Summary
Data regarding the impact of hepatitis C (HCV) therapy on incidence of type 2 diabetes mellitus are limited. We used the data from the longitudinal Chronic Hepatitis Cohort Study—drawn from four large US health systems—to investigate how response to HCV treatment impacts the risk of subsequent diabetes. Among HCV patients without a history of type 2 diabetes mellitus or hepatitis B, we investigated the incidence of type 2 diabetes from 12 weeks post-HCV treatment through December 2015. Cox proportional hazards models were used to test the effect of treatment status (sustained virologic response [SVR] or treatment failure) and baseline risk factors on the development of diabetes, considering any possible risk factor-by-SVR interactions, and death as a competing risk. Among 5127 patients with an average follow-up of 3.7 years, diabetes incidence was significantly lower among patients who achieved SVR (231/3748; 6.2%) than among patients with treatment failure (299/1379; 21.7%; adjusted hazard ratio [aHR] = 0.79; 95% CI: 0.65–0.96). Risk of diabetes was higher among African American and Asian American patients than White patients (aHR = 1.82 and 1.75, respectively; P < .05), and among Hispanic patients than non-Hispanics (aHR = 1.86). Patients with BMI ≥ 30 and 25–30 (demonstrated higher risk of diabetes aHR = 3.62 and 1.72, respectively; P < .05) than those with BMI < 25; patients with cirrhosis at baseline had higher risk than those without cirrhosis (aHR = 1.47). Among a large US cohort of patients treated for HCV, patients who achieved SVR demonstrated a substantially lower risk for the development of type 2 diabetes mellitus than patients with treatment failure.
Keywords: insulin resistance, race, treatment failure, virologic suppression
1. INTRODUCTION
Data regarding the impact of hepatitis C (HCV) therapy on incidence of type 2 diabetes mellitus (T2D) are limited. European studies from the interferon era of HCV therapy suggested that viral suppression and clearance greatly reduce the risk of future insulin resistance and T2D.1–3 In contrast, other studies have found no significant difference in the incidence of T2D or insulin resistance between treated and untreated HCV patients.4,5 There remains a lack of current data regarding the effect of HCV- related complications and treatment outcomes on the incidence of T2D in the United States, particularly among racially diverse populations.
We evaluated the impact of HCV treatment response, the presence of cirrhosis and other factors on the incidence of T2D using longitudinal data from the Chronic Hepatitis Cohort Study (CHeCS), a geographically and racially diverse cohort of over 13 000 patients from four large US health systems.
2. METHODS
2.1. Patient population
CHeCS is an observational multicenter study that includes adult (≥18 years) chronic hepatitis C patients from four large health systems. The study follows all guidelines of the US Department of Health and Human Services regarding the protection of human subjects. Protocols are reviewed annually by the institutional review board at each of the study sites: Geisinger Clinic, Danville, Pennsylvania; Henry Ford Health System, Detroit, Michigan; Kaiser- Permanente Hawai’i, Honolulu, Hawai’i; and Kaiser- Permanente Northwest, Portland, Oregon. CHeCS study methods have been previously described.6 Briefly, electronic administrative data and electronic health records for patients ≥18 years who received health services at any study site between 1 January 2006 and 31 December 2015 were used to identify study candidates; eligibility was confirmed during medical chart abstraction.
For this analysis, patients were included if they had received HCV treatment prior to 31 December 2015. Patients were excluded if they had hepatitis B co- infection or pre- existing diabetes, which was defined as the presence of an International Classification of Diseases, version 9 or 10 (ICD9/10) diagnosis code for type 1 diabetes (ICD- 9- CM code 250.x1/x3 and ICD- 10- CM code E10.xxxx) or type 2 diabetes (ICD- 9- CM code 250.x0/x2 and ICD- 10- CM code E11.xxxx) in their electronic health record. “Index date” was defined as 12 weeks after the end of a patient’s most recent course of HCV therapy. Prior HCV treatment history was captured as a baseline covariate.
2.2. Antiviral HCV therapy and response
Detailed antiviral medication data (drug name and start/stop dates) were collected via chart abstraction for the patient’s most recent course of HCV therapy. Combination therapy was identified when the multiple hepatitis drugs were administered concomitantly. Data on routine HCV RNA quantification tests were obtained via the electronic health record. Patients were classified as having achieved SVR if laboratory tests collected ≥12 weeks post- therapy showed undetectable viral RNA loads; otherwise, the patients were classified as having treatment failure (TF).
2.3. Baseline covariates and possible risk factors
Baseline data (at the start of a patient’s most recent course of HCV therapy) included the following: demographic information (age, sex, race/ethnicity, estimated median annual household income and insurance status); HCV genotype; Charlson/Deyo comorbidity score (calculated from inpatient, outpatient and claims data for 12 months prior to the index date); laboratory test results for imputation of the Fibrosis- 4 (FIB4) score (based on our validated classification categories of ≤1.21, 1.21– 5.88, >5.88 or “unknown”); alanine aminotransferase (ALT) levels (<45 U/L, ≥45 U/L or “unknown”); and HIV co- infection.
Baseline cirrhosis status was determined using data for 2 years prior to the start date of a patient’s most recent course of HCV therapy. Due to the observational nature of this study, the availability of cirrhosis data varied. Roughly 20% of our sample had liver biopsy or Fibroscan data collection, and 60–70% had laboratory data for the calculation of FIB4. Cirrhosis data were sometimes inconsistent between the various sources for an individual patient. To overcome this variation, we implemented the following hierarchical classification algorithm to identify cirrhosis: (i) decompensated cirrhosis identified using our validated Classification and Regression Tree model7; (ii) F4 liver biopsy or Fibroscan >12.5; (iii) FIB4 > 5.888; and (iv) the presence of ICD9/10 diagnosis codes for cirrhosis.
2.4. Statistical analysis
Time- to- event outcomes included the incidence of T2D (defined using ICD9/10 codes) or death. Follow- up began after the achievement of SVR or, for treatment- failure patients, the date 12 weeks after HCV treatment initiation. Patients were followed until the outcome of interest or 10 years of follow- up. Cox proportional hazards regression was used to estimate the impact of SVR and other factors on the risk of T2D; death was included using a competing risk approach. To assess the influence of additional risk factors on any potential SVR effect, we began the analysis by testing each individual risk factor effect, considering any possible risk factor-by-SVR interactions. Variables demonstrating either individual effects or interactions with SVR (P < .05) were included in the multivariable model; those with significant effects (P < .05) or interactions with SVR after adjustment for other covariates were retained in the final model using a forward model selection approach. Study site was included in all multivariable analyses as an adjustment variable. Because FIB4, ALT and cirrhosis are highly correlated, they were fitted into multivariable models separately to avoid confounding effects.
3. RESULTS
Among 14 312 patients with confirmed HCV in the CHeCS cohort, 7556 (53%) had received treatment and were hepatitis B- negative. Of these 7556 patients, 1048 were excluded for pre- existing diabetes, 809 for the ongoing antiviral treatment or unknown SVR status and 572 for having their last encounter date occur before the index date. This resulted in an analytic sample of 5127 treated patients; of these, 3748 (73%) achieved SVR (44% were treated with a direct- acting all- oral antiviral [DAA] regimen). A total of 530 incident cases of T2D (10.3%) were observed during a mean follow- up period of 3.7 years.
Table 1 presents the characteristics of patients at the time of last treatment initiation, as well as the risk associated with baseline covariates and the impact of SVR on the development of T2D. Overall, 60% of patients were male and 72% were White, with an average age of 59. Baseline age, sex, race, body mass index (BMI), Hispanic ethnicity, the presence of cirrhosis at baseline, and SVR all had individual effects on incidence of T2D. After multivariable modelling, six variables (age, race, BMI, cirrhosis at baseline, Hispanic ethnicity and SVR) were retained in the final model. No risk factor- by- SVR interactions were detected after adjustment.
Table 1.
Association of baseline covariates with T2D incidence: univariate effects and variable by interaction with SVR
| Variable | Response | Sample (n = 5127) | Hazard ratio | P- value | P- value for interaction with SVR |
|---|---|---|---|---|---|
| Treatment status | Failure | 1379 (27%) | Reference | .0003 | NA |
| SVR | 3748 (73%) | 0.706 | |||
| Sex | Female | 2043 (40%) | Reference | .1931 | .041 |
| Male | 3084 (60%) | 1.131 | |||
| Age, y | <40 | 624 (12%) | Reference | .0001 | .289 |
| 40 < 50 | 1242 (24%) | 2.121 | |||
| 50 < 60 | 1886 (37%) | 2.36 | |||
| ≥60 | 1375 (27%) | 2.341 | |||
| Race | White | 3714 (72%) | Reference | <.001 | .480 |
| African American |
881 (17%) | 1.92 | |||
| ASINPI | 297 (6%) | 1.76 | |||
| Unknown | 235 (5%) | 1.131 | |||
| Hispanic/Latino | No | 4116 (80%) | Reference | .0062 | .257 |
| Yes | 213 (4%) | 1.87 | |||
| Unknown | 798 (16%) | 1.121 | |||
| Household income, US $ | <15K | 106 (2%) | Reference | .409 | .022 |
| 15 < 30K | 785 (15%) | 1.182 | |||
| 30 < 50K | 2154 (42%) | 0.949 | |||
| 50 < 75K | 1327 (26%) | 0.913 | |||
| ≥75K | 465 (9%) | 0.815 | |||
| Missing | 290 (6%) | 1.083 | |||
| BMI, kg/m2 | <25 | 939 (18%) | Reference | <.0001 | .127 |
| 25– 30 | 1272 (25%) | 1.797 | |||
| ≥30 | 1405 (27%) | 3.814 | |||
| Missing | 1511 (29%) | 3.018 | |||
| HCV genotype | 1 | 3201 (62%) | Reference | .1877 | .102 |
| 2 | 568 (11%) | 0.921 | |||
| 3 | 448 (9%) | 0.672 | |||
| Other/Unknown | 910 (18%) | 0.902 | |||
| HIV co-infected | No | 4978 (97%) | Reference | .5969 | .468 |
| Yes | 149 (3%) | 1.144 | |||
| FIB4 | ≤1.21 | 898 (18%) | Reference | .0149 | .525 |
| 1.21–5.88 | 2258 (44%) | 1.56 | |||
| >5.88 | 468 (9%) | 1.709 | |||
| Unknown | 1503 (29%) | 1.58 | |||
| ALT levels (U/L) | <45 | 1178 (23%) | Reference | .079 | .901 |
| ≥45 | 2972 (58%) | 1.261 | |||
| Unknown | 977 (19%) | ||||
| Prior treatment | No | 3882 (76%) | Reference | .018 | .692 |
| Yes | 1245 (24%) | 1.297 | |||
| Multiple comorbidities | No | 3211 (63%) | Reference | .0035 | .084 |
| Yes | 1916 (37%) | 1.323 | |||
| Cirrhosis | No | 3750 (73%) | Reference | <.0001 | .216 |
| Yes | 1377 (27%) | 1.55 |
ASINPI, Asian American, American Indian, or Pacific Islander; BMI, body mass index; FIB4, Fibrosis- 4 Index; HCV, hepatitis C virus; SVR, sustained virologic response; T2D, type 2 diabetes mellitus.
3.1. Effect of SVR on incidence of T2DM
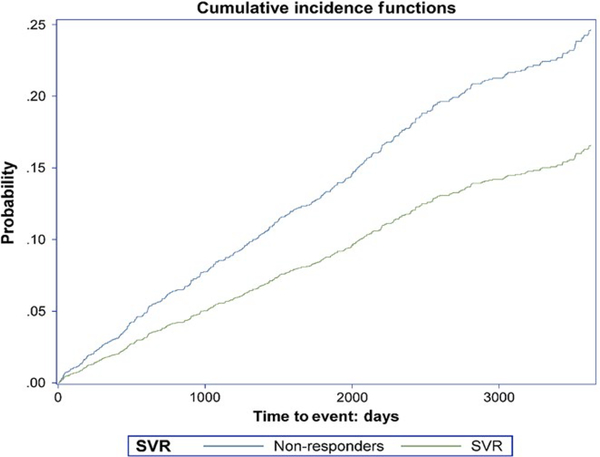
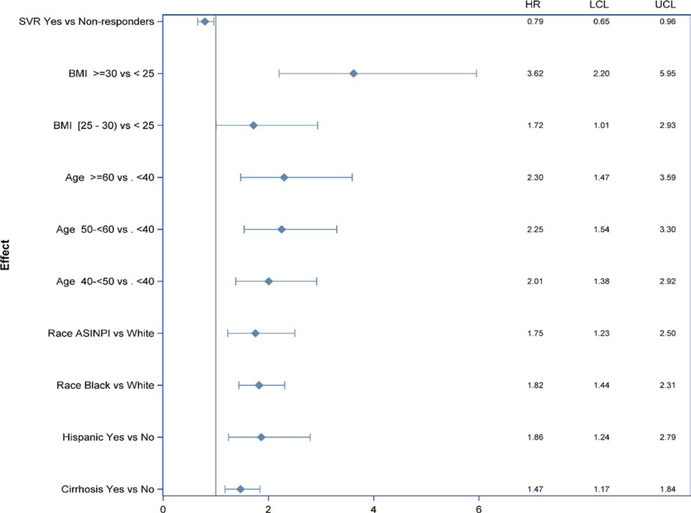
After adjustment for baseline covariates, SVR reduced the risk of T2D by 21% (aHR = 0.79, 95% CI: 0.65– 0.96, P = .02; Figures 1 and 2). There was no interaction between SVR and other risk factors, indicating an independent effect of SVR on incidence of T2D.
FIGURE 1.
Cumulative incidence of type 2 diabetes in patients who did and did not achieve sustained virologic response (SVR)
FIGURE 2.
Multivariate model of the impact of baseline covariates on the risk of type 2 diabetes
3.2. Effect of additional risk factors on incidence of T2DM
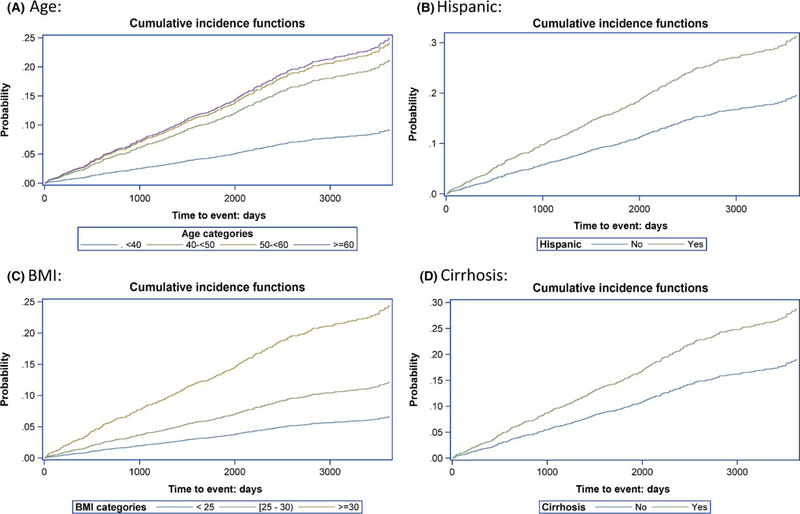
Higher BMI was associated with higher risk of incident T2D; in patients with BMI ≥ 30, the risk of T2D was almost 4 times higher than in patients with BMI <25 (aHR = 3.6; 95% CI: 2.2– 5.95; Figure 2). Race was also associated with the risk of T2D; African American and Asian American, American Indian, or Pacific Islander (ASINPI) patients demonstrated risks 1.82- fold and 1.75- fold higher, respectively, than the White patients. There was no significant difference in the risk for T2D between African American and ASINPI patients. Risk increased with age. Hispanics had an almost two-fold higher risk than non- Hispanics. Patients with baseline cirrhosis demonstrated 1.5- fold higher risk of T2D than those without cirrhosis (Figure 3). The sensitivity analysis showed a nonsignificant effect of ALT on T2D development (P = 0.08).
FIGURE 3.
Cumulative incidence of type 2 diabetes in patients by age, BMI, cirrhosis status and hispanic ethnicity
4. DISCUSSION
In a large US cohort of patients treated for HCV, we found that the achievement of SVR independently reduced the risk of T2D. There were no SVR- by- covariate interactions detected, meaning the SVR effect was consistent across patient demographic characteristics and clinical conditions at the time of treatment, including serum alanine aminotransferase (ALT) levels.
We observed an independent effect of HCV- related cirrhosis on increasing T2D incidence after adjusting for other covariates as well as SVR status. This effect has been reported previously; univariate analyses in a smaller European study (n = 365) showed that increasing fibrosis score was a risk factor for the development of T2D.2 We did not observe a significant effect of ALT in either univariate or multivariate analyses. It is possible, however, that the lack of a significant finding was a consequence of a relatively large proportion (20%) of missing ALT data and/or the strong correlation between ALT and cirrhosis, which may have diminished the observed effect of ALT.
Race has been previously identified as a factor associated with increased the risk of T2D in the general US population and among patients with HCV in Europe.9,10 Our results are consistent with these findings, although the effects we observed were larger than those reported in other studies; the risk of T2D was 92% higher among African American patients and 75% higher among ASINPI patients compared with Whites, after adjusting for BMI and other covariates.
Some studies have suggested that sex and HCV genotype are associated with the risk of T2D, but these results have not been consistent.9 In our study, sex was a significant risk factor in univariate analysis, but was not significant after adjusting for other covariates. HCV genotype was not significantly associated with T2D incidence in either univariate or multivariate analyses.
Our study has some limitations. Data available to calculate baseline BMI and to impute FIB4 score were incomplete, given our reliance on electronically collected observational data as well as the inclusion of some patients treated beginning in the early 1990s. Additionally, our study was largely limited to the interferon era of HCV treatment. However, the CHeCS “dynamic” sampling design, which adds new patients to the cohort at regular intervals while continuing to follow the existing patients, has allowed us to begin the preliminary analyses of the impact of DAA regimens on the incidence of T2D.
Debate remains regarding whether and how HCV infection might increase the risk of T2D.11,12 Although some studies have found that T2D occurs more frequently among subsets of HCV- infected versus uninfected individuals,13–15 other studies suggest that observed increases in the risk of T2D may be a consequence of HCV- related elevation in ALT,16,17 perhaps further confounded by high BMI and/or cirrhosis.18 We found that cirrhosis, but not baseline ALT, independently increased the risk of T2D in all treatment groups. Although we observed that successful HCV treatment reduced the risk of future diabetes, our analysis could not evaluate whether this risk reduction resulted from viral eradication, from subsequent reductions in inflammation or fibrosis, or through some other mechanism. Future analyses may help elucidate these mechanisms.
Additionally, although we observed that the absence of successful antiviral therapy increases HCV patients’ risk of T2D, a number of studies have suggested that T2D and insulin resistance reduce response to antiviral therapy, particularly interferon- based treatments.19–22 This two- way association illustrates the complex relationship between T2D, HCV and SVR, and may have introduced bias into the observed effect of SVR on the risk of T2D. We excluded patients previously diagnosed with T2D from our analysis, but due to the observational nature of our study, comprehensive identification of each patient with potentially elevated glucose and insulin resistance was not feasible. To address this issue, we performed a sensitivity analysis of patients with available glucose assessments, excluding those with fasting or random glucose levels greater than 110 mg/dl. Exclusion of these patients produced results similar to our main analysis.
Another limitation is that our assessment of the association between independent baseline covariates and the risk of T2D incidence was restricted to treated patients. Given the absence of variable- by- SVR interactions and the increasing uptake of DAA treatment in the HCV patient population, we expect that our observations regarding the impact of race and cirrhosis on the development of T2D may be generalizable to a broader population of patients with HCV.
In conclusion, among a geographically and racially diverse cohort of more than 5000 patients from US healthcare systems, successful HCV treatment was associated with significant reductions in the incidence of T2D. African American and ASINPI race as well as the presence of cirrhosis appear to increase the risk of developing T2D among those without SVR. Therefore, patients with these risk factors should be monitored closely for T2D prevention and care.
ACKNOWLEDGEMENTS
The CHeCS Investigators include the following investigators and sites: Scott D. Holmberg, Eyasu H. Teshale, Philip R. Spradling, Anne C. Moorman, Jian Xing and Yuna Zhong - Division of Viral Hepatitis, National Centers for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, Georgia; Stuart C. Gordon, David R. Nerenz, Mei Lu, Lois Lamerato, Jia Li, Loralee B. Rupp, Nonna Akkerman, Nancy Oja- Tebbe, Talan Zhang, Sheri Trudeau and Yueren Zhou - Henry Ford Health System, Detroit, Michigan; Joseph A. Boscarino, Zahra S. Daar and Robert E. Smith; Center for Health Research, Geisinger Health System, Danville, Pennsylvania; Yihe G. Daida, Connie Mah Trinacty and Carmen P. Wong - The Center for Health Research, Kaiser Permanente- Hawaii, Honolulu, Hawaii; and Mark A. Schmidt and Judy L. Donald - The Center for Health Research, Kaiser Permanente- Northwest, Portland, OR.
Funding information
Henry Ford Health System receives funding for CHeCS from the Centers for Disease Control and Prevention and from Gilead Sciences. CHeCS was previously funded by the CDC Foundation on May 2016, which received the grants from AbbVie; Genentech, A Member of the Roche Group; Gilead Sciences; Janssen Pharmaceuticals, Inc.; and Vertex Pharmaceuticals; the past partial funders include Bristol- Myers Squibb. Granting corporations do not have access to CHeCS data and do not contribute to data analysis or writing of the manuscripts.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations:
- ASINPI
Asian, American Indian, or Pacific Islander
- BMI
body mass index
- CHeCS
Chronic Hepatitis Cohort Study
- FIB4
Fibrosis-4 Index
- HCV
hepatitis C virus
- SVR
sustained virologic response
- T2D
type 2 diabetes mellitus
Footnotes
CONFLICT OF INTEREST
Stuart C. Gordon receives grant/research support from AbbVie Pharmaceuticals, Conatus, CymaBay, Exalenz BioScience, Gilead Pharmaceuticals, Intercept Pharmaceuticals and Merck. He is also a consultant/advisor for Abbvie, CVS Caremark, Gilead, Intercept and Merck. The other authors have no potential conflict of interests.
REFERENCES
- 1.Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–744. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Gomez M, Fernandez-Rodriguez CM, Andrade RJ, et al. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48:721–727. [DOI] [PubMed] [Google Scholar]
- 3.Simo R, Lecube A, Genesca J, Esteban JI, Hernandez C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462–2466. [DOI] [PubMed] [Google Scholar]
- 4.Aghemo A, Prati GM, Rumi MG, et al. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology. 2012;56:1681–1687. [DOI] [PubMed] [Google Scholar]
- 5.Giordanino C, Bugianesi E, Smedile A, et al. Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol. 2008;103:2481–2487. [DOI] [PubMed] [Google Scholar]
- 6.Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56:40–50. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Mei J, Li H, et al. Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products. Bioresour Technol. 2017;228:62–68. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Gordon SC, Rupp LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21:930–937. [DOI] [PubMed] [Google Scholar]
- 9.Hammerstad SS, Grock SF, Lee HJ, Hasham A, Sundaram N, Tomer Y. Diabetes and hepatitis C: a two- way association. Front Endocrinol (Lausanne). 2015;6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gastaldi G, Goossens N, Clement S, Negro F. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: a review. J Adv Res. 2017;8:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruhl CE, Menke A, Cowie CC, Everhart JE. Relationship of hepatitis C virus infection with diabetes in the U.S. population. Hepatology. 2014;60:1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. [DOI] [PubMed] [Google Scholar]
- 14.Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50–56. [DOI] [PubMed] [Google Scholar]
- 15.Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community- based longitudinal study. Am J Epidemiol. 2007;166:196–203. [DOI] [PubMed] [Google Scholar]
- 16.Lin YJ, Shaw TG, Yang HI, et al. Chronic hepatitis C virus infection and the risk for diabetes: a community- based prospective study. Liver Int. 2017;37:179–186. [DOI] [PubMed] [Google Scholar]
- 17.Montenegro L, De Michina A, Misciagna G, Guerra V, Di Leo A. Virus C hepatitis and type 2 diabetes: a cohort study in southern Italy. Am J Gastroenterol. 2013;108:1108–1111. [DOI] [PubMed] [Google Scholar]
- 18.Cusi K The relationship between hepatitis C virus infection and diabetes: time for a divorce? Hepatology. 2014;60:1121–1123. [DOI] [PubMed] [Google Scholar]
- 19.Dai CY, Huang JF, Hsieh MY, et al. Insulin resistance predicts response to peginterferon- alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009;50:712–718. [DOI] [PubMed] [Google Scholar]
- 20.Deltenre P, Louvet A, Lemoine M, et al. Impact of insulin resistance on sustained response in HCV patients treated with pegylated interferon and ribavirin: a meta- analysis. J Hepatol. 2011;55:1187–1194. [DOI] [PubMed] [Google Scholar]
- 21.Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci. 2009;54:2699–2705. [DOI] [PubMed] [Google Scholar]
- 22.Fattovich G, Covolo L, Pasino M, et al. The homeostasis model assessment of the insulin resistance score is not predictive of a sustained virological response in chronic hepatitis C patients. Liver Int. 2011;31:66–74. [DOI] [PubMed] [Google Scholar]