Abstract
Introduction
Methods for efficiently identifying cognitive decline and Alzheimer's disease (AD) are a critical unmet need. The goal of this work was to validate novel online study partner (SP)-reported outcomes to identify cognitive decline in older adults.
Methods
In older adults enrolled in the Brain Health Registry, we analyzed associations between SP-reported cognitive decline, measured by the Everyday Cognition Scale, and either (1) participant cognition, assessed by Cogstate Brief Battery or (2) participant-reported diagnosis of mild cognitive impairment or AD.
Results
We found strong associations between SP-reported Everyday Cognition Scale and both Cogstate scores and participant diagnosis. The associations were cognitive domain specific, dependant on participant diagnosis, and were stronger in spouse dyads and those who knew each other longer.
Discussion
Collecting SP-reported data online from a large cohort is feasible. Results support the construct validity of our approach, which has the potential to facilitate clinical AD and aging research.
Keywords: Alzheimer's disease, Mild cognitive impairment, Neuropsychological tests, Cognitive neuropsychology in dementia, Study partner-reported outcomes, Informant-reported outcomes, Cognitive decline, Activities of daily living, Online registry, Brain Health Registry
Highlights
-
•
Online study partner (SP)-reported decline is associated with cognition.
-
•
Associations are cognitive domain specific.
-
•
Online SP-reported cognitive decline is associated with participant diagnosis.
-
•
Individuals at risk for mild cognitive impairment have greater SP-reported decline.
-
•
These aforementioned associations depend on diagnosis and dyad relationship.
1. Introduction
Methods for efficiently identifying those at risk for and with Alzheimer's disease (AD) are needed to better understand disease mechanisms, facilitate clinical trial recruitment, and improve cognitive screening in geriatric health care [1]. Older adults with preclinical and prodromal AD are often underdiagnosed and not treatment seeking, which underscores the need for screening strategies that can be deployed widely in a cost-efficient manner [2].
In older adults, decline in cognition and functional abilities are important indicators of incipient dementia. Functional and cognitive decline are associated with AD pathology [3], [4], [5] and are predictors of future disease progression [6]. Furthermore, preservation of everyday function is important to patients and their families, and decline is associated with high caregiver burden [7], [8]. Cognitive and functional decline can be sensitively and reliably measured by asking a collateral source or study partner (SP). SP report is an integral component of many AD clinical studies, and can discriminate between, and predict progression from, cognitively normal status to mild cognitive impairment (MCI) [9], [10], [11], [12].
SP report of decline is also reliably associated with magnitude of cognitive impairment determined from neuropsychological assessment [13], [14]. The same relationship is not observed as consistently for self-reported cognitive decline, where factors such as depressed mood [15], [16], [17] and lack of awareness associated with dementia limits its usefulness [14], [18], [19]. SP report of impairment is associated moderately with abnormal levels of AD biomarkers, such as positron emission tomography amyloid burden and hippocampal volume [3], [20], [21], [22]. Thus, SP ratings of functional and cognitive abilities potentially provide a sound basis for early identification of the disease. Furthermore, use of SP report provides the opportunity to identify decline in adults reluctant to seek care themselves, has low influence from cultural or educational backgrounds, and can be applied widely in the community without requiring any formal clinical setting [23], [24].
A number of validated SP-reported instruments exist, including the Clinical Dementia Rating Scale [25], a semistructured interview incorporating self-report and SP report, which has been adopted as the standard benchmark to detect dementia [25], [26]. SP report cognitive and functional questionnaires administered in clinic or by phone, including the Everyday Cognition Scale (ECog) [27], [28], [29] and shorter screening instruments, have been developed and validated, with some studies also considering their utility in the context of remote assessment [12], [13], [30], [31]. Thus, SP report has great potential for both identifying early dementia and dementia risk, and ultimately improving health care outcomes in older adults.
Despite the enormous growth of internet use among older adults [32], little research has been directed at developing methods for exploiting Web-based instruments for identification of dementia [2], [33], [34]. Recently, we developed an online SP-rated assessment of cognitive and functional abilities and applied this within the Brain Health Registry (BHR), a global, internet-based registry with over 57,000 participants that aims to facilitate AD and other brain disease research. The BHR Caregiver and Study Partner Portal (CASPP) is a Web-based tool launched that allows an SP for each BHR participant to separately register and answer questions about the participant online, with data linkage between the SP and participant. In a large cohort of dyads comprising older adult participants in BHR and their SPs, we tested the hypotheses that (1) online SP-reported data collection from a large cohort is feasible, including in participants likely to have MCI and AD; (2) online SP report of participant cognitive decline is significantly associated with objective measures of participant cognition; and (3) online SP report is significantly associated with participant diagnosis.
2. Methods
2.1. Brain Health Registry
BHR is a public Web site and registry. Participants register, sign informed consent, and perform tasks online including questionnaires and neuropsychological tests [35]. Participants in this study came from two sources: (1) they were recruited to join BHR from the general public between April 2014 and February 2018; or (2) they were participants enrolled in the Imaging Dementia-Evidence for Amyloid Scanning study who were invited by email to join BHR between February 2017 and February 2018. All participants from both sources who provided data necessary to perform analyses were included. The CASPP [35], [36], [37] within the BHR Web site allows an SP of a BHR participant to separately register, consent, and complete questionnaires (see also Supplementary materials).
2.2. Everyday Cognition Scale
SPs complete the Everyday Cognition-Informant version (SP-ECog) within the CASPP, consisting of 39 items assessing the participant's capability to perform everyday tasks in comparison to activity levels 10 years prior, including activities that map to cognitive abilities across six domains [27]. The BHR uses an adapted version of the ECog in an online survey form, where the text of all items is identical to the paper version. Alzheimer's Disease Neuroimaging Initiative (ADNI) ECog-SP version data were collected according to the ADNI protocol and downloaded from the Laboratory of Neuro Imaging ADNI Web site (http://adni.loni.usc.edu/) in October 2017.
