Abstract
Background and Purpose
Although intracellular zinc accumulation has been shown to contribute to neuronal death following cerebral ischemia, the mechanism by which zinc keeps on accumulating to cause severe brain damage remains unclear. Herein the dynamic cause-effect relationships between zinc accumulation and ROS production during cerebral ischemia/reperfusion are investigated.
Methods
Rats were treated with zinc chelator, ROS scavenger, mitochondria-targeted ROS inhibitor, or NADPH oxidase inhibitor during a 90-minute middle cerebral artery occlusion. Cytosolic labile zinc, ROS level, cerebral infarct volume, and neurological functions were assessed after ischemia/reperfusion.
Results
Zinc and ROS were colocalized in neurons, leading to neuronal apoptotic death. Chelating zinc reduced ROS production at 6 and 24h after reperfusion, whereas eliminating ROS reduced zinc accumulation only at 24h. Furthermore suppression of mitochondrial ROS production reduced the total ROS level and brain damage at 6h after reperfusion, but did not change zinc accumulation, indicating that ROS is produced mainly from mitochondria during early reperfusion and the initial zinc release is upstream of ROS generation following ischemia. Suppression of NADPH oxidase decreased ROS generation, zinc accumulation and brain damage only at 24h after reperfusion, indicating that the majority of ROS is produced by NADPH oxidase at later reperfusion time.
Conclusions
This study provides the direct evidence that there exists a positive feedback loop between zinc accumulation and NADPH oxidase-induced ROS production, which greatly amplifies the damaging effects of both. These findings reveal that different ROS-generating source contributes to ischemia-generated ROS at different time, underscoring the critical importance of spatial and temporal factors in the interaction between ROS and zinc accumulation, and the consequent brain injury, following cerebral ischemia/reperfusion.
Keywords: brain ischemia, middle cerebral artery occlusion, zinc, reactive oxygen species
Introduction
Zinc (Zn2+) homeostasis plays an important role in normal brain functions. Normally, zinc is tightly regulated in the brain; disturbance of zinc homeostasis, however, has been found to be involved in neurological diseases.1 Previously studies showed that toxic influx of zinc may be a key mechanism underlying selective neuronal death after transient global ischemic insults.2,3 We reported recently that focal cerebral ischemia induced abnormally high concentration of Zn2+ accumulation in the neurons, resulting in neuronal apoptosis.4 Treatment with zinc chelator N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN) even at 30min after reperfusion reduced the accumulation of intracellular zinc, leading to a significant reduction in infarction volume, improved functional outcomes, as well as dramatic improvement in the survival rate, indicating that high level of zinc accumulation is an important causal factor for neuronal death after stroke.4 However, the reason why zinc keeps on accumulating after cerebral ischemia/reperfusion and produces severe brain damage remains to be elucidated.
Extensive evidence indicates that cerebral ischemia results in an elevated oxidative stress due to generation of reactive oxygen species (ROS) including superoxide anions, hydrogen peroxide, and hydroxyl radicals. ROS generation has been reported to come from various sources. The traditional view of pathology-associated ROS is that they are produced by mitochondria, cyclooxygenase, monoamine oxygenase, and xanthine oxidase, and these sources have indeed been linked to a number of neurological diseases. Recent studies suggest that NADPH oxidase is a major source of neuronal ROS production in hypoglycemia and other conditions.5,6 Although ROS formation has been demonstrated to be of particular pathophysiological significance in ischemic stroke,7 the sources, mechanisms, and time course of ROS generation during ischemia/reperfusion are not clearly understood.
ROS and intracellular zinc are intimately related. On the one hand, ROS production can lead to oxidation of proteins such as metallothionein III (MT III) that normally binds the metal. Although zinc itself is redox-inactive, MT III is readily oxidized due to the very low redox potential of its thiols (−366 mV), thereby releasing zinc in cytoplasmic compartments. On the other hand, not only can zinc impair mitochondrial function, leading to excessive ROS production, but it can also activate a variety of extra-mitochondrial ROS-generating signaling cascades.1,8 Zinc could act on mitochondria to increase superoxide production,9 and induce neuronal NADPH oxidase activation in neuronal cultures.10 Various neuroprotective measures against zinc toxicity attenuate zinc-induced increases in ROS in parallel. Zinc chelation prevents the translocation of p47phox and p67phox subunits of NADPH oxidase from the cytoplasm to the plasma membrane after glucose deprivation.5 Our recent study showed that excess zinc promoted ROS production under hypoxic conditions, leading to cell death.11 However, the temporal order and the cause-effect relationships between zinc accumulation and ROS production in ischemic brain remain unclear.
To investigate the interaction between zinc and ROS in the ischemic brain and to explore the dynamic relationships of cytosolic labile zinc accumulation in relation to various ROS-generating systems during cerebral ischemia/reperfusion, a rat model of focal cerebral ischemia/reperfusion was used in this study. Zinc accumulation and ROS production in the penumbra tissue were measured. We also investigated whether chelating zinc would decrease ROS level in the ischemic tissue and whether decreasing ROS level by ROS scavenger, mitochondria-targeted ROS inhibitor, or NADPH oxidase inhibitor would reduce intracellular zinc level in the ischemic tissue and the subsequent brain damage.
Materials and Methods
This article adheres to the AHA Journals implementation of the Transparency and Openness Promotion Guidelines. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Rat Model of Focal Cerebral Ischemia/Reperfusion
Animal protocols were approved by the Institutional Animal Care and Use Committee of Xuanwu Hospital of Capital Medical University and in accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (280–300g) were anesthetized with 3.5% enflurane in N2O:O2 (70%:30%). Focal cerebral ischemia was induced using the modified intraluminal filament method. Female rats were not used since the current study was designed to provide the proof of concept for the interaction of zinc with ROS. The animals underwent 90min of right middle cerebral artery occlusion (MCAO) and then were reperfused for 6 or 24h by withdrawal of the filament, as we previously described.4 Success of MCAO model was assessed by observing animal circling to the non-ischemic side at the end of ischemia, and further confirmed by 2,3,5-triphenyltetrazolium chloride (TTC) staining at the end of reperfusion.
Drug Administration and Experimental Groups
TPEN (Sigma-Aldrich, St. Louis, MO) was dissolved in 10% dimethylsulfoxide (DMSO) to a final concentration of 5mmol/L. TPEN at a dose of 15mg/kg i.p., selected based on our previous study,4 was administered to the rats 30min before MCAO. EUK-134 (Sigma-Aldrich), a synthetic ROS scavenger having both Mn-SOD and catalase activity, was dissolved in 0.1% DMSO. EUK-134 at 10mg/kg, s.c., total of three doses were administered: 30min before MCAO, 3 and 8h after reperfusion.12 Mitochondria-targeted ROS inhibitor R(+) pramipexole [R(+)PPX] (Sigma-Aldrich), a synthetic aminobenzothiazol derivative that blocks permeability transition pores, restores the integrity of mitochondrial membranes and limits ROS production, was dissolved in saline. R(+)PPX at 1mg/kg, i.p., total of five doses were administered: 8h and 30min before MCAO, immediately after reperfusion, 8 and 16h after reperfusion.12,13, The NADPH oxidase inhibitor diphenyliodonium (DPI) (Sigma-Aldrich) at 0.2mg/kg was i.p. administered 30min before MCAO.14
Rats were assigned randomly to 6 groups using a random number table: vehicle-treated sham-operated group, vehicle-treated MCAO group, TPEN-treated MCAO group, EUK-134-treated MCAO group, R(+)PPX-treated MCAO group, and DPI-treated MCAO group. Each group was further divided into 2 subgroups according to different reperfusion time (6 and 24h) (n=8 in each subgroup). A sample size of 8 animals per subgroup was a priori calculated based on previous research from our group.4,15 Six rats with unsuccessful MCAO were excluded from this study. No rats died due to surgical or stroke complications.
Measurement of Neurological Deficits
At 6 and 24h after reperfusion, neurological deficits were assessed blindly with 3 methods: Zea-Longa score,4 Ludmila Belayev16 and forelimb foot-fault-placing test.4
Tissue Collection, Determination of Infarct Volume, and Penumbra Identification
Rats were decapitated at the end of 6 or 24h reperfusion, brains harvested and sectioned into three 2-mm thick coronal slices from a 6-mm thick region 5mm away from the tip of the frontal lobe. The first and third slices were incubated in 1% TTC (Sigma-Aldrich) solution at 37°C for 20min to measure the infarct volume as previously described4 by investigators blinded to the experimental groups. The remaining brain tissues were snap-frozen in liquid nitrogen and stored at −80°C. Coronal brain sections (20μm) were obtained using a cryostat (CM1900, Leica) and mounted onto gelatin-coated glass slides for histological staining.
Penumbral tissue is operationally defined as the ischemic area that will undergo apoptosis without treatment but is rescued with treatment. The differences in infarct area between drug-treated MCAO groups and vehicle-treated MCAO group were considered as the penumbra as our previous studies described.15
Staining for Detecting Labile Zinc
Brain tissue sections were stained with the zinc-specific membrane-permeable fluorescent dyes Newport Green (NG) (N7990, Invitrogen, Eugene, OR) as previously described.4 Sections were incubated with NG (10μmol/L in PBS; pH, 7.4) for 3min in the dark, then rinsed in saline.
Evaluation of ROS Formation
ROS was detected using an oxidant-sensing fluorescent probe, 2′,7′-dichlorofluorescin diacetate (H2DCF-DA) (Sigma-Aldrich).10,12 Brain sections were incubated in 10 μM H2DCF-DA for 20min, then rinsed in PBS.
Superoxide generation was determined by fluorescent-labeled dihydroethidium (DHE) (Sigma-Aldrich) staining.17 Brain sections were stained with 100μmol/L DHE in PBS for 90min at room temperature.
For quantitative analysis of immunostaining, five brains for each experimental subgroup were measured. Every 4th section was selected (total=6 sections per brain). The number of NG-, H2DCF-DA- or DHE-fluorescent cells in three fields (200× magnification) randomly placed within penumbra tissue was counted under a fluorescence microscope (Nikon 80i) by a technician who was blinded to group assignment.
Costaining of Cytosolic Labile Zinc with ROS
Colocalization of Zinc and ROS was performed to determine the relationship between Zn2+ accumulation and ROS production. Slices were first incubated with DHE. After rinsing in PBS, the sections were incubated with NG. The nuclei were stained with 4′6-diamidino-2-phenylindole (DAPI).
Costaining of Cytosolic ROS and Neuronal Cell Death
Colocalization of ROS and neuron-specific nuclear protein (NeuN) or terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) was performed to determine the relationship between ROS production and neuronal cell death. Slices were first incubated with H2DCF-DA for 20min. NeuN immunofluorescence staining with mouse anti-NeuN polyclonal antibody (1:100; Chemicon, Temecula, CA) as the primary antibody, or TUNEL staining with In Situ Cell Death Detection Kit-POD (Roche, San Francisco, CA) was then performed.4
Statistical Analysis
Results are reported as mean±SD. The difference between means was assessed by the ANOVA and post hoc least significant difference/Tamhane T2 tests for multiple comparisons, with P<0.05 considered statistically significant.
Results
1. Cytosolic labile zinc accumulation was colocalized with ROS production and associated with neuronal cell death in ischemic rats
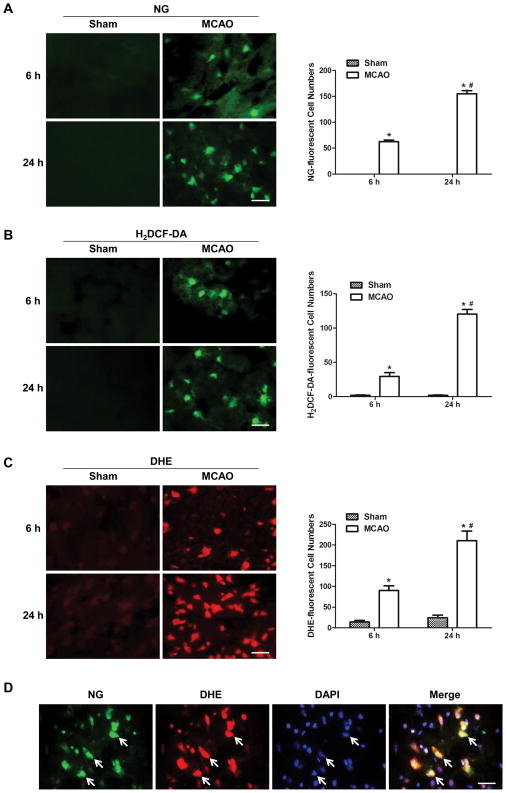
To investigate the interaction between cytosolic labile zinc accumulation and ROS formation following cerebral ischemia, selective zinc-specific fluorescence indicator NG and ROS fluorescence indicator H2DCF-DA were used. There was none or few NG-fluorescent and H2DCF-DA-fluorescent cells in the sham group, whereas a drastic reperfusion time-dependent increase of both NG-fluorescent cells and H2DCF-DA-fluorescent cells was observable in the penumbra tissue section at 6 and 24h after reperfusion (Figures 1A&B), indicating that cytosolic labile zinc level and ROS formation kept on increasing after ischemia, at least up to 24h. Similar results were obtained by using another ROS fluorescent dye, DHE (Figure 1C). We also investigated the spatial relationship between zinc accumulation and ROS formation. NG-positive cells were found to colocalize with DHE-positive cells (Figure 1D), indicating that cytosolic labile zinc accumulation and ROS production occurred in the same cells. These findings suggest that cytosolic labile zinc accumulation may be associated with ROS production in the ischemic brain.
Figure 1.
Cytosolic labile zinc accumulation and ROS formation increased with time after ischemia and colocalized in the penumbra tissue of MCAO rats. Representative staining with NG (A), a fluorescent Zn2+ indicator; H2DCF-DA (B), a fluorescent ROS indicator, and DHE (C), another fluorescent ROS indicator, of brain sections at 6 and 24h after reperfusion. Quantification of NG-, H2DCF-DA- and DHE-stained cells are shown to the right of the corresponding images. Data are means±SD (n=5). *P<0.05 vs sham group, #P<0.05 vs vehicle-treated MCAO 6h group. (D) Double staining of ischemic brain sections of ischemic rats after 24-hour reperfusion with NG (green) and DHE (red). White arrows indicate cells labeled with NG, DHE, or double labeled with both. Bars=20μm.
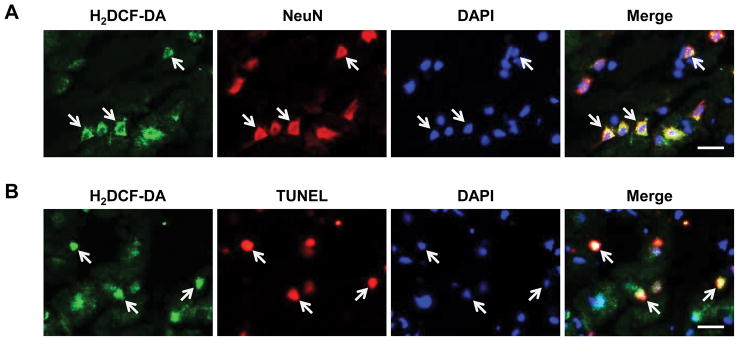
We further investigated the cell-type specificity and outcome of the interaction between ROS formation and zinc accumulation. Figure 2A shows that H2DCF-DA-positive cells were largely colocalized with NeuN-positive cells and Figure 2B shows that the majority of H2DCF-DA-stained cells displayed TUNEL positive. These results suggest that ischemia-induced ROS formation occurred in neurons, which may promote neuronal death following ischemia. Taken together, our findings indicate that focal ischemia induced a high level of zinc accumulation and ROS production in the neurons, resulting in neuronal death.
Figure 2.
ROS production in neurons is associated with neuronal cell death in the penumbra tissue of ischemic rats. (A) Double staining of ischemic brain sections of rats in MCAO group after 24-hour reperfusion with H2DCF-DA (green) and neuron-specific NeuN (red). White arrows indicate cells labeled with H2DCF-DA, NeuN, or double labeled with both. (B) Double-staining of ischemic brain sections after 24-hour reperfusion with H2DCF-DA (green) and TUNEL (red). White arrows indicate cells labeled with H2DCF-DA, TUNEL, or double labeled with both. Bars=20μm.
2. Chelating zinc by TPEN reduced ROS formation and brain damage in ischemic rats
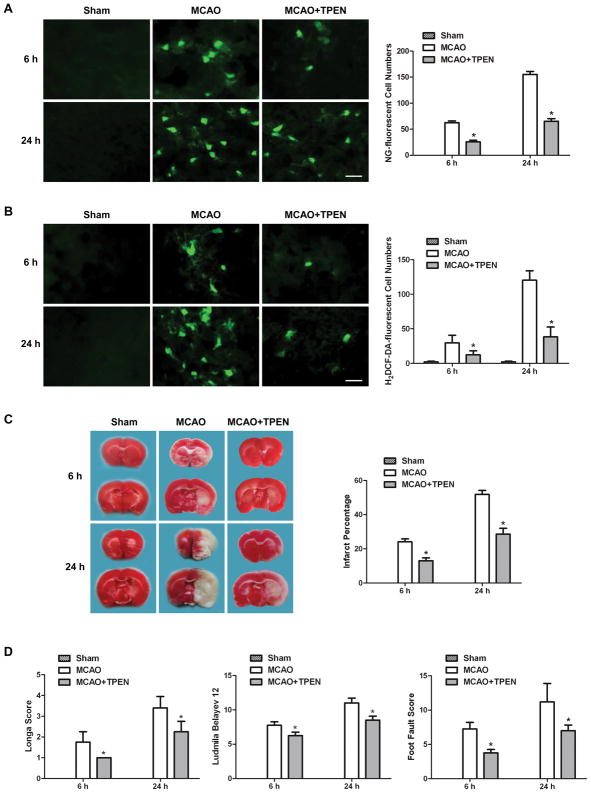
To examine the interaction between cytosolic labile zinc accumulation and ROS production, zinc chelator or ROS inhibitor were used. Firstly, zinc chelator TPEN was used to remove cytosolic labile zinc in MCAO rats to probe whether zinc accumulation contributes to ROS production. The results indicate that chelating zinc with TPEN not only reduced the reperfusion time-dependent increase of NG-fluorescent cells in the penumbra of MCAO rat (Figure 3A), but more importantly, decreased the H2DCF-DA-fluorescent cells as well (Figure 3B). These findings demonstrate that zinc accumulation significantly contributed to ROS formation in ischemic brain.
Figure 3.
TPEN treatment significantly decreased cytosolic labile zinc and ROS accumulation in ischemic brain tissue, and reduced the ischemic brain injury at 6 and 24h after reperfusion. TPEN was injected intraperitoneally 30min before MCAO. (A) Effect of TPEN on zinc accumulation as indicated by NG-stained cells. (B) Effect of TPEN on ROS formation as indicated by H2DCF-DA-stained cells. Bars=20μm. (C) Effect of TPEN on cerebral infarction volume, calculated by TTC-stained coronal sections. (D) Effect of TPEN on neurological assessment and motor function, assessed by neurological deficit scores and foot-fault-placing test. Data are means±SD (n=5 for A&B; n=8 for C&D). *P<0.05 vs vehicle-treated MCAO group.
Next we investigated whether chelating zinc reduces ischemic brain damage. TPEN treatment significantly reduced MCAO-induced cerebral infarct at 6 and 24h after reperfusion (Figure 3C). This marked reduction in infarct volume could not be attributed to hypothermia or other alterations of physiological parameters because these parameters were essentially unchanged between sham and ischemic groups (Table 1 in the online-only Data Supplement). We also investigated neurological outcome of chelating zinc in ischemic rats using 3 independent methods. Treatment with TPEN significantly decreased neurological deficit scores, improved the behavioral deficits and enhanced the functional recovery compared with vehicle-treated MCAO rats (Figure 3D). These results demonstrate that chelating zinc by TPEN reduces brain damage in ischemic rats.
3. Eliminating ROS by EUK-134 decreased brain damage in ischemic rats, but only reduced the cytosolic labile zinc accumulation at 24h after reperfusion
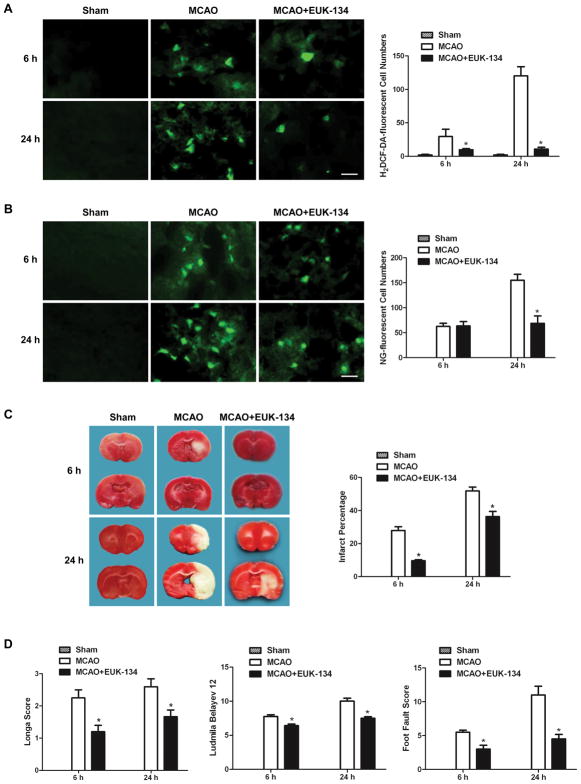
SOD and catalase are important endogenous enzymes in scavenging ROS in ischemic brain. To investigate the effect of ROS production on cytosolic labile zinc accumulation, a synthetic ROS scavenger having both Mn-SOD and catalase activity, EUK-134, was used. Figure 4A shows that EUK-134 mostly eliminated the reperfusion time-dependent increase of H2DCF-DA-fluorescent cells in the penumbra tissue of MCAO rats. Interestingly, the NG-fluorescent cells in the penumbra of EUK-134-treated MCAO rat did not change at 6h after reperfusion, but decreased markedly at 24h after reperfusion (Figure 4B), suggesting that ROS had little effect on zinc accumulation at 6h after reperfusion, but contributed significantly to zinc accumulation at 24h after reperfusion in ischemic rats.
Figure 4.
EUK-134 treatment decreased ROS level and reduced ischemic brain injury at 6 and 24h after reperfusion, but cytosolic labile zinc accumulation was reduced only at later reperfusion time. EUK-134 was injected subcutaneously 30min before MCAO as well as 3 and 8h after reperfusion. (A) Effect of EUK-134 on ROS formation as indicated by H2DCF-DA-stained cells. (B) Effect of EUK-134 on zinc accumulation as indicated by NG-stained cells. Bars=20μm. (C) Effect of EUK-134 on cerebral infarction volume, calculated by TTC-stained coronal sections. (D) Effect of EUK-134 on neurological assessment and motor function, assessed by neurological deficit scores and foot-fault-placing test. Data are means±SD (n=5 for A&B; n=8 for C&D). *P<0.05 vs vehicle-treated MCAO group.
The MCAO-induced cerebral infarct was significantly reduced by EUK-134 treatment at both 6 and 24h after reperfusion (Figure 4C). EUK-134 treatment also significantly decreased neurological deficit scores, improved the behavioral deficits and enhanced the functional recovery (Figure 4D). These results suggest that ROS was only involved in zinc accumulation at late phase of reperfusion, and that scavenging ROS by EUK-134 reduces ischemic brain damage.
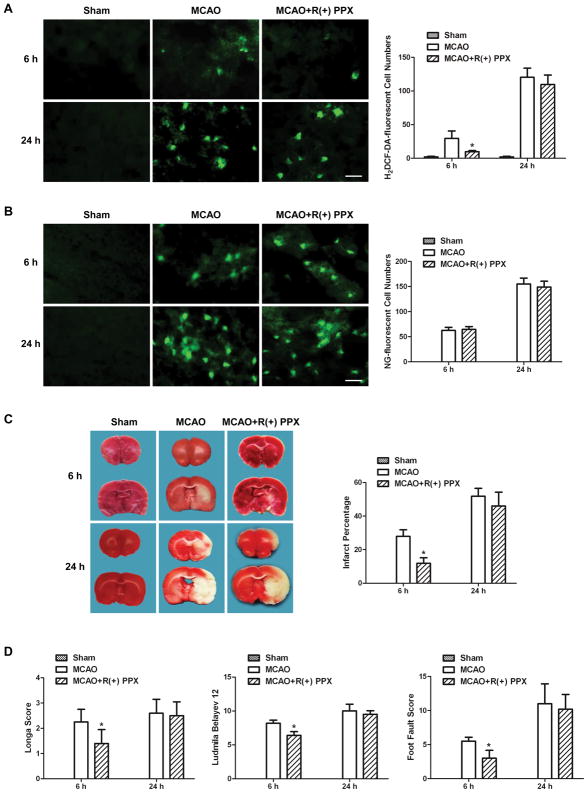
4. Suppression of mitochondrial ROS production by R(+)PPX did not change cytosolic labile zinc accumulation, but reduced brain damage in ischemic rats at 6h after reperfusion
To investigate the effect of ROS produced by mitochondria on cytosolic labile zinc accumulation, mitochondria-targeted ROS inhibitor R(+)PPX was used. As shown in Figure 5A, R(+)PPX reduced the H2DCF-DA-fluorescent cells significantly in the penumbra of MCAO rat at 6h, but not at 24h, after reperfusion, suggesting that ROS is mainly produced from mitochondria at 6h after reperfusion. At 24h after reperfusion, the majority of ROS in the penumbra of MCAO rat is produced from pathways other than mitochondria. The NG-fluorescent cells in the penumbra of R(+)PPX-treated MCAO rat did not change at 6 or 24h after reperfusion (Figure 5B), suggesting the mitochondrial ROS production had no effect on zinc accumulation in ischemic brain.
Figure 5.
R(+)PPX treatment decreased ROS formation and reduced the ischemic brain injury only at 6h after reperfusion, but had no effect on the cytosolic labile zinc accumulation. R(+)PPX was injected intraperitoneally 8h and 30min before MCAO, immediately after reperfusion, and 8 and 16h after reperfusion. (A) Effect of R(+)PPX on ROS formation as indicated by H2DCF-DA-stained cells. (B) Effect of R(+)PPX on zinc accumulation as indicated by NG-stained cells. Bars=20μm. (C) Effect of R(+)PPX on cerebral infarction volume, assessed by TTC-stained coronal sections. (D) Effect of R(+)PPX on neurological assessment and motor function, assessed by neurological deficit scores and foot-fault-placing test. Data are means±SD (n=5 for A&B; n=8 for C&D). *P<0.05 vs vehicle-treated MCAO group.
The MCAO-induced cerebral infarct was significantly reduced by R(+)PPX treatment at 6h after reperfusion (Figure 5C). Meanwhile, neurological deficit scores, behavioral deficits and functional recovery of ischemic rats were also ameliorated (Figure 5D). Surprisingly, at 24h after reperfusion, the infarct volume and neurological deficits as well as behavioral dysfunction in MCAO rats were not affected by R(+)PPX treatment (Figs. 5C&5D). These unexpected findings indicate that the mitochondria-targeted antioxidants R(+)PPX reduced brain damage in ischemic rats only at 6h after reperfusion, consistent with the earlier finding (Figure 5A) that mitochondria-generated ROS occurs only at early phase (i.e., 6h), but not at later phase of reperfusion (i.e., 24h).
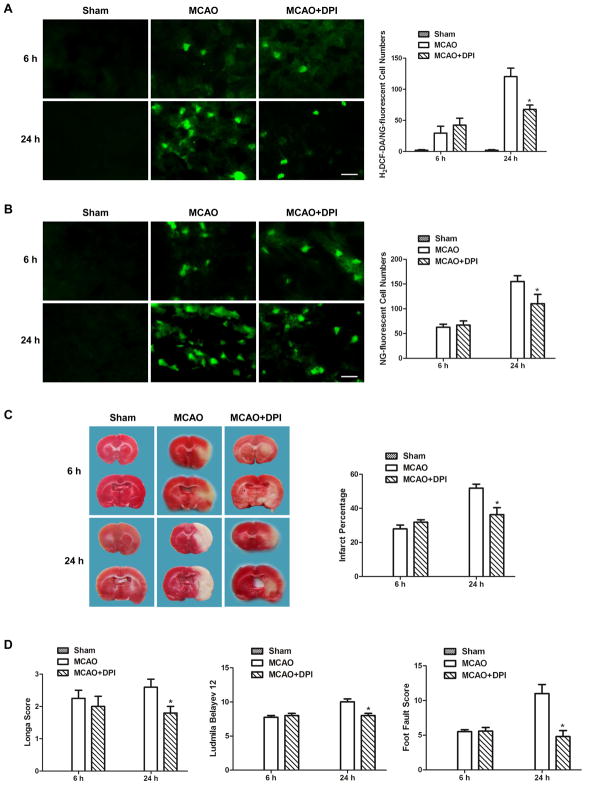
5. Suppression of NADPH oxidase-derived ROS production reduced cytosolic labile zinc accumulation and brain damage at 24h after reperfusion
In order to investigate the effect of ROS produced by NADPH oxidase on cytosolic labile zinc accumulation in cerebral ischemia, NADPH oxidase inhibitor DPI was used. As shown in Figure 6A, DPI only reduced the H2DCF-DA-fluorescent cells in the penumbra of MCAO rat at 24h, but not at 6h, after reperfusion, suggesting that ROS produced through NADPH oxidase pathway is mainly at 24h after reperfusion. At 6h after reperfusion, the ROS in the penumbra of MCAO rat is produced from pathways other than NADPH oxidase. Similarly, the NG-fluorescent cells in the penumbra of DPI-treated MCAO rat did not change at 6h after reperfusion, but decreased significantly at 24h after reperfusion (Figure 6B), suggesting that the ROS produced by NADPH oxidase contributes to zinc accumulation at 24h after reperfusion in ischemic rats.
Figure 6.
DPI treatment decreased ROS formation, cytosolic labile zinc accumulation, and ischemic brain injury only at 24h after reperfusion. DPI was injected intraperitoneally 30min before MCAO. (A) Effect of DPI on ROS formation as indicated by H2DCF-DA-stained cells. (B) Effect of DPI on zinc accumulation as indicated by NG-stained cells. Bars=20μm. (C) Effect of DPI on cerebral infarction volume, assessed by TTC-stained coronal sections. (D) Effect of DPI on neurological assessment and motor function, assessed by neurological deficit scores and foot-fault-placing test. Data are means±SD (n=5 for A&B; n=8 for C&D). *P<0.05 vs vehicle-treated MCAO group.
Compared with vehicle-treated MCAO rats, treatment with DPI significantly reduced MCAO-induced cerebral infarct, decreased neurological deficit scores, improved the behavioral deficits and enhanced the functional recovery at 24h, but not at 6h, after reperfusion (Figures 6C&D). The NADPH oxidase inhibitor DPI reduced ischemic brain damage only at 24h after reperfusion, supporting the idea that ROS produced through NADPH oxidase pathway was mainly at 24h after reperfusion.
Discussion
The present study investigated the interaction between zinc and ROS following transient focal cerebral ischemia, and explored the dynamic relationships of cytosolic labile zinc accumulation in relation to ROS-generating systems during the course of ischemia/reperfusion. We demonstrated that ischemia-triggered zinc release promoted mitochondrial ROS production, which was the major ROS source at early time after reperfusion (6h). Furthermore, mitochondrial ROS production had no influence on zinc accumulation, indicating the critical importance of spatial and temporal factors in the interaction between ROS and zinc accumulation. At 24h after reperfusion, activation of neuronal NADPH oxidase, which is the major ROS source at this time, led to further zinc accumulation, and zinc accumulation led to further ROS production, indicating that there exists a positive feedback loop between zinc accumulation and NADPH oxidase-induced ROS production in the ischemic brain, which contributes to subsequent neuronal death and brain injury. Although both zinc and ROS have been shown to contribute to brain injury following ischemia, the present study provides the first direct evidence that zinc increases ROS while ROS increases zinc, a “zinc-ROS-zinc” positive feedback loop to amplify the damaging effect of both, thus causing greater damage to the brain.
Previous in vitro study showed that eliminating ROS by EUK-134 almost completely blocked all the consequences of Zn2+ treatment. Zinc chelation prevents ROS production and neuronal death after hypoglycemia.18 However, there is little in vivo evidence to prove whether labile cytosolic zinc accumulation is a cause or an effect of ROS production in ischemic brain injury. TPEN is a specific zinc chelator that is neuronal cell membrane permeable and that can bind and remove intra- and extracellular labile zinc. Our previous studies demonstrate that 15mg/kg TPEN treatment effectively reduced labile zinc generated in brain tissue following cerebral ischemia/reperfusion.4 In this in vivo study, chelation of zinc by TPEN decreased ROS generation at both 6 and 24h after reperfusion. However, eliminating ROS by EUK-134 had no effect on zinc accumulation at 6h, but reduced zinc accumulation at 24h after reperfusion, indicating that zinc promotes ROS production, while ROS production only contributes to zinc accumulation in neurons at later reperfusion time (24h). These results suggest that initial zinc release is the upstream of ROS production in the penumbra regions following cerebral ischemia/reperfusion.
It is reported that ROS production in hippocampal neurons during oxygen-glucose deprivation and chemical ischemia is primarily generated by the mitochondrial respiratory chain.6 After focal ischemia, reperfusion was associated with partial recovery of mitochondrial respiratory function during the first hour then followed by deterioration between 2 and 4h,19 suggesting that mitochondria probably is a major source of ROS production several hours after reperfusion. In this study, mitochondria-targeted neuroprotectant R(+)PPX reduced ROS formation and brain damage at 6h after reperfusion (Figures 5A&C), while DPI, the inhibitor of NADPH oxidase, has little effect on ROS production at 6h after reperfusion (Figure 6A), suggesting that ROS produced at 6h after reperfusion is mainly from mitochondria.
Beside mitochondrial respiratory chain, recent studies aimed at determining the source of superoxide production in the neurons suggest that NADPH oxidase is a major source of superoxide production after hypoglycemia and in a cell culture model of ischemia/reperfusion.5,6,20 We observed a small decrease of ROS production at 24h after reperfusion by mitochondria-targeted ROS inhibitor R(+)PPX (Figure 5A), but the most profound inhibition was seen after inhibition of NADPH oxidase by DPI (Figure 6A), suggesting that the majority of the ROS signal seen at 24h after reperfusion is generated by the NADPH oxidase.
Prior studies showed that oxidant-induced mobilization of endogenous Zn2+ already in neurons could interfere with mitochondrial function.8,21 Zn2+ accumulates rapidly in neurons and contributes to consequent mitochondrial dysfunction and cell death.22 Our recent study showed that ischemia induced zinc accumulation in mitochondria. Treatment with TPEN stabilized the mitochondrial membrane potential and reduced cytochrome c release in the penumbra after cerebral ischemia,15 supporting the idea that mitochondria may be the important targets of Zn2+in ischemia. The present study shows that chelating zinc reduced ROS formation and brain damage in ischemic rats at 6h after reperfusion (Figures 3A–D), indicating that zinc promotes mitochondrial ROS production following cerebral ischemia. However, suppression of mitochondrial ROS production did not change cytosolic labile zinc accumulation at 6h after reperfusion (Figure 5B), supporting the idea that zinc release is the upstream of mitochondrial ROS production following cerebral ischemia/reperfusion. At 24h after reperfusion, TPEN reduced ROS formation and ischemic brain damage. Meanwhile, DPI reduced cytosolic labile zinc accumulation and brain damage in ischemic rats, indicating that there exists a positive feedback between zinc accumulation and NADPH oxidase-induced ROS production which contributes to subsequent neuronal death and brain injury following ischemia. Although it is well known that there are multiple sources contributing to ischemia-generated ROS, we demonstrate here that different sources contribute at different time, particularly in terms of interaction with zinc.
We previously reported that in cerebral ischemic rats, there was a dramatically elevated level of zinc accumulation in microvessels, showing the direct interaction of zinc on ischemic microvessels. Zinc accumulation in microvessels activated the superoxide/matrix metalloproteinase-9/-2 pathway, leading to the loss of tight junction proteins and death of endothelial cells in microvessels themselves.23 NADPH oxidases are key enzymes in endothelial cells. Thus, although the current study was focused on neuronal injury, it is highly likely that the proposed pathways would be similar in endothelial cells as well.
Taken together, the present study delineates for the first time an ordered sequence and dynamic relationship between zinc and ROS that lead to neuronal death and brain injury after cerebral ischemia. Cerebral ischemia leads to zinc release, which in turn activates mitochondrial ROS production. Further zinc accumulation activates neuronal NADPH oxidase. ROS produced by NADPH oxidase leads to further increased zinc accumulation, resulting cell death and ischemic brain injury. These findings provide a novel mechanism explaining cerebral ischemia damage and implicate “zinc-ROS-zinc” as an effective and viable new target for treating ischemic stroke.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (81620108011, 81571175, 81171242), and US National Institutes of Health (P30GM103400).
Footnotes
Disclosures
None.
References
- 1.McCord MC, Aizenman E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front Aging Neurosci. 2014;6:77. doi: 10.3389/fnagi.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 3.Canzoniero LM, Turetsky DM, Choi DW. Measurement of intracellular free zinc concentrations accompanying zinc-induced neuronal death. J Neurosci. 1999;19:RC31. doi: 10.1523/JNEUROSCI.19-19-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Pan R, Li S, Luo Y, Yan F, Yin J, et al. Chelating intracellularly accumulated zinc decreased ischemic brain injury through reducing neuronal apoptotic death. Stroke. 2014;45:1139–1147. doi: 10.1161/STROKEAHA.113.004296. [DOI] [PubMed] [Google Scholar]
- 5.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gursoy-Ozdemir Y, Can A, Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- 8.Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, et al. Modulation of mitochondrial function by endogenous Zn2+pools. Proc Natl Acad Sci U S A. 2003;100:6157–6162. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Preferential Zn2+ influx through Ca2+-permeable AMPA/kainate channels triggers prolonged mitochondrial superoxide production. Proc Natl Acad Sci U S A. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan R, Timmins GS, Liu W, Liu KJ. Autophagy mediates astrocyte death during zinc-potentiated ischemia-reperfusion injury. Biol Trace Elem Res. 2015;166:89–95. doi: 10.1007/s12011-015-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boscolo A, Starr JA, Sanchez V, Lunardi N, DiGruccio MR, Ori C, et al. The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: the importance of free oxygen radicals and mitochondrial integrity. Neurobiol Dis. 2012;45:1031–1041. doi: 10.1016/j.nbd.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danzeisen R, Schwalenstoecker B, Gillardon F, Buerger E, Krzykalla V, Klinder K, et al. Targeted antioxidative and neuroprotective properties of the dopamine agonist pramipexole and its nondopaminergic enantiomer SND919CL2x [(+)2-amino-4,5,6,7-tetrahydro-6-propylamino-benzathiazole dihydrochloride] J Pharmacol Exp Ther. 2006;316:189–199. doi: 10.1124/jpet.105.092312. [DOI] [PubMed] [Google Scholar]
- 14.Dong J, Chen P, Wang R, Yu D, Zhang Y, Xiao W. NADPH oxidase: a target for the modulation of the excessive oxidase damage induced by overtraining in rat neutrophils. Int J Biol Sci. 2011;7:881–891. doi: 10.7150/ijbs.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong W, Qi Z, Liang J, Shi W, Zhao Y, Luo Y, et al. Reduction of zinc accumulation in mitochondria contributes to decreased cerebral ischemic injury by normobaric hyperoxia treatment in an experimental stroke model. Exp Neurol. 2015;272:181–189. doi: 10.1016/j.expneurol.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 17.Zhao H, Wang R, Tao Z, Gao L, Yan F, Gao Z, et al. Ischemic postconditioning relieves cerebral ischemia and reperfusion injury through activating T-LAK cell-originated protein kinase/protein kinase B pathway in rats. Stroke. 2014;45:2417–2424. doi: 10.1161/STROKEAHA.114.006135. [DOI] [PubMed] [Google Scholar]
- 18.Suh SW, Garnier P, Aoyama K, Chen Y, Swanson RA. Zinc release contributes to hypoglycemia-induced neuronal death. Neurobiol Dis. 2004;16:538–545. doi: 10.1016/j.nbd.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda S, Katsura KI, Tsuchidate R, Siesjo BK. Secondary bio-energetic failure after transient focal ischaemia is due to mitochondrial injury. Acta Physiol Scand. 1996;156:149–150. doi: 10.1046/j.1365-201X.1996.449170000.x. [DOI] [PubMed] [Google Scholar]
- 20.Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT, et al. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- 21.Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E, et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 22.Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J Neurosci. 2009;29:1105–1114. doi: 10.1523/JNEUROSCI.4604-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi Z, Liang J, Pan R, Dong W, Shen J, Yang Y, et al. Zinc contributes to acute cerebral ischemia-induced blood-brain barrier disruption. Neurobiol Dis. 2016;95:12–21. doi: 10.1016/j.nbd.2016.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.