Abstract
Baxdela (delafloxacin) for treatment of acute bacterial skin and skin structure infections
INTRODUCTION
Skin and soft tissue infections (SSTI) have become widespread in community and hospital settings. These types of infections can range from mild, which can be self-treated, to severe, life-threatening infections that contribute to mortality. 1 In the past decade, there has been a three-fold increase in hospitalizations due to SSTI.2,3 The emergence of Community-Acquired Methicillin-Resistant Staphylococcus aureus (CA-MRSA) in the United States has led to a rise in hospital admissions and emergency department (ED) visits by patients with SSTI.4 There has been a shift from hospital-acquired to community-acquired MRSA purulent SSTI, yet only 38% of empiric treatment regimens prescribed in the ED have included agents with activity against MRSA.1,3
In October 2013, the FDA published a guidance document to classify more severe and complicated SSTI that would likely require inpatient treatment with parenteral antibiotics.5 The newly assigned nomenclature introduced the term Acute Bacterial Skin and Skin Structure Infections (ABSSSI). ABSSSI can be defined as three categories of infections: cellulitis/erysipelas, wound infection, and major cutaneous abscess. The minimum lesion size to be considered an ABSSSI is 75 cm2. Common pathogens that may cause ABSSSI include Streptococcus pyogenes and Staphylococcus aureus (most commonly MRSA).5 Less common bacterial pathogens are Enterococcus faecalis or gram-negative bacteria.
ABSSSI are managed by surgical intervention (incision and drainage), culture and sensitivity, and antimicrobial therapy. The recommended agents for ABSSSI when MRSA is suspected are: vancomycin, linezolid, daptomycin, lipoglycopeptides (such as telavancin), tigecycline, and ceftaroline. Empiric coverage for gram-negative organisms is not recommended. The risk of polymicrobial infection is increased in sites of infection that are avascular (such as diabetic foot ulcers). In these cases, broad-spectrum antibiotics with activity against gram-positive and gram-negative pathogens, such as beta lactam/beta lactamase inhibitors, tigecycline, or moxifloxacin, are warranted.6
Baxdela (delafloxacin, Melinta Therapeutics, Inc.) is a novel fluoroquinolone (FQ) that was approved in 2017 for the treatment of ABSSSI, including MRSA SSTI. It is currently being studied for additional indications including community-acquired respiratory tract infections, urinary tract infections (UTIs), and sexually transmitted diseases due to Neisseria gonorrhoeae.7 This article reviews the pharmacology, pharmacokinetics, drug interactions, clinical efficacy, dosage and administration, safety profile, and place in therapy of Baxdela.
PHARMACOLOGY
FQs are synthetic antibiotics that inhibit bacterial DNA synthesis, thereby exhibiting bactericidal activity. These antibiotics act on two bacterial enzymes: DNA gyrase and topoisomerase IV. Inhibition of DNA gyrase prevents the relaxation of supercoiled DNA, averting the transcription and translation of DNA. Inhibition of topoisomerase IV prevents separation of replicated chromosomes during cell division. Some FQs have more affinity for one enzyme over another, which translates clinically to differences in spectrum of activity.8 In gram-negative bacteria, DNA gyrase is more susceptible to inhibition by FQs, whereas in gram-positive bacteria, FQs target topoisomerase IV.9,10
In contrast, delafloxacin binds with equal affinity to and forms a complex with both DNA gyrase and topoisomerase IV, therefore exhibiting good activity against gram-negative, gram-positive, anaerobic, and atypical pathogens. (See Table 1 for delafloxacin’s spectrum of activity.) An additional advantage to dual inhibition is that delafloxacin confers less resistance compared to other FQs.9
Table 1.