2.3. Self-reported diagnosis
Self-reported diagnosis of MCI and AD was obtained from the BHR medical history questionnaire question, “Please indicate whether you currently have or have had any of the following conditions in the past.”
2.4. Cogstate Brief Battery
This is a computerized cognitive assessment battery that has been validated under supervised [38], [39] and unsupervised [40], [41], [42], [43] conditions in a variety of patient populations. It consists of four cognitive tests using playing card stimuli: (1) detection (detection task to measure processing speed), a measure of psychomotor function and information processing speed that uses a simple reaction time paradigm; (2) identification (identification task to measure attention), a measure of visual attention that uses a choice reaction time paradigm. The primary outcome for detection task to measure processing speed and identification task to measure attention is reaction time in milliseconds for correct responses normalized using a logarithmic base 10 transformation, with a possible range of 2.00 to 6.00; (3) One Card Learning (OCL), a measure of visual learning and memory that uses a pattern separation paradigm; and (4) One-Back Test (ONB), a measure of working memory that uses an n-back paradigm. The primary outcome variable for OCL and ONB is proportion of correct responses normalized using an arcsine transformation, with a possible range of 0 to 1.57.
2.5. Diagnostic risk group classification
Participants were assigned to one of two likely diagnostic groups based on the following criteria: (1) higher likelihood of being cognitively unimpaired (Likely CU)—Cogstate ONB and OCL scores within one standard deviation (SD) of the age- and education-adjusted mean; no self-reported MCI. Those with self-reported MCI, AD, or dementia were excluded. (2) Higher likelihood of having MCI (Likely MCI)—Cogstate ONB or OCL score greater than 1.5 with standard deviations less than the adjusted mean, self-reported subjective memory concern (SMC), and age older than 60 years. SMC was evaluated by asking participants, “Are you concerned that you have a memory problem?”. Participants with self-reported AD or dementia were excluded from this analysis.
2.6. Statistics and data analysis
We tested the a priori hypotheses that (1) online SP-ECog scores are not significantly different from ADNI ECog scores for CU; (2) online SP-ECog scores are lower (indicating less functional decline) than in ADNI for MCI and AD, as we assumed that BHR participants considered to have MCI/AD are higher functioning compared with ADNI participants in these diagnostic groups; (3) SP-reported ECog is associated with online Cogstate scores, diagnosis of MCI and AD, and diagnostic risk group. Exploratory analyses included the contributions of participant diagnosis and dyad relationship to the previously mentioned associations. Ordinary least squares regression or logistic regression (binomial family) procedures were used. Data for each cognitive test were modeled separately and included SP-ECog, participant age, gender, online participant Geriatric Depression Scale score [44], and education as covariates. Education was parameterized as a three-level factor (less than, equal to, or more than 4 years of college). Geriatric Depression Scale scores omitted response to the question, “Do you feel you have more problems with memory than most?”, because this question is closely related to outcome measures of cognitive status. Model fits were inspected by an analysis of the residuals. We also considered an interaction between SP-ECog and two dyad relationship variables: (1) number of years the dyad has known each other, parameterized as a two-level factor of less than 50 years or greater than 50 years; and (2) whether the dyad comprised spouses.
Estimates, errors, P values, and R2 values reported are from multivariable models. Welch's unpaired t tests were used to determine differences between ADNI and BHR ECog means in each diagnostic group; t tests (difference in means) or χ2 tests (differences in proportions) were used to determine differences between participants with and without an SP. Effect sizes (fx) were calculated as Cohen's d for t tests, φ coefficient for χ2 tests, or the difference in means divided by the standardized SD (the square root of the average SD between groups) for Welch's unpaired t tests. For multivariable linear regression models, fx was calculated as the variable estimate divided by the residual error in the model. Effect size, fx, was categorized as small (≤0.2), medium (0.21–0.79), or large (≥0.8) according to Ref. [45]. All analyses were done using R v3.1.1 (www.r-project.org).
3. Results
3.1. Participants and SPs used in this study
Between June 2016 and February 2018, 14,797 BHR participants were asked to identify an SP (see Supplementary material). Of those, 5778 (39%) participants indicated that they had no potential SP, and 8990 (61%) identified a potential SP who was then invited to join the CASPP. Of invited potential SPs, 4463 (45%) enrolled and signed online consent, 4411 (45%) have not yet enrolled, and 116 (1%) declined to participate. Of all enrolled SPs, 3584 have an associated BHR participant age 55 years and older; this cohort was used for the analyses described herein (Table 1). Compared with BHR participants older than 55 years who indicated that they did not have a potential SP, participants with an enrolled SP were slightly older and more educated, with a higher percentage of Caucasians and males. There were significantly more participants with MCI and AD in the group with an SP versus the group without (Table 1).
Table 1.
Characteristics of BHR participants with and without study partners
| BHR participants with study partners | BHR participants without study partners | |
|---|---|---|
| Total N | 3584 | 4108 |
| Demographics | ||
| Age, mean ± SD | 66.6 ± 7.0 | 65.7 ± 7.0∗ |
| Female (%) | 2323 (64.8%) | 3106 (76.9%)∗ |
| Years education (mean ± SD) | 16.7 ± 2.2 | 16.1 ± 2.2∗ |
| Caucasian (%) | 3283 (91.6%) | 3602 (87.7%)∗ |
| Subjective memory concern | 2041 (57.0%) | 2273 (55.3%) |
| Self-report diagnosis | ||
| Completed medical history | 3478 | 3899∗ |
| Self-reported diagnosis of MCI | 433 (12.4%) | 301 (7.7%)∗ |
| Self-reported diagnosis of AD | 187 (5.4%) | 68 (1.7%)∗ |
| Dyad relationship | ||
| Spouses | 2851 (79.5%) | N/A |
| Years known (mean ± SD) | 36.72 ± 14.0 | N/A |
Abbreviations: AD, Alzheimer's disease; BHR, Brain Health Registry; MCI, mild cognitive impairment; SD, standard deviation.
NOTE. Diagnosed MCI and diagnosed AD are based on self-report of diagnosis in a BHR Medical History questionnaire.
