ABSTRACT
Non-small cell lung cancer (NSCLC) accounts for the majority of all lung cancer cases, which is the leading cause of cancer deaths worldwide. IL-17░A, the major effector cytokine derived from Th17 cells, is a key cytokine in tumor pathogenesis and modulates tumor progression. We aimed to identify whether IL-17░A derived from Th17 cells promotes the progression of NSCLC. Here we found that the level of Th17 cells was increased in NSCLC and IL-17░A was mainly produced by CD4+ cells (Th17 cells) in NSCLC. IL-17░A enhanced the migration, invasion and stemness of NSCLC via STAT3/NF-κB/Notch1 signaling. Blockade of this signaling inhibited the migration, invasion and stemness of NSCLC mediated by IL-17░A. Th17 cells in NSCLC were closely associated with poor prognosis of NSCLC patients. Our results indicated that Th17 cell-derived IL-17░A plays an important role in tumor progression of NSCLC via STAT3/NF-κB/Notch1 signaling. Therefore, therapeutic strategies against this pathway would be valuable to be developed for NSCLC treatment.
KEYWORDS: Tumor microenvironment, Th17 cells, interleukin-17A (IL-17A), non-small cell lung cancer (NSCLC), tumor progression
Introduction
Non-small cell lung cancer (NSCLC) accounts for the majority of all lung cancer cases,1 which is the leading cause of cancer deaths worldwide.2 The unfavorable outcome could be at least partially attributed to the poor understanding of NSCLC tumor microenvironment. The complex tumor microenvironment comprises immune cells, fibroblasts, blood and lymphatic vessels,3 which promotes the proliferation, invasion and metastasis of tumor cells, and contributes to disease progression.4 T helper (Th) 17 cells are a subtype of Th cell, which have been shown highly pro-inflammatory and to contribute to autoimmune disease.5,6 Interleukin (IL)-6 and TGF-β can promote the differentiation of Th17 cells through STAT3.7-9 The orphan retinoic acid nuclear receptor (ROR) family transcription factor RORγt is essential for Th17 development and function.10 IL-17 family is composed of IL-17░A-F, among them, IL-17░A (also termed IL-17) is the major effector cytokine derived from Th17 cells.
Recently, it has been shown that IL-17░A was detected in several tumors.11,12 The evidences indicate that tumor development is associated with Th17 cells and IL-17.13-15 The adoptive transfer of Th17 cells induced by TGF-β and IL-6 promoted tumor growth in a CD39-dependent manner.13 IL-17 promotes tumor progression by enhancing angiogenesis in a mouse model of breast cancer.14 Therefore, we consider that IL-17░A is a key cytokine in tumor pathogenesis and modulates tumor progression.
However, the evidence to indicate the protumoral activity of IL-17░A in NSCLC is not clear. The aim of this study is to evaluate whether IL-17░A derived from Th17 cells promotes the progression of NSCLC. Our results indicate that Th17 cell-derived IL-17░A can promote the invasion, migration and cancer stem cell-like properties via STAT3/NF-κB/Notch1 signaling in NSCLC.
Results
The level of Th17 cells was increased in NSCLC
To determine the relationship between Th17 cells and NSCLC, Th17 cell frequencies in peripheral blood from NSCLC patients and healthy donor were analyzed by flow cytometry (Fig. 1░A). CD3+CD4+IL-17░A+IFN-γ−cell population was defined as Th17 cells as previously described.16,17 The percentage of Th17 cells in peripheral blood from NSCLC patients was significantly higher than that from healthy donor (P<0.001, Fig. 1B). IL-17░A mRNA level was also significantly higher in peripheral blood from NSCLC patients than that from healthy donor (P<0.001, Fig. S1░A). Th17 cell frequency in NSCLC tissues was significantly higher than that in non-tumor tissues (P<0.05, Fig. 1░C). The mRNA level of IL-17░A was significantly higher in NSCLC tissues than that in non-tumor tissues (P<0.05, Fig. S1B). Moreover, Th17 cell frequency in tumor tissue was increased compared to that in peripheral blood from NSCLC patients (P<0.01, Fig. 1D). The relationships between Th17 cell frequency and clinical parameters were shown in Table 1. In addition, we detected the expression of Th17 cells in tumor tissues and non-tumor tissues from NSCLC patients by immunofluorescence. Tumor-infiltrated CD4+ T cells exhibited high-level production of IL-17░A (Fig. 1E). Therefore, these results indicate that the level of Th17 cells is increased in NSCLC, and IL-17░A is mainly produced by CD4+ cells (Th17 cells) in NSCLC.
Figure 1.
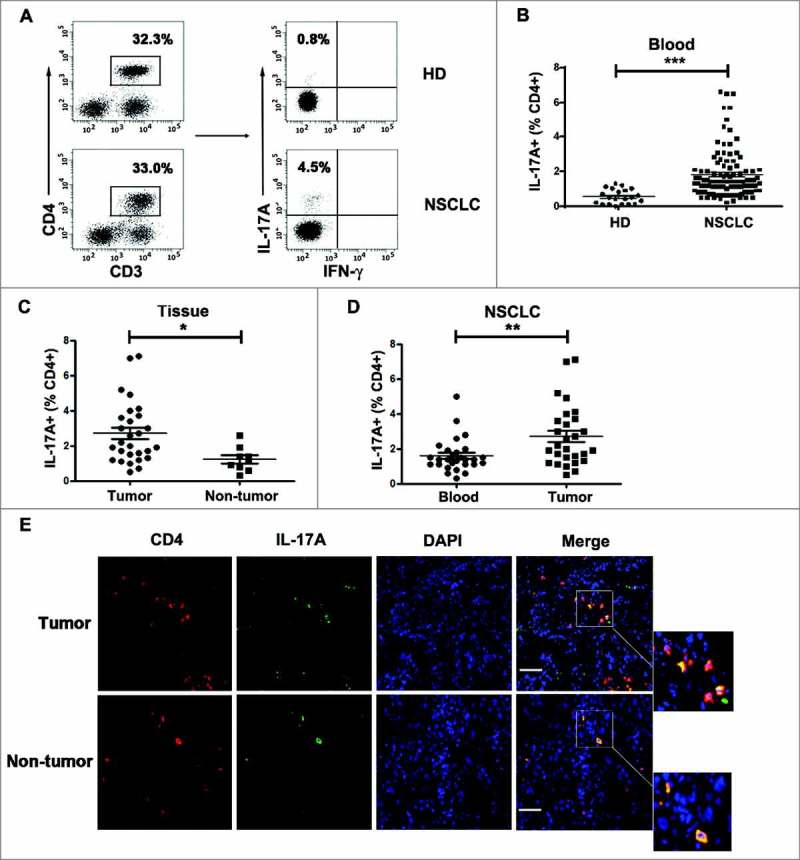
The level of Th17 cells in NSCLC was increased. (A) CD3+CD4+IL-17░A+IFN-γ−Th17 cells from peripheral blood were analyzed by flow cytometry in NSCLC patients and healthy donors. (B) Comparison of Th17 cell phenotypes in peripheral blood from NSCLC patients and healthy donors represented as scatter. (C) Comparison of Th17 cell frequencies in NSCLC tissues and non-tumor tissues represented as scatter. (D) Comparison of Th17 cell frequencies in peripheral blood and tumor tissues from NSCLC patients represented as scatter. (E) Tumor tissues and non-tumor tissues from NSCLC patients were subjected to double immunofluorescence for CD4 (red), IL-17░A (green) and DAPI (blue). *, ** and *** indicate P < 0.05, P < 0.01 and P< 0.001, respectively. Scale bar represents 50 μm.
Table 1.
Correlations between Th17 expression and clinicopathological parameters
| Characteristics | Number | P value | |
| Age at diagnosis | <65 years old | 30 | 30 |
| ≥65 years old | 20 | 0.8662 | |
| Gender | Male | 35 | |
| Female | 15 | 0.9409 | |
| Histological type | Adenocarcinoma | 25 | |
| Squamous carcinoma | 20 | 0.2449 | |
| Large cell carcinoma | 5 | ||
| Tumor grade | Good | 8 | |
| Moderate | 21 | ||
| Poor | 21 | 0.0203 | |
| TNM stage | I | 18 | |
| II | 14 | ||
| III | 11 | 0.0340 | |
| IV | 7 | ||
| Lymph node metastasis | Positive | 28 | |
| Negative | 22 | 0.0110 | |
| Smoking habit | Yes | 21 | |
| No | 29 | 0.9686 |
IL-17░A promotes the migration and invasion of NSCLC cells
Whether IL-17░A derived from Th17 cells has the effect on NSCLC progression, the migration and invasion assays were firstly performed in vitro. After the treatment of rhIL-17░A in vitro, the migration activity of A549 and H460 cells was significantly increased compared to control (P<0.05, Fig. 2░A and B). Moreover, the invasion activity of A549 and H460 cells with the treatment of rhIL-17░A was also increased compared to untreated group in vitro (P<0.05, Fig. 2░C and D). Then, the effect of IL-17░A on epithelial-mesenchymal transition (EMT) and angiogenesis of NSCLC was evaluated in vitro. We detected the expression of N-cadherin (mesenchymal-related gene) in A549 and H460 cells with or without rhIL-17░A treatment by western blotting and qPCR. The results showed that the expression of N-cadherin was significantly higher in cells with rhIL-17░A treatment than that in untreated groups (P<0.05, Fig. 2E and F). Snail and vimentin, two other EMT-related genes, were investigated by western blotting and qPCR as well. The expressions of snail and vimentin were significantly higher in cells with rhIL-17░A treatment than that in untreated groups (P<0.05, Fig. S2░A-C). Next, the effect of IL-17░A on angiogenesis of NSCLC was evaluated. First, the expression of IL-17 receptor on tumor tissues and endothelium of blood vessels was detected. The results showed that IL-17 receptor expression in NSCLC tissues was significantly higher than that in non-tumor tissues (P<0.05, Fig. S2D). Similarly, the expression of IL-17 receptor in primary endothelium from NSCLC tissues was significantly higher than that from non-tumor tissues (P<0.05, Fig. S2E). Then we found tube formation was enhanced with rhIL-17░A treatment than that in untreated groups (P<0.05, Fig. S2░F). Further, the relationship between IL-17░A and CD31, which is the biomarker of angiogenesis, was investigated. We found that IL-17░A expression was associated with CD31 (P<0.05, Fig. S2G). Lastly, the expression of CD31 was investigated in NSCLC tissues by immunofluorescence. CD31 was highly or lowly expressed in NSCLC tissues in correspondence with high or low expression of IL-17░A (Fig. 2G). The results suggest that IL-17░A promotes the migration and invasion of NSCLC cells.
Figure 2.
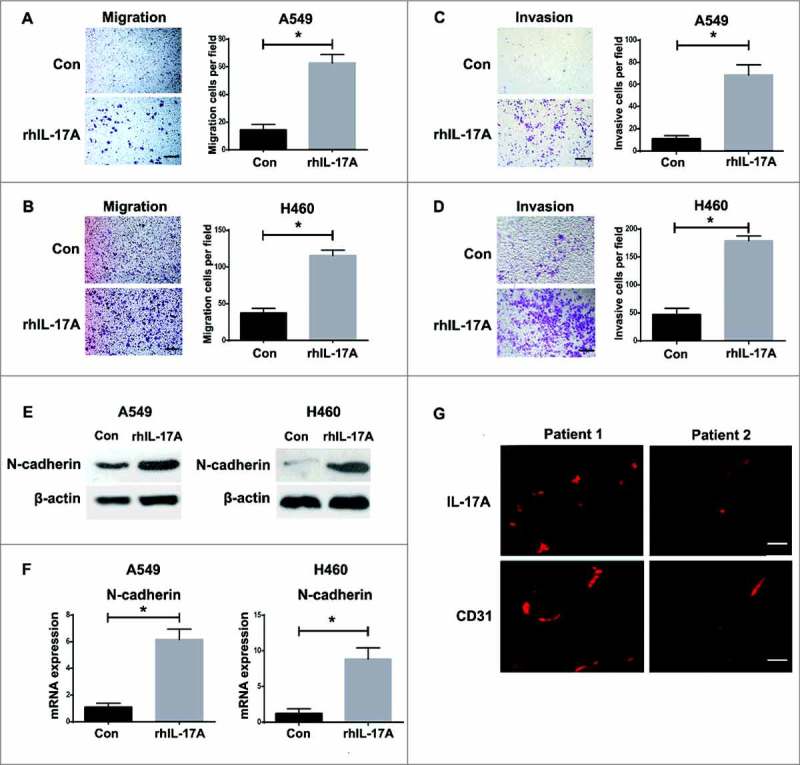
IL-17░A promoted the migration and invasion of NSCLC cells in vitro. The migration activities of A549 (A) and H460 (B) cells, and the invasion activities of A549 (C) and H460 (D) cells before and after treatment of rhIL-17░A was analyzed using transwell assay. One representative analysis is shown. Data are presented as a histogram. (E) The expression of N-cadherin in A549 and H460 cells before and after treatment of rhIL-17░A was analyzed using western blotting. (F) The relative expression of N-cadherin in A549 and H460 cells before and after treatment with rhIL-17░A was analyzed using qPCR. (G) The expressions of IL-17░A and CD31 in tumor tissues from NSCLC patients were detected by immunofluorescence. * indicates P < 0.05. Scale bar represents 50 μm.
The STAT3/NF-κB/Notch1 signaling was critical for IL-17░A-induced migration and invasion in NSCLC cells
Recent study showed that IL-17░A could promote the transition from chronic pancreatitis to pancreatic cancer through stimulating STAT3 activation.18 It is shown that tumorigenesis capacity was mediated by NF-κB signaling in ovarian cancer.19 It is also demonstrated that IL-17 could induce Notch1 activation in oligodendrocyte progenitor cells that enhanced proliferation and inflammatory gene expression.20 Furthermore, Notch1 signaling pathway is associated with cancer stem cell (CSC)-like properties in tumors.21 To determine whether the STAT3/NF-κB/Notch1 signaling is involved in IL-17░A-induced migration and invasion in NSCLC, the expression of phospho-STAT3, phospho-p65 and cleavage-Notch1 in A549 and H460 cells treated with rhIL-17░A was investigated by western blotting. The results showed that rhIL-17░A increased phospho-STAT3, phospho-p65 and cleavage-Notch1 in A549 and H460 cells (Fig. 3░A, Fig. S3░A-C). Then, we investigated whether STAT3/NF-κB/ Notch1 depletion would affect IL-17░A-mediated tumor progression in NSCLC. rhIL-17░A enhanced the migration and invasion activities in A549 and H460 cells treated with or without DMSO, but could not do so in A549 and H460 cells treated with STAT3, or NF-κB, or Notch1 inhibitors, respectively (Fig. 3B-G). The result was confirmed with STAT3, or NF-κB, or Notch1 knockdown, respectively (Fig. S3D-H). In addition, IL-17░A-mediated high level of N-cadherin expression in NSCLC cells was blocked in A549 and H460 cells treated with signaling inhibitors compared to cells treated with or without DMSO (Fig. 3░H). All of these results demonstrate that STAT3/NF-κB/Notch1 signaling is critical for IL-17░A-induced migration and invasion in NSCLC cells.
Figure 3.
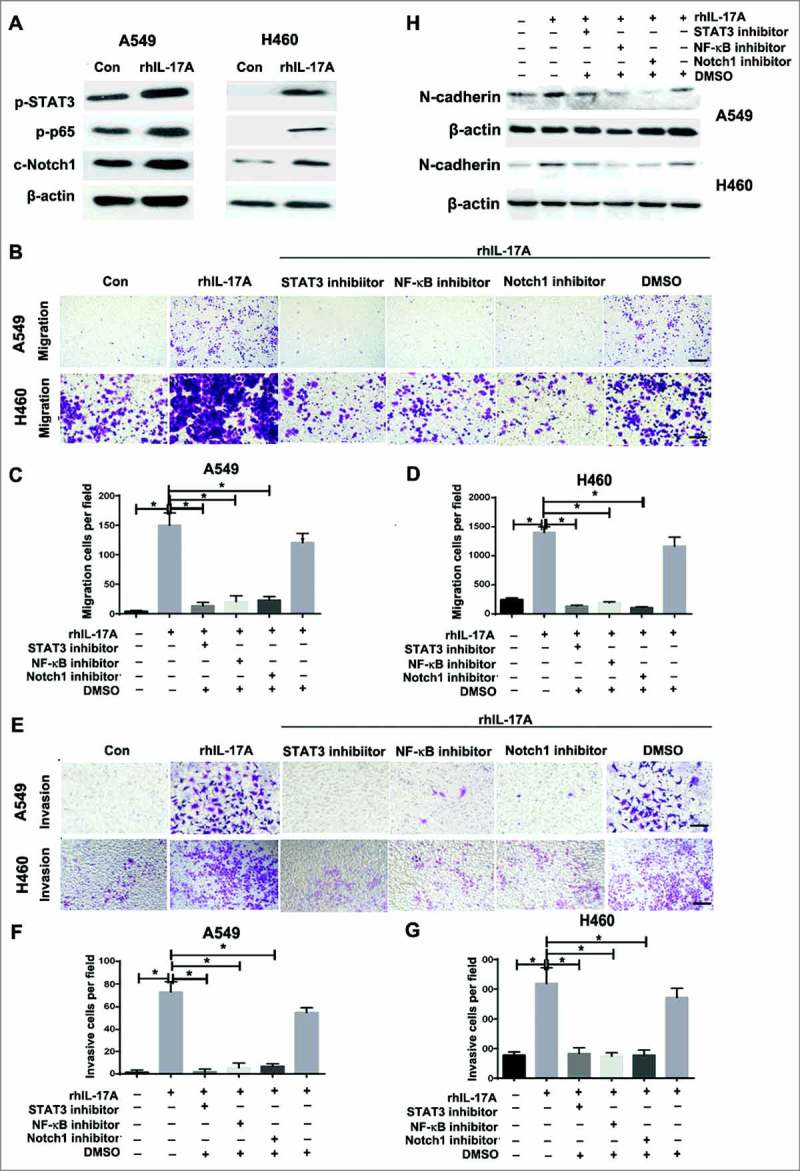
The STAT3/NF-κB/Notch1 signaling was critical for IL-17░A-induced migration and invasion in NSCLC cells. (A) The activation of STAT3, NF-κB and Notch1in A549 and H460 cells treated with rhIL-17░A was analyzed using western blotting. (B) The migration activities of A549 and H460 cells treated with or without rhIL-17░A and STAT3, or NF-κB, or Notch1 inhibitor were assessed by transwell assay. One representative analysis is shown. The data from A549 (C) and H460 (D) cells are presented as histogram. (E) The invasion activities of A549 and H460 cells treated with or without rhIL-17░A and these molecular inhibitors were assessed by transwell assay. One representative analysis is shown. The data from A549 (F) and H460 (G) cells are presented as a histogram. (H) The expression of N-cadherin in A549 and H460 cells treated with or without rhIL-17░A and STAT3, or NF-κB, or Notch1 inhibitor was analyzed using western blotting. * indicates P < 0.05. Scale bar represents 50 μm.
IL-17░A promoted the CSC-like properties of NSCLC cells
Stemness is an important characteristic of tumor progression. To determine the effect of IL-17░A on the stemness of NSCLC, sphere formation assay was firstly investigated in vitro. The sphere formation was enhanced in A549 and H460 cells treated with rhIL-17░A compared to untreated cells (P<0.01, Fig. 4░A and B). In addition, the expression of CSC-related gene Oct4 in A549 and H460 cells treated with rhIL-17░A was significantly higher than that in untreated cells (Fig. 4░C). Nanog and Sox2, two other CSC-related genes, were significantly higher with rhIL-17░A treated than that in untreated cells (Fig. S4░A-C). After the treatment with docetaxel, the apoptosis of A549 and H460 cells was significantly increased compared to control (P<0.05, Fig. 4D and E). Moreover, the apoptosis of A549 and H460 cells with the treatment of docetaxel was blocked by rhIL-17░A in vitro (Fig. 4D and E), indicating that IL-17░A induces the resistance of NSCLC cells.
Figure 4.
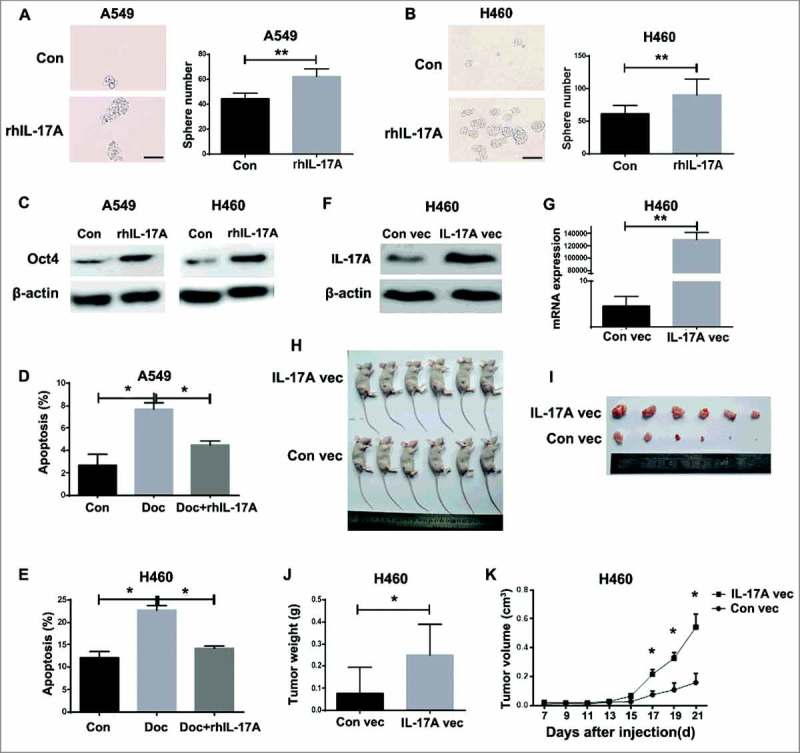
IL-17░A promoted the CSC-like properties of NSCLC cells. A549 (A) and H460 (B) cells were cultured with rhIL-17░A for 7 days, and then collected for sphere assay. One representative photomicrograph is shown. Data are presented as a histogram. (C) The expression of Oct4 in A549 and H460 cells before and after treatment of rhIL-17░A was analyzed using western blotting. The apoptosis of A549 (D) and H460 (E) cells treated with or without docetaxel and rhIL-17░A was analyzed by flow cytometry. IL-17░A expression in IL-17░A-expressing and control vectors-tranfected H460 cells was analyzed by western blotting (F) and qPCR (G). (H) IL-17░A promotes NSCLC tumor growth in vivo. IL-17░A expressing A549 cells were injected subcutaneously into nude mice (n = 6 mice/group). Tumor volumes were measured at 21 days after cell implantation. (I) Lump images of xenograft tumors that were formed in nude mice at 21 days after cell implantation. (J) The result of tumor weights at 21 days after cell implantation was shown as histogram. (K) Tumor growth curve from 7 to 21 days after cell implantation was shown as statistical graph. * indicates P < 0.05. Scale bar represents 100 μm.
To further evaluate whether IL-17░A is required for CSC-like properties in NSCLC, stable IL-17░A expression in H460 cells was established using IL-17░A-expressing plasmid (P<0.01, Fig. 4░F and G). The results showed that tumor volumes in IL-17░A-expressing H460 cell group were significantly higher than those in control group (P<0.05, Fig. 4░H, I and K), and the tumor weights in IL-17░A-expressing H460 cell group were increased as well (P<0.05, Fig. 4░J), suggesting that IL-17░A enhanced tumor growth in NSCLC. Collectively, IL-17░A promotes the CSC-like properties in NSCLC cells.
Blockade of STAT3/NF-κB/Notch1 signaling inhibited NSCLC cell stemness promoted by IL-17░A
To examine if the STAT3/NF-κB/Notch1 signaling is required for IL-17░A-induced CSC-like properties in NSCLC cells, the blockade of this signaling in A549 and H460 cells was investigated. The results showed that the enhancing activity of sphere forming in A549 and H460 cells induced by IL-17░A was reversed by the treatment of STAT3, or NF-κB, or Notch1 inhibitors, respectively (Fig. 5░A, B and C). The result was also confirmed with STAT3, or NF-κB, or Notch1 knockdown, respectively (Fig. S5░A-C). After the treatment with STAT3, or NF-κB, or Notch1 inhibitor, Oct4 expression in A549 and H460 cells treated with rhIL-17░A was decreased (Fig. 5D). In addition, IL-17░A-mediated resistance in NSCLC cells was blocked in A549 and H460 cells treated with signaling inhibitors compared to cells treated with DMSO (Fig. 5E and F). Taken together, STAT3/NF-κB/Notch1 signaling is critical for IL-17░A-induced CSC-like properties in NSCLC cells, and blockade of this signaling inhibits the stemness of NSCLC cells mediated by IL-17░A.
Figure 5.
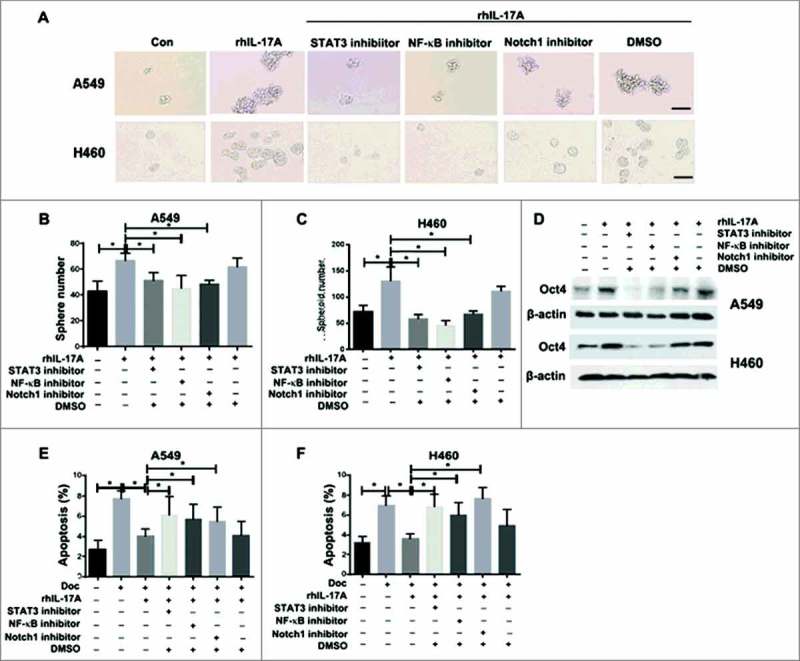
Blockade of STAT3/NF-κB/Notch1 signaling inhibited NSCLC stemness promoted by IL-17░A. (A) After the treatment with STAT3░or NF-κB or Notch1 inhibitor, the sphere forming of A549 and H460 cells induce by rhIL-17░A was blocked. One representative photomicrograph is shown. The results from A549 (B) and H460 (C) cells were shown as histogram. (D) The expression of Oct4 in A549 and H460 cells treated with or without rhIL-17░A and these molecular inhibitors were assessed by western blotting. The apoptosis of A549 (E) and H460 (F) cells treated with or without rhIL-17░A and these molecular inhibitors were assessed by transwell assay. * indicates P < 0.05. Scale bar represents 100 μm.
Th17 cells were associated with poor prognosis in NSCLC patients
Th17 cells were reported associated with a poor clinical outcome in glioblastoma recently.22 So the relationship between Th17 frequency and clinical parameters (TNM stage, tumor grade and lymph node metastasis), and the impact of Th17 cells on therapeutic effect and survival were evaluated in NSCLC patients. The level of Th17 frequency in peripheral blood was increased in patients with II/III TNM stage (P<0.05, Fig. 6░A), poor tumor grade (P<0.05, Fig. 6B), and positive lymph node metastasis (P<0.05, Fig. 6░C). After chemotherapy treatment (cisplatin+ docetaxel), the percentage of Th17 cells was significantly increased in patients with progressive disease (PD) (P<0.05, Fig. 6D), decreased in patients with partial responses (PR) (P<0.01, Fig. 6E), and no significant difference in patients with stable disease (SD) (P>0.05, Fig. 6░F). Upon IL-17░A expression from immunohistochemistry results, patients were grouped as “high” or “low” using the respective median (2.8) as a cut-off point (Fig. 6G). NSCLC patients with dense infiltration of Th17 cells had a worse overall survival (OS) (P<0.05, Fig. 6░H). Taken together, Th17 cells in NSCLC are closely associated with poor prognosis in NSCLC patients, and could be served as a potential biomarker and target for prognosis and treatment of NSCLC.
Figure 6.
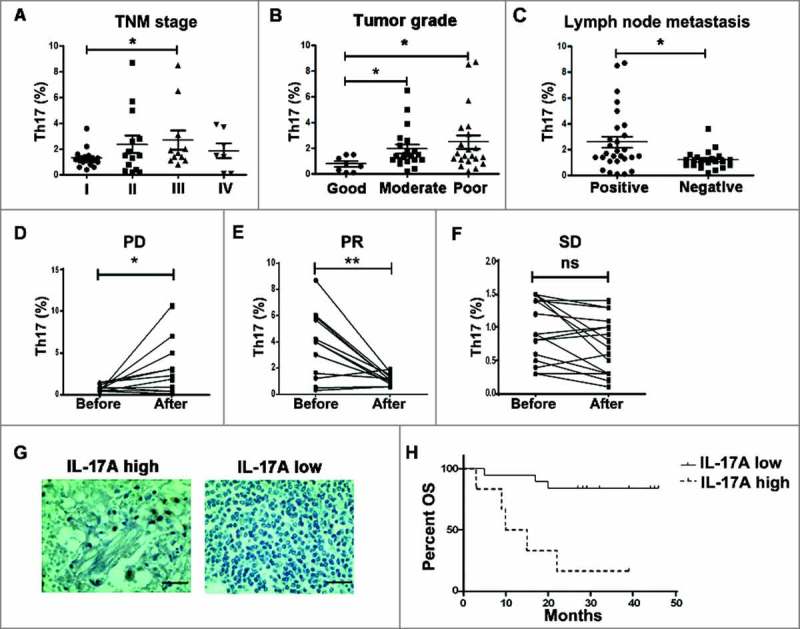
The level of Th17 cells was associated with poor survival in NSCLC patients. The relationship between Th17 frequency and TNM stage (A), tumor grade (B) and lymph node metastasis (C) of NSCLC patients, respectively. The changes of Th17 cell frequency in peripheral blood from NSCLC patients before and after chemotherapy with the status of PD (D), PR (E) and SD (F). (G) IL-17░A expression in tumor tissues of NSCLC was analyzed by immunohistochemistry. (H) Kaplan-Meier survival curves for NSCLC patients with lower and higher IL-17░A expression (immunohistochemistry analysis). *, ** and ns indicate P < 0.05, P < 0.05 and non-significance, respectively. Scale bar represents 50 μm.
Discussion
The link between inflammation and tumorigenesis is well known, studies have implicated many inflammatory components, such as IL-6, as a key player in tumor development, growth, and metastasis.23 Recently, it is shown that the numbers of Th17 cells and expression of IL-17░A are increased in several tumors.24,25 The combination of IL-6 and TGF-β induces IL-17-producing cells (Th17). Moreover, IL-6 can use TGF-β produced by thymus-derived natural regulatory T cells (nTregs) to convert them to Th17 cells. However, IL-2 and TGF-β induce naive T cells to become forkhead/winged helix transcription factor (Foxp3) positive regulatory cells (iTregs), which are resistant to Th17 conversion by IL-6.9 Although numerous studies have been reported that IL-17░A was involved in inducing and mediating inflammatory responses,26,27 the role of IL-17░A in cancer initiation, growth, and metastasis was very controversial.28-30 Therefore, we consider that IL-17░A is an important cytokine in tumor progression. In this study, we aimed to evaluate the role of IL-17░A derived from Th17 cells in tumor progression of NSCLC.
In this study, the percentage of Th17 cells in peripheral blood from NSCLC patients was significantly higher than that from healthy donor, and Th17 cells in NSCLC were closely associated with therapeutic effect and prognosis of NSCLC patients. Other studies have also proven that Th17 cells and IL-17░A were correlated with worse prognosis in cancer patients. Vizio et al. found that increased expression of IL-17 predicted a poor response to chemotherapy and adverse prognosis in pancreatic carcinoma.31 Yamada et al. reported that Th17 cell abundance and IL-17 expression were also elevated in gastric cancer and indicated a poor prognosis.32 In addition, high level of IL-17 expression and tumor-infiltrating Th17 cells were associated with worse prognosis in patients with invasive ductal carcinoma, colorectal and lung cancer patients.33-35
Abundant evidences have indicated that tumor development is closely correlated with IL-17. IL-17 could promote angiogenesis in tumor, mainly by stimulating surrounding fibroblasts and endothelial cells to produce proangiogenic factors such as VEGF.36,37 Furthermore, IL-17-transfected HCC cells significantly promoted angiogenesis, neutrophil recruitment and tumor growth in vivo.38 Recently, Treg enrichment in cancer patients correlated with the increase of IL-17░A expression which induced tumor invasiveness.39 In addition, IL-17 promoted mammary tumor progression by changing tumor cell behavior and eliciting tumorigenic neutrophils recruitment.33 IL-17 contributed to ovarian cancer malignancy through promoting CD133+ CSCs self-renewal.40 In this study, we found that IL-17░A promoted the migration and invasion of NSCLC by transwell assays, enhanced N-cadherin expression in NSCLC cells and CD31 expression in xenograft tumors. Moreover, our data showed that rhIL-17░A enhanced the sphere formation of NSCLC cells, the expression of Oct4 and resistance in NSCLC cells, and tumor growth in NSCLC, indicating that IL-17░A promotes the CSC-like properties in NSCLC cells. Taken together, IL-17░A promotes the migration, invasion and stemness to induce tumor progression in NSCLC.
The signaling mediated IL-17░A-induced tumor progression is reported by several studies. Celine et al. found that IL-17 functions through the novel REG3β/JAK2/STAT3 signaling pathway to promote the transition from chronic pancreatitis to pancreatic cancer.18 The effect of IL-17 on tumor progression was operated through AKT signaling activation, which resulted in IL-6 production. Then, IL-6 activated JAK2/STAT3 signaling and subsequently up-regulated IL-8, MMP2, and VEGF expression.38 According to the results from other studies, IL-17 also could promote tumor growth through stimulating production of IL-6 and STAT3 activation.41,42 In addition, IL-17 not only promoted sphere formation of ovarian in vitro, but also enhanced the tumorigenesis in vivo, by p38 MAPK and NF-κB activation.40 Meanwhile, IL-17 could induce Notch1 activation in oligodendrocyte progenitor cells that enhanced proliferation and inflammatory gene expression.20 Pardoll et al. showed that maintenance of NF-κB activity in tumors requires Stat3.43 Pfeffer et al. showed that STAT3 and p65 regulated Notch1 expression in adherent CSCs by directly binding to the Notch1 promoter.44 In our study, we found that IL-17 significantly induced STAT3 and NF-κB phosphorylation and Notch1 cleavage in NSCLC cells. These results indicated that STAT3, NF-κB and Notch1 activation play an important role in NSCLC progression.
Our study supports the principle that IL-17░A/STAT3/NF-κB/Notch1 may participate in NSCLC progression. Thus, targeting IL-17░A/STAT3/NF-κB/Notch1-mediated signaling pathway in tumors may provide a novel approach to controlling tumor growth. There are several approaches available for blocking this signaling. It is shown that IL-17 blockade significantly reduced the tumor volume and inhibited tumor growth in TC-1 tumor model.33 Tumor growth was inhibited in IL-17−/− mice in another B16 melanoma model.42 IL-17░A-neutralizing or IL-17RA blocking antibodies (secukinumab, ixekizumab, and brodalumab), already proven effective in autoimmune disorders such as psoriasis, psoriatic arthritis, and ankylosing spondylitis,45 could be used as potential neo-adjuvants in the treatment of early, non-metastatic colorectal cancer.17 Furthermore, agents targeted STAT3/NF-κB/Notch1 signaling for cancer treatment are used in preclinical and clinical trials. Niclosamide, an inhibitor of STAT3, has the potential to target the IL-6-STAT3-AR pathway to inhibit migration and invasion in advanced prostate cancer.46 1-O-acetylbritannilactone, a Chinese traditional medicine, has been performed to have the activity of anticancer with decreased NF-κB expression.47 A clinical phase I trial was to investigate that enoticumab, a fully human IgG1 monoclonal antibody that binds human Delta-like Ligand 4 and disrupts Notch-mediated signaling, was tolerated, and the antitumor activity included two partial responses and 16 patients with stable disease.48 Similarly, our data showed that blockage of STAT3/NF-κB/Notch1 signaling inhibited the CSC-like properties in NSCLC cells, indicating that STAT3/NF-κB/Notch1 signaling could be served as the potential therapeutic targets of NSCLC.
In summary, high level of IL-17░A derived from Th17 cells in NSCLC enhanced the migration, invasion and stemness of NSCLC cells via STAT3/NF-κB/Notch1 signaling, which could be blocked by these signaling molecular inhibitors. Therefore, therapeutic strategies that target this pathway could represent an effective method for NSCLC treatment.
Materials and methods
Patients and samples
From February 2014 to March 2015, 110 patients with NSCLC from The First Affiliated Hospital of Zhengzhou University were enrolled. These patients were subjected to routine laboratory diagnosis, and the samples were analyzed using conventional cytology. In addition, 41 NSCLC patients were enrolled from May 2014 to June 2015 and administrated with cisplatin (75░mg/m2) plus docetaxel (30░mg/m2 on day 1, 8) every three weeks for 4 cycles. Samples from all these patients’ peripheral blood were obtained. Samples from 21 healthy donors and 9 non-tumor patients were used as control. All patients gave the written informed consent. The whole consent procedure was in accordance with the standards defined by Institutional Review Boards of The First Affiliated Hospital of Zhengzhou University (approval number 2013-LW-1201).
Flow cytometry analysis
Fresh human peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque (Huajing Biology) density gradient centrifugation, and stimulated with 1░mg/mL phorbol-12-myristate-13-acetate (PMA, Sigma, #P1585) and 1░mg/mL ionomycin (Sigma, #I9657) in the presence of Brefeldin-A (BFA, BioLegend, #420601) for 5░h. Cells were stained with anti-human CD3 (BD, #555332), CD4 (BD, #550631), and then intracellular staining for IFN-γ (BD, #557643) and IL-17░A (BD, #580437) was performed as described.49 Cells were analyzed using flow cytometry (BD, FACS CantoII, USA).
Immunofluorescence
The protocol of immunofluorescence is described elsewhere. Mouse anti-CD4 (Abcam, #ab846), rabbit anti-IL-17░A (PeproTech, #500-P07) and mouse anti-CD31 (R&D, #BBA7) were used as the primary antibodies. The secondary antibodies of Alexa Fluor 555-conjugated goat anti mouse IgG (Life technology, #A21422), Dylight 488-conjugated donkey anti-rabbit IgG (Thermo Fisher, #A21206), and Dylight 555-conjugated goat anti-rabbit IgG (Thermo Fisher, #A21430) were used in immunofluorescence. The nuclear was stained with 40,6-diamidino-2-phenylindole (DAPI, Solarbio, #C0065). Images were analyzed using a fluorescent microscope (200×, Leica, DMi8, Germany).
Migration and invasion assay
A transwell system (Corning, #3422) was used to analyze cell migration and invasion. A549░or H460 cells (1×104 cells/mL) were harvested and suspended in serum-free RMPI-1640. The suspension was seeded onto the upper chamber of the transwell system. Recombinant human IL-17░A (rhIL-17░A) was suspended in serum-free RMPI-1640 at the concentration of 100░ng/mL in the bottom wells. For blocking STAT3/NF-κB/Notch1 pathway with inhibitors, STAT3 inhibitor (Niclosamide, Sigma, #N3510), NF-κB inhibitor (PDTC, MCE, # HY-18738) and Notch1 inhibitor (LY411575, MCE, # HY-50752) were added respectively in the bottom 30 minutes before rhIL-17░A was added. For blocking STAT3/NF-κB/Notch1 pathway with siRNAs, A549 and H460 cells were transfected with siRNAs 24 hours before rhIL-17░A was added. In addition, cells were seeded onto Matrigel matrix chambers (Corning, #354248) following the manufacturer's instructions in invasion assay. The cells were incubated with 5% CO2 for 48░h at 37░°C, then stained with crystal violet and counted under a microscope (100×). Triplicate in-dependent experiments were performed.
Western blotting
Quantified protein lysates were measured with a Protein BCA Assay Kit (Thermo Fisher, #23228) according to the manufacturer's instructions. The proteins in lysates were resolved on SDS-PAGE gels, transferred onto PVDF membranes (Millipore, #ISEEQ00010), and immunoblotted with anti-human N-cadherin (Cell signaling Technology, #13116), snail (Cell signaling Technology, #3879), vimentin (Cell signaling Technology, #5741), p-STAT3 (Cell signaling Technology, #9145), p-p65 (Cell signaling Technology, #3033), cleaved-Notch1 (Cell signaling Technology, #4147), Oct4 (Cell signaling Technology, #2750), Nanog (Cell signaling Technology, #4903), Sox2 (Cell signaling Technology, #3579), IL-17░A (PeproTech, #500-P07) and anti-human β-actin antibody (ProteinTech, #20536-1-AP-50).
RNA extraction and quantitative real-time polymerase chain reaction (qPCR)
For detecting IL-17░A mRNA level in peripheral blood, total RNA was extracted from PBMC of healthy donors and NSCLC patients by TRIzol reagent (Takara, #9109) according to the manufacturer's instructions.. For detection of IL-17░A and IL-17R mRNA in tissues, total RNA was extracted from tumor tissues and non-tumor tissues of NSCLC patients. Total RNA was extracted from rhIL-17░A-treated A549 and H460 cells to investigated EMT and CSC-related genes. The first-strand cDNA was synthesized from 1░µg of total RNA using Prime Script™ RT reagent kit (Takara, #RR047░A). qPCR was performed using SYBR Premix Ex Taq II (Takara, #RR820░A) and assessed by Agilent Mx3005P. The abundance of mRNA for each gene of interest was normalized to GAPDH. Details of the primer sequences used for qPCR are listed in Table S1.
Cell sorting
To obtain CD31+ and CD31− endothelial cells, Fresh tumor and non-tumor tissues were cut into small pieces and digested in RPMI 1640 (Gibco, USA) supplemented with 0.25% trypsin (Gibco), 0.002% DNaseI (Gibco), and 20% fetal bovine serum (FBS, Gibco) at 37° C for 20░min. Dissociated cells were filtered through a 40░µm mesh. Also, CD31 MicroBeads (Miltenyi, # 130-091-935) were used to enrich for endothelial cells through magnetic cell separation as per manufacturer's protocol. Total RNA was extracted from CD31+ and CD31− endothelial cells and IL-17R mRNA expression was detected by qPCR.
Tube-Forming Assay
Ice-cold growth factor-reduced basement membrane matrix (Matrigel; Corning, #354230) was added at 64░ul per well to precooled 96-well plates and allowed to polymerize at 37░°C for 30 minutes. Human umbilical vein endothelial cells (HUVEC, 3×104 cells/well) in 100░ul of EGM-2 MV basal medium with or without 100░ng/mL IL-17░A were plated onto the gel surface and incubated at 37░°C for 5░h. Cell rearrangement and tube formation were visualized by microscopy (200×, Leica, DMi8, Germany).
Sphere formation assay
A549░or H460 cells were resuspended in DMEM/F12 medium (Gibco, #11330032) with 4░µg/mL heparin (Solarbio, #H8270), B27 (1:50, Gibco, #17504044), 20░ng/mL EGF (PeproTech, #AF-100-15-100), 20░ng/mL bFGF (PeproTech, #AF-100-18B) in 24-well ultra-low cluster plates (Corning, #3473). The cells were treated with or without rhIL-17░A (PeproTech, #200-17). For blocking STAT3/NF-κB/Notch1 pathway with inhibitors, STAT3 inhibitor, NF-κB inhibitor and Notch1 inhibitor were added respectively 30 minutes before rhIL-17░A was added. For blocking STAT3/NF-κB/Notch1 pathway with siRNAs, A549 and H460 cells were transfected with siRNAs 24 hours before rhIL-17░A was added. After culturing for 7 days, the numbers of spheres were counted.
Chemotherapy resistance assay
1×105 A549░or H460 cells were treated with 100░ng/mL of rhIL-17░A for 0.5░h. Then docetaxel (Sangon Biotech, #A606561) was added to the wells at a final concentration of 1μg/mL for 24░h. Cells untreated with docetaxel and rhIL-17░A were used as control group. In the assay of inhibitors’ treatment, A549░or H460 cells were treated with rhIL-17░A and inhibitors for 0.5░h, then 1μg/mL docetaxel was added. Lastly, the apoptosis percentage was analyzed by flow cytometry.
Transfected cell line
To evaluate the effect of IL-17░A on tumor growth, H460 cells were stably transfected with IL-17░A-expressing vector (Sangon Biotech) to induce the overexpression of IL-17░A in H460 cells. The control vector was used to generate the control H460 cells. To evaluate the signaling of IL-17░A-induced tumor progression in NSCLC, A549 cells and H460 were transfected with vectors containing an STAT3-specific siRNA to knock down STAT3 expression, NF-κB-specific siRNA to knock down NF-κB expression, Notch1-specific siRNA to knockdown Notch1 expression, respectively. A549 and H460 cells were also transfected with a vector containing a scramble siRNA as a control. The sequences of the siRNAs used are as follows. STAT3: 5′- UGGCCCAAUGGAAUCAGCUACAGCA-3′, NF-κB: 5’-GAGAGGAGCACAGA UACCACCAAGA-3’and Notch1: 5’-GACGAUUGUCCAGGAAACAACUGCA-3’.
In vivo xenograft experiments
Animal protocols were approved by the Review Board of the First Affiliated Hospital
of Zhengzhou University. Severe combined immunodeficient mice (female, 4-6-week old) were purchased from the Chinese Academy of Medical Sciences. Mice were housed and maintained in laminar flow cabinets under specific pathogen-free conditions. For xenograft experiments, 4×106 H460 cells transfected with IL-17-expressing or control vector were resuspended in 1░ml PBS and injected into each mice subcutaneously. By visual observation and palpation, engrafted mice were inspected every 2 days for tumors. At the 21 days after cell transplantation, mice were sacrificed by cervical dislocation and tumors were isolated for analysis.
Immunohistochemistry
Formalin-fixed paraffin-embedded sections were provided by the Department of Pathology of our hospital, and confirmed by histopathological results. The protocol of immunohistochemistry is described elsewhere.50 Rabbit anti-IL-17░A (PeproTech, #500-P07) was used as primary antibody. Three fields of images per sample were taken. Non-immune rabbit IgG at the same dilution was used as negative control. Photos were recorded under a microscope (200×, Leica, DMi8, Germany).
Statistical analysis
All the data from quantitative assays were expressed as mean ± standard deviation by GraphPad Prism 5 software. Statistical analyses were performed using t-test or one-way analysis of variance. Kaplan-Meier curves in combination with log-rank tests were used to compare the survival of patients in different groups. The difference was considered statistically significant when P< 0.05.
Figure S1. The level of IL-17░A mRNA in NSCLC was increased. (A) Comparison IL-17░A mRNA level in peripheral blood mononuclear cells from NSCLC patients and healthy donors represented as scatter. (B) Comparison the level of IL-17░A mRNA in NSCLC tissues and non-tumor tissues represented as scatter. *, ** and *** indicate P < 0.05, P < 0.01 and P< 0.001, respectively.
Figure S2. IL-17░A promoted EMT-related genes in vitro. (A) The expressions of snail and vimentin in A549 and H460 cells before and after treatment of rhIL-17░A were analyzed using western blotting. (B-C) The relative expressions of snail and vimentin in A549 and H460 cells before and after treatment of rhIL-17░A were analyzed using qPCR. (D) Comparison of IL-17 receptor mRNA level in NSCLC tissues and non-tumor tissues represented as scatter. (E) Comparison of IL-17 receptor mRNA level in primary endothelium from NSCLC tissues and non-tumor tissues represented as scatter. (F) Tube formation was shown with or without rhIL-17░A treatment. (G) The correlation between IL-17░A and CD31 was analyzed. * indicates P < 0.05. Scale bar represents 50 μm.
Figure S3. IL-17░A promoted migration and invasion through STAT3/NF-κB/Notch1 signaling in NSCLC cells. (A-C) The expressions of STAT3, NF-κB and Notch1in A549 and H460 cells treated with rhIL-17░A were analyzed and shown as histogram. (D-F) The transfection efficiency of A549 and H460 cells treated with STAT3/NF-κB/Notch1 siRNAs was confirmed by qPCR. (G-H) The migration and invasion activities of A549 and H460 cells treated with or without rhIL-17░A and these molecular siRNAs were assessed by transwell assay. One representative analysis is shown. The data from A549 and H460 cells are presented as a histogram. * indicates P < 0.05. Scale bar represents 50 μm.
Figure S4. IL-17░A promoted the CSC-related genes in vitro. (A) The expressions of Nanog and Sox2 in A549 and H460 cells before and after treatment of rhIL-17░A were analyzed using western blotting. (B-C) The relative expressions of Nanog and Sox2 in A549 and H460 cells before and after treatment of rhIL-17░A were analyzed using qPCR. * indicates P < 0.05.
Figure S5. Knockdown of STAT3/NF-κB/Notch1 signaling inhibited NSCLC stemness promoted by IL-17░A. (A) After knockdown of STAT3░or NF-κB or Notch1 signaling, the sphere forming of A549 and H460 cells induce by rhIL-17░A was blocked. One representative photomicrograph is shown. The results from A549 (B) and H460 (C) cells were shown as histogram. * indicates P < 0.05. Scale bar represents 100 μm.
Supplementary Material
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (No.81171986, No. 81602024, No. 81771781), Funding from State's Key Project of Research and Development Plan (No. 2016YFC1303500), Research Grant from the Ministry of Public Health (No.201501004), Fundings for Creative Research Team of Henan Province, Creative Research Team of Higher Education of Henan Province.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male: female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. PMID:15900604 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016; 893:1–19. doi: 10.1007/978-3-319-24223-1_1. PMID:26667336 [DOI] [PubMed] [Google Scholar]
- 3.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. PMID:18039110 [DOI] [PubMed] [Google Scholar]
- 4.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–44. doi: 10.1200/JCO.2008.18.9068. PMID:19470939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. PMID:19132915 [DOI] [PubMed] [Google Scholar]
- 6.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98. doi: 10.1056/NEJMra0707449. PMID:19710487 [DOI] [PubMed] [Google Scholar]
- 7.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. PMID:18164222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong C. Targeting Th17 cells in immune diseases. Cell Res. 2014;24(8):901–903. doi: 10.1038/cr.2014.92. PMID:25022899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng SG, Wang J, Horwitz DA. Cutting Edge: Foxp3+CD4+CD25+ Regulatory T cells induced by IL-2 and TGF-β are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. PMID:18490709 [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing Y, Deng Z, Yao Z, Tsun A, Li B. FOXP3 and RORγt: Transcriptional regulation of Treg and Th17. Int Immunopharmacol. 2011;11(5):536–42. doi: 10.1016/j.intimp.2010.11.008. PMID:21081189 [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17- mediated tumour growth. Nature. 2012;491:254–8. doi: 10.1038/nature11465. PMID:23034650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reppert S, Boross I, Koslowski M, Tureci O, Koch S, Lehr HA, Finotto S. A role for T-bet-mediated tumour immune surveillance in anti–IL-17A treatment of lung cancer. Nat Commun. 2011;2:600. doi: 10.1038/ncomms1609. PMID:22186896 [DOI] [PubMed] [Google Scholar]
- 13.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, Ladoire S, Derangere V, Vincent J, Masson D, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 2012; 36:362–73. doi: 10.1016/j.immuni.2011.12.019. PMID:22406269 [DOI] [PubMed] [Google Scholar]
- 14.Du JW, Xu KY, Fang LY, Qi XL. Interleukin-17, produced by lymphocytes, promotes tumor growth and angiogenesis in a mouse model of breast cancer. Mol Med Rep. 2012;6:1099–102. doi: 10.3892/mmr.2012.1036. PMID:22922996 [DOI] [PubMed] [Google Scholar]
- 15.De Simone V, Pallone F, Monteleone G, Stolfi C. Role of TH17 cytokines in the control of colorectal cancer. Oncoimmunology. 2013;2(12):e26617. doi: 10.4161/onci.26617. PMID:2449854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, Peng L, Yu P, Zhao Y, Shi Y, Mao X, Chen W, Cheng P, Wang T, Chen N, et al. Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J Clin Immunol. 2012;32(6):1332–9. doi: 10.1007/s10875-012-9718-8. PMID:22760549 [DOI] [PubMed] [Google Scholar]
- 17.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. doi: 10.1038/ni1254. PMID:16200070 [DOI] [PubMed] [Google Scholar]
- 18.Loncle C, Bonjoch L, Folch-Puy E, Lopez-Millan MB, Lac S, Molejon MI, Chuluyan E, Cordelier P, Dubus P, Lomberk G, et al. IL-17 functions through the novel REG3β-JAK2-STAT3 inflammatory pathway to promote the transition from chronic pancreatitis to pancreatic cancer. Cancer Res. 2015;75(22):4852–62. doi: 10.1158/0008-5472.CAN-15-0896. PMID:26404002</bib> [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu LW, et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early Colorectal Tumorigenesis. Immunity. 2014;41(6):1052–63. doi: 10.1016/j.immuni.2014.11.009. PMID:25526314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Zhang CJ, Martin BN, Bulek K, Kang Z, Zhao J, Bian G, Carman JA, Gao J, Dongre A, et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat Commun 2017;8:15508. doi: 10.1038/ncomms15508. PMID:28561022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Ye X, Bhattacharya R, Boulbes DR, Fan F, Xia L, Ellis LM. A disintegrin and metalloproteinase domain 17 regulates colorectal cancer stem cells and chemosensitivity via Notch1 Signaling. Stem Cells Transl Med 2016; 5(3):331–8. doi: 10.5966/sctm.2015-0168. PMID:26744411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madkouri R, Kaderbhai CG, Bertaut A, Truntzer C, Vincent J, Aubriot-Lorton MH, Farah W, Limagne E, Ladoire S, Boidot R, et al. Immune classification with cytotoxic CD8 and Th17 infiltrates are predictors of clinical prognosis in glioblastoma. Oncoimmunology. 2017;6(6):e1321186. doi: 10.1080/2162402X.2017.1321186. PMID:28680758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313–23. doi: 10.1038/onc.2010.109. PMID:20400974 [DOI] [PubMed] [Google Scholar]
- 24.Chen D, Hu Q, Mao C, Jiao Z, Wang S, Yu L, Xu Y, Dai D, Yin L, Xu H. Increased IL-17-producing CD4(+) T cells in patients with esophageal cancer. Cell Immunol. 2012;272:166–174. doi: 10.1016/j.cellimm.2011.10.015. PMID:22082565 [DOI] [PubMed] [Google Scholar]
- 25.Hu J, Mao Y, Li M, Lu Y. The profile of Th17 subset in glioma. Int. Immunopharmacol. 2011;11:1173–1179. doi: 10.1016/j.intimp.2011.03.015. PMID:21473956 [DOI] [PubMed] [Google Scholar]
- 26.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. PMID:17201677 [DOI] [PubMed] [Google Scholar]
- 27.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. PMID:15485625 [DOI] [PubMed] [Google Scholar]
- 28.Martin-Orozco N, Dong C. The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr Opin Investig Drugs. 2009;10:543–9. PMID:19513943 [PubMed] [Google Scholar]
- 29.Ngiow SF, Smyth MJ, Teng MW. Does IL-17 suppress tumor growth? Blood. 2010;115:2554–5. doi: 10.1182/blood-2009-11-254607. PMID:20339108 [DOI] [PubMed] [Google Scholar]
- 30.Ji Y, Zhang W. Th17 cells: positive or negative role in tumor? Cancer Immunol Immunother. 2010;59:979–87. doi: 10.1007/s00262-010-0849-6. PMID:20352428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vizio B, Novarino A, Giacobino A, Cristiano C, Prati A, Ciuffreda L, Montrucchio G, Bellone G. Potential plasticity of T regulatory cells in pancreatic carcinoma in relation to disease progression and outcome. Exp Ther Med. 2012;4:70–78. doi: 10.3892/etm.2012.553. PMID: 23060925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada Y., Saito H., and Ikeguchi M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J Surg Res 2012; 178:685–691. doi: 10.1016/j.jss.2012.07.055. PMID: 22940035 [DOI] [PubMed] [Google Scholar]
- 33.Benevides L, da Fonseca DM, Donate PB, Tiezzi DG, De Carvalho DD, de Andrade JM, Martins GA, Silva JS. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 2015;75(18):3788–99. doi: 10.1158/0008-5472.CAN-15-0054. PMID:26208902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reppert S, Boross I, Koslowski M, Türeci Ö, Koch S, Lehr HA, Finotto S. A role for T-bet-mediated tumour immune surveillance in anti–IL-17A treatment of lung cancer. Nat Commun. 2011;2:600. doi: 10.1038/ncomms1609. PMID:22186896 [DOI] [PubMed] [Google Scholar]
- 35.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–71. doi: 10.1158/0008-5472.CAN-10-2907. PMID:21303976 [DOI] [PubMed] [Google Scholar]
- 36.Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol Lett. 2005;98:189–193. doi: 10.1016/j.imlet.2004.11.012. PMID:15860217 [DOI] [PubMed] [Google Scholar]
- 37.Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor alpha-induced elaboration of proangiogenic factors from fibroblasts. Immunol Lett. 2004;93:39–43. doi: 10.1016/j.imlet.2004.01.014. PMID:15134897 [DOI] [PubMed] [Google Scholar]
- 38.Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, et al. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. doi: 10.1186/1476-4598-10-150. PMID: 22171994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benevides L, Cardoso CR, Tiezzi DG, Marana HR, Andrade JM, Silva JS. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor. Eur J Immunol. 2013;43:1518–28. doi: 10.1002/eji.201242951. PMID:23529839 [DOI] [PubMed] [Google Scholar]
- 40.Xiang T, Long H, He L, Han X, Lin K, Liang Z, Zhuo W, Xie R, Zhu B. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene. 2015;34(2):165–76. doi: 10.1038/onc.2013.537. PMID:24362529 [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 2009; 206(7):1457–64. doi: 10.1084/jem.20090207. PMID:19564351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70:10112–10120. doi: 10.1158/0008-5472.CAN-10-0775. PMID:21159633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15(4):283–93. doi: 10.1016/j.ccr.2009.02.015. PMID:19345327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garner JM, Fan M, Yang CH, Du Z, Sims M, Davidoff AM, Pfeffer LM. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor κB signaling in glioblastoma cancer stem cells regulates the Notch pathway. J Biol Chem. 2013;288(36):26167–76. doi: 10.1074/jbc.M113.477950. PMID:23902772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis 2013; 72(Suppl2), ii116–ii123. doi: 10.1136/annrheumdis-2012-202371. PMID:23253932 [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Lou W, Armstrong C, Zhu Y, Evans CP, Gao AC. Niclosamide suppresses cell migration and invasion in enzalutamide resistant prostate cancer cells via Stat3-AR axis inhibition. Prostate 2015; 75(13):1341–53. doi: 10.1002/pros.23015. PMID:25970160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang F, Li H, Qiao JO. 1-O-acetylbritannilactone combined with gemcitabine elicits growth inhibition and apoptosis in A549 human non small cell lung cancer cells. Mol Med Rep. 2015;12(4):5568–5572. doi: 10.3892/mmr.2015.4042. PMID:26151622 [DOI] [PubMed] [Google Scholar]
- 48.Chiorean EG, LoRusso P, Strother RM, Diamond JR, Younger A, Messersmith WA, Adriaens L, Liu L, Kao RJ, DiCioccio AT, et al. A Phase I First-in-human study of Enoticumab (REGN421), a Fully Human Delta-like Ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors. Clin Cancer Res. 2015;21(12):2695–2703. doi: 10.1158/1078-0432.CCR-14-2797. PMID:25724527 [DOI] [PubMed] [Google Scholar]
- 49.Li L, Yang L, Wang L, Wang F, Zhang Z, Li J, Yue D, Chen X, Ping Y, Huang L, et al. Impaired T cell function in malignant pleural effusion is caused by TGF-β derived predominantly from macrophages. Int J Cancer. 2016;139(10):2261–2269. doi: 10.1002/ijc.30289. PMID:27459735 [DOI] [PubMed] [Google Scholar]
- 50.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, et al. Macrophage-derived Wnt opposes notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. PMID:22388089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