Delafloxacin Spectrum of Activity
| Gram-Positive | Gram-Negative | Atypical | Anaerobes |
|---|---|---|---|
|
S. aureus (including MRSA) S. epidermidis S. pneumoniae E. faecalis E. faecium Coagulase (-) staphylococci |
H. influenzae M. catarrhalis N. gonorrhoeae K. pneumoniae P. aeruginosa H. pylori Enterobacter sp. E. coli |
Mycoplasma sp. L. pneumophila Chlamydia sp. Ureaplasma sp. |
C. difficile B. fragilis Prevotella sp. C. perfringens |
MRSA = Methicillin-Resistant Staphylococcus aureus
There are a few unique structural modifications that increase the potency of delafloxacin compared to other FQs. One of the major modifications is the change in the substituent on C-7 (Figure 1)11, making the molecule weakly acidic (pKa 5.5). Therefore, the molecule will be neutral in an acidic environment, which enables good transmembrane penetration and higher concentrations in the bacterium.12,13 This property becomes important in the treatment of ABSSSI, where the infection sites typically have an acidic pH. Once the antibiotic penetrates into the bacteria, the pH is neutral. The change in the pH will cause the antibiotic to become ionized and remain in the bacteria to exert its bactericidal effect.13 Other modifications include an electronegative chlorine atom on C-8, as well as a large substitution on N-1, which increases the molecular surface area, thereby further enhancing the potency of delafloxacin.14
Figure 1.
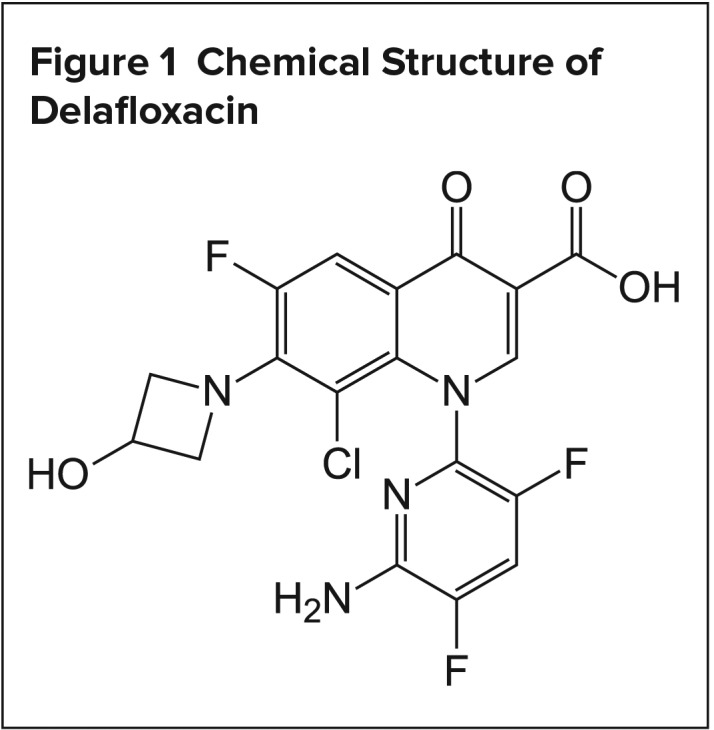
Chemical Structure of Delafloxacin
PHARMACOKINETICS
The pharmacokinetic parameters of delafloxacin have been evaluated in healthy patients. Delafloxacin has a volume distribution (Vd) of 35 L at steady state, which is similar to the total water volume of the body. The drug is primarily excreted renally (65%) in unchanged form, and about 28% is excreted in the feces (unchanged). Clearance is reduced in patients with moderate to severe renal impairment.15 It is metabolized via phase II reactions, primarily glucoronidation. Delafloxacin does not undergo CYP-mediated metabolism, which is an advantage over other FQs that have interactions with medications that undergo oxidative metabolism.16 Delafloxacin has been studied in patients with mild, moderate, and severe hepatic dysfunction and was found to be safe and efficacious in those patient populations.17 The bioavailability of orally administered delafloxacin is 58.8%. Giving oral tablets with high-fat meals or milk reduces the Cmax by 20%, while the area under the plasma drug concentration-time curve (AUC) remains unchanged. Because the activity of FQ is dependent on total exposure, delafloxacin can be administered without regard to food.18
PHARMACODYNAMICS
FQs, as a class, display concentration-dependent bactericidal activity.19 The pharmacodynamic index that best correlates with efficacy is free-drug AUC to MIC ratio (fAUC/MIC).20 In a murine-infection model, the median delafloxacin fAUC/MIC required to achieve a 1-log 10 reduction in bacterial load was 14.3 for both Methicillin-sensitive S. aureus (MSSA) and MRSA isolates (MICs range, 0.004–0.8 mg/L).21 Based on this pharmacodynamic target, Bhavnani et al. conducted a pharmacokinetic/pharmacodynamic (PK/PD) analysis to estimate the probability of target attainment with delafloxacin in humans. At the MIC90 of 0.25 mcg/mL for S. aureus, percentage probabilities of attaining fAUC/MIC associated with 1-log killing was 96.9% on day 1 and 93.1% on day 4.22
DOSAGE