Bold values indicate statistical significance of P < .001.
P < .001 compared with participants with study partners.
3.2. SP-reported cognitive decline using the online ECog
We compared SP-ECog scores between the online BHR sample and ECog scores collected in clinic in ADNI. BHR ECog scores were significantly different from ADNI for all diagnostic groups (Table 2). However, for CU and MCI, the effect sizes were less than 0.2. For the AD group, BHR ECog scores were significantly lower, with an effect size of 1.10, indicating less SP report of decline than ADNI scores (Table 2).
Table 2.
Comparison of ECog scores in ADNI and BHR
| Diagnostic group | ADNI |
BHR |
P value | Effect size | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |||
| CU | 298 | 1.20 | 0.29 | 2354 | 1.25 | 0.34 | .006 | 0.09 |
| MCI | 376 | 1.69 | 0.65 | 250 | 1.82 | 0.67 | .02 | 0.16 |
| AD | 364 | 3.03 | 0.65 | 65 | 2.22 | 0.44 | <.001 | 1.10 |
Abbreviations: AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; BHR, Brain Health Registry; CU, cognitively unimpaired; ECog, Everyday Cognition Scale; MCI, mild cognitive impairment; SD, standard deviation.
NOTE. Bold values indicate statistical significance of P < .001.
3.3. Associations between SP-ECog and cognition
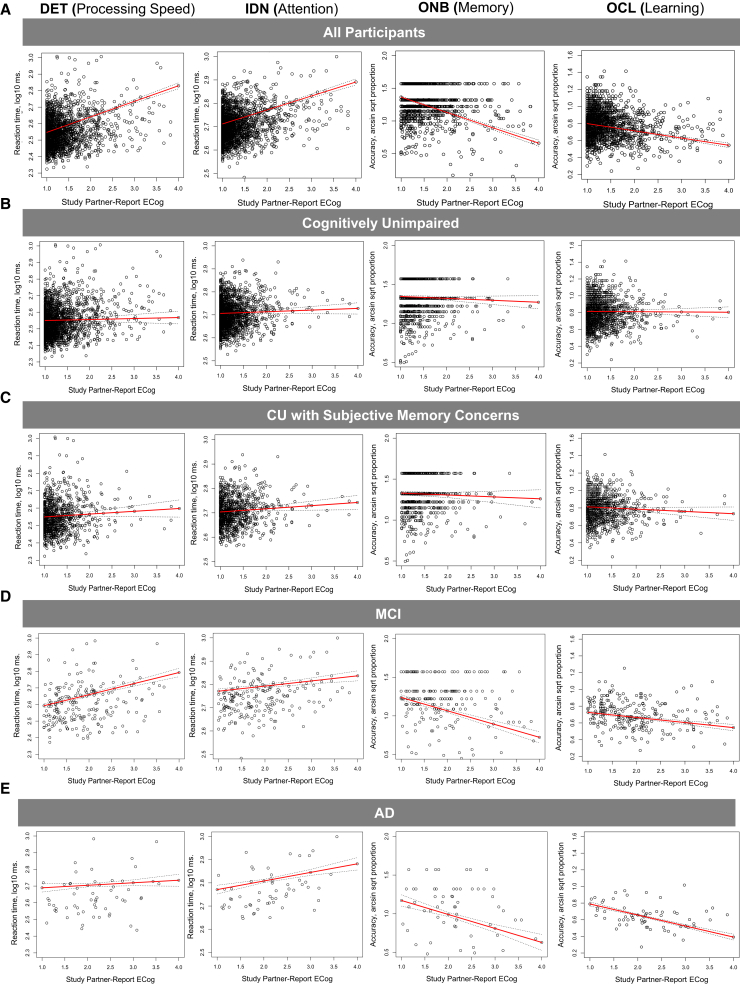
We measured associations between SP-ECog and participant Cogstate scores. In the entire cohort, SP-ECog was significantly but weakly associated with Cogstate processing speed (Table 3). We then measured the associations separately in each diagnostic group and in those with self-reported SMCs. In the CU group, there were no significant associations between SP-ECog and any Cogstate test. In the MCI group, SP-ECog was significantly associated with processing speed and attention. In the AD group, SP-ECog was significantly associated with all Cogstate tests. In the SMC group, SP-ECog was significantly associated with Cogstate memory and learning. In spouse dyads and those who had known each other longer, we found significantly stronger associations between Cogstate score and SP-ECog in a number of diagnostic subgroups (Table 3). Regression analyses are visualized in Fig. 1.
Table 3.
Associations between SP-ECog and participant Cogstate scores
| Cogstate subtest | Estimate | Standard Error | P value | Residual error | Effect size | R2 | Other significant variables | Significant interactions with SP-ECog |
|---|---|---|---|---|---|---|---|---|
| Whole cohort, n = 2693 | ||||||||
| DET (time) | 0.04 | 0.01 | .003 | 0.09 | 0.44 | 0.292 | Spouse, YK | |
| IDN (time) | 0.01 | 0.01 | .562 | 0.07 | 0.08 | 0.255 | Spouse, YK | |
| ONB (accuracy) | −0.04 | 0.04 | .235 | 0.25 | 0.17 | 0.245 | Spouse, YK | |
| OCL (accuracy) | −0.05 | 0.02 | .010 | 0.14 | 0.37 | 0.249 | Edu | Spouse |
| CU only, n = 2354 | ||||||||
| DET (time) | 0.00 | 0.01 | .900 | 0.09 | −0.02 | 0.047 | Gender∗ | YK |
| IDN (time) | 0.00 | 0.01 | .690 | 0.06 | 0.06 | 0.042 | ||
| ONB (accuracy) | −0.04 | 0.03 | .207 | 0.20 | 0.20 | 0.034 | ||
| OCL (accuracy) | −0.04 | 0.02 | .092 | 0.15 | 0.27 | 0.065 | Edu | Spouse |
| MCI only, n = 250 | ||||||||
| DET (time) | 0.23 | 0.03 | <.001 | 0.09 | 2.46 | 0.340 | Gender,† Edu | Spouse, YK |
| IDN (time) | 0.05 | 0.03 | .01 | 0.08 | 0.70 | 0.254 | Gender,† Edu | Spouse, YK |
| ONB (accuracy) | 0.12 | 0.09 | .186 | 0.28 | 0.44 | 0.340 | Gender,∗ Edu | Spouse |
| OCL (accuracy) | −0.02 | 0.05 | .658 | 0.14 | 0.15 | 0.259 | Gender,∗ Edu | |
| AD only, n = 65 | ||||||||
| DET (time) | 0.11 | 0.02 | <.001 | 0.07 | 1.62 | 0.519 | Age, gender,∗ Edu, GDS | YK |
| IDN (time) | 0.13 | 0.01 | <.001 | 0.05 | 2.48 | 0.442 | Age, gender,∗ Edu | |
| ONB (accuracy) | −0.22 | 0.06 | <.001 | 0.22 | 1.00 | 0.194 | ||
| OCL (accuracy) | −0.09 | 0.02 | <.001 | 0.08 | 1.14 | 0.397 | Gender,∗ Edu | Spouse, YK |
| CU with subjective memory concern, n = 1582 | ||||||||
| DET (time) | 0.01 | 0.02 | .538 | 0.08 | 0.14 | 0.098 | ||
| IDN (time) | 0.01 | 0.01 | .218 | 0.05 | 0.28 | 0.139 | Gender∗ | Spouse |
| ONB (accuracy) | −0.11 | 0.05 | .020 | 0.20 | 0.53 | 0.076 | ||
| OCL (accuracy) | −0.08 | 0.03 | .005 | 0.13 | 0.64 | 0.224 | Edu | YK |
Abbreviations: AD, Alzheimer's disease; CU, cognitively unimpaired; DET, detection task to measure processing speed; Edu, education level; GDS, Geriatric Depression Scale; IDN, identification task to measure attention; MCI, mild cognitive impairment; OCL, One Card Learning; ONB, One-Back Test; SP-ECog, Study partner-reported Everyday Cognition scale score; Spouse, spouse partners; YK, number of years that the dyad has known each other.
NOTE. The “Other significant variables” column includes all variables with P < .05 and effect size >0.2 in the multivariable models. Significant dyad relationship variables includes those dyad relationship variables with a significant interaction with SP-ECog, P < .05, and effect size >0.20.
Bold values indicate statistical significance of P < .001.
Females score worse than males.
Males score worse than females.
Fig. 1.
Associations between participant Cogstate scores and SP-ECog. Scatterplots showing associations between SP-ECog and Cogstate scores on four Cogstate Brief Battery subtests (DET, IDN, ONB, and OCL; shown in different columns) in (A) all participants; (B) CU participants with no SMCs; (C) CU participants with SMCs; (D) participants with self-reported previous diagnosis MCI; or (E) participants with self-reported previous diagnosis of AD. Solid red lines are linear regression lines, and dotted black lines show the upper and lower confidence intervals, for multivariable regression models. Abbreviations: AD, Alzheimer's disease; CU, cognitively unimpaired; DET, detection task to measure processing speed; IDN, identification task to measure attention; MCI, mild cognitive impairment; ONB, One-Back Test; OCL, One Card Learning; SMC, subjective memory concern; SP-ECog, study partner-reported Everyday Cognition scale score.
3.4. Associations between SP-ECog and diagnosis
We next determined whether SP-ECog could predict participant self-report diagnosis, or an elevated risk for MCI, as defined by BHR data of the participant (see Section 2).
3.4.1. Associations between SP-ECog and self-reported diagnosis
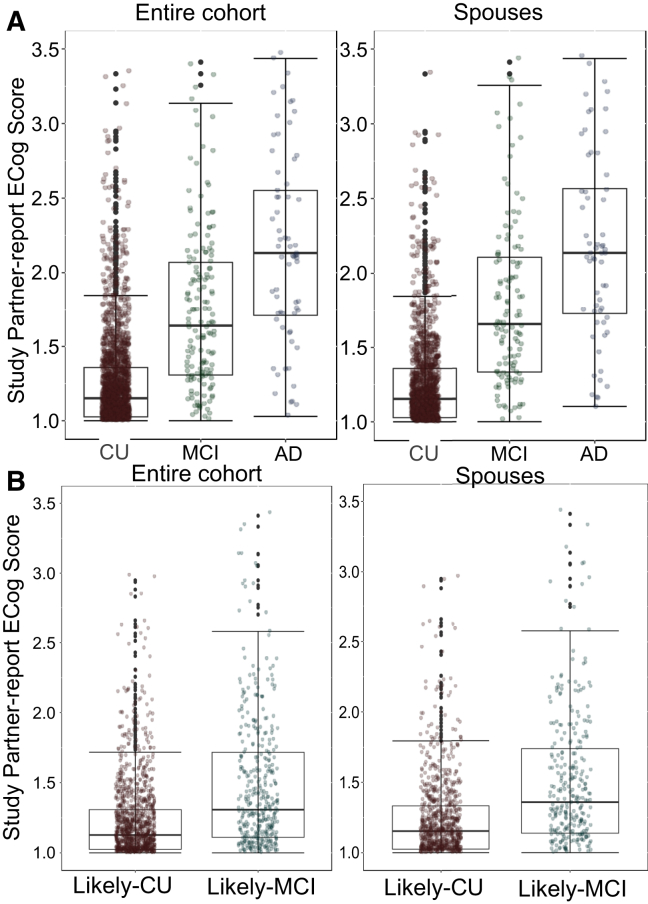
SP-ECog was significantly associated with self-reported diagnosis of MCI but not AD (Fig. 2A, Table 4). The association between SP-ECog and diagnosis was significantly stronger in spouse partners compared with nonspouse partners (P = .02 for MCI, P = .05 for AD). In a subset of participants consisting only of spouse dyads, we found a significant association between SP-ECog and diagnosis of both MCI and AD. (Table 4).
Fig. 2.
Associations between SP-ECog and participant diagnosis. Box and whisker plots showing SP-ECog in (A) participants of different diagnostic groups (CU, MCI, and AD) and (B) participants in different diagnostic risk categories (Likely CU and Likely MCI). Left column panels include all participants; right column panels include only spouse dyads. Abbreviations: AD, Alzheimer's disease; CU, cognitively unimpaired; MCI, mild cognitive impairment; SP-ECog, study partner-reported Everyday Cognition scale score.
Table 4.
Associations between SP-ECog and participant diagnosis or diagnostic risk group
| Diagnostic group | Estimate (log odds ratio) | Standard error | P value | Odds ratio (CI) |
|---|---|---|---|---|
| CU versus MCI | ||||
| Whole cohort | 1.25 | 0.38 | <.001 | 3.49 (1.64–7.41) |
| Spouses | 2.22 | 0.24 | <.001 | 9.20 (5.80–14.58) |
| CU versus AD | ||||
| Whole cohort | 0.90 | 0.78 | .25 | 2.45 (0.53–11.34) |
| Spouses | 1.58 | 0.28 | <.001 | 4.87 (2.79–8.51) |
| MCI versus AD | ||||
| Whole cohort | 0.46 | 0.94 | .62 | 1.59 (0.25–9.99) |
| Spouses | 1.98 | 0.28 | <.001 | 7.28 (4.21–12.59) |
| Likely CU versus Likely MCI | ||||
| Whole cohort | 0.45 | 0.39 | .26 | 1.57 (0.72–3.39) |
| Spouses | 1.19 | 0.25 | <.001 | 3.28 (2.00–5.39) |
Abbreviations: AD, Alzheimer's disease; CI, confidence interval; CU, cognitively unimpaired; GDS, Geriatric Depression Scale; MCI, mild cognitive impairment; SP-ECog, study partner-reported Everyday Cognition scale score.
NOTE. Results of the SP-ECog estimate in binomial logistic regression models including participant age, gender, education, and GDS score; as well as dyad relationship.
Bold values indicate statistical significance of P < .001.
3.4.2. Associations between SP-ECog and diagnostic risk group
Reliance on self-report diagnosis may fail to identify those with MCI because of lack of awareness about diagnosis [46]. We therefore assigned participants to two diagnostic risk groups based on age, Cogstate scores, and self-reported SMCs. Participants were categorized as more likely to be cognitively unimpaired (Likely CU), or at a higher risk for MCI (Likely MCI) (see Section 2). Of the 2693 participants who provided enough information for classification, 489 (18%) were classified as Likely MCI, 1575 (58%) were classified as Likely CU, and 629 (23%) were excluded because they did not fit the criteria for either classification. Agreement between self-report MCI and Likely MCI classification was 82%; the remaining 18% participants were assigned to the Likely MCI group but did not self-report MCI. SP-ECog was not significantly associated with diagnostic risk group in the whole cohort (Fig. 2B, Table 4). Advanced age (odds ratio [OR] = 1.17), more depressive symptoms (OR = 1.18), and lower education (OR = 3.66) were significantly associated with Likely MCI classification (Table 4). In spouse dyads, there was a significant association between higher (worse) SP-ECog scores and Likely MCI classification (Table 4).
4. Discussion
The major findings are as follows: (1) it is feasible to collect data online from older adults and their SPs, including older adults likely to have MCI and AD; (2) online SP-reported cognitive decline is comparable to that collected in clinic and is associated with objectively defined participant cognition; (3) the associations are stronger in participants with self-reported SMCs, MCI, and AD than in CU participants; (4) associations are domain specific, depending on the diagnostic group of participants, with the strongest associations found in processing speed and learning; (5) online SP-ECog is associated with self-reported diagnosis of MCI and increased MCI risk; in spouse dyads, SP-ECog is associated with participant diagnosis of MCI and AD; and (6) dyad relationship plays a crucial role in several of the associations measured previously, with stronger associations in spouses and dyads who have known each other longer. Taken together, the results support the construct validity of online SP-reported data using the BHR. Validated online SP assessment tools have the potential for high impact on the AD and cognitive aging fields by facilitating clinical trial recruitment and screening, as well as by improving screening of older adults for cognitive problems in various health care settings.
We found significant associations between online SP-ECog and participant online Cogstate scores. This is consistent with previous findings using in-clinic or telephone-based SP report, demonstrating associations between neuropsychological test scores and SP-reported cognitive decline in AD, MCI, and CU [13], [14], [47]. The supervised version of the Cogstate Brief Battery has been well validated [38], [39], and there is accumulating evidence for the reliability, sensitivity to disease state, and validity of the unsupervised version [40], [41], [42], [48], [49], including in participants with MCI and AD [49], and in BHR [43]. Our cohort is large, which could result in statistically significant associations (P < .05) that are not clinically meaningful. Therefore, we mainly evaluated the strength of associations based on fx, and the amount of variance that could be explained by the model (R2) [45]. Online SP-ECog was moderately associated with participant processing speed (fx = 0.44, R2 = 0.292) and learning (fx = 0.37, R2 = 0.249) in the whole cohort, which included CU, MCI, and AD participants.
Because our cohort is primarily (84%) comprising CU older adults, many of who have SPs who report no cognitive decline in the participant, we hypothesized that the contributions of impaired participants were driving these associations. In support of this hypothesis, we found no significant associations between SP-ECog and Cogstate scores in a subset of only CU participants. Conversely, we found strong associations in MCI and AD participants. In MCI, the strongest associations were between SP-ECog and processing speed (fx = 2.46, R2 = 0.340) and attention (fx = 0.67, R2 = 0.254). In AD, associations were robust across cognitive domains with effect sizes between 1.00 and 2.48, and R2 between 0.194 and 0.519. Interestingly, in CU with SMC, we found small to moderate associations between SP-ECog and Cogstate memory (fx = 0.53, R2 = 0.076) and learning (fx = 0.005, R2 = 0.224), but not other cognitive domains. Thus, in AD, SPs are able to detect changes in function that map to multiple cognitive domains, whereas in SMC and MCI the domains are limited. In MCI, SP-reported changes map to processing speed and attention, whereas in SMC, SP-reported changes map to learning and memory. This finding provides evidence of construct validity, because SMCs are specific to memory, whereas impairments in MCI are likely to encompass multiple domains. Our data also support previous studies demonstrating that SP-ECog, collected in clinic, is associated with memory and executive function [14], [50]. Thus these findings agree with past studies using traditional, in-clinic or by-phone SP report, supporting the construct validity of our online approach.
We found significant associations between SP-reported ECog and participant self-reported diagnosis of MCI, in agreement with previous in-clinic studies showing that SP can discriminate between CU and MCI [9], [11]. Contrasting findings using in-clinic SP report, online SP-ECog did not significantly predict self-report AD diagnosis, suggesting that online SP-ECog alone is not sufficient to categorize participants into diagnostic groups. This may be because of several factors. Our AD group is relatively small compared with other diagnostic groups, and is likely to be more heterogeneous than an AD cohort in an in-clinic study. Furthermore, the low burden of SP participation in BHR compared with traditional in-clinic studies may make potential SPs likely to agree to join BHR even if they do not know the associated participant very well and/or do not see them often. If this hypothesis is correct, we should find higher associations between SP-ECog and diagnosis in spouse dyads, which is what we find. Higher (worse) SP-ECog scores are associated with MCI and AD diagnosis, as well as higher risk for MCI, in spouses. Further evidence for the importance of dyad relationship comes from our finding that, in spouses and those who have known each other longer, the associations between SP-ECog and Cogstate are stronger (Table 3). Ultimately, online SP-report information may be best used in combination with other remotely collected data, and taking into account variability associated with dyad relationship, as a tool to categorize participants by diagnosis.
We have not confirmed the clinical diagnosis of BHR participants, which raises concerns about the accuracy of self-report, especially in participants with MCI and dementia [46] who may lack awareness of their diagnosis. Our results identifying Likely MCI participants using Cogstate scores and SMCs support underdiagnosis or a lack of diagnostic awareness of MCI; 18% of participants were classified as Likely MCI, but failed to endorse MCI diagnosis in their medical history questionnaire. On the other hand, we did not find evidence of over-report of MCI diagnosis. No participants who endorsed MCI failed to meet criteria for Likely MCI (Section 3.4.2). This issue can only be fully addressed in a population with BHR data linked to confirmed clinical diagnosis; such data will be available in the near future in cohorts coenrolled in both BHR and in-clinic studies [35].
This study allowed us to uncover important new information about online registry cohorts. SP-reported ECog scores in ADNI and BHR were significantly different across diagnostic groups, but the difference for CU and MCI is unlikely to be meaningful because of low effect size (CU, fx = 0.09; MCI, fx = 0.18). Thus, the data are similar between BHR and ADNI despite important differences between the two cohorts, such as the method of ECog administration and participant selection. For the AD group, BHR had lower (better) ECog scores compared with ADNI. This is not surprising considering the assumed selection bias for less impaired participants in an online registry, in which participants must be functional enough to use a computer and complete tasks online. Another important feature of our registry cohort uncovered in this study is gender bias. The general BHR cohort comprised 73.9% females [35]. Compared with those with an SP, the group without an SP had a significantly higher proportion of females (Table 1). This raises the possibility that online SP-report data will exclude detection of cognitive decline in females, who are at greater risk for developing dementia [33], [51]. Although analysis of the contributions of participant and SP gender on SP-report outcomes is outside the scope of the present work, it will be crucial to address such contributions in future studies.
Limitations of our study that affect generalizability include lack of racial/ethnic diversity, high education levels, and restriction to participants who can successfully complete tasks online. SPs of individuals unable or reluctant to volunteer online data are not represented. However, our results suggest that in the future, online SP-ECog could be used to help identify those with cognitive problems, even in the absence of cognitive data from the participant himself/herself. This model requires that an SP be able to initiate registry contact without the participant, a capability that we plan to enable in the near future. Finally, clinical diagnosis of MCI or AD may include SP report of cognitive impairment [52], which could confound the association between SP-ECog and diagnosis.
5. Conclusions
Online SP-ECog is significantly associated with participant cognition and self-reported diagnosis. The associations depend on diagnostic group and dyad relationship. If validated, BHR SP data have many potentially high-impact applications in the aging and AD fields, including using such data alone or in combination with other, remotely collected data for routine screening of older adults in health care and research settings. Because BHR can be accessed remotely by a large number of individuals, it could also be used for longitudinal monitoring in clinical trials, lessening the in-clinic burden of participation on SPs, which is a known barrier to participation [53], [54]. These results are a first step to address the feasibility and validity of online SP data in BHR.
Research in Context.
-
1.
Systematic Review. The authors reviewed the literature using PubMed and by personal communication with authors. Past literature demonstrates associations between in-clinic study partner (SP)-reported data and both participant cognition and diagnosis. Remote collection and analysis of SP-reported data from a large Internet-based cohort is novel.
-
2.
Interpretation: Our findings support the feasibility and validity of our approach. They are consistent with past findings using in-clinic data, and also provide novel information about online registry cohorts, including those with mild cognitive impairment and Alzheimer's disease (AD). Validation of our approach has the potential to facilitate future AD and aging clinical research.
-
3.
Future directions: Future studies will extend these findings by (A) further validating the online approach by linking online data and in-clinic data, including clinical diagnosis; (B) assessing the relative contributions of SP report and additional variables to predicting outcomes; (C) measuring associations between online SP-reported data and additional variables, including AD biomarkers.
Acknowledgments
This work was funded by the National Institutes of Health (NIH) (K01AG055692), the Larry L. Hillblom Foundation (2015-A-011-NET), the Patient-Centered Outcomes Research Institute (PPRN-1501-26817), the California Department of Public Health (16-10054), and the Alzheimer's Drug Discovery Foundation (20150802).
