Rosa26−ZsGreen1, as indicated by Safranin-O staining from the Ihh d/d (D–a and D–b, bottom) and the age-matched controls (Ihh +/+) (D–a and D–b, top). A similar finding was found in the femur and tibia from deleted Ihh mice (right) compared with the littermates (left) (D–c). (E) Early and abnormal ossification. Von Kossa staining indicates an early and abnormal bone formation occurs in the medullary cavities in mutant mice while the growth plate is already developing very well at P10 tibia control mice. (E–a, E–b) No digital joint formation at P5 and P10 in Ihh-deleted mice. (E–b) Growth plate already forms in control mice at P10 but not in mutant mice. (E-c) Early mineralization and no growth plate formation in Ihh deleted mice from femur and tibia.
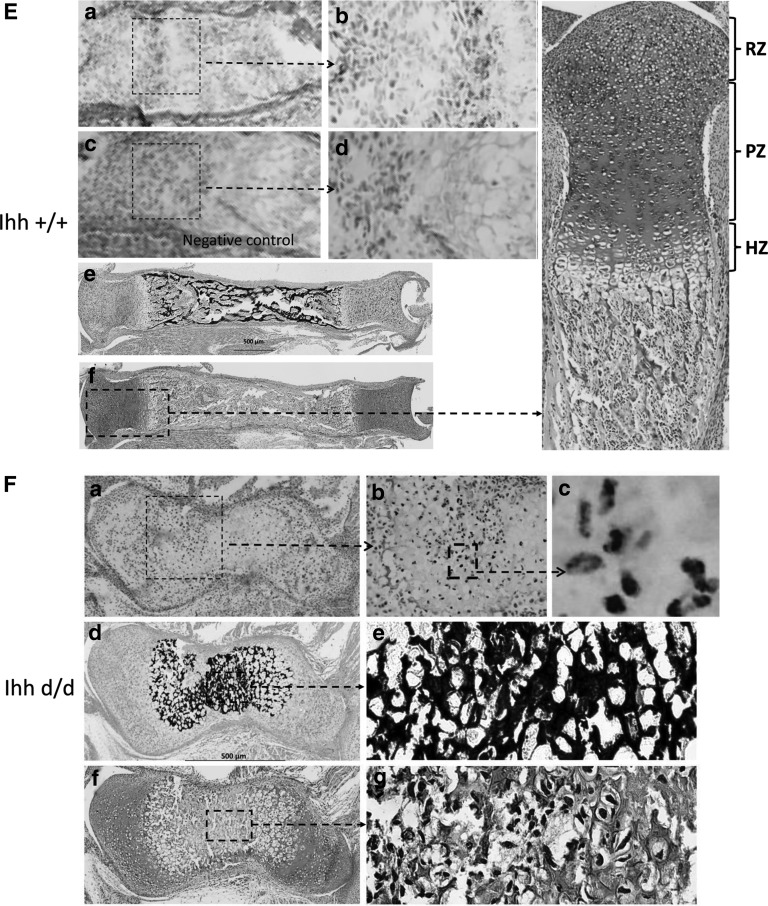
Osteocalcin is produced by osteoblasts and is generally regarded as a marker of bone formation, while TRAP staining is often known as a bone resorption indicator [25]. Immunohistochemistry results showed a positive osteocalcin staining detected in the chondro-osseous junction of the growth plate and cortical bone in Ihh +/+ mice, while abundant osteocalcin-positive staining occupied the whole medullary cavities and the space previously filled with hypertrophic chondrocytes in the Ihh d/d mice at P5. (Fig. 3A, B). A positive staining of TRAP was also observed in the whole mutant bone tissues (Fig. 3C, D). We next examined the osteoclast number in the same area in the chondro-osseous junction of tibia and found that the osteoclast number in ihh d/d mice was lower than in control mice (Supplementary Fig. S1). In in situ hybridization on paraffin sections prepared from tibia at P0, a strong collagen X mRNA was found in the whole medullary cavities in the mutant tibia (Fig. 3F), while collagen X mRNA was only presented in the prehypertrophic zone in control (Fig. 3E). Hypertrophic chondrocytes are located at the hypertrophic zone without ossification in control mice, while whole cartilage template is von Kossa-positive staining in mutant mice. Moreover, improper ossification and osteogenesis with the complete loss of normal growth plates lead to severely disrupted bone formation and abnormal structures, resulting in the unsegmented phenotype (no digital joint formation) in Ihh mutant mice.
FIG. 3.
A strong osteocalcin and TRAP staining in mutant bones at P5. Immunohistochemical analyses using anti-osteocalcin (osteoblast marker, A, B) and TRAP staining (osteoclast marker, C, D) on foot, femur, and tibia sections at P5. Osteocalcin and TRAP staining-positive cells are located in the chondro-osseous junction of the growth plate in the control mice, while abundant positive cells are observed in the whole medullary cavities in the mutant mice. In situ hybridization on paraffin sections prepared from tibia at P0 (E, F). A strong collagen X mRNA was found in the whole medullary cavities in the mutant tibia (F), while collagen X mRNA was only presented in prehypertrophic zone in control (E). Hypertrophic chondrocytes are located at hypertrophic zone without ossification in control mice (E), while whole cartilage template is von Kossa-positive staining in mutant mice (E–a, b) In situ hybridization results of Ihh +/+; (E–c, d) negative control; (E–e) Von Kossa staining results of Ihh +/+; (E–f) Safranin-O staining results of Ihh +/+; (F–a, b, c) In situ hybridization results of Ihh d/d; (F–d, e) Von Kossa staining results of Ihh d/d; (E–f, g) Safranin-O staining results of Ihh d/d. (F). HZ, hypertrophy zone; PZ, proliferation zone; RZ, reserve zone; TRAP, tartrate-resistant acid phosphatase.
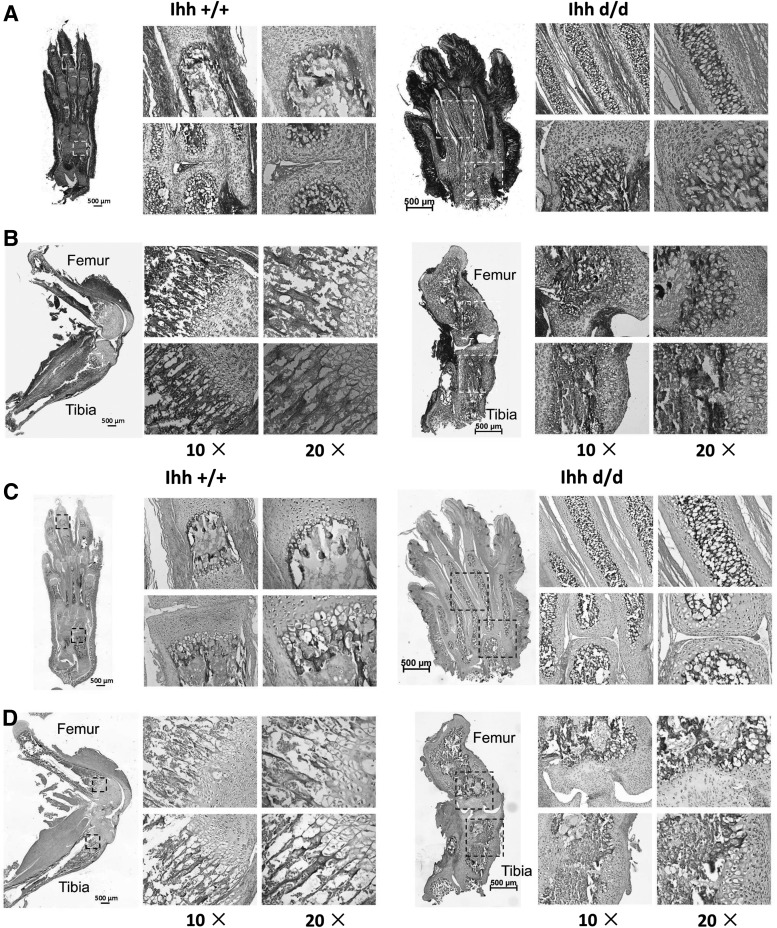
Deleted Ihh results in more and disorganized trabecular formation
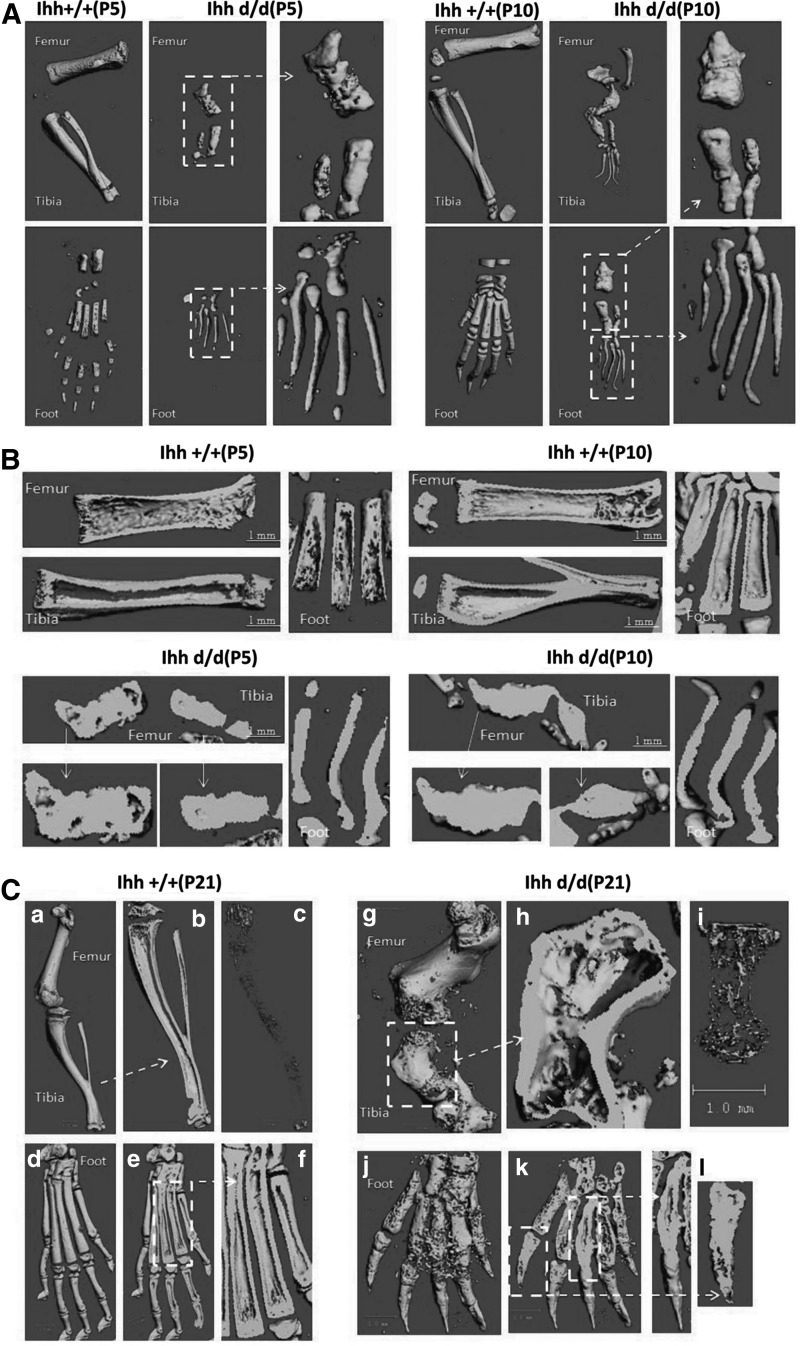
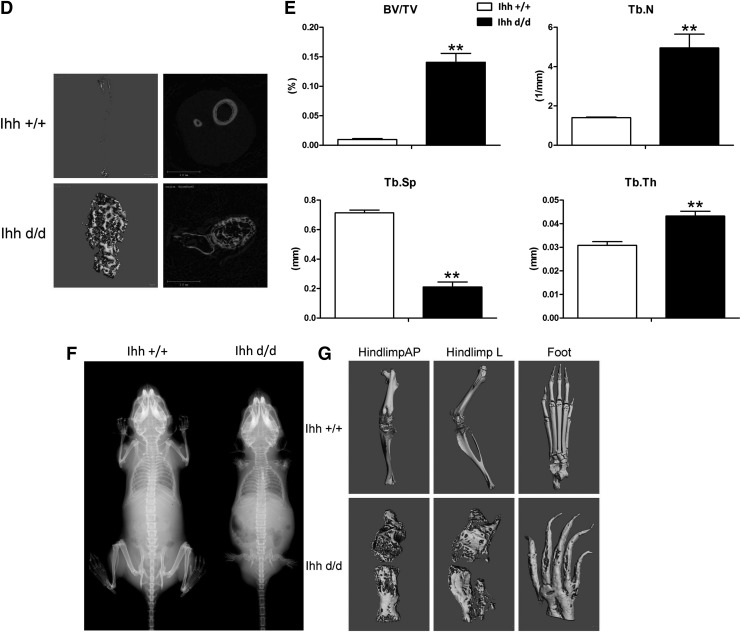
Micro-CT 3D images revealed that deleted Ihh in Prx1-Cre;Ihhfl/fl;Rosa26−ZsGreen1 mice had more and disorganized trabecular architecture and expanded marrow cavities, compared with the control mice (Fig. 4A–D). Bone histomorphometric analyses indicated that the bone volume/tissue volume (BV/TV), Tb.N, and Tb.Th in the distal femur were significantly higher in Ihh d/d mice than in control mice, whereas the trabecular spacing (Tb.Sp) was significantly lower in mutant mice than in control mice (Fig. 4E). Both X-ray and micro-CT showed no digital joint formation at P42 in mutant mice (Fig. 4F, G).
FIG. 4.
Micro-CT showed abnormal and more bone filled the whole medullary cavities (days 5, 10, and 21 after birth). (A) Deleted Ihh in mesenchyme has a short and wide limb as well as no digital joint formation. (B) The medullary cavities were well developed in the WT mice (top) while they were filled completely with bone (bottom). (C) A similar result was found at P21. (C–a, b) Ihh +/+ mice tibia micro-CT results; (C–c) Ihh +/+ mice 3D reconstruction of the tibia trabecular bone. (C–d, e, f) Ihh +/+ mice foot micro-CT results; (C–g, h) Ihh d/d mice tibia micro-CT results; (C–i) Ihh d/d mice 3D reconstruction of the tibia trabecular bone. (C–j, k, l) Ihh d/d mice foot micro-CT results. (D) The more trabecular bone was found in the deleted Ihh mice compared with the littermate control at P21. (E) Bone histomorphometric analyses. **P < 0.01; n = 6. (F, G) X-ray and micro-CT further confirmed no digital joint formation at P42 in mutant mice. BV/TV, bone volume per total volume; micro-CT, microcomputed tomography; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
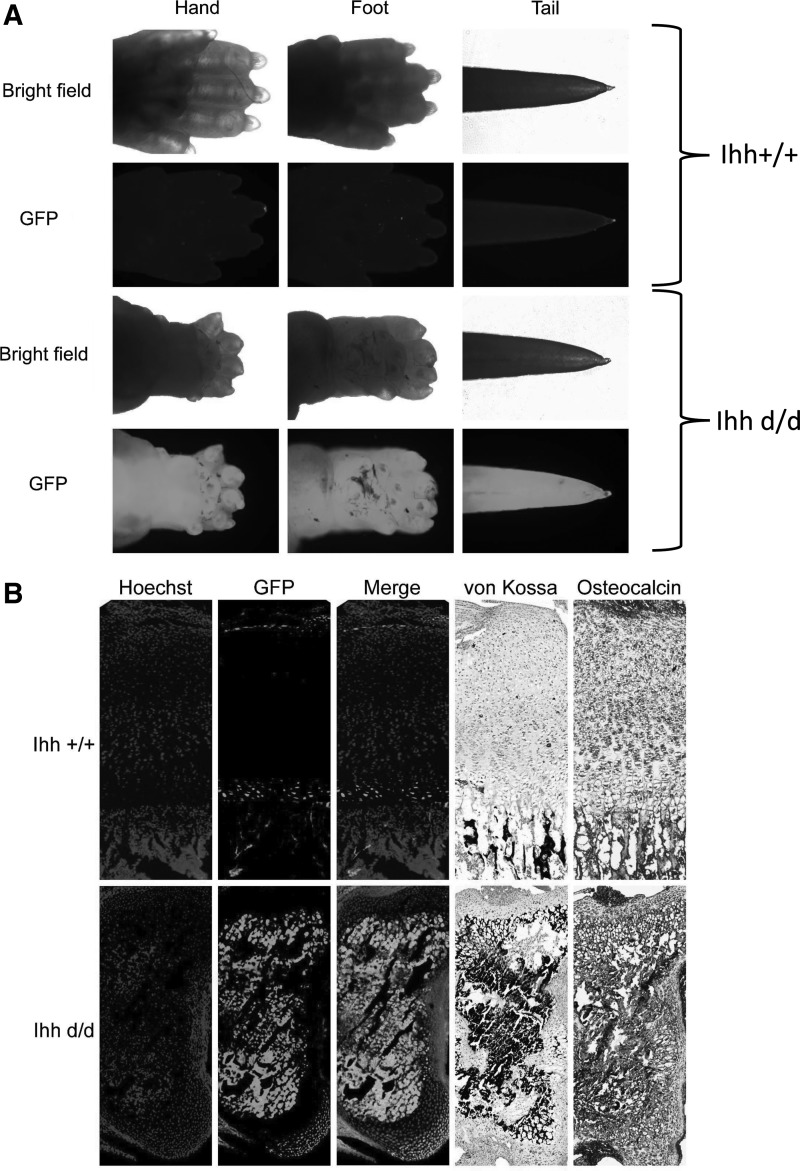
Deleted Ihh/GFP-positive cells overlapped with von Kossa staining and osteocalcin-positive staining area
By establishing Prx1-Cre;Ihhfl/fl;Rosa26−ZsGreen1 mice to specifically track deleted Ihh limb mesenchymal cells, we can track the fate of deleted Ihh mesenchymal cells using fetal tibia growth plate. Deleted Ihh mice can be easily identified by short limb and GFP color (Fig. 5A). Deleted Ihh/EGFP-positive mesenchymal cells were distributed in the whole bone marrow cavity and overlapped with von Kossa-positive area where anti-osteocalcin staining was also positive (Fig. 5B). This further proves that these cells can survive the mesenchyme-to-bone transition directly.
FIG. 5.
Deleted Ihh in limb mesenchymal cells can differentiate directly into bone cells, resulting in intermediate cartilage scaffold ossification at P5. EGFP-positive signals were easily detected in the foot and tail in mutant mice, while the controls showed weak autofluorescence and no EGFP signal (A). Genetic cell fate mapping studies confirmed that deleted Ihh/EGFP-positive mesenchymal cells distribute among the whole bone marrow cavity and overlapped with the von Kossa and osteocalcin staining-positive area (B). EGFP, enhanced green fluorescent protein.
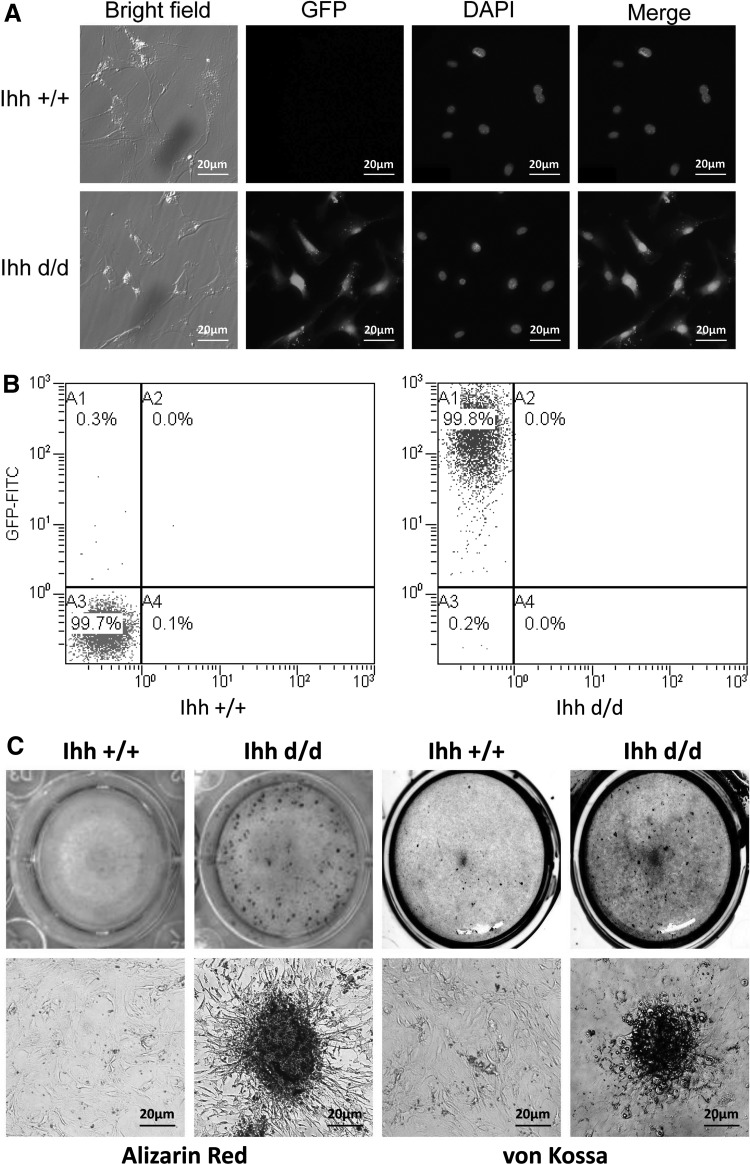
Deleted Ihh/GFP-positive cells isolated from Prx1-Cre;Ihhfl/fl;Rosa26−ZsGreen1 newborn mice have osteogenic differentiation in vitro
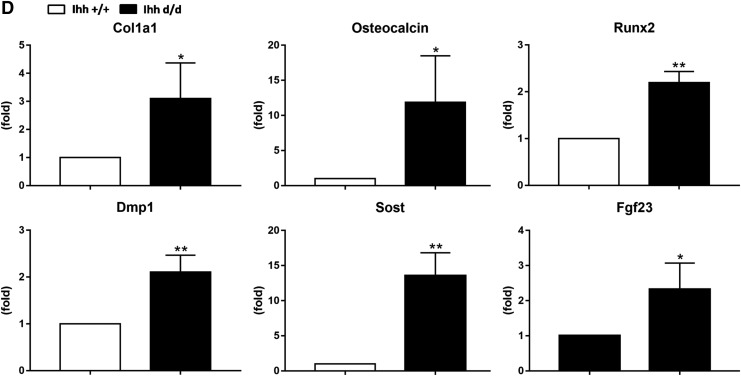
To prove that deleted Ihh in the mesenchyme promotes osteogenic differentiation and mineralization, we isolated and tracked the deleted Ihh/EGFP-expressing mesenchyme cells from newborn rib cage by fluorescence-activated cell sorting (FACS), and cultured in vitro to determine whether deleted Ihh has osteogenic differentiation ability (Fig. 6A). EGFP expression cells were assessed by microscopy (Fig. 6A) and flow cytometry analysis (Fig. 6B). In the Ihh d/d group, 99.8% were GFP-positive cells. Strong Alizarin red and von Kossa staining was detected in the Ihh d/d group compared with the control group over time in 3 weeks of culture under no osteogenesis induction condition (Fig. 6C). The mRNA levels of osteoblast markers col1a1, osteocalcin, runx2 and osteocyte markers dmp1, sost, fgf23 were significantly higher in the Ihh d/d mice than that in the Ihh +/+ mice. Collectively, these data suggest that limb mesenchyme-specific deletion of Ihh cells isolated from Prx1-Cre;Ihhfl/fl;Rosa26−ZsGreen1 newborn mice has osteogenic differentiation.
FIG. 6.
Deleted Ihh in the mesenchyme promotes osteogenic differentiation and mineralization in vitro. (A) GFP-positive cells isolated from P6 murine costal tissue. (B) Flow cytometry analysis confirmed that majority of the costal cells differentiate from deleted Ihh/EGFP-positive mesenchyme cells. (C) Alizarin red and von Kossa staining indicates the presence of a mineralizing extracellular matrix over time in 3 weeks of culture under no osteogenesis induction condition. (D) Real-time PCR shows deleted Ihh in the limb mesenchyme increases bone formation marker genes. **P < 0.01, *P < 0.05.
Discussion
During the vertebrate limb development, the limb mesenchyme cells form an intermediate cartilage scaffold first, which then develops into the growth plate and is replaced by bone [1]. Although Ihh plays a critical role during endochondral ossification and synovial joint formation [5,6], its role from the mesenchyme cells to the earlier stages of chondrogenesis and growth plate formation is unclear. To do so, we ablated Ihh in the mesenchyme cells. Overall, Ihh-deficient mice are smaller than corresponding control mice, displayed through the marked short limbs. At the microstructural levels, the abnormal ossification of the Ihh mutant mice had disorganized trabecular formation, pathological matrix calcification, and no growth plate and phalange joint formation.
On establishing a genotype and phenotype relationship in the Ihh mutant mice, our subsequent study revealed that the deleted Ihh-GFP-positive cells derived from the mesenchyme cells overlap with von Kossa-positive area where osteocalcin staining, a typical osteoblast marker, is also positive. This indicates that these deleted Ihh mesenchyme cells can transform into bone cells. Our in vitro experiment further reveals that the deleted Ihh/GFP-positive cells isolated from Prx1-Cre;Ihhfl/fl;Rosa26−ZsGreen1 newborn mice have osteogenic differentiation by showing a positive Alizarin red and von Kossa staining, and enhanced Col1a1, osteocalcin, and Runx2. An early closure of the growth plate has been reported by Amano et al. [2,17] in the Prx1-Cre;Ihhfl/fl mice, and they demonstrate that Ihh and PTH1R signaling in limb mesenchyme is essential to regulate digit structure development. They believe an initial cartilaginous fusion in digits and the initial cartilage gradually resorbed and replaced by bone are specific mechanisms that lead to the loss of epiphyseal growth plate. During this replacement process, chondrocyte differentiation seems to occur in a spatially abnormal manner, resulting in growth failure in digit bones [2,17]. In this study, we demonstrate for the first time that the deleted Ihh from mesenchyme induces an early ossification of intermediate cartilage scaffold by promoting the hypertrophic process, which prevents growth plate formation and results in dwarfish mice. The new finding explains why there is no growth plate formation in these mutant mice. Furthermore, our finding is consistent with recent work that identified a novel mechanism by which hypertrophic chondrocytes can directly transform into bone cells [26–28].
Chondrogenesis and osteogenesis are one continuous developmental and lineage-defined biological process [29] and both are derived from limb mesenchyme [15]. In endochondral ossification, hypertrophic chondrocytes secrete extracellular matrix, which eventually becomes mineralized and causes the elongation of the bone [30]. By providing crucial local signals from prehypertrophic and hypertrophic chondrocytes to both chondrocytes and preosteoblasts, Ihh couples chondrogenesis to osteogenesis in endochondral bone development [31] and can eventually lead to skeletal abnormalities when abnormal. Our data indicate that Ihh plays a critical role from the mesenchymal cells to chondrogenesis/osteogenesis.
The interzone is essential for joint formation because its removal by microdissection led to joint ablation and fusion of bones [32]. It has been reported that Ihh produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone [33]. In our study, we noticed that these mice do not form phalange joints. One possible explanation is that the absence of Ihh signaling in the limb mesenchymal cells induced an early ossification of the intermediate cartilage scaffold, which could not further form interzone and growth plate. Thus, there is no digital joint formation.
We also used conditional knockout Col2a1-CreERT2; Ihhfl/fl mice to investigate the mechanism of deleting Ihh results in complete loss of growth plate. After injecting tamoxifen (TM) at P0 to delete Ihh, the growth plate of mutant mice was checked at different specific times. The von Kossa staining confirmed that functional knockout of the Ihh gene causes future growth plate cartilage scaffold to mineralize at P10, leading to lack of normal growth plate and secondary ossification center (unpublished data). This further supports that the deleted Ihh results in a loss of growth plate due to abnormal ossification of the cartilage scaffold.
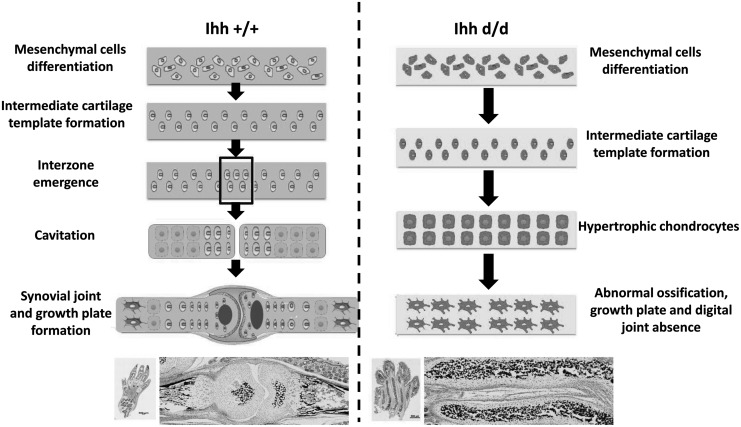
In summary, we present novel data regarding the function of Ihh in limb mesenchymal cells and its contribution to growth plate and synovial joint formation. Ihh deletion on mesenchyme cells results in the intermediate cartilage scaffold ossification, which prevents growth plate and phalange joint formation causing short limb and dwarfish phenotypes (Fig. 7).
FIG. 7.
A model of deleted Ihh in mesenchyme promotes chondrocyte hypertrophy and pathological matrix calcification. The normal process of the synovial join formation (left). Deleted Ihh in limb mesenchymal cells accelerates hypertrophy and abnormal mineralization, inducing intermediate cartilage scaffold ossification directly, prevents growth plate and digit joint formation, and results in dwarfish phenotype (right).
Supplementary Material
Acknowledgments
The project was supported by grant R01AR059142 from NIH/NIAMS, NSFC 81572098, 81772415, and 81601949, SXNSF 20150313012-6, and SSCC2016-118. This work was also supported by Visiting Scholarship of Shanxi Scholarship Council of China. We thank Dr. Olin D Liang for providing Rosa26-ZsGreen1 mice. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Long F. and Ornitz DM. (2013). Development of the endochondral skeleton. Cold Spring Harb Perspect Biol 5:a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano K, Densmore MJ. and Lanske B. (2015). Conditional deletion of Indian hedgehog in limb mesenchyme results in complete loss of growth plate formation but allows mature osteoblast differentiation. J Bone Miner Res 30:2262–2272 [DOI] [PubMed] [Google Scholar]
- 3.Lai LP. and Mitchell J. (2005). Indian hedgehog: its roles and regulation in endochondral bone development. J Cell Biochem 96:1163–1173 [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Andre P, Ye L. and Yang YZ. (2015). The hedgehog signalling pathway in bone formation. Int J Oral Sci 7:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moskalewski S, Hyc A, Jankowska-Steifer E. and Osiecka-Iwan A. (2013). Formation of synovial joints and articular cartilage. Folia Morphol (Warsz) 72:181–187 [DOI] [PubMed] [Google Scholar]
- 6.St-Jacques B, Hammerschmidt M. and McMahon AP. (1999). Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13:2072–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronenberg HM. (2003). Developmental regulation of the growth plate. Nature 423:332–336 [DOI] [PubMed] [Google Scholar]
- 8.Ma RS, Zhou ZL, Luo JW, Zhang H. and Hou JF. (2013). The Ihh signal is essential for regulating proliferation and hypertrophy of cultured chicken chondrocytes. Comp Biochem Physiol B Biochem Mol Biol 166:117–122 [DOI] [PubMed] [Google Scholar]
- 9.Gao B, Guo J, She C, Shu A, Yang M, Tan Z, Yang X, Guo S, Feng G. and He L. (2001). Mutations in IHH, encoding Indian hedgehog, cause brachydactyly type A-1. Nat Genet 28:386–388 [DOI] [PubMed] [Google Scholar]
- 10.Gao B, Hu J, Stricker S, Cheung M, Ma G, Law KF, Witte F, Briscoe J, Mundlos S, et al. (2009). A mutation in Ihh that causes digit abnormalities alters its signalling capacity and range. Nature 458:1196–1200 [DOI] [PubMed] [Google Scholar]
- 11.Ma G, Yu J, Xiao Y, Chan D, Gao B, Hu J, He Y, Guo S, Zhou J, et al. (2011). Indian hedgehog mutations causing brachydactyly type A1 impair Hedgehog signal transduction at multiple levels. Cell Res 21:1343–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohba S, Kawaguchi H, Kugimiya F, Ogasawara T, Kawamura N, Saito T, Ikeda T, Fujii K, Miyajima T, et al. (2008). Patched1 haploinsufficiency increases adult bone mass and modulates Gli3 repressor activity. Dev Cell 14:689–699 [DOI] [PubMed] [Google Scholar]
- 13.Mak KK, Bi Y, Wan C, Chuang PT, Clemens T, Young M. and Yang Y. (2008). Hedgehog signaling in mature osteoblasts regulates bone formation and resorption by controlling PTHrP and RANKL expression. Dev Cell 14:674–688 [DOI] [PubMed] [Google Scholar]
- 14.Razzaque MS, Soegiarto DW, Chang D, Long F. and Lanske B. (2005). Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol 207:453–461 [DOI] [PubMed] [Google Scholar]
- 15.Logan M, Martin JF, Nagy A, Lobe C, Olson EN. and Tabin CJ, EN Olson (2002). Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33:77–80 [DOI] [PubMed] [Google Scholar]
- 16.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amano K, Densmore M, Fan Y. and Lanske B. (2016). Ihh and PTH1R signaling in limb mesenchyme is required for proper segmentation and subsequent formation and growth of digit bones. Bone 83:256–266 [DOI] [PubMed] [Google Scholar]
- 18.Lufkin T, Mark M, Hart CP, Dollé P, LeMeur M. and Chambon P. (1992). Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature 359:835–841 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wei X, Browning S, Scuderi G, Hanna LS. and Wei L. (2017). Targeted designed variants of alpha-2-macroglobulin (A2M) attenuate cartilage degeneration in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Arthritis Res Ther 19:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ. and Luo Y. (2012). RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ. and Müller R. (2010). Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486 [DOI] [PubMed] [Google Scholar]
- 22.Gosset M, Berenbaum F, Thirion S. and Jacques C. (2008). Primary culture and phenotyping of murine chondrocytes. Nat Protoc 3:1253–1260 [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O'Keefe RJ. and Chen D. (2007). Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis 45:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ. and Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 25.Hlaing TT. and Compston JE. (2014). Biochemical markers of bone turnover—uses and limitations. Ann Clin Biochem 51:189–202 [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Tsang KY, Tang HC, Chan D. and Cheah KS. (2014). Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A 111:12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, von der Mark K, Henry S, Norton W, Adams H. and de Crombrugghe B. (2014). Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 10:e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J, de Crombrugghe B, Hinton RJ. and Feng JQ. (2015). Chondrocytes directly transform into bone cells in mandibular condyle growth. J Dent Res 94:1668–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jing Y, Jing J, Ye L, Liu X, Harris SE, Hinton RJ. and Feng JQ. (2017). Chondrogenesis and osteogenesis are one continuous developmental and lineage defined biological process. Sci Rep 7:10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS. and Mirams M. (2008). Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol 40:46–62 [DOI] [PubMed] [Google Scholar]
- 31.Chung UI, Schipani E, McMahon AP. and Kronenberg HM. (2001). Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest 107:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holder N. (1977). An experimental investigation into the early development of the chick elbow joint. J Embryol Exp Morphol 39:115–127 [PubMed] [Google Scholar]
- 33.Maeda Y, Nakamura E, Nguyen MT, Suva LJ, Swain FL, Razzaque MS, Mackem S. and Lanske B. (2007). Indian hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci U S A 104:6382–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.