The recommended dose of delafloxacin for ABSSSI is 300 mg intravenously (IV) every 12 hours or 450 mg orally (PO) every 12 hours. For patients with an eGFR of 15–29 mL/min, the IV dose should be adjusted to 200 mg every 12 hours. The IV formulation of delafloxacin contains sulphobutylether-B-cyclodextrin (SBECD) as an inactive ingredient. SBECD may increase serum creatinine and accumulate in patients with renal impairment. The oral dose does not need to be renally adjusted.11 The use of delafloxacin is not recommended for patients with an eGFR < 15 mL/min or for patients on hemodialysis. Both IV and PO dosage forms should not be administered concomitantly with preparations containing multivalent cations such as calcium, magnesium, aluminum, and iron. Based on phase 2 and 3 clinical trials, the duration of delafloxacin therapy for the treatment of ABSSSI has ranged from 5 to 14 days, depending on clinical improvement. (See Table 2 for delafloxacin dosing.) One ongoing phase 3 trial is evaluating a regimen of delafloxacin 300 mg IV twice daily for three days, followed by 450 mg PO twice daily for a total of 5 to 14 days.23
Table 2.
Delafloxacin Dosing
| Route of Administration | Dose | Renal Dosing Recommendations | |
|---|---|---|---|
| eGFR 30–89 ml/min | eGFR 15–29 ml/min | ||
| PO | 450 mg PO q12 h | No adjustment needed | No adjustment needed |
| IV | 300 mg IV q12 h* | 200 mg IV q12 h | |
| IV to PO | Switch 300 mg IV to 450 mg PO q12 h | 200 mg IV switch to 450 mg PO q12 h | |
Infuse over 1 hour
CLINICAL TRIALS
The clinical efficacy and safety of delafloxacin in treating SSTI was studied in two phase 2 trials and two phase 3 trials.
Phase II Trials
The efficacy and tolerability of delafloxacin (dosed at 300 mg IV and 450 mg IV every 12 hours) was compared to tigecycline (100 mg IV for one dose, followed by 50 mg IV every 12 hours) in 150 patients for 5 to 14 days.24 This was a randomized, double-blind, multi-center study. Randomization was stratified by infection types: cellulitis (36%), abscess (33%), and wound infection (31%). Clinical cure was defined as the complete resolution of baseline signs or symptoms or improvement, such that no additional antibiotics were needed. S. aureus was the most common pathogen isolated (96 isolates), of which 68 isolates (71%) were MRSA. The MIC90 values for delafloxacin and tigecycline were 0.06 μg/ml and 0.12 μg/ml, respectively. In the clinically evaluable (CE) population, the clinical cure rates were 94.3% for delafloxacin 300 mg, 92.5% for delafloxacin 450 mg, and 91.2% for tigecycline at the test-of-cure (TOC) visit (14–21 days after the final dose of drug), demonstrating that delafloxacin had comparable efficacy to tigecycline.
A second randomized, double-blind phase 2 clinical trial compared the efficacy of delafloxacin (300 mg IV q12 hours) to linezolid (600 mg IV q12 hours) and vancomycin (15 mg/kg IV q12 hours) in 256 patients with ABSSSI.25 Randomization was stratified by infection category: cellulitis/erysipelas, wound infection, major cutaneous abscess, or burn infection. The study’s primary endpoint was to measure clinical response, such as cure (complete resolution), improved (near resolution with no additional antibiotics needed), failure (additional antibiotics needed), and indeterminate (assessment incomplete), at the follow-up visit in the intent-to-treat (ITT) population. The cure rate of delafloxacin (57/81; 70.4%) was comparable to linezolid (50/77; 64.9%) and was statistically better than vancomycin (53/98; 54.1%; P = 0.031). Using a digital measurement, delafloxacin had a significantly greater percentage decrease in erythema compared to vancomycin at follow-up, −96.4% versus −84.5%; P = 0.028). MRSA was the most common pathogen isolated and clinical cure rates were similar among the treatment arms. The MIC90 values for delafloxacin, linezolid, and vancomycin were 0.12, 2, and 0.5 μg/ml, respectively. Also, the cure rate was statistically higher in the obese patients (body mass index (BMI) ≥ 30 kg/m2) who received delafloxacin (78.8%) versus vancomycin (48.8%); P = 0.009.
