Abstract
We present a 58-year-old gentleman who initially presented to the otolaryngology clinic with new onset epistaxis revealing a palpable facial mass that was subsequently biopsied revealing metastatic renal cell carcinoma. We hope to present an interesting case highlighting the rarity of this disease and unusual presentation in which the presence of the primary renal cell carcinoma was recognized only after biopsy.
Keywords: Metastatic renal cell carcinoma, Paranasal sinuses
Introduction
Renal cell carcinoma (RCC) is the most common primary tumor of the renal parenchyma and represents approximately 3% of all malignancies [1], [2], [3], [4]. RCC is known to metastasize to unusual sites and has been reported to metastasize to the sinonasal region. Clinically, patients present with either nasal obstruction or epistaxis due to the vascularity of the mass.
Clinical history
A 58-year-old gentleman was referred to the otolaryngology clinic for evaluation of epistaxis and a midline anterior facial mass. The patient reported swelling between his eyes and forehead for a month prior to presentation with new left epistaxis requiring nasal packing.
On physical exam, it was noted that there was a palpable soft tissue mass overlying the forehead and mid face that appeared to involve the medial canthus bilaterally. No skin changes, scar or palpable bone was identified on physical exam.
Nasal endoscopy was performed at the time of presentation and was notable for a vascularized soft tissue mass occupying the superior half of the nasal cavity with obliteration of the olfactory cleft. The left anterior nasal cavity was occluded with packing material and inspissated secretions in the left inferior turbinate.
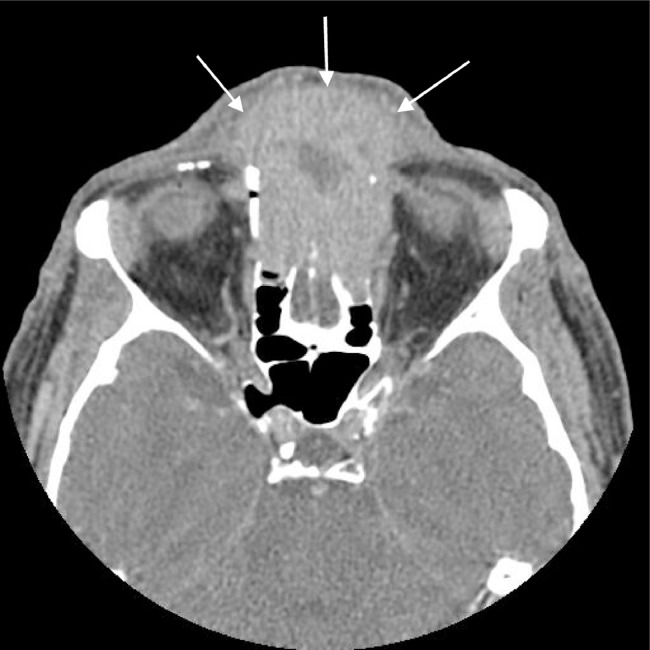
Imaging from an outside hospital was performed and showed a heterogenous enhancing 4.2 × 3.9 × 5.5 cm mass arising from the nasal cavity and extending into anterior cranial fossa with regions of low attenuation. There was erosion of the cribriform plate and crista galli with further invasion into the frontal sinus and extraconal fat of both orbits. No calcifications were identified in the mass (Fig. 1).
Fig. 1.
Axial contrast-enhanced computed tomography through the orbits shows an expansile enhancing soft tissue mass occupying the ethmoid sinuses and soft tissues of the anterior face. This image also demonstrates erosion of the anterior ethmoid air cells and nasal bones.