Data collection and sharing for this project was funded by the Alzheimer's DiseaseNeuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.;Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.trci.2018.09.008.
Supplementary data
Supplementary Materials
References
- 1.Watson J.L., Ryan L., Silverberg N., Cahan V., Bernard M.A. Obstacles and opportunities in Alzheimer's clinical trial recruitment. Health Aff (Millwood) 2014;33:574–579. doi: 10.1377/hlthaff.2013.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott T.J., O'Connor C.A., Link A.N., Beauliew T.J. Economic analysis of opportunities to accelerate Alzheimer's R&D. N Y Acad Sci. 2014;1313:17–34. doi: 10.1111/nyas.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okonkwo O.C., Alosco M.L., Griffith H.R., Mielke M.M., Shaw L.M., Trojanowski J.Q. Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: Normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol. 2010;67:688–696. doi: 10.1001/archneurol.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall G.A., Lorius N., Locascio J.J., Hyman B.T., Rentz D.M., Johnson K.A. Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer's disease spectrum. J Alzheimers Dis. 2014;41:719–728. doi: 10.3233/JAD-132768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lilamand M., Cesari M., del Campo N., Cantet C., Soto M., Ousset P.J. Brain amyloid deposition is associated with lower instrumental activities of daily living abilities in older adults. Results from the MAPT study. J Gerontol A Biol Sci Med Sci. 2016;71:391–397. doi: 10.1093/gerona/glv155. [DOI] [PubMed] [Google Scholar]
- 6.Farias S.T., Mungas D., Reed B.R., Harvey D., DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall G.A., Rentz D.M., Frey M.T., Locascio J.J., Johnson K.A., Sperling R.A. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2011;7:300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauphinot V., Delphin-Combe F., Mouchoux C., Dorey A., Bathsavanis A., Makaroff Z. Risk factors of caregiver burden among patients with Alzheimer's disease or related disorders: A cross-sectional study. J Alzheimers Dis. 2015;44:907–916. doi: 10.3233/JAD-142337. [DOI] [PubMed] [Google Scholar]
- 9.Marshall G.A., Zoller A.S., Kelly K.E., Amariglio R.E., Locascio J.J., Johnson K.A. Everyday cognition scale items that best discriminate between and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res. 2014;11:853–861. doi: 10.2174/1567205011666141001120903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donovan N.J., Amariglio R.E., Zoller A.S., Rudel R.K., Gomez-Isla T., Blacker D. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22:1642–1651. doi: 10.1016/j.jagp.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomaszewski Farias S., Mungas D., Harvey D.J., Simmons A., Reed B.R., Decarli C. The measurement of everyday cognition: Development and validation of a short form of the Everyday Cognition scales. Alzheimers Dement. 2011;7:593–601. doi: 10.1016/j.jalz.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amariglio R.E., Donohue M.C., Marshall G.A., Rentz D.M., Salmon D.P., Ferris S.H. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: The Alzheimer's Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 2015;72:446–454. doi: 10.1001/jamaneurol.2014.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y., Pang W.S., Lim L.B., Yang Y.H., Morris J.C., Hilal S. The informant AD8 is superior to participant AD8 in detecting cognitive impairment in a memory clinic setting. J Alzheimers Dis. 2013;35:159–168. doi: 10.3233/JAD-122026. [DOI] [PubMed] [Google Scholar]
- 14.Rueda A.D., Lau K.M., Saito N., Harvey D., Risacher S.L., Aisen P.S. Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer's disease. Alzheimers Dement. 2015;11:1080–1089. doi: 10.1016/j.jalz.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon J.S., Charness N., Boot W.R., Czaja S.J., Rogers W.A. Depressive symptoms as a predictor of memory complaints in the PRISM sample. J Gerontol B Psychol Sci Soc Sci. 2017:1–10. doi: 10.1093/geronb/gbx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yates J.A., Clare L., Woods R.T., Matthews F.E. Cognitive Function and Ageing Study Wales. Subjective Memory Complaints are involved in the relationship between mood and mild cognitive impairment. J Alzheimers Dis. 2015;48:S115–S123. doi: 10.3233/JAD-150371. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez-Huete A., Del Barrio A., Riva E., Campo P., Toledano R., Franch O. Subjective evaluation of mood and cognitive functions in a general neurology clinic: Patients versus informants. J Clin Neurol. 2017;13:259–264. doi: 10.3988/jcn.2017.13.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farias S.T., Mungas D., Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: Dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry. 2005;20:827–834. doi: 10.1002/gps.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherling C.S., Wilkins S.E., Zakrezewski J., Kramer J.H., Miller B.L., Weiner M.W. Decreased self-appraisal accuracy on cognitive tests of executive functioning is a predictor of decline in mild cognitive impairment. Front Aging Neurosci. 2016;8:120. doi: 10.3389/fnagi.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidoni E.D., Honea R.A., Burns J.M. Neural correlates of impaired functional independence in early Alzheimer's disease. J Alzheimers Dis. 2010;19:517–527. doi: 10.3233/JAD-2010-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall G.A., Olson L.E., Frey M.T., Maye J., Becker J.A., Rentz D.M. Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement Geriatr Cogn Disord. 2011;31:443–450. doi: 10.1159/000329543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrotin A., Mormino E.C., Madison C.M., Hayenga A.O., Jagust W.J. Subjective cognition and amyloid deposition imaging: A Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razavi M., Tolea M.I., Margrett J., Martin P., Oakland A., Tscholl D.W. Comparison of 2 informant questionnaire screening tools for dementia and mild cognitive impairment: AD8 and IQCODE. Alzheimer Dis Assoc Disord. 2014;28:156–161. doi: 10.1097/WAD.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr D.B., Gray S., Baty J., Morris J.C. The value of informant versus individual's complaints of memory impairment in early dementia. Neurology. 2000;55:1724–1726. doi: 10.1212/wnl.55.11.1724. [DOI] [PubMed] [Google Scholar]
- 25.Morris J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 26.Morris J.C., McKeel D.W., Jr, Fulling K., Torack R.M., Berg L. Validation of clinical diagnostic criteria for Alzheimer's disease. Ann Neurol. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- 27.Farias S.T., Mungas D., Reed B.R., Cahn-Weiner D., Jagust W., Baynes K. The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeffer R.I., Kurosaki T.T., Harrah C.H., Jr, Chance J.M., Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 29.Jorm A.F., Scott R., Cullen J.S., MacKinnon A.J. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med. 1991;21:785–790. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- 30.Galvin J.E., Roe C.M., Powlishta K.K., Coats M.A., Muich S.J., Grant E. The AD8: A brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 31.Li C., Neugroschl J., Luo X., Zhu C., Aisen P., Ferris S. The utility of the cognitive function instrument (CFI) to detect cognitive decline in non-demented older adults. J Alzheimers Dis. 2017;60:427–437. doi: 10.3233/JAD-161294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pew Research Center, Mobile fact sheet. Internet Sci Tech, 2017.
- 33.Alzheimer'sAssociation 2018 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;14:367–429. [Google Scholar]
- 34.Feldman H.H., Haas M., Gandy S., Schoepp D.D., Cross A.J., Mayeux R. Alzheimer's disease research and development: A call for a new research roadmap. Ann N Y Acad Sci. 2014;1313:1–16. doi: 10.1111/nyas.12424. [DOI] [PubMed] [Google Scholar]
- 35.Weiner M.W., Nosheny R., Camacho M., Truran-Sacrey D., Mackin R.S., Flenniken D. The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. 2018;14:1063–1076. doi: 10.1016/j.jalz.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nosheny R., Flenniken D., Camacho M., Ulbricht A., Fockler J., Insel P. Clinical Trials for Alzheimer's Disease. 2016. The Brain Health Registry Caregiver and Study Partner Portal to facilitate Alzheimer's clinical trials. San Diego, CA. [Google Scholar]
- 37.Nosheny R., Camacho M., Flenniken D., Ulbricht A., Fockler J., Insel P. Alzheimer's Association International Conference. 2017. Validation of study partner reported outcomes collected online using the Brain Health Registry. London, UK. [Google Scholar]
- 38.Maruff P., Thomas E., Cysique L., Brew B., Collie A., Snyder P. Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- 39.Maruff P., Lim Y.Y., Darby D., Ellis K.A., Pietrzak R.H., Snyder P.J. Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer's disease. BMC Psychol. 2013;1:30. doi: 10.1186/2050-7283-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuiper J.S., Oude Voshaar R.C., Verhoeven F.E.A., Zuidema S.U., Smidt N. Comparison of cognitive functioning as measured by the Ruff Figural Fluency Test and the CogState computerized battery within the LifeLines Cohort Study. BMC Psychol. 2017;5:15. doi: 10.1186/s40359-017-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cromer J.A., Harel B.T., Yu K., Valadka J.S., Brunwin J.W., Crawford C.D. Comparison of cognitive performance on the Cogstate Brief Battery when taken in-clinic, in-group, and unsupervised. Clin Neuropsychol. 2015;29:542–558. doi: 10.1080/13854046.2015.1054437. [DOI] [PubMed] [Google Scholar]
- 42.Sumner J.A., Hagan K., Grodstein F., Roberts A.L., Harel B., Koenen K.C. Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depress Anxiety. 2017;34:356–366. doi: 10.1002/da.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott Mackin R., Insel P.S., Truran D., Finley S., Flenniken D., Nosheny R.L. Unsupervised online neuropsychological test performance for individuals with MCI and dementia: Results from The Brain Health Registry. Alzheimers Dement (Amst) 2018 doi: 10.1016/j.dadm.2018.05.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 45.Cohen D. 2nd ed. Lawrence Erlbaum Associates; New York, NY: 1988. Statistical Power Analyses for the Behavioral Sciences. [Google Scholar]
- 46.Stites S.D., Karlawish J., Harkins K., Rubright J.D., Wolk D. Awareness of mild cognitive impairment and mild Alzheimer's disease dementia diagnoses associated with lower self-ratings of quality of life in older adults. J Gerontol B Psychol Sci Soc Sci. 2017;72:974–985. doi: 10.1093/geronb/gbx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rattanabannakit C., Risacher S.L., Gao S., Lane K.A., Brown S.A., McDonald B.C. The cognitive change index as a measure of self and informant perception of cognitive decline: Relation to neuropsychological tests. J Alzheimers Dis. 2016;51:1145–1155. doi: 10.3233/JAD-150729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mielke M.M., Machulda M.M., Hagen C.E., Edwards K.K., Roberts R.O., Pankratz V.S. Performance of the CogState computerized battery in the Mayo Clinic Study on Aging. Alzheimers Dement. 2015;11:1367–1376. doi: 10.1016/j.jalz.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maruff P.S., Schembri A., Petersen R.C., Aisen P.A., Weiner M.W., Albala B. Acceptability, usability, validity, an sensitivity of cognition assessed in unsupervised settings. Alzheimers Dement. 2017;13:P596–P597. [Google Scholar]
- 50.Brown P.J., Devanand D.P., Liu X., Caccappolo E. Alzheimer's Disease Neuroimaging Initiative. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68:617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen R.C., Lopez O., Armstrong M.J., Getchius T.S.D., Ganguli M., Gloss D. Author response: Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;91:373–374. doi: 10.1212/WNL.0000000000006042. [DOI] [PubMed] [Google Scholar]
- 53.Grill J.D., Zhou Y., Elashoff D., Karlawish J. Disclosure of amyloid status is not a barrier to recruitment in preclinical Alzheimer's disease clinical trials. Neurobiol Aging. 2016;39:147–153. doi: 10.1016/j.neurobiolaging.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rollin-Sillaire A., Breuilh L., Salleron J., Bombois S., Cassagnaud P., Deramecourt V. Reasons that prevent the inclusion of Alzheimer's disease patients in clinical trials. Br J Clin Pharmacol. 2013;75:1089–1097. doi: 10.1111/j.1365-2125.2012.04423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials