Abstract
Introduction
Evidence indicates that associations between diet and Alzheimer's disease may occur through biomarker pathways such as amyloid-β (Aβ); however, few studies have investigated dietary/Aβ relationships, and no study has investigated this relationship in women.
Methods
Dietary patterns were extrapolated for 115 participants from the Women's Health Aging Project. Aβ deposition was measured via in vivo F-18 florbetaben positron emission tomography scanning.
Results
Participants were, on average, aged 70 years (±2.63 SD), had 13 years of education (±3.57 SD), a BMI of 28 kg/m2 (±5.46 SD), and a daily energy intake of 5161 kJ (±1679.03 SD). Four dietary patterns were identified: high fat, Mediterranean, junk food, and low fat. Adherence to the junk food diet was a significant predictor of Aβ deposition (β = .10, P = .03).
Discussion
This study highlights the potential of diet to influence neurodegenerative disease and as a potential modifiable lifestyle risk factor for Alzheimer's disease.
Keywords: Biomarkers, Alzheimer's disease, Neuropathology, β-amyloid protein, Diet, Nutrition, Dietary pattern, Factor analysis, Women
Highlights
-
•
This is the first study to investigate dietary/cerebral Aβ relationships in women only.
-
•
Four dietary patterns extrapolated by principal component analysis.
-
•
Adherence to the junk food pattern is a significant predictor of Aβ deposition.
-
•
These findings support the notion of diet as a modifiable risk factor for AD.
1. Introduction
Diet may play a substantial role in the Alzheimer's disease (AD) symptomatology and offer great potential for nonpharmacological prevention. Epidemiological evidence has suggested increased adherence to a Mediterranean diet [1], low glycemic index [2], [3], and higher consumption of omega-3 polyunsaturated fatty acids [4] were associated with a decrease in AD biomarker burden. Systematic review found 50 out of 64 studies revealed an association between diet and AD incidence [5]; however, only one study has used a priori analysis to analyze dietary associations with the hallmark cerebral protein implicated in AD, β-amyloid (Aβ). In this study, dietary pattern analysis identified a pattern characterized by a higher intake of fresh fruit, vegetables, whole grains, fish, and low-fat dairies, and a lower intake of sweets, fried potatoes, processed meat, and butter was negatively associated with in vivo cerebral Aβ [6].
Furthermore, male and mixed cohort studies predominate the research, and to date, no study has investigated this relationship specifically in women. Women are more likely than men to develop AD [7], have a higher penetrance for the apolipoprotein ε-4 (APOE-ε4) allele [8], and are more likely to progress from mild cognitive impairment to AD [8]. Impacts of higher male mortality, vascular risk factors, and the postmenopausal loss of estrogenic neuroprotection suggest females are 1.5 times more likely to develop AD than men [9]. Given sex differences in AD risk, research is needed for those at greater risk of disease.
Studies investigating in vivo AD biomarkers are needed to clarify how nutrition promotes healthy brain aging and to identify neuroprotective patterns for those at the greatest risk of AD. The objectives of this study were to identify dietary patterns using an a priori approach and investigate their associations with Aβ deposition in healthy aging Australian women. We previously reported on a lack of a relationship between a healthy Mediterranean diet and Aβ deposition [10] and hypothesized this was due to limitations of the self-reported food frequency questionnaire in measuring the potentially beneficial phytochemicals in olive oil. Given high-fat, high-glycemic diets have been associated with increased AD biomarker burden, we hypothesized that a dietary pattern characterized by high-fat, high-sugar content would be associated with an increase in cerebral Aβ pathology.
2. Methods
2.1. Study population
Participants were sought from the 2012 follow-up of the Women's Health Ageing Project (WHAP), an epidemiologically sourced prospective study of healthy aging Australian women. WHAP is an extension of the Melbourne Women's Midlife Health Project. Briefly, 438 women within the Melbourne metropolitan area were identified by random digit dialing in 1991. Women were eligible for the cohort if they were Australian-born, aged 45–55 years, had menstruated in the three months before recruitment, and were not taking estrogen-containing hormone replacement therapy. In 2012, participants were re-contacted and invited to participate in a late-life health study. Clinical assessments were conducted on 252 participants by trained field researchers. The clinical assessments included a battery of validated measures of physical health, sociodemographics, lifestyle, cognitive function, psychological health, and biomarkers. A complete methodology has been published elsewhere [11].
2.2. Diet
Participants completed a validated food frequency questionnaire entitled the Dietary Questionnaire for Epidemiological Studies Version 2 (DQES v2) [12]. The DQES v2 incorporates 80 food items with frequency response options on 74 of these items. The DQES v2 covers five types of dietary intake: cereals/sweets/snacks, dairy/meat/fish, fruits, vegetables, and alcoholic beverages. Data collected by the DQES v2 was used to calculate daily energy and nutrient intakes by the Cancer Council of Victoria based on Australian nutrient composition data from NUTTAB95, collated via the Composition of Foods, Australia [13]. Individuals were removed if their energy intake was reported as below 3000 kJ/day or above 20,000 kJ/day. All food items were reported in grams per day and placed into 33 food groups defined a priori (Table 1) that was similar to those used by others [14], [15]. Dietary patterns were extrapolated from food groupings using iterated principal factor analysis with oblique varimax rotation due to the presumed intercollinearity and nonindependence of dietary patterns.
Table 1.
Food groupings from Dietary Questionnaire for Epidemiological Studies Version 2 (DQES v2)
| Food group | Items in the DQES v2 |
|---|---|
| Whole grains | All bran, bran flakes, high fiber white bread, muesli, multigrain bread, porridge, rye bread, Weet-Bix, wholemeal bread |
| Refined grains | Corn flakes, crackers, pasta, rice, white bread |
| Red meats | Beef, lamb, pork, veal |
| Processed meats | Bacon, salami, sausages |
| Poultry | Chicken |
| Takeaway foods | Hamburger, meat pies, pizza |
| Fried fish | Fried fish |
| Other fish | Fish (nonfried), tinned fish |
| Fried potatoes | Chips (French fries) |
| Other potato | Potatoes |
| Yellow or red vegetables | Capsicum, carrots, pumpkin |
| Legumes | Baked beans, green beans, other beans, peas, tofu |
| Cruciferous vegetables | Broccoli, cabbage, cauliflower |
| Leafy green vegetables | Lettuce, spinach |
| Other vegetables | Bean sprouts, beetroot, celery, cucumber, garlic, mushrooms, onion, zucchini |
| Tomato | Tomatoes |
| Fresh fruit | Apples, apricots, avocado, bananas, mango, melon, oranges, peaches, pears, pineapple, strawberries |
| Canned fruit | Tinned fruit |
| Cakes, biscuits, sweet pastries | Cakes, sweet biscuits |
| Low-fat dairy products | Flavored milk drink, low-fat cheese, reduced fat milk, ricotta cheese, cottage cheese, skim milk |
| Full-fat dairy products | Cream cheese, firm cheese, full-cream milk, hard cheese, ice cream, soft cheese, yoghurt |
| Soya milk | Soya milk |
| Confectionery | Chocolate |
| Added sugar | Jam, sugar |
| Crisps | Crisps |
| Nuts | Nuts, peanut butter |
| Eggs | Eggs |
| Fruit juice | Fruit juice |
| Saturated spreads | Butter, butter-margarine blends, margarine |
| Unsaturated spreads | Monounsaturated margarine, polyunsaturated margarine |
| Alcohol–beer | Heavy beer, light beer |
| Alcohol–wine | Red wine, white wine |
| Alcohol–spirits | Fortified wines, spirits |
2.3. Imaging
In the 2012 follow-up, all WHAP participants were offered the opportunity to have cerebral imaging. Aβ deposition was measured via in vivo F-18 Florbetaben positron emission tomography (PET) at the Austin Health Centre for PET in Victoria, Australia. Participants received 250 MBq of 18F-florbetaben intravenously, with a 20-minute acquisition commencing 90 minutes after injection. Standardized uptake values (SUVs) were calculated for all brain regions examined, and standard uptake value ratios (SUVRs) were generated by normalizing regional SUVs by the cerebellar cortex with atrophy correction from structural magnetic resonance imaging. Neocortical SUVR, a global index of Aβ burden, is expressed as the average SUVR of the area-weighted mean. Area-weighted means were calculated for each participant by averaging the frontal, superior parietal, lateral temporal, lateral occipital, and anterior and posterior cingulate regions. This protocol has been described elsewhere [11].
2.4. Covariates
Age (in years), education (in years), and body mass index (BMI) were collected as part of the clinical assessments in 2012. Total energy intake in kilojoules was calculated by the Cancer Council of Victoria from the DQES v2. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Savings Score was used as a valid indicator of cognitive ability [16]—it has been suggested as the most reliable index in differentiating cognitively normal individuals from AD [17]. Participants' APOE genotype was determined by direct sequencing and were dichotomized as an APOE ε4 carrier (APOE ε2/ε4, APOE ε3/ε4, and APOE ε4/ε4) or a noncarrier. Adherence to identified dietary patterns was converted from weighted factor loadings to binary adherence to minimize the intracorrelations between variables. All analyses were adjusted for age in years, education in years, energy intake (kJ/day), cognition (CERAD Savings), and binary presence of the APOE ε4 allele.
2.5. Statistical analysis
All analyses were conducted in STATA software on Windows operating system. Complete data were available for 115 WHAP participants, and there were no significant differences between the included (n = 115) and excluded (n = 137) cohorts. PET SUVRs displayed a positive skew that was rectified using 1/square transformation; therefore, results should be interpreted as inverse coefficient derivatives. Generalized linear models were used to assess associations between Aβ deposition and dietary patterns scores. Generalized linear models were adjusted for age in years, education in years, cognition (CERAD Savings), and binary presence of the APOE ε4 allele.
3. Results
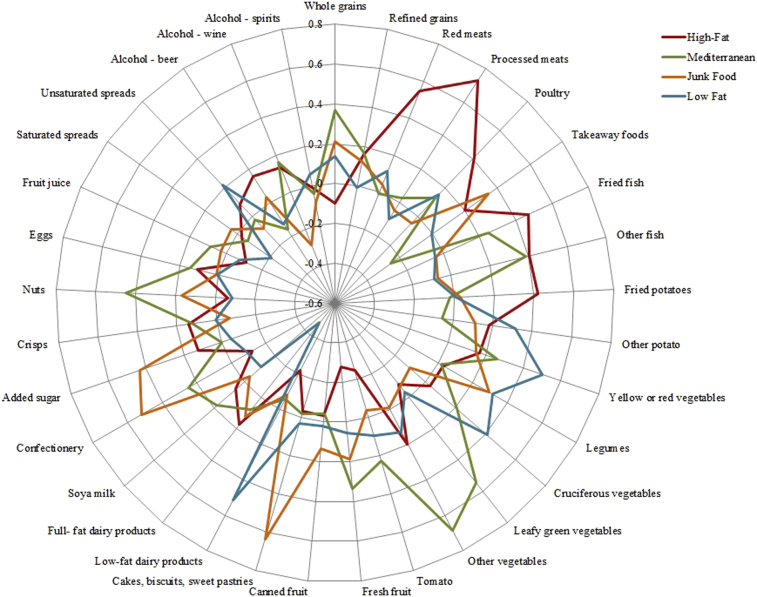
Four dietary patterns were identified: high fat, Mediterranean, junk food, and low fat. Factor loadings (Table 2 and Fig. 1) indicated that the high-fat diet loaded heavily on food groups such as processed meats, fried fish, red meats, fried potatoes, and poultry. The Mediterranean style diet loaded chiefly on whole grains, vegetables, nuts, fish, and wine as the main source of alcohol. The unhealthy junk food pattern was characterized by high consumption of takeaway foods, added sugar, confectionary and cakes, biscuits, and sweet pastries, whereas the low-fat diet loaded heavily on low-fat dairy products, vegetables, and unsaturated spreads.
Table 2.
Dietary pattern factor loadings for all WHAP participants in 2012 (n = 224)
| Variable | High fat | Mediterranean | Junk food | Low fat |
|---|---|---|---|---|
| Eigenvalue | 3.03802 | 2.13623 | 1.80738 | 1.55595 |
| Variance | 2.15252 | 2.00648 | 1.58108 | 1.39511 |
| Proportion | 0.3164 | 0.2949 | 0.2324 | 0.2051 |
| Whole grains | 0.3668 | 0.2096 | 0.1387 | |
| Refined grains | 0.1598 | 0.1673 | 0.1247 | |
| Red meats | 0.5464 | 0.1128 | ||
| Processed meats | 0.7281 | |||
| Poultry | 0.4130 | 0.1302 | 0.1549 | |
| Takeaway foods | 0.2021 | −0.2543 | 0.3427 | |
| Fried fish | 0.4662 | 0.2473 | ||
| Other fish | 0.4029 | 0.3867 | ||
| Fried potatoes | 0.4195 | |||
| Other potato | 0.1803 | 0.1127 | 0.3143 | |
| Yellow or red vegetables | 0.1675 | 0.2594 | 0.1480 | 0.5034 |
| Legumes | 0.2923 | 0.3134 | ||
| Cruciferous vegetables | 0.2117 | −0.1027 | 0.4114 | |
| Leafy green vegetables | 0.5466 | |||
| Other vegetables | 0.1961 | 0.6869 | 0.1286 | |
| Tomato | −0.2459 | 0.2255 | ||
| Fresh fruit | −0.2786 | 0.3381 | 0.1887 | |
| Canned fruit | 0.1337 | |||
| Cakes, biscuits, sweet pastries | 0.6350 | |||
| Low-fat dairy products | −0.2222 | 0.5117 | ||
| Full-fat dairy products | 0.1754 | 0.1255 | −0.4775 | |
| Soya milk | 0.1843 | −0.1101 | ||
| Confectionery | −0.1195 | 0.2484 | 0.5211 | |
| Added sugar | 0.1263 | 0.4327 | ||
| Crisps | 0.1405 | 0.1229 | ||
| Nuts | 0.4485 | 0.1703 | ||
| Eggs | 0.1083 | 0.1448 | ||
| Fruit juice | −0.1134 | |||
| Saturated spreads | −0.2113 | |||
| Unsaturated spreads | 0.2165 | |||
| Alcohol–beer | 0.1540 | −0.1618 | −0.1272 | |
| Alcohol–wine | 0.1345 | 0.1586 | −0.2868 |
Rotated factor loadings for iterated principal factor analysis with oblique promax rotation. Blanks represent absent loadings (<0.1). Alcohol–spirits not shown due to not loading (>0.1) on any factor.
Fig. 1.
Spider diagram of factor loadings by dietary patterns.
Participants' characteristics are found in Table 3. Participants in the Mediterranean diet group (n = 31) displayed the highest level of education (14.10 ± 3.87 years), highest CERAD Savings score (72.97 ± 31.07), and lowest level of Aβ deposition (PET SUVR 1.0834 ± 0.14). Daily energy intake was highest in the high-fat group (5443.46 ± 2116.50 kJ/day) and lowest in the Mediterranean group (4677.26 ± 1242.79 kJ/day). Significant group differences were observed in education, energy intake, and CERAD Savings and were therefore adjusted for in all generalized linear models.
Table 3.
Descriptive statistics for the included participants grouped by adherence to dietary patterns identified using IPFA
| Variable | High fat (n = 24) | Mediterranean (n = 31) | Junk food (n = 24) | Low fat (n = 35) | Total (n = 115) |
|---|---|---|---|---|---|
| Age (in years) | 69.79 ± 2.42 | 69.45 ± 2.23 | 70.41 ± 3.19 | 69.57 ± 2.70 | 69.76 ± 2.63 |
| Education (in years) | 12.88 ± 3.67 | 14.10 ± 3.87 | 11.50 ± 2.96 | 12.63 ± 3.36 | 12.84 ± 3.57 |
| BMI | 28.58 ± 6.75 | 27.43 ± 5.48 | 27.14 ± 5.58 | 29.29 ± 4.26 | 28.18 ± 5.46 |
| Energy (kJ/day) | 5443.46 ± 2116.50 | 4809.79 ± 1145.51 | 6035.40 ± 1993.21 | 4677.26 ± 1242.79 | 5160.53 ± 1679.03 |
| APOE positive, n (%) | 9 (37.5) | 9 (29.0) | 9 (37.5) | 10 (28.57) | 37 (32.46) |
| CERAD Savings Score (%) | 72.93 ± 18.08 | 72.97 ± 31.07 | 65.57 ± 28.76 | 63.34 ± 28.62 | 68.45 ± 27.52 |
| PET SUVR (raw) | 1.1296 ± 0.1539 | 1.0835 ± 0.1427 | 1.2150 ± 0.2458 | 1.1300 ± 0.2336 | 1.1352 ± 0.2026 |
| PET SUVR (transformed) | 0.8185 ± 0.1770 | 0.8829 ± 0.1646 | 0.7389 ± 0.2174 | 0.8446 ± 0.2043 | 0.8273 ± 0.1959 |
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; PET, positron emission tomography; SUVR, standard uptake value ratio.
NOTE. If not otherwise described, data are presented as mean ± standard deviation of the mean.
Adherence to the junk food diet (Table 4) was a significant predictor of Aβ deposition (β = .10, P = .036) as was binary presence of the APOE ε4 allele (β = .11, P = .004). No significant interaction effects were observed in the combined effect of diet and APOE ε4 on Aβ deposition (P = .59). All other dietary patterns were not associated with Aβ deposition. Age, education, and cognition were also not significantly associated with Aβ deposition.
Table 4.
Generalized linear model for independent variables (PET SUVR) and four dietary patterns identified using iterative principal factor analysis. (95% CIs shown; n = 114)
| PET SUVR | Coefficient | Std. Err. | P | CI lower | CI higher |
|---|---|---|---|---|---|
| High fat | −0.00705 | 0.04372 | .872 | −0.09273 | 0.07864 |
| Mediterranean | 0.06390 | 0.04349 | .142 | −0.02135 | 0.14915 |
| Junk food | −0.09740 | 0.04511 | .031 | −0.18582 | −0.00898 |
| Low fat | 0.02338 | 0.03962 | .555 | −0.05428 | 0.10103 |
| Age (in years) | −0.00120 | 0.00702 | .864 | −0.01495 | 0.01256 |
| Education (in years) | 0.00139 | 0.00502 | .781 | −0.00845 | 0.01125 |
| BMI | −0.00076 | 0.00326 | .816 | −0.00714 | 0.00563 |
| Energy (kJ/day) | −0.00001 | 0.00001 | .309 | −0.00003 | 0.00001 |
| APOE Presence | −0.10916 | 0.03919 | .005 | −0.18598 | −0.03233 |
| CERAD Savings Score | 0.00125 | 0.00065 | .054 | −0.00002 | 0.00252 |
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CI, confidence interval; PET, positron emission tomography; SUVR, standard uptake value ratio.
NOTE. Bold indicates statistical significance (P < .05). CIs are for coefficient. Analysis adjusted for age in years, education in years, cognition (CERAD Savings score), and binary presence of the APOE ε4 allele.
4. Discussion
In this cross-sectional study in Australian women, adherence to the junk food was a significant predictor of cerebral Aβ deposition. These results suggest that higher adherence to a high-fat, high-sugar style diet may be associated with an increased deposition of AD biomarkers and a higher risk for disease.
We observed similar cognitive status between dietary groups. However, women adhering to the Mediterranean dietary pattern displayed significantly higher cognitive scores than the other dietary groups. In the longitudinal Nurse's Health Study, women with higher Mediterranean diet adherence had significantly higher overall cognitive status [18]. Given evidence for the cardiovascular determinants of cognitive decline [19], [20], there is clear evidence for an inverse relationship between Mediterranean diet adherence and cognition; however, the cross-sectional nature of this study limits our ability to address this relationship.
Our results contribute to the growing body of evidence linking diet with AD. A high-glycemic diet has been associated with greater amyloid burden in the brain [2] and cerebrospinal fluid measures [3], [21], [22]. A principal component analysis on nutrient intake patterns showed consumption of omega-3 fatty acids, zinc, vitamin B-12, and vitamin D was associated with decreased amyloid deposition [6], [23]. Consumption of omega-3 fatty acid supplementation has been shown to be related to tau (phosphorylated and total) and amyloid biomarkers of AD in cerebrospinal fluid [24]. Serum docosahexaenoic acid has also been inversely associated with cerebral amyloid burden [25].
Research has established that diets with higher consumption of sugar, carbohydrates, and high-glycemic foods are associated with impaired glucose metabolism [26]. Disrupted glucose metabolism affects the production and clearance of Aβ and tau phosphorylation [27], and both insulin resistance [28] and type-2 diabetes [29] are risk factors for AD. Several animal studies have illustrated that a high-fat diet causes brain Aβ accumulation in wild-type rabbits [30] and transgenic mice [31], [32]. Furthermore, human APOE isoforms have been shown to modulate glucose and metabolic pathways, with the APOE ε3/ε4 variants showing markedly reduced glucose uptake and metabolism in mouse models [33]. APOE ε2 brains demonstrated a more robust metabolic profile than APOE ε3/ε4, suggesting a physiological mechanism for its protective role against AD [33].
We speculate that the relationship observed between a high-fat, high-sugar diet and increased cerebral Aβ deposition may be modulated by impaired glucose metabolism in this female-only cohort. We believe our results suggest an impaired glucose metabolic pathway interacting with an APOE-Aβ physiological mechanism. Research has been shown that APOE ε4 confers a greater risk in women than men [8]. Women with a single APOE ε4 allele have up to a four-fold increase in risk when compared with women homozygous for APOE ε3; however, men with a single APOE ε4 allele have little to no increase in risk [34]. Given animal model evidence for an APOE-mediated glucose metabolism [33], females may experience greater AD risk due to a mechanistic action in their glucose metabolism. Further research is required to elucidate the physiological mechanisms that underpin this relationship, for example, to replicate animal evidence of glucose metabolism in human models of APOE ε4 isoforms.
Our findings strengthen the hypothesis of diet being a modifiable risk factor for AD by linking amyloid deposition with an unhealthy-type diet in a female-only cohort. These findings suggest a metabolic pathway linking diet with cerebral Aβ deposition and should motivate investigations into dietary impacts on glucose metabolism by variations in presence of the APOE ε2/ε3/ε4 alleles.
Research in Context.
-
1.
Systematic review: The authors previously conducted a systematic review that highlighted the paucity of research regarding dietary adherence and biomarkers of Alzheimer's disease. This review was conducted in accordance with PRISMA guidelines (PROSPERO: CRD42017076389) searching MEDLINE, PubMed, PsycINFO, Google Scholar, and SCOPUS databases.
-
2.
Interpretation: Our findings contribute to the growing body of evidence linking diet with Alzheimer's disease. We speculate that the relationship observed between a high-fat, high-sugar diet and increased cerebral β-amyloid deposition is affected by impaired glucose metabolism. These findings suggest an apolipoprotein E (APOE)–mediated glucose metabolic pathway.
-
3.
Future directions: Our research suggests a metabolic pathway linking diet with cerebral Aβ deposition and should motivate investigations into dietary impacts on glucose metabolism and Alzheimer's disease biomarker deposition by variations in presence of the APOE ε2/ε3/ε4 alleles.
Acknowledgments
The authors would like to acknowledge the contribution of the participants and their supporters who have contributed their time and commitment for over 20 years to the university. A full list of all researchers contributing to the project and the membership of our Scientific Advisory Board is available at http://www.medrmhwh.unimelb.edu.au/Research/WHAP.html.
Funding sources: Funding for the Healthy Aging Program (HAP) has been provided by the National Health and Medical Research Council [NHMRC grants 547600, 1032350, and 1062133], Ramaciotti Foundation, Australian Healthy Aging Organisation, the Brain Foundation, the Alzheimer's Association [NIA320312], Australian Menopausal Society, Bayer Healthcare, Shepherd Foundation, Scobie and Claire Mackinnon Foundation, Collier Trust Fund, J.O. & J.R. Wicking Trust, Mason Foundation, and the Alzheimer's Association of Australia. Inaugural funding was provided by VicHealth and the NHMRC. The Principal Investigator of HAP (C.Sz.) is supported by the National Health and Medical Research Council. The authors thank Professor Graham Giles of the Cancer Epidemiology & Intelligence Division, Cancer Council Victoria, for permission to use the DQES v2. E.H. would like to acknowledge support from the Australian Government Research Training Program Scholarship.
Ethical approval: This study received approval from the University of Melbourne Human Research Ethics Committee (HREC: 1750632.1, 010528, 010411, 1034765, and 1647448) and was carried out in accordance with the Declaration of Helsinki. All participants signed written informed consent before participation.
C.S. has provided clinical consultancy and been on scientific advisory committees for the Australian Commonwealth Scientific and Industrial Research Organization, Alzheimer's Australia, University of Melbourne, and other relationships that are subject to confidentiality clauses. She has been a named chief investigator on investigator-driven collaborative research projects in partnership with Pfizer, Merck, Bayer, and GE. She may accrue revenues from patent in pharmacogenomics prediction of seizure recurrence. The other authors have no conflict of interest to report.
References
- 1.Merrill D.A., Siddarth P., Raji C.A., Emerson N.D., Rueda F., Ercoli L.M. Modifiable risk factors and brain positron emission tomography measures of amyloid and tau in nondemented adults with memory complaints. Am J Geriatr Psychiatry. 2016;24:729–737. doi: 10.1016/j.jagp.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor M.K., Sullivan D.K., Swerdlow R.H., Vidoni E.D., Morris J.K., Mahnken J.D. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106:1463–1470. doi: 10.3945/ajcn.117.162263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer-Carter J.L., Green P.S., Montine T.J., VanFossen B., Baker L.D., Watson G.S. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68:743–752. doi: 10.1001/archneurol.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu Y., Schupf N., Cosentino S.A., Luchsinger J.A., Scarmeas N. Nutrient intake and plasma beta-amyloid. Neurology. 2012;78:1832–1840. doi: 10.1212/WNL.0b013e318258f7c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusufov M., Weyandt L.L., Piryatinsky I. Alzheimer's disease and diet: A systematic review. Int J Neurosci. 2017;127:161–175. doi: 10.3109/00207454.2016.1155572. [DOI] [PubMed] [Google Scholar]
- 6.Berti V., Murray J., Davies M., Spector N., Tsui W.H., Li Y. Nutrient patterns and brain biomarkers of Alzheimer's disease in cognitively normal individuals. J Nutr Health Aging. 2015;19:413–423. doi: 10.1007/s12603-014-0534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pike K.E., Ellis K.A., Villemagne V.L., Good N., Chételat G., Ames D. Cognition and beta-amyloid in preclinical Alzheimer's disease: Data from the AIBL study. Neuropsychologia. 2011;49:2384–2390. doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Altmann A., Tian L., Henderson V.W., Greicius M.D. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viña J., Lloret A. Why women have more Alzheimer's disease than men: Gender and mitochondrial toxicity of amyloid-β peptide. J Alzheimer's Dis. 2010;20:527–533. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- 10.Hill E., Szoeke C., Dennerstein L., Campbell S., Clifton P. Adherence to the Mediterranean Diet is not Related to Beta-Amyloid Deposition: Data from the Women's Healthy Ageing Project. J Prev Alzheimers Dis. 2018;5:137–141. doi: 10.14283/jpad.2018.12. [DOI] [PubMed] [Google Scholar]
- 11.Szoeke C., Coulson M., Campbell S., Dennerstein L. Cohort profile: Women's Healthy Ageing Project (WHAP): A longitudinal prospective study of Australian women since 1990. Women's Midlife Heal. 2016;2:5. doi: 10.1186/s40695-016-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles G.G., Ireland P.D. The Cancer Council of Victoria; Melbourne, Australia: 1996. Dietary Questionnaire for Epidemiological Studies (Version 2) [Google Scholar]
- 13.Thomas S., Corden M. Australian Government Publishing Service; Canberra, Australia: 1977. Metric Tables of Composition of Australian Foods. [Google Scholar]
- 14.Ambrosini G.L., Fritschi L., De Klerk N.H., Mackerras D., Leavy J. Dietary patterns identified using factor analysis and prostate cancer risk: A case control study in Western Australia. Ann Epidemiol. 2008;18:364–370. doi: 10.1016/j.annepidem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosini G.L., Oddy W.H., Robinson M., O'Sullivan T.A., Hands B.P., De Klerk N.H. Adolescent dietary patterns are associated with lifestyle and family psycho-social factors. Public Health Nutr. 2009;12:1807–1815. doi: 10.1017/S1368980008004618. [DOI] [PubMed] [Google Scholar]
- 16.Lamberty G.J., Kennedy C.M., Flashman L.A. Clinical utility of the CERAD word list memory test. Appl Neuropsychol. 1995;2:170. doi: 10.1080/09084282.1995.9645357. [DOI] [PubMed] [Google Scholar]
- 17.Welsh K.A., Butters N., Mohs R.C., Beekly D., Edland S., Fillenbaum G. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 18.Samieri C., Okereke O.I., Devore E., Grodstein F. Long-term adherence to the mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr. 2013;143:493–499. doi: 10.3945/jn.112.169896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grodstein F. Cardiovascular risk factors and cognitive function. Alzheimers Dement. 2007;3:S16–S22. doi: 10.1016/j.jalz.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Fotuhi M., Hachinski V., Whitehouse P.J. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 21.Hanson A.J., Bayer-Carter J.L., Green P.S., Montine T.J., Wilkinson C.W., Baker L.D. Effect of apolipoprotein E genotype and diet on apolipoprotein E lipidation and amyloid peptides: Randomized clinical trial. JAMA Neurol. 2013;70:972–980. doi: 10.1001/jamaneurol.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker L.D., Bayer-Carter J.L., Skinner J., Montine T.J., Cholerton B.A., Callaghan M. High-intensity physical activity modulates diet effects on cerebrospinal amyloid-β levels in normal aging and mild cognitive impairment. J Alzheimer's Dis. 2012;28:137–146. doi: 10.3233/JAD-2011-111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosconi L., Murray J., Davies M., Williams S., Pirraglia E., Spector N. Nutrient intake and brain biomarkers of Alzheimer's disease in at-risk cognitively normal individuals: A cross-sectional neuroimaging pilot study. BMJ Open. 2014;4:e004850. doi: 10.1136/bmjopen-2014-004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freund Levi Y., Vedin I., Cederholm T., Basun H., Faxen Irving G., Eriksdotter M. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer's disease: The OmegAD study. J Int Med. 2014;275:428–436. doi: 10.1111/joim.12166. [DOI] [PubMed] [Google Scholar]
- 25.Yassine H.N., Feng Q., Azizkhanian I., Rawat V., Castor K., Fonteh A.N. Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol. 2016;73:1208–1216. doi: 10.1001/jamaneurol.2016.1924. [DOI] [PubMed] [Google Scholar]
- 26.Livesey G., Taylor R., Hulshof T., Howlett J. Glycemic response and health—a systematic review and meta-analysis: Relations between dietary glycemic properties and health outcomes–. Am J Clin Nutr. 2008;87:258S–268S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 27.Sato N., Morishita R. The roles of lipid and glucose metabolism in modulation of β-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front Aging Neurosci. 2015;7:199. doi: 10.3389/fnagi.2015.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuzaki T., Sasaki K., Tanizaki Y., Hata J., Fujimi K., Matsui Y. Insulin resistance is associated with the pathology of Alzheimer disease The Hisayama Study. Neurology. 2010;75:764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- 29.Huang C.-C., Chung C.-M., Leu H.-B., Lin L.-Y., Chiu C.-C., Hsu C.-Y. Diabetes mellitus and the risk of Alzheimer's disease: A nationwide population-based study. PLoS One. 2014;9:e87095. doi: 10.1371/journal.pone.0087095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparks D.L., Scheff S.W., Hunsaker J.C., III, Liu H., Landers T., Gross D.R. Induction of Alzheimer-like β-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 31.Refolo L.M., Pappolla M.A., Malester B., LaFrancois J., Bryant-Thomas T., Wang R. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 32.Ho L., Qin W., Pompl P.N., Xiang Z., Wang J., Zhao Z. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 33.Keeney J.T.-R., Ibrahimi S., Zhao L. Human ApoE isoforms differentially modulate glucose and amyloid metabolic pathways in female brain: Evidence of the mechanism of neuroprotection by ApoE2 and implications for Alzheimer's disease prevention and early intervention. J Alzheimer's Dis. 2015;48:411–424. doi: 10.3233/JAD-150348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease - A meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]