Abstract
Institutional rearing is associated with deficits in executive functions, such as inhibitory control, and may contribute to later externalizing behavior problems. In the current study, we explored the impact of institutional rearing on attention in the context of inhibiting a planned action. As part of the Bucharest Early Intervention Project (BEIP), children were randomized to either remain in the institutions in which they lived (Care as Usual Group) or be placed into foster family homes (Foster Care Group). We also recruited age and gender matched never-institutionalized (NIG) children from the Bucharest community. We examined differences in behavioral and Event Related Potentials (ERPs) during a go-no-go task when children were 12 years old. Results revealed that the ever-institutionalized group (CAUG and FCG combined) showed slower reaction times, worse performance accuracy, larger P2 activation, and smaller (less negative) N2 activation than the NIG group. Results of a moderation analysis revealed that children who spent more time in institutions and had small N2s showed more externalizing symptoms. These results have implications for the design of treatment approaches for previously institutionalized children with externalizing behavior problems.
Keywords: Early Institutionalization, Inhibiting a Planned Action, Externalizing Behavior, Event-Related Potentials, BEIP
A large number of children reared in institutions exhibit signs of externalizing disorders (e.g., Humphreys, Gleason, Drury, Miron, Nelson, Fox, et al., 2015; Merz & McCall, 2010; Stevens, Sonuga-Barke, Kreppner, Beckett, Castle, Colvert, et al., 2008; Wiik, Loman, Van Ryzin, Armstrong, Essex, Pollak, et al., 2011; Zeanah, Egger, Smyke, Nelson, Fox, Marshall, et al, 2009). However, not all institutionally reared children go on to develop externalizing behavior problems (e.g., Humphreys, Gleason, Drury, Miron, Nelson, Fox, & Zeanah, 2015; Merz & McCall, 2010; Wiik, et al., 2011; also see review by Troller-Renfree, Zeanah, Nelson, & Fox, 2017). For example, Merz & McCall (2010) found that roughly between 20% and 35% of previously institutionalized children, primarily from Russia and Romania, compared with 10% of non-deprived adopted children in the US, showed externalizing behavior problems. Similarly, Wiik, et al. (2011) found that roughly 16% of previously institutionalized children adopted internationally, compared to 4% for non-adopted children, showed externalizing behavior problems. Additionally, the levels of externalizing behavior problems outlined by Wiik and colleagues (2011), as well as by Merz & McCall (2010) for previously institutionalized children is considerably higher than the 7.1% mean level of externalizing problems for non-previously institutionalized children (across 9 countries) found by Crijnen, Achenbach, and Verhulst (1997). Therefore, investigators have started to examine which factors might moderate the association between early institutional rearing and externalizing behavior (e.g., McDermott, Troller-Renfree, Vanderwert, Nelson, Zeanah, & Fox, 2013; Troller-Renfree, Nelson, Zeanah, & Fox, 2016). For example, Troller-Renfree, et al. (2016) found that brain activation associated with error processing moderated the association between amount of time spent in the institution and externalizing behaviors.
Additionally, several studies have shown that early institutional rearing contributes to deficits in executive functions (Bos, Fox, Zeanah, & Nelson, 2009; Bruce, Tarullo, Gunnar, 2009; Merz & McCall, 2011; Pollak, Nelson, Schlaak, Roeber, Wewerka, Wiik, et al., 2010; Tibu, Sheridan, McLaughlin, Nelson, Fox, & Zeanah, 2016), including inhibitory control (e.g., McDermott, Westerlund, Zeanah, Nelson, & Fox, 2012; Pollak, et al., 2010), i.e., the ability to inhibit a planned action (Schachar et al., 1995), and that deficits in inhibitory control are associated with externalizing behavior problems (Huijbregts, Warren, de Sonneville, & Swaab-Barneveld, 2008; Schachar, Mota, Logan, Tannock, & Klim, 1999; Schachar, Tannock, Marriott, & Logan, 1995; Tibu, Sheridan, McLaughlin, Nelson, Fox, & Zeanah, 2016). Thus, the current study examined if deficits in the ability to inhibit a planned action and perturbations in the neural correlates that contribute to inhibitory control might moderate the association between institutional rearing and externalizing symptoms.
To efficiently inhibit a planned action, several attention mechanisms need to be applied (as discussed in Aron, 2007; Cisek, 2007; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). To examine these attentional mechanisms, in the current study we measured three components of the event-related potential (ERP)—the P2, N2, and P3—in the context of a task that requires inhibition of a planned action (go-no-go task). ERPs were used to decompose the neural chronometry underlying the inhibition of a planned action because of their excellent temporal specificity (Kappenman & Luck, 2012; Hillyard & Anllo-Vento, 1998).
Previous studies have associated P2 activation, a mediofrontal ERP found in adults roughly 200–300 ms after stimulus onset, with attentional orienting (Kanske, Plitschka, & Kotz, 2011; Maeno, Gjini, Iramina, Eto, & Ueno, 2004; Van Voorhis & Hillyard, 1977). Furthermore, Loman and colleagues found that in the context of a go-no-go task previously institutionalized children had larger P2s for no-go trials compared to go trials but that this effect was not evident for non-institutionalized children (Loman, Johnson, Westerlund, Pollak, Nelson, & Gunnar, 2014). Thus, we predicted that P2 activation would be enhanced in previously institutionalized children and moderate the association between institutional rearing and externalizing symptoms; specifically, that institutionalized children with large P2s would show the most externalizing symptoms.