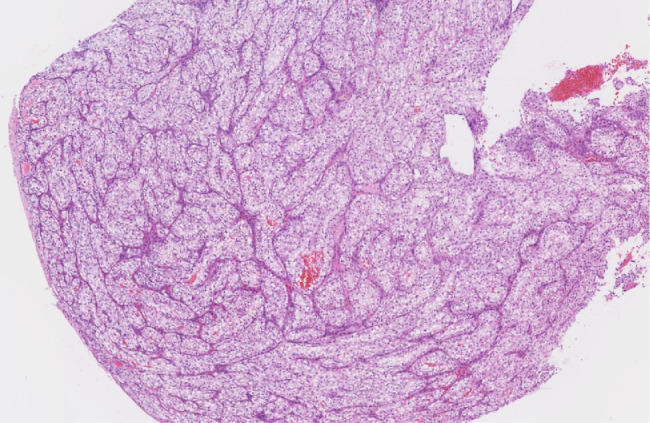
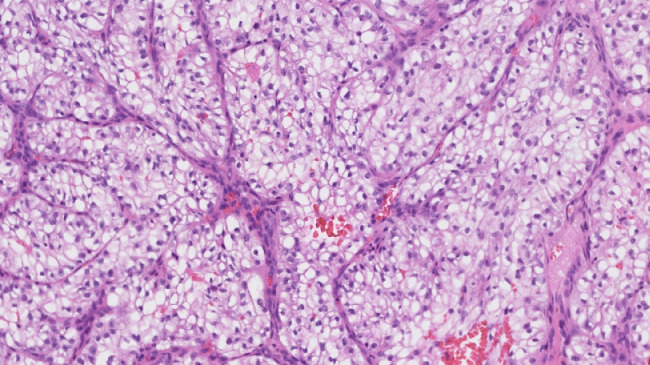
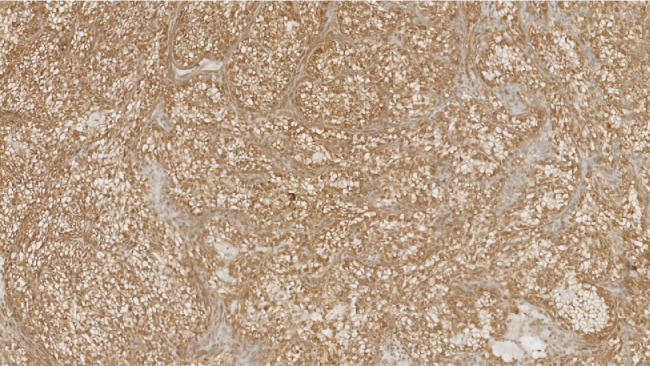
It was decided to perform an intraoperative biopsy due to the vascularity of the lesion. In the operating room, multiple biopsies were taken, which was complicated by bleeding requiring local topical management to control. The patient's postoperative course was uneventful. The final pathology demonstrated carcinoma with clear cell variant and immunohistochemical profile consistent with metastatic RCC; morphologically heterogeneous group clear and/or eosinophilic cytoplasm cells with network of small, thin-walled, chicken wire vasculature; and clusters of variably cohesive large epithelioid cells with large, eccentric, and irregular nuclei, and prominent nucleoli (Figs. 2 and 3). Clear cell RCC will commonly express epithelial markers such as AE1 and/or AE3, CAM 5.2, and epithelial membrane antigen, which were all positive in our case. Consequently, this immunohistochemical profile was consistent with metastatic RCC (Fig. 4).
Fig. 2.
Low-power hematoxylin and eosin image of the lesion demonstrates heterogeneous grouped clear and/or eosinophilic cytoplasm cells with networks of small, thin-walled, ``chicken wire'' vasculature.
Fig. 3.
High-power hematoxylin and eosin of the lesion demonstrates heterogeneous group clear and/or eosinophilic cytoplasm cells with network of small, thin-walled, ``chicken wire'' vasculature. Clusters of variably cohesive large epithelioid cells with large, eccentric, and irregular nuclei, and prominent nucleoli.
Fig. 4.
Positive renal cell carcinoma immunohistochemical stain supportive of metastatic renal cell carcinoma.
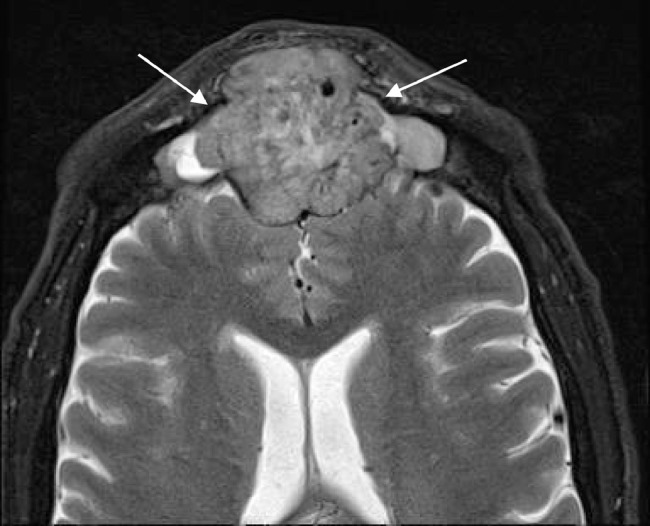
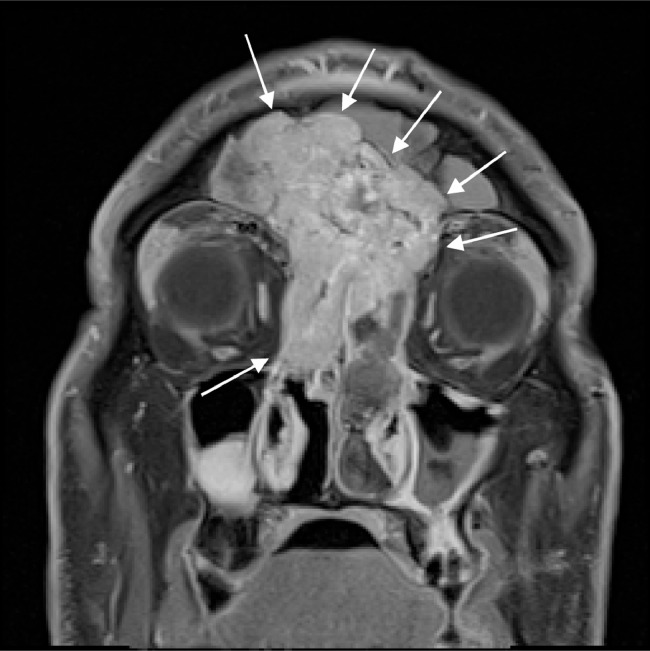
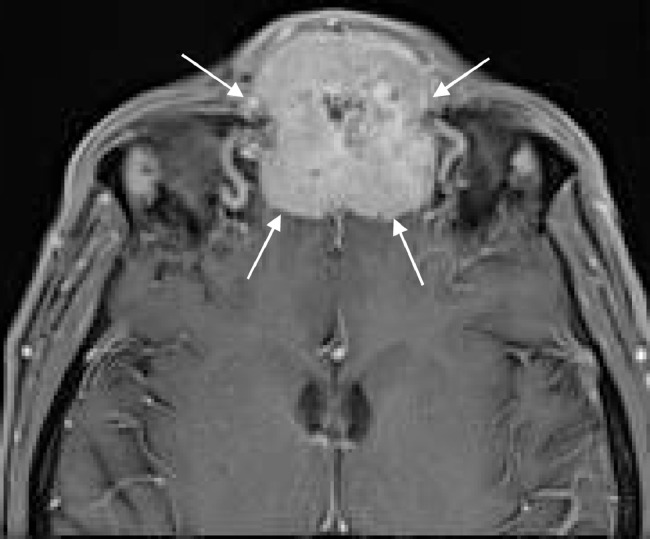
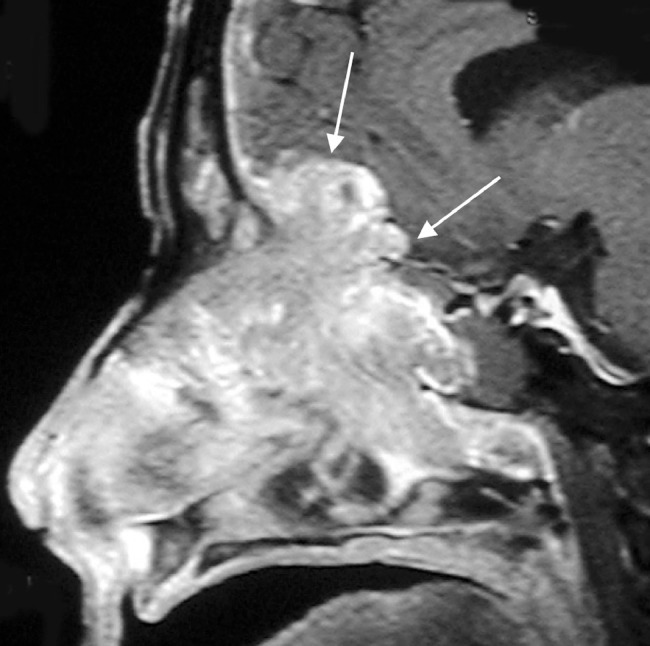
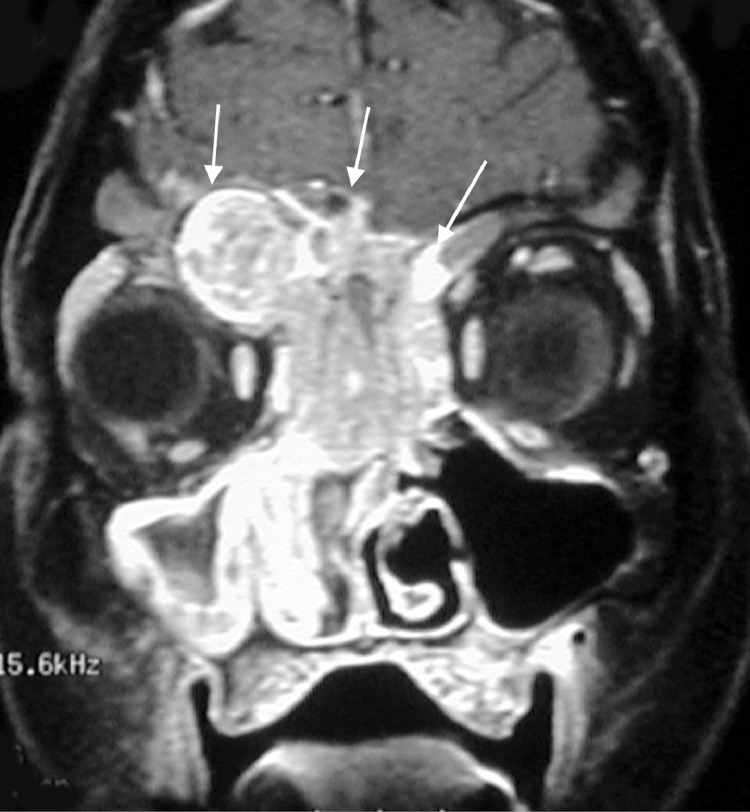
Postbiopsy contrast-enhanced magnetic resonance imaging (MRI) demonstrated a large avidly enhancing heterogeneous mass with multiple flow voids centered within the superior nasal cavity and involving the ethmoid and frontal sinus. The enhancement infiltrated through the cribriform plate and posterior frontal sinus with extension into the anterior cranial fossa and extra-axial anterior frontal lobe (Fig. 5, Fig. 6, Fig. 7).
Fig. 5.
Axial T2-weighted image shows a heterogeneous mass in the frontal sinus with extension through the posterior wall and involving the extra-axial space of the anterior cranial fossa. Note the presence of several flow voids indicating vascularity.
Fig. 6.
Coronal postcontrast T1W image shows a large infiltrating enhancing mass centered in the sinonasal cavity and eroding through the cribriform plate and ethmoid sinus. Enhancing soft tissue is also extending into the left extraconal orbital fat.
Fig. 7.
Axial postcontrast T1WI again shows an avidly enhancing mass extending through the frontal sinus and touching the dura of the frontal lobes.
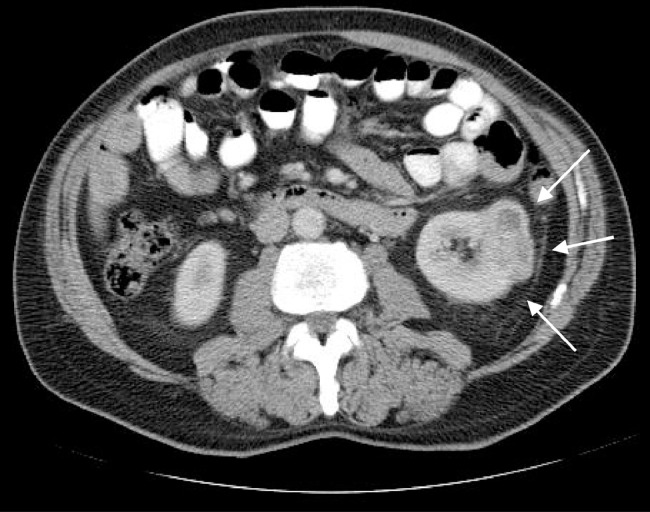
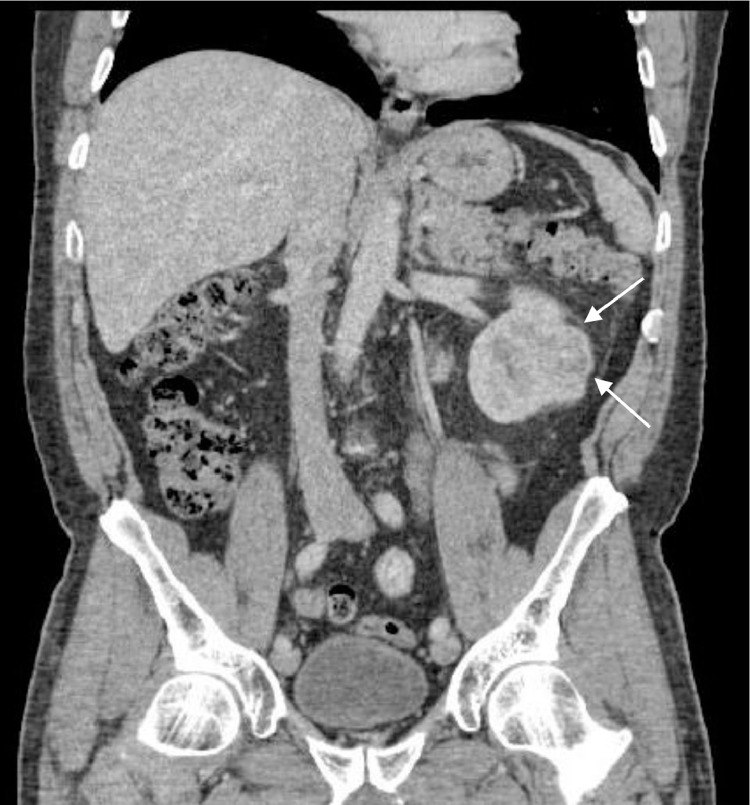
As a result, a computed tomography (CT) examination of the chest, abdomen, and pelvis was recommended, which did indeed show a 3.0-cm enhancing mass at the interpole of the left kidney consistent with RCC (Figs. 8 and 9).
Fig. 8.
Axial contrast-enhanced CT of the abdomen shows an exophytic enhancing mass at the interpole of the kidney indicating the site of primary malignancy.
Fig. 9.
Coronal contrast-enhanced CT of the abdomen shows the solid enhancing mass at the interpole of the left kidney indicating the site of primary malignancy.
The patient was referred to urology for evaluation of resection of the primary tumor. Additionally, palliative radiation therapy to the skull base was offered to provide local disease control and reduce the bleeding; however, the patient preferred systemic therapy and was subsequently referred to medical oncology to discuss systemic options.
Discussion
RCC comprises approximately 3% of all primary malignancies, while primary sinonasal neoplasms compromise <1% of all malignancies [1], [2], [3], [4]. The most common sites of metastasis of RCC are in the lung at 74%, liver at 41%, and bone at 42% [1], [4], [5], [6]. Although not as common, RCC has been reported to metastasize to the head and neck. This usually manifests as disease in the thyroid gland; however, the sinonasal cavity and paranasal sinuses have been described. As these tumors are known to be hypervascular, epistaxis is a common clinical presentation. RCC is classically known to metastasize to unusual locations with a median time of 15 months after nephrectomy. Literature reviews of 46 cases of metastatic tumors to the head and neck region, 4 of which were located in the nasal and/or paranasal sinuses and 1 in the maxillary sinus [2], [3], [4].
The sinonasal cavity is composed of the nasal cavity, maxillary sinus, ethmoid sinus, frontal sinus, and sphenoid sinus. As a result, various epithelial types are present including squamous mucosa, respiratory mucosa, and olfactory mucosa. The submandibular nodes drain the anterior sinonasal tract and retropharyngeal nodes drain the posterior tract. The differential diagnosis for masses in this region includes angiofibroma, inverted papilloma, squamous cell carcinoma, adenocarcinoma, or metastasis. Malignant features include tumor expansion, erosion, remodeling, expansion of the sphenopalatine foramen, and pterygopalatine fossa [1], [3], [4], [5], [6], [7]. Olfactory neuroblastomas arise from neuroepithelial cells of the olfactory mucosa with possibility for lymph node metastasis and distant metastasis. Characteristic imaging features of olfactory neuroblastomas include a large mass occupying the nasal cavity including the cribriform plate and can extend into the adjacent paranasal sinuses or anterior cranial fossa and difficult to reliably differentiate from other tumors occupying the paranasal sinuses. As this tumor grows slowly, it tends to invade into adjacent membrane giving a characteristic appearance of appearance of this tumor is involvement of the lamina papyrecea, cribriform plate, and fovea ethmoidalis with intratumoral cysts (Figs. 10 and 11) [8], [9], [10], [11].
Fig. 10.
Sagittal contrast-enhanced T1W image of the nasal cavity shows an enhancing mass invading through the cribriform plate and anterior cranial fossa with intracranial extension.
Fig. 11.
Coronal contrast-enhanced T1W image of the nasal cavity shows an enhancing mass centered in the paranasal sinuses and extending superiorly into the anterior cranial fossa.
Traditionally, contrast-enhanced CT is the imaging test of choice to delineate vascularity, extent, invasion, and skull base involvement [7]. Characteristic findings in metastatic RCC include avid enhancement without calcification due to high tumor vascularity. Intralesional calcifications are more often seen in adenocarcinoma, olfactory neuroblastoma, inverted papilloma, fibrous dysplasia, osteomas, osteosarcomas, and cartilaginous tumors [1], [2], [3], [4], [5], [6], [7]. Further, high-grade malignancies tend to show extensive bony destruction. MRI provides greater soft tissue resolution compared to CT. Malignant tumors are usually characterized by T2-weighted high signal with variable T1-weighted signal intensity [7]. Low Apparent Diffusion Coefficient (ADC) indicates hypercellularity, hemorrhage, or necrosis. Reported ADC values of 0.87 are most commonly associated with malignancy [7]. Perineural spread and dural invasion should specifically be sought on MRI. Management is typically based on symptomatology and those symptoms requiring intervention such as epistaxis. A case series demonstrated 30% complete response and 50% partial response with endoscopic resection [1]. Local control of head and neck metastatic disease is often performed with preoperative embolization prior to resection [1]. Gross total resection with microscopic negative margins was the greatest predictor of overall survival and progression free survival, while involvement of the cavernous sinuses or internal carotid artery require complicated operative management [10], [11].
Conclusion
In conclusion, we present an interesting case of metastatic RCC recognized only after biopsy of a hypervascular nasal cavity mass. Although RCC is not a common primary malignancy, it has a propensity to metastasize to unusual locations including the paranasal sinuses and nasal cavity.
References
- 1.Mahajan R., Mayappa N., Prashanth V. Metastatic renal cell carcinoma presenting as nasal mass: case report and review of literature. Indian J Otolaryngol Head Neck Surg. 2016;68(3):374–376. doi: 10.1007/s12070-015-0959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechara G.R., Resende Junior J.A.D., Gouveia H.A., de Souza T.A. Metastasis to paranasal sinuses as the first presenting sign of renal cell carcinoma. Open J Urol. 2012;2:28–31. [Google Scholar]
- 3.Remenschneider A., Sadow P., Lin D., Gray S. Metastatic renal cell carcinoma to the sinonasal cavity: a case series. J Neurol Surg Rep. 2013;74:67–72. doi: 10.1055/s-0033-1346972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechara G.R., Resende Junior J.A.D., Gouveia H.A., de Souza T.A. Metastasis to paranasal sinuses as the first presenting sign of renal cell carcinoma. Open J Urol. 2012;2:28–31. [Google Scholar]
- 5.Sountoulides P., Metaxa L., Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: a review of case reports. J Med Case Rep. 2011;5:429. doi: 10.1186/1752-1947-5-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescher A., Brors D. Metastases to the paranasal sinuses: case report and review of the literature. Laryngorhinootologie. 2001;80:583–594. doi: 10.1055/s-2001-17835. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi M., Kato H., Tomita H., Mizuta K., Aoki M., Hara A. Imaging characteristics of malignant sinonasal tumors. J Clin Med. 2017;6:116. doi: 10.3390/jcm6120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampath P., Park M., Huang D., Deville C., Cortez S., Chougule P. Esthesioneuroblastoma (olfactory neuroblastoma) with hemorrhage: an unusual presentation. Skull Base. 2006;16:169–173. doi: 10.1055/s-2006-939677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ow T., Bell D., Kupferman M., DeMonte F., Hanna E. Esthesioneuroblastoma. Neurosurg Clin N Am. 2013;24:51–65. doi: 10.1016/j.nec.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Bak M., Wein R. Esthesioneuroblastoma: a contemporary review of diagnosis and management. Hematol Oncol Clin N Am. 2012;26:1185–1207. doi: 10.1016/j.hoc.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 11.DeMonte F. Management considerations for malignant tumors of the skull base. Neurosurg Clin N Am. 2013;24:1–10. doi: 10.1016/j.nec.2012.08.003. [DOI] [PubMed] [Google Scholar]