Phase III Trials
The PROCEED studies were two phase 3, multi-center, stratified, randomized, double-blind trials that assessed the efficacy of delafloxacin versus vancomycin plus aztreonam in adults with ABSSSI. The first trial compared intravenous delafloxacin 300 mg versus vancomycin 15 mg/kg plus aztreonam 2 g each given twice daily for 5 to 14 days.23 Aztreonam was discontinued if gram-negative organisms were not present in baseline cultures. To be included in the study, patients had to have a diagnosis of ABSSSI classified as cellulitis/erysipelas, wound infection, major cutaneous abscess, or burn infection with ≥ 75 cm2 of erythema and ≥ 2 signs of systemic infection. The primary efficacy endpoint was the response at 48 to 72 hours (± 2), defined as ≥ 20% reduction in erythema of the ABSSSI lesion. The secondary endpoints were investigator-assessed clinical cure (resolution of symptoms) at the follow-up (FU) visit (day 14 ± 1) and late follow-up (LFU) visit (days 21–28). A total of 660 patients were included in the study and stratified by infection type. The mean duration of treatment with delafloxacin was 5 days, and 5.5 days for the vancomycin/aztreonam group. The mean duration for aztreonam was 2 days. The majority of the patients were men (62.9%) and Caucasian (91.1%) with a mean age of 45.8 (± 14.2) years. S. aureus was the most common pathogen identified (n = 324, 66.1%), of which the majority of these isolates were MRSA (n = 169, 52.2%). Most of the patients had either cellulitis/erysipelas (n = 232, 38.8%) or a wound infection (n = 232, 35.2%). A smaller percentage of patients presented with a major cutaneous abscess (n = 167, 25.3%) or burn infection (n = 5, 0.8%). Delafloxacin met non-inferiority criteria, with similar objective response rates across both treatment arms for the primary endpoint. The percentage of responders in the delafloxacin group was 78.2% and 80.9% in the vancomycin/aztreonam group. The cure rates were comparable at FU (52% vs. 50.5%) and at LFU (70.4% vs. 66.6%) for the delafloxacin and vancomycin/aztreonam arms, respectively. In those patients with MRSA, microbiological eradication was 100% and 98.5% in the delafloxacin and vancomycin/aztreonam group, respectively. Of note, delafloxacin (71.7%) had a higher cure rate at LFU compared to the vancomycin/aztreonam group (57.4%) in obese patients (BMI ≥ 30 kg/m2).
The second phase 3 trial randomized 850 patients to receive either IV delafloxacin (n = 423) 300 mg twice daily for three days, with a mandatory blinded switch to delafloxacin 450 mg PO twice daily, or vancomycin (15 mg/kg) plus aztreonam (n = 427) 2 g IV twice daily for 5 to 14 days.26 As in the previous phase 3 trial, aztreonam could be discontinued if the baseline cultures did not reveal gram-negative pathogens. The inclusion criteria for this study were the same as for the previous phase 3 ABSSSI clinical trial.23 Again, as in the earlier clinical trial, the primary endpoint was the response rate at 48 to 72 hours (± 2), defined as ≥ 20% reduction in ABSSSI lesion size, with no further antibiotics. The secondary endpoint was cure rate (complete resolution of symptoms) at the FU and LFU visits. Most of the patients presented with cellulitis/erysipelas (n = 408, 48%), wound infections (n = 223, 26.2%), or major cutaneous abscesses (n = 212, 24.9%), and seven patients had a burn infection (0.8%). Once again, delafloxacin met non-inferiority criteria, with similar 48–72-hour responses observed in the delafloxacin arm (83.7%) and the vancomycin/aztreonam arm (80.6%). S. aureus, once again, was the most common pathogen. Of those isolates, MRSA was identified in about 32.1% of the patients compared to MSSA (66.3%). When comparing the per-pathogen microbiological response between both treatment arms, it was similar.
Adverse Effects
The most commonly observed adverse reactions have been gastrointestinal (GI)-related, such as nausea, vomiting, or diarrhea. 23–26 In a phase 2 trial comparing two doses of delafloxacin (300 mg or 450 mg) to tigecycline, the tigecycline group had more GI events (mostly nausea). A convulsion was reported in one patient in the 450-mg delafloxacin group, and was thought to be related to the drug.24 One phase 3 trial comparing delafloxacin to vancomycin/aztreonam found that the incidence of serious adverse events was equal in the two groups (3.7%).23 The occurrence of adverse events leading to discontinuation of the medication was lower in the delafloxacin group compared to the vancomycin/aztreonam group (0.9% vs. 4.3%). One patient experienced a hypoglycemic event and two patients experienced hyperglycemia in the delafloxacin group. Blood glucose was monitored in patients for 12 hours post-dose in both groups and no significant differences were reported. In another phase 3 trial comparing oral delafloxacin to vancomycin/aztreonam, similar results were found, with the most common treatment-emergent adverse events related to delafloxacin being nausea (7.7%) and diarrhea (7.7%). Common adverse events seen in the pooled phase 3 trials for ABSSI can be found in Table 3.
Table 3.
Common Adverse Events Seen in the Pooled Phase 3 Trials
| Type of Adverse Event | Delafloxacin n = 741, n (%) | Vancomycin/Aztreonam n = 751, n (%) |
|---|---|---|
| Diarrhea | 59 (8%) | 29 (4%) |
| Nausea | 56 (7.6%) | 47 (6.3) |
| Headache | 24 (3.2%) | 41 (5.5%) |
| Transaminase elevation | 22 (3%) | 28 (3.7%) |
| Vomiting | 17 (2.3%) | 18 (2.4%) |
Twelve-lead ECGs were performed as part of the trial protocol’s safety assessments and there were no abnormal findings. A randomized, double-blind, placebo-controlled, four-period crossover study conducted among 52 patients assessed the effects of delafloxacin, at therapeutic (300 mg IV) and supratherapeutic (900 mg IV) doses, on the corrected QT interval (QTc) compared to moxifloxacin (400 mg). Delafloxacin was found to have no clinically meaningful increase in QTc, while moxifloxacin produced predicted increases in the QTc. Based on this formal QTc study, QTc prolongation may not be of concern with delafloxacin as compared to other FQs.27 QTc prolongation was not reported in any of the trials. Phototoxicity also does not seem to be of concern with delafloxacin compared to other FQs. Phototoxicity was not seen with delafloxacin compared with lomefloxacin in a phase 1 trial.28
WARNINGS AND PRECAUTIONS
The FDA has placed a boxed warning regarding the risk of tendonitis, tendon rupture, peripheral neuropathy, CNS effects, and exacerbation of myasthenia gravis for all FQs. Although these adverse reactions have not been reported in clinical trials for delafloxacin, it is recommended to immediately discontinue delafloxacin if a patient experiences any of these adverse reactions.11 There have been no reports of C. difficile infections in the safety data. Nevertheless, FQs are considered to have a higher risk of causing C. difficile-associated diarrhea (CDAD) compared to other antibiotics. 29 Therefore, a precaution is included for CDAD with the prolonged use of delafloxacin.
SUPPLY/STORAGE/STABILITY/COST
Intravenous delafloxacin is supplied as a sterile, lyophilized powder in single-dose vials of 300 mg (packaged in cartons of 10). The tablet formulation contains 450 mg of delafloxacin (supplied in bottles of 20 tablets). The injection formulation should be stored at 20° to 25°C (68°–77°F). Once reconstituted, the powder may be stored refrigerated or at controlled room temperature for up to 24 hours.11 The average wholesale price (AWP) of a 450-mg bottle of 20 tablets is $1,620.00 ($81/tablet). The AWP for the 300-mg intravenous 10-vial pack is $1,590.00 ($159/vial).
PATIENT COUNSELING
Instruct your patients to report symptoms of tendonitis, tendon rupture, or peripheral neuropathy. Educate your patients on symptoms of CDAD, such as persistent diarrhea with antibiotic use. Remind patients that oral delafloxacin tablets should be taken two hours before or six hours after magnesium- or aluminum-containing antacids or products containing iron or zinc. Common adverse effects may include nausea, vomiting, headache, and diarrhea.11
PLACE IN THERAPY
Delafloxacin possesses several pharmacological properties that advantageously differentiate it from other FQs. These differences include a broad spectrum of antimicrobial coverage, dual-targeting enzyme activity on DNA gyrase and topoisomerase IV, the absence of drug–drug interactions, a diminished effect on QTc interval, and heightened antibacterial activity in an acidic environment. Currently, delafloxacin is approved for the treatment of ABSSSI, as it showed non-inferiority in efficacy compared to standard-of-care therapies. As stated earlier, ABSSSI are mostly caused by gram-positive bacteria. Delafloxacin’s broad-spectrum antimicrobial coverage is likely unnecessary for this indication. With this in mind, Melinta Therapeutics is pursuing additional FDA indications for delafloxacin. Currently, there are ongoing phase 3 studies evaluating delafloxacin for the treatment of severe community-acquired bacterial pneumonia and complicated UTIs. Both of these indications may expand the use of delafloxacin in outpatient or inpatient settings. The availability of oral and intravenous formulations of delafloxacin provides the ability to transition patients from IV to oral therapy, which can decrease the cost of care. This may be beneficial in MRSA infections such as bacteremia or bone infections that require prolonged use of an antimicrobial agent. Unfortunately, there is a paucity of data at this time and more randomized controlled trials are warranted to establish efficacy for the aforementioned indications.
Footnotes
Disclosure: The authors report no commercial or financial interests in regard to this article.
REFERENCES
- 1.Pallin DJ, Egan DJ, Pelletier AJ, et al. Increased U.S. emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51(3):291–298. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Itani KMF, Merchant S, Lin SJ, et al. Outcomes and management costs in patients hospitalized for skin and skin-structure infections. Am J Infect Control. 2011;39(1):42–49. doi: 10.1016/j.ajic.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Kaye KS, Patel DA, Stephens JM, et al. Rising United States hospital admissions for acute bacterial skin and skin structure infections: recent trends and economic impact. PLoS ONE. 2015;10(11):e0143276. doi: 10.1371/journal.pone.0143276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frazee BW, Lynn J, Charlebois ED, et al. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45(3):311–320. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration, Center for Drug Evaluation and Research Guidance for Industry. Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment 2013 Draft Guidance. [Accessed December 3, 2017]. Available at: https://www.fda.gov/downloads/Drugs/Guidances/ucm071185.pdf.
- 6.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. FDA approves Baxdela to treat acute bacterial skin and skin structure infections (ABSSSI) Jun 17, 2017. [Accessed December 3, 2017]. Available at: https://www.accessdata.fda.gov/drug-satfda_docs/nda/2017/208610Orig1s000,208611Orig1s000Approv.pdf.
- 8.Aldred K, Kerns R, Osheroff N. Mechanism of quinolone action and resistance. Biochemistry. 2014;53(10):1565–1574. doi: 10.1021/bi5000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilius AM, Shen LL, Hensey-Rudloff D, et al. In vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinolone. Antimicrob Agents Chemother. 2003;47(10):3260–3269. doi: 10.1128/AAC.47.10.3260-3269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby GA. Mechanisms of resistance to quinolones. Clin Infect Dis. 2005;41(suppl 2):S120–S126. doi: 10.1086/428052. [DOI] [PubMed] [Google Scholar]
- 11.Baxdela [package insert] Lincolnshire, IL: Melinta Therapeutics Inc; 2017. [Google Scholar]
- 12.Flamm R, Rhomberg P, Huband M, Farrell D. In vitro activity of delafloxacin tested against isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 2016;60(10):6381–6385. doi: 10.1128/AAC.00941-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemaire S, Tulkens PM, VanBambeke F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55(2):649–658. doi: 10.1128/AAC.01201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candel FJ, Peñuelas M. Delafloxacin: design, development and potential place in therapy. Drug Des Devel Ther. 2017;11:881–891. doi: 10.2147/DDDT.S106071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoover R, Hunt T, Benedict M, et al. Safety, tolerability, and pharmacokinetic properties of intravenous delafloxacin after single and multiple doses in healthy volunteers. Clin Ther. 2016;38(1):53–65. doi: 10.1016/j.clinthera.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Paulson SK, Wood-Horrall RN, Hoover R, et al. The pharmacokinetics of the CYP3A substrate midazolam after steady-state dosing of delafloxacin. Clin Ther. 2017;39(6):1182–1190. doi: 10.1016/j.clinthera.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Hoover R, Marbury TC, Preston RA, et al. Clinical pharmacology of delafloxacin in patients with hepatic impairment. J Clin Pharmacol. 2017;57(3):328–335. doi: 10.1002/jcph.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoover R, Hunt T, Benedict M, et al. Single and multiple ascending-dose studies of oral delafloxacin: effects of food, sex, and age. Clin Ther. 2016;38(1):39–52. doi: 10.1016/j.clinthera.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Almer LS, Hoffrage JB, Keller EL, et al. In vitro and bactericidal activities of ABT-492, a novel fluoroquinolone, against gram-positive and gram-negative organisms. Antimicrob Agents Chemother. 2004;48(7):2771–2777. doi: 10.1128/AAC.48.7.2771-2777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connors KP, Kuti JL, Nicolau DP. Optimizing antibiotic pharmacodynamics for clinical practice. Pharmaceut Anal Acta. 2013;4:214. [Google Scholar]
- 21.Burak E, Bortolon E, Molstad D, et al. Pharmacokinetics and pharmacodynamics of delafloxacin in S aureus murine thigh infection models. Proceedings of the Forty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. 2009; Washington, D.C: American Society for Microbiology; Poster A1-1941. [Google Scholar]
- 22.Bhavnani SM, Zhang L, Ambrose PG, et al. Population pharmacokinetic and pharmacokinetic-pharmacodynamic target attainment analyses for delafloxacin to support dose selection for the treatment of patients with acute bacterial skin and skin structure infections. Proceedings of IDWeek 2017; San Diego, CA. 2017; Arlington, VA: The Infectious Diseases Society of America; Poster 1851. [Google Scholar]
- 23.Pullman J, Gardovskis J, Farley B, et al. Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a phase 3, double-blind, randomized study. J Antimicrob Chemother. 2017;72(12):3471–3480. doi: 10.1093/jac/dkx329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riordan WO, Mehra P, Manos P, et al. A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis. 2015;30:67–73. doi: 10.1016/j.ijid.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821–829. doi: 10.1093/jac/dkv411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riordan WO, McManus AM, Teras J, et al. A global phase 3 study of delafloxacin compared to vancomycin/aztreonam in patients with acute bacterial skin and skin structure infections [published online October 26, 2016] Open Forum Infectious Diseases. 2016;3(suppl 1):S1347. doi: 10.1093/ofid/ofw172.1050. [DOI] [Google Scholar]
- 27.Litwin JS, Benedict MS, Thorn MD, et al. A thorough QT study to evaluate the effects of therapeutic and supratherapeutic doses of delafloxacin on cardiac repolarization. Antimicrob Agents Chemother. 2015;59(6):3469–3473. doi: 10.1128/AAC.04813-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson J, Lawrence L, Paulson S, et al. Assessment of phototoxicity potential of delafloxacin in healthy male and female subjects: a phase I study. Proceedings of the Fifty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy with the International Society of Chemotherapy’s International Congress of Chemotherapy and Infection; San Diego, CA. 2015; Washington, DC: American Society of Microbiology; Poster F-1198a. [Google Scholar]
- 29.Dingle KE, Didelot X, Quan TP, et al. Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet. 2017;17(4):411–421. doi: 10.1016/S1473-3099(16)30514-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