Studies have associated N2 activation—a mediofrontal ERP found in adults roughly 250–350 ms after stimulus onset—with various aspects of cognitive control, including response monitoring or response conflict (Bartholow, Pearson, Dickter, Sher, Fabiani, Gratton, 2005; Dimoska, Johnstone, & Barry, 2006; Donkers & van Boxtel, 2004; Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003; van Veen & Carter, 2002). Loman and colleagues (2014) also examined the impact of institutionalization on N2 amplitudes and found that N2s were smaller (less negative) for institutionalized children compared to non-institutionalized children. Additionally, Troller-Renfree, et al., (2016), using the same sample, found that another mediofrontal ERP also associated with response processing (the ERN) moderated the association between amount of time-spent-in-the-institution and externalizing behavior problems. Therefore, we predicted that institutionally reared children with smaller N2s would show the most externalizing symptoms but that this would not be the case for institutionally reared children with large N2s, i.e., that the N2 would moderate the association between time-spent-in-the-institution and externalizing symptoms.
Lastly, the P3 —an ERP component that peaks in adults roughly at 300 ms after stimulus onset—has been associated with context updating in working memory (Donchin, 1981; for a review see Polich, 2012). McDermott et al. (2012) found deficient P3 activation for previously institutionalized and foster care children compared to never institutionalized children in the context of a go-no-go task. Thus, we predicted that children who were institutionally reared would exhibit smaller P3 amplitudes and that children with small P3s would show the most externalizing symptoms.
The goals of the current study are to determine: 1) if removal from an institution and subsequent placement in foster care early in life impacts neural activation underlying one’s ability to inhibit planned actions, at age 12 years, and 2) if patterns of neural activation (P2, N2, and P3) underlying the ability to inhibit a planned action moderates the association between amount of time-in-institution and externalizing symptoms. These questions were explored in the context of the Bucharest Early Intervention Project (BEIP), the first randomized control trial of a foster care intervention for institutionalized children (for details see Zeanah, Nelson, Fox, Smyke, Marshall, Parker, & Koga, 2003).
Method
Participants
Participants were part of the Bucharest Early Intervention Project (BEIP; Zeanah, et al., 2003), a randomized controlled trial comparing the effects of foster care as an alternative to institutional care for young children abandoned at birth and placed in institutions. For a detailed breakdown of the history, design, and implementation of this study, please see Zeanah, et al., 2003. This study assessed 136 children between the ages of 6 and 31 months who were institutionalized in Bucharest, Romania, and who at that time had spent at least half of their lives living in an institution. After initial assessment of all children, half of the institutionalized sample (n = 68; 33 boys and 35 girls) was randomly assigned to continued institutional care (care as usual, CAU) and the other half of the sample (n= 68, 34 boys and 34 girls) was assigned to foster care (FC; see Zeanah et al., 2003, for a full description of the sample). Both groups (CAUG and FCG) were followed systematically through 12 (mean = 12.63, SD = .55) years of age. Additionally, a separate never institutionalized group (NIG) of age- and gender-matched children (n = 52, 23 boys and 29 girls; mean age = 12.68, SD = .39) from the Bucharest area were recruited as a comparison group. The current study presents ERP and clinical symptom data collected at 12 years of age and includes 144 participants (CAUG: male 26, female 21; FCG: male 26, female 23; NI: male 22, female 26). Participants were excluded from this study for a number of reasons, including that the child did not participate in the 12-year visit (11 CAUG, 12 FCG), go-no-go data was missing (8 CAUG, 6 FCG, 4 NIG), and because ERPs were comprised of too few artifact free trials (2 CAUG, 1 FCG). Missing data was compared to analyzed data for differences in gender, χ2(1, N = 188) = 3.05, p = .08, age, t(156) = −.18, p = .86, ethnicity, χ2(3, N = 188) = 4.73, p = .19, group status, χ2(2, N = 188) = 10.06, p = .007, and severity of externalizing symptoms, t(157) = −.58, p = .56. For the significant group status analysis, fewer NIG children had missing data for the 12-year visit than CAUG and FCG children.
Measures
Diagnostic Interview Schedule for Children, 4th Edition; DSM-IV (DISC; Shaffer et al., 2000). The DISC is a structured psychiatric interview assessment tool that is both reliable and valid for children 6 years and older. The DISC probes current symptom levels, duration or persistence, age of onset, and functional impairment. For the NIG and FCG groups, the parent report was obtained from the mother if available, otherwise fathers provided the report. For the CAUG children, an institutional caregiver who worked with the child regularly and knew them well reported on the child’s behavior. For more information on how the DISC was administered within the BEIP study, please see Humphreys, et al. (2015). For the current study, only the Attention-Deficit/Hyperactivity Disorder (ADHD), Oppositional Defiant Disorder (ODD), and Conduct Disorder (CD) modules were used. The Mean internal consistency values for our study was .77.
MacArthur Health and Behavior Questionnaire (HBQ; Luby, Heffelfinger, Measelle, Ablow, Essex, Dierker, et al., 2002). The HBQ parent report is a reliable and valid measure of the physical and mental health of young children. This measure yields dimensional ratings of current functioning in the domains of 1) emotional and behavioral symptomatology, 2) physical health, 3) social adaption, and 4) school adaptation, to comprise 18 subscales covering a number of behavior problems, including ADHD and externalizing symptoms. The Mean internal consistency values for our study was .88. For the current study, we used the same reporter for the CAUG, FCG, and NIG children as described above for the DISC.
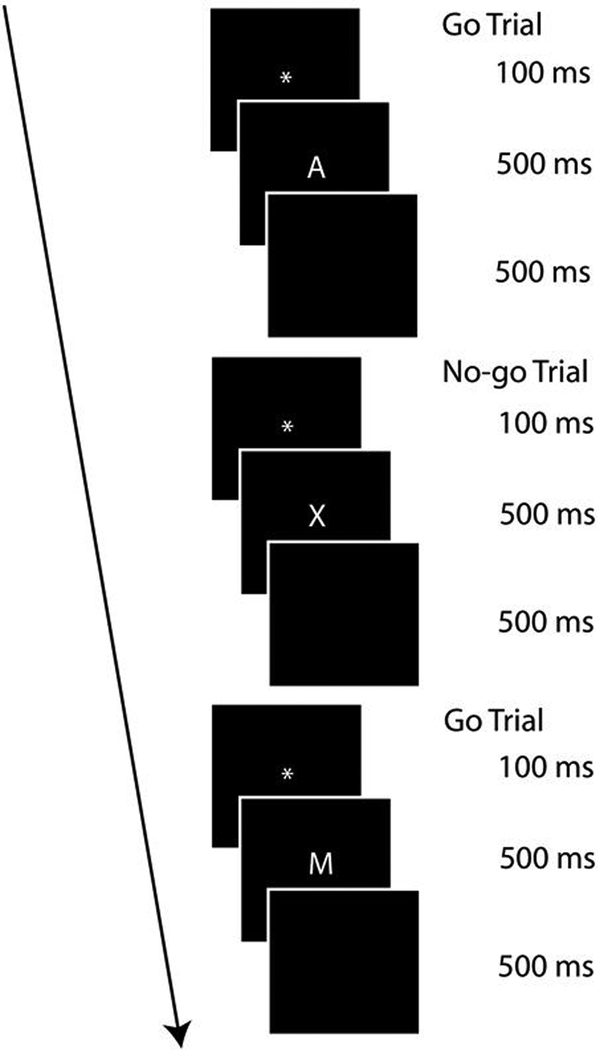
Go-no-go Task (McDermott et al., 2012). The current task was a modified version of the traditional letter go-no-go task (Conners, 2000) and was presented on a 17-in computer monitor using E-Prime software (Psychological Software Tools, Pittsburgh, PA; Schneider, Eschman, & Zuccolotto, 2002). The timing and appearance of the traditional go-no-go task was altered slightly to make it appropriate for an ERP task (see Figure 1). Stimuli were shown on a black screen and consisted of single letters presented in white (170-point size upper case, Times New Roman). The current task consisted of 70% go trials and 30% no-go trials. This ratio of go to no-go trials ensures a response prepotency (i.e., requiring enhanced response control for the no-go trials). This ratio of trials occurred pseudo randomly (each participant got the same trial order) within 2 blocks of 140 trials each. Throughout the task, no-go trials were always separated by at least one go trial. Prior to these 140 trials, each block consisted of a string of 20 go trials (not included in percentages above) to train participants to have a response prepotency, thereby making it more difficult to inhibit responding when a no-go stimulus was presented. The total number of trials presented was 320 (not including the 10-trial practice block). Each trial consisted first of a fixation image (100 ms) followed by a stimulus image (500 ms) and a blank black screen (500 ms). All no-go trials presented the letter “X”. Go trials consisted of all other letters except the letter “K”.
Figure 1.
Go-no-go task diagram showing three trials
EEG data collection and analyses
EEG was recorded using a 64-channel Geodesic Sensor Net and sampled at 250 Hz, using EGI software (Net Station; Electrical Geodesic, Inc., Eugene, OR [data were also processed using Net Station]). Once the impedance values for all EEG channels were reduced to below 50 kΩ, data acquisition began. During recording, all channels were referenced to Cz and after acquisition, data were re-referenced using an average reference.
Data were filtered using a FIR bandpass filter with a low pass frequency of 30 Hz and a high pass frequency of .3 Hz. To best capture eye blink artifacts, the threshold was set to 140 μV (peak-to-peak) and all trials in which this threshold was violated were excluded from analyses. Furthermore, signal activation change (peak-to-peak) exceeding 125 μV across the entire segment and fast transits exceeding a difference (peak-to-peak) of 100 μV across 2 samples (8 ms) were marked as bad and interpolated. After automatic artifacting was completed, participant data were visually inspected to ensure artifact criteria were applied appropriately.
Procedure
After consent and assent were attained, children were seated in a chair 96 cm from the computer screen. The electrode sensor net was applied and the go-no-go task was administered. Children played a 10-trial long practice block to ensure proficiency of the task. Participants moved on to the actual task if they reached 60% accuracy. All participants reached this criterion and no participants had to repeat the practice block.
Data analyses
Waveforms for correct go and no-go trials were segmented into epochs from 200 ms before to 600 ms after stimulus onset and baseline corrected for the 200 ms preceding stimulus onset. Inspection of the grand-averaged ERP waveform indicated that mediofrontal P2 activation was maximal between 210 and 310 ms, mediofrontal N2 activation was maximal between 315 and 415 ms, and parietal P3 activation was maximal between 300 and 550 ms after stimulus onset; thus, peak activation was exported for these time windows. To eliminate trials characterized by attentional lapses or chronic non-responding, no-go trials that did not have a correct go trial preceding and following them were removed from analyses. Due to this strict criterion, the mean number of trials comprising correct no-go ERPs was 44.47 (SD = 13.90; range = 11–75). Mean number of trials comprising correct go ERPs was 134.33 (SD = 30.16; range = 28–182). Traditionally, N2 activation has been analyzed as no-go activation minus go activation (e.g., Falkenstein, Hoormann, & Hohnsbein, 1999; Mathalon, Whitfield, & Ford, 2003). However, there is recent evidence that conducting difference scores on ERPs brings about bias (Meyer, Lerner, De Los Reyes, Laird, & Hajcak, 2017). Therefore, we removed go activation from no-go activation using linear regression and analyzed the standardized residuals.
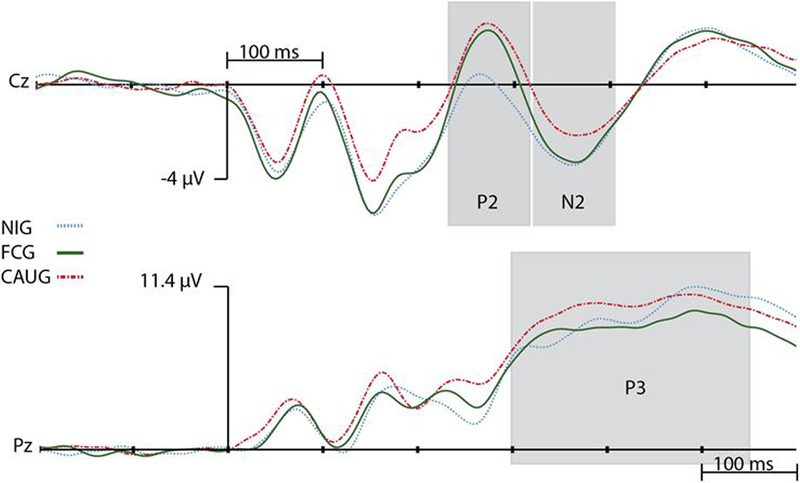
Visualization of the correct go and no-go stimulus-locked waveforms revealed clear N1, P2, and N2 components for mediofrontal electrodes and a clear P3 at parietal electrodes (see Figure 2). Consistent with previous literature (e.g., Falkenstein, et al., 1999; Gajewski, Stoerig, & Falkenstein, 2008; Nieuwenhuis, Yeung, & Cohen, 2004), P2 and N2 activation was exported for electrodes Cz and FCz, and the average activation across these electrodes was analyzed to limit the number of analyses performed, while the P3 was analyzed at electrode Pz.
Figure 2.
Correct no-go activation waveforms (μV). Positive activation is up.
Preliminary Analyses
To determine if potential gender differences might affect analyses, a priori t-tests were run (see Table 1). Gender was controlled for (added as a covariate) in all analyses that showed significant gender differences in Table 1.
Table 1.
Gender differences with means and standard deviations
| Variable Name | Gender Differences | Male | Female |
|---|---|---|---|
| Go P2 amplitudes | t(142) = −.79, p = .43 | 1.89 (1.89) | 1.47(1.47) |
| No-go P2 amplitudes | t(142) = .64, p = .52 | 3.53(4.03) | 3.93(3.42) |
| No-go (with go regressed out) N2 amplitudes | t(142) = −.80, p = .43 | .05(1.00) | −.07(.74) |
| Go P3 amplitudes | t(142) = −1.21, p = .23 | 10.22(4.66) | 9.34(4.11) |
| No-go P3 amplitudes | t(142) = −2.34, p = .02* | 13.81(5.66) | 11.76(4.82) |
| Go reaction times | t(142) = .34, p = .39 | 361.23(46.96) | 367.99(46.15) |
| No-go reaction times | t(142) = −.55, p = .58 | 322.74(64.66) | 317.51(47.03) |
| Go performance accuracy | t(142) = 1.04, p = .30 | .95(.06) | .96(.05) |
| No-go performance accuracy | t(142) = 2.15, p = .03* | .67(.13) | .72(.13) |
| CD Symptoms | t(142) = −1.14, p = .26 | .07(.87) | −.08(.76) |
| ODD Symptoms | t(142) = −1.83, p = .07 | .13(.88) | −.13(.82) |
| ADHD Symptoms | t(142) = −3.42, p = .001*L | .23(.93) | −.24(.72) |
Equal variances not assumed (significant Levene’s test)
Significant Gender differences
CD = Conduct Disorder; ODD = Oppositional Defiant Disorder;
ADHD = Attention Deficit Hyperactivity Disorder
Because there is considerable debate about what is the best ERP data extraction procedure, i.e., mean or maximum/minimum values, all analyses were conducted on both types of data. Patterns of results were the same across both types of data but maximum/minimum yielded slightly stronger effects, so these were reported.
To prevent potential Type I errors, we limited the number of analyses conducted on externalizing variables by making three composite scales measuring ADHD, ODD, and CD symptoms. The ADHD symptom composite consisted of HBQ inattention and impulsivity variables, and DISC inattention, hyperactivity, and impulsivity variables. All variables were standardized and then averaged together. The same procedure was applied to comprise the ODD and CD symptom composite scales. The ODD composite scale was comprised of HBQ oppositional defiant, DISC ODD mood, and DISC ODD defiant scales. Lastly, the CD composite scale was comprised of HBQ conduct and DISC violent CD. See below for a correlation table between these symptom composite scales and both ERP and behavioral measures (see Table 2).
Table 2.
Pearson correlations between all clinical, ERP, and behavioral measures
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. CD | -- | 0.61** | .59** | .03 | .02 | .006 | −.14 | .14 | .09 | .08 | .005 | .01 |
| 2. ODD | -- | .74** | .10 | .12 | −.24** | −.22* | .13 | .03 | .06 | .03 | .07 | |
| 3. ADHD | -- | .009 | .06 | −.24** | −.24** | .09 | −.006 | −.03 | −.02 | .000 | ||
| 4. Go RT | -- | .70** | −.23** | .44** | .08 | .03 | .008 | −.10 | −.08 | |||
| 5. No-go RT | -- | −.38** | .22** | −.008 | −.05 | −.08 | −.02 | −.04 | ||||
| 6. Go Acc. | -- | .28** | −.10 | −.007 | −.01 | .13 | .15 | |||||
| 7. No-go Acc | -- | .04 | .13 | .15 | .04 | −.03 | ||||||
| 8. N2 | -- | .15 | .39** | .10 | .16 | |||||||
| 9. Go P2 | -- | −83** | −.07 | −.09 | ||||||||
| 10. No-go P2 | -- | −.03 | −.03 | |||||||||
| 11. Go P3 | -- | .69** | ||||||||||
| 12. No-go P3 | -- |
Significant < or = .05;
Significant < or = .05
Acc = performance accuracy; RT = reaction times; N2 = no-go N2 with go removed
CD = conduct disorder; ODD = oppositional defiant disorder; ADHD = attention deficit hyperactivity disorder
Each subsection of the results section is organized starting with our intent-to-treat analyses (Little & Yau, 1996; t-test examining differences between FCG and CAUG groups) to assess results of the randomized controlled trail (RCT), followed by group difference analyses (t-test examining differences between ever institutionalized and never institutionalized children), and finally, moderation analyses examining if ERP amplitudes moderated the association between time-in-institution and externalizing behavior symptoms. Time-in-institution was calculated as percent time that each child had lived in an institution at age 12. Reduced t-test degrees of freedom shown below are due to violation of the homogeneity of variance assumption. Additionally, to determine if ERP activation moderated the association between time-in-institution and externalizing behavior symptoms, we conducted a number of hierarchical linear regression analyses. Percent-time-in-institution (independent variable) and ERP activation (moderator) were entered in step one. The interaction term between time-in-institution and ERP activation was entered in step two. The dependent variables consisted of three composite symptom variables: conduct disorder (CD), oppositional defiant disorder (ODD), and attention deficit hyperactivity disorder (ADHD), which were entered into separate analyses.
Ethical considerations
Parents or guardians provided consent at each time point and children assented to participate in this study at 12 years of age. All children assented to each procedure.
This study received IRB approval from Boston Children’s Hospital (protocol number 10-04-0185; with the University of Maryland relying on this protocol) and Tulane University (protocol number 196018). Ethical dimensions of the study have been discussed by us (Nelson, Fox, & Zeanah, 2014; Zeanah, Fox, & Nelson, 2012; Zeanah, Koga, Simion, Stanescu, Tabacaru, Fox, & Nelson, 2006a,b) and by others (Miller, 2009; Millum & Emmanuel, 2007; Rid, 2012).
Results
Behavioral Data
Intent-to-treat.
We conducted independent samples t-tests to explore reaction time and performance accuracy differences between our CAUG and FCG groups. Results revealed significant differences only for go reaction times, t(85) = 2.46, p = .02, CAUG Mean = 381.18, FCG Mean = 356.46, indicating that the FC group had faster reaction times than the CAU group. There were no intervention effects for no-go reaction times or go and no-go performance accuracy: t(94) = 1.78, p = .08, CAUG M = 338.19, FCG M = 315.27; t(94) = −.49, p = .62, CAUG M = .94, FCG M = .94; t(94) = .67, p = .50, CAUG M = .70, FCG M = .68.
Group differences.
To ascertain the differential behavioral performance for ever institutionalized (EI) children, i.e., children in the CAU and FC groups combined, compared with NIG children, we conducted independent samples t-tests for go and no-go reaction times, as well as go and no-go performance accuracy. Results revealed worse go performance accuracy, t(135) = −5.26, p < .001, EIG M = .94, NIG M = .98, and slower erroneous reactions times (no-go trials), t(139) = 2.26, p = .03, EIG M = 326.49, NIG M = 307.62, for the EI group compared to the NI group. The reaction time results suggest that errors for the EIG children occur later than for the NIG children in the cognitive sequence required to inhibit a planned action. Non-significant results were found for no-go performance accuracy, t(119) = −.37, p = .72, EIG M = .69, NIG M = .70, and go reaction times, t(122) = 1.64, p = .10, EIG M = 368.56, NIG M = 356.41.
P2 Results
Intent-to-treat.
We conducted independent samples t-tests to explore differences in go and no-go P2 activation between our CAU and FC groups. Results revealed non-significant effects for go, t(94) = .77, p = .44, CAUG M = 2.30, FCG M = 1.79, and no-go, t(94) = .02, p = .98, CAUG M = 4.33, FCG M = 4.31, trials.
Group differences.
To explore group differences in P2 activation between EIG and NIG children, we conducted separate t-tests for go and no-go trials. Results revealed larger no-go P2s for the EI group than the NI group, t(142) = 2.78, p = .006, EIG M = 4.32, NIG M = 2.52. For go trials, results were not significant, t(142) = 1.89, p = .06, EIG M = 2.04, NIG M = .99.
Moderational analyses.
Next, to determine if no-go P2 amplitude moderated the association between time-in-institution and externalizing behavior symptoms, a number of moderation analyses were conducted (as outlined above). Results revealed a main effect of time-in-institution on both CD, β = .29, t(92) = 2.85, p = .005, and ODD, β = .27, t(92) = 2.56, p = .01, but not ADHD, β = .09, t(92) = .87, p = .39. Additionally, no main effect of P2 was found for any of the externalizing measures: CD, β = .002, t(92) = .02, p = .98; ODD, β = −.04, t(92) = −.41, p = .68; ADHD, β = −.12, t(92) = −1.12, p = .24. Lastly, no significant interaction was found between time-in-institution and P2 on CD, β = .15, t(92) = 1.44, p = .15; ODD, β = −.05, t(92) = −.48, p = .64, or ADHD, β = .01, t(92) = .10, p = .92.
N2 Results
Intent-to-treat.
We conducted an independent samples t-test to explore if no-go activation (with go activation regressed out) differed between our CAU and FC groups. This t-test did not yield a significant difference, t(94) = .73, p = .47, CAUG M = .17, FCG M = .03.
Group differences.
To explore group differences in N2 activation (no-go with go regressed out), we conducted a t-test comparing EIG children with NIG children. Results revealed that the EI group had less negative N2s than the NI group, t(142) = 2.01, p = .05, EIG M = .10, NIG M = −.21.
Moderational analyses.
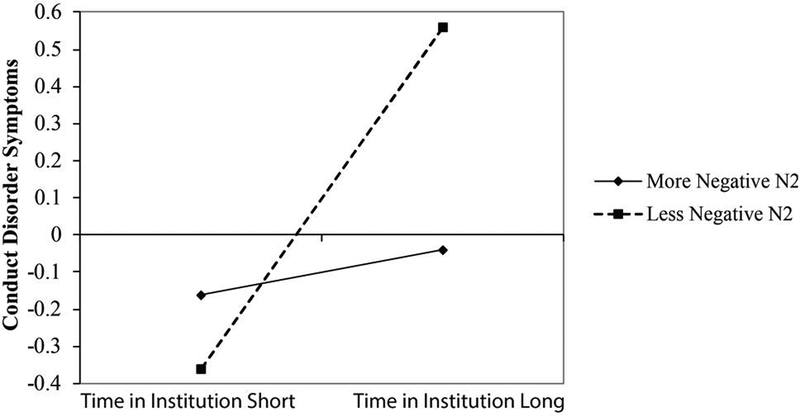
Next, to determine if N2 activation (no-go with go regressed out) moderated the association between time-in-institution and externalizing behavior symptoms, we conducted a number of hierarchical linear regression analyses. Results revealed a main effect of time-in-institution on both CD, β = .26, t(92) = 2.61, p = .01, and ODD, β = .26, t(92) = 2.44, p = .02, but not ADHD, β = .06, t(92) = .56, p = .57. Additionally, no main effect of N2 was found for any of the externalizing measures: CD = β = .10, t(92) = 1.03, p = .31; ODD = β = .07, t(92) = 68, p = .50; ADHD = β = .06, t(92) = .57, p = .57. Lastly, a significant interaction was found between time-in-institution and N2 on CD, β = .20, t(92) = 1.98, p = .05 (see Figure 3). However, no such interaction was found for the ODD, β = −.05, t(92) = −.44, p = .66, or ADHD, β = .08, t(92) = .76, p = .45, composite variables.
Figure 3.
Moderation plot showing that the association between time in the institution and conduct-disorder-like symptoms is moderated by N2 (no-go with go regressed out) activation. Activation is average of Cz and FCz.
To decompose the interaction between percent-time-in-institution and N2 activation on CD, simple slopes were tested by re-calculating N2s into new variables representing high and low N2 activation, and running additional regression analyses using the re-calculated scores, as suggested by Aiken, et al. (1991). Follow-up analyses showed that when N2 activation was less negative, time-in-institution was related to CD symptoms, β = .51, t(92) = 3.69, p<.001. However, when N2 activation was more negative, time-in-institution was unrelated to CD symptoms, β = .02, t(92) = .10, p=.92. Thus, children who stayed in the institution for a large amount of time and had small (less negative) N2s, displayed higher levels of CD symptoms.
P3 Results
Intent-to-treat.
We conducted two independent samples t-tests to explore differences in go and no-go P3 activation between our CAU and FC groups. Results revealed no significant P3 differences: go, t(94) = .13, p = .89, CAUG M = 9.76, FCG M = 9.64; no-go, t(94) = .64, p = .52, CAUG M = 12.96, FCG M = 12.23.
Group differences.
To explore group differences in P3 activation between EIG and NIG children, we conducted separate t-test on no-go and go activation. No significant Group effects were found for either go, t(142) = −.36, p = .72, EIG M = 9.70, NIG M = 9.98, or no-go, t(142) = −.71, p = .48, EIG M = 12.59, NIG M = 13.26, trials. Because we found no group differences in P3 activation, we did not conduct moderation analyses on P3s.
Discussion
We examined whether children with a history of psychosocial deprivation showed differential amounts of neural activation underlying the ability to inhibit a planned action, and if amounts of neural activation moderated the association between institutional rearing and symptoms of externalizing behavior. To inhibit a planned action, a number of attention mechanisms need to be deployed (Aron, 2007; Cisek, 2007; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004), the extent of which can be measured using ERPs.
Previous studies suggest that P2 activation might be a neural marker underlying attentional orienting (Kanske, Plitschka, & Kotz, 2011; Maeno, Gjini, Iramina, Eto, & Ueno, 2004; Van Voorhis & Hillyard, 1977). Loman, et al., (2013) found that previously institutionalized children had larger P2s for no-go trials than go trials but that never institutionalized children did not show this differentiation. Consistent with the works of Loman, et al. (2013), our group analyses found that the ever-institutionalized group (EIG; CAUG and FCG combined) recruited more no-go P2 activation, had worse performance accuracy, and slower erroneous reaction times than the NI group. Together, these results suggest that the CAU and FC groups may be orienting their attention towards important cues inefficiently compared to the NI group, and that their inefficient attention orienting may in part contribute to their worse behavioral performance.
Once attention has been oriented toward an event that might require action, then it becomes necessary to determine if and what action needs to be initiated, potentially leading to a conflict between response strategies. The N2 has been associated with response conflict or response monitoring (Bartholow, et al., 2005; Dimoska, et al., 2006; Donkers & van Boxtel, 2004; Nieuwenhuis, et al., 2003; van Veen & Carter, 2002). Consistent with previous literature (Kishiyama, et al., 2008; Loman, et al., 2013; McDermott, et al., 2012; McFarlane, et al., 2005), we found that early severe psychosocial deprivation, such as that generally found in institutions, impacts patterns of N2 activation. We found that the EI group had smaller (less negative) N2s than the NI group. If we examine these results in the context of our P2 results, it may be that the EI group performs poorly (low accuracy and slow reaction times) in part because they have difficulty orienting their attention to an important response cue (the no-go X) and then subsequently have limited conflict processing (response conflict/conflict monitoring) resources making it difficult to initiate the correct response in a timely manner.
We also examined if institutional rearing altered P3 activation, an ERP component that has been associated with context updating in working memory (e.g., Donchin, 1981; for a review see Polich, 2012). Previous studies have shown variable results, with some showing clear effects of institutional rearing on P3s (e.g., Evren-Guler, et al., 2012), while others, like our study, did not (e.g., Loman, et al., 2013). These conflicting results might be due to the different tasks used. Studies that showed P3 effects, compared to those that did not, used tasks with a memory component, e.g. old vs. new picture recognition (Evren-Guler, et al., 2012; Loman et al., 2013).
We also were interested to see if ERP measures reflecting commonly-studied attention processes moderated the well-established association between institutional rearing and externalizing behavior. Indeed, the N2 moderated this association but not the P2 or P3, suggesting that it is the combination of extended placement in the institution and insufficient N2 activation, reflecting inadequate conflict processing, that leads to more conduct-disorder-like symptoms. Previous studies have linked N2s with activation in the anterior cingulate cortex (ACC), ventral prefrontal cortex (vPFC), and dorsolateral prefrontal cortex (DLPFC; Bocquillon, Bourriez, Palmero-soler, Molaee-Ardekani, Derambure, & Dujardin, 2014; Lamm, Walker, Degnan, Henderson, Pine, McDermott, & Fox, 2014; Lamm, White, McDermott, & Fox, 2012; Lavric, Pizzagalli, & Forstmeier, 2004; Pandey, Tang, Roopesh, Stimus, Rangaswamy, & Porjesz, 2012). Furthermore, for children who experienced severe early adversity, such as institutionalization, compared with typically-developing children, studies have found reduced ACC and prefrontal activation (Cohen, Grieve, Hoth, Paul, Sweet, Tate, et al., 2006; Escobar, Huepe, Decety, Sedeno, Messow, Baez, et al., 2014; Kim, Kim, Jin, Im, & Lee, 2018), reduced prefrontal cortical thickness (Dannlowski, Stuhrmann, Beutelman, Zwanzger, Lenzen, Grotegerd, et al., 2012; McLaughlin, Sheridan, Winter, Fox, & Nelson, 2014), and abnormal white matter connectivity (Bick, Zhu, Stamoulis, Fox, Zeanah, & Nelson, 2015; Eluvathingal, Chugani, Behen, Juhasz, Muzik, Maqbook, et al., 2006; Hanson, Adluru, Chung, Alexander, Davidson, & Pollak, 2013; Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012) and that duration of adversity may impact the severity of neural deficit (Bick, et al., 2015; Hodel, Hunt, Cowell, van den Heuvel, Gunnar, & Thomas, 2015). Additionally, deficits in these prefrontal brain regions have been associated with externalizing behavior problems (e.g., Best, Williams, & Coccaro, 2002; Lamm, Granic, Zelazo, & Lewis, 2011; Lotze, Veit, Anders, & Birbaumer, 2007; New, Buchsbaum, Hazlett, Goodman, Koenigsberg, Lo, et al., 2004; Rubia, Smith, Halari, Matsukura, Mohammad, Taylor, et al., 2009). Thus, it is likely that our moderating effect reflects the combined effects of early adversity, i.e., extended placement in the institution, and individual differences in these neural deficits. These results speak to the need to limit the duration of institutional rearing and suggest that treatment of externalizing behavior problems might incorporate training in conflict processing.
Limitations
This study had a number of limitations. First, we used only caregiver reports to establish externalizing behavior problems. However, given the impairment level of our sample, self-reporting is likely not meaningful. Second, as with most ERP studies of children, our study analyzed ERP components based on relatively low numbers of trials (mean no-go trial count was 44). We found no group differences in no-go trial count, CAUG vs. FCG: t(94) = 1.16, p = .25, CAUG M = 45.04, FCG M = 41.63; EIG vs. NIG: t(142) = −1.43, p = .15, EIG M = 43.30, NIG M = 46.81; thus, trial count should not have biased our results.
Conclusions
The current study explores the impact of adverse rearing environments on several attention mechanisms underlying the ability to inhibit a planned action. Results indicate that early institutional rearing impacts both P2 and N2 activation, reflecting attention orienting and conflict processing, and that deficits in N2 activation moderate the association between institutional rearing and antisocial behavior. Future research should explore if these attention deficits can be ameliorated with treatment.
Acknowledgments
Funding for this study was provided by the John D and Catherine T MacArthur Foundation, the Binder Family, and the National Institute of Mental Health (MH091363) to C. A. N. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or NIH.
References
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions. Sage. [Google Scholar]
- Aron AR (2007). The neural basis of inhibition in cognitive control. The Neuroscientist, 13, 1–15. doi: 10.1177/1073858407299288 [DOI] [PubMed] [Google Scholar]
- Bocquillon P, Bourriez JL, Palmero-Soler E, Molaee-Ardekani B, Derambure P, & Dujardin K (2014). The spatiotemporal dynamics of early attention processes: a high-resolution electroencephalographic study of N2 subcomponent sources. Neuroscience, 271, 9–22. [DOI] [PubMed] [Google Scholar]
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, & Gratton G (2005). Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology, 42(1), 33–42. [DOI] [PubMed] [Google Scholar]
- Best M, Williams JM, & Coccaro EF (2002). Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proceedings of the National Academy of Sciences, 99(12), 8448–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, Zhu T, Stamoulis C, Fox NA, Zeanah C, & Nelson CA (2015). A randomized clinical trial of foster care as an Intervention for early institutionalization: long term Improvements in white matter microstructure. JAMA pediatrics, 169(3), 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos KJ, Fox N Zeanah CH, & Nelson CA (2009). Effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioral Neuroscience, 3, 1–7. doi: 10.3389/neuro.08.016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P (2007). Cortical mechanisms of action selection: the affordance competition hypothesis. Philosophical Transactions of The Royal Society Biological Sciences, 362, 1585–1599. doi: 10.1098/rstb.2007.2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Grieve SG, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud L, McCaffery J, Hitsman B, Niaura R, Clark CR, MacFarlane A, Bryant R, Gordon E, & Williams LM (2006). Early life stress and morphometry of the adult andterior cingulate cortex and caudate nuclei. Biological Psychiatry, 59, 975–982. [DOI] [PubMed] [Google Scholar]
- Conners CK, Staff MHS, Connelly V, Campbell S, MacLean M, & Barnes J (2000). Conners’ continuous performance Test II (CPT II v. 5). Multi-Health Syst Inc, 29, 175–96. [Google Scholar]
- Crijnen AAM, Achenbach TM, & Verhulster FC (1997). Comparisons of problems reported by parents of children in 12 cultures: Total problems, externalizing, and internalizing. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 1269–1277. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, … & Lindner C (2012). Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological psychiatry, 71(4), 286–293. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, & Barry RJ (2006). The auditory-evoked N2 and P3 components in the stop-signal task: indices of inhibition, response-conflict or error-detection?. Brain and cognition, 62(2), 98–112. [DOI] [PubMed] [Google Scholar]
- Donchin E (1981). Surprise!… surprise?. Psychophysiology, 18(5), 493–513. [DOI] [PubMed] [Google Scholar]
- Donkers FC, & Van Boxtel GJ (2004). The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and cognition, 56(2), 165–176. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Chugani DC, & Makki M (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics, 117, 2093–2100. [DOI] [PubMed] [Google Scholar]
- Escobar MJ, Huepe D, Decety J, Sedeno L, Messow MK, Baez S, … & Schröeder J (2014). Brain signatures of moral sensitivity in adolescents with early social deprivation. Scientific reports, 4, 5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evren-Güler O, Hostinar CE, Frenn KA, Nelson CA, Gunnar MR, & Thomas KM (2012). Electrophysiological evidence of altered memory processing in children experiencing early deprivation. Developmental science, 15(3), 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, & Hohnsbein J (1999). ERP components in Go/Nogo tasks and their relation to inhibition. Acta psychologica, 101(2), 267–291. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Stoerig P, & Falkenstein M (2008). ERP—correlates of response selection in a response conflict paradigm. Brain research, 1189, 127–134. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, & Pollak SD (2013). Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development, 84, 1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA & Anllo-Vento L (1998). Event-related brain potentials in the study of visual selective attention. Proceeds of the National Academy of Sciences, 95, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, & Thomas KM (2015). Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage, 105, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SCJ, Warren AJ, de Sonneville LMJ, Swaab-Barneveld H (2008). Hot and cool forms of inhibitory control and externalizing behavior in children of mothers who smoked during pregnancy: An exploratory study. Journal of Abnormal Child Psychology, 36, 323–333. doi: 10.1007/s10802-007-9180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Gleason MM Drury, Miron D, Nelson CA, Fox NA, & Zeanah CH (2015). Effects of institutional rearing and foster care on psychopathology at age 12 years in Romania: follow-up of an open, randomized controlled trial. Lancet Psychiatry, 2, 625–634. doi: 10.1016/S2215-0366(15)00095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Plitschka J, & Kotz SA (2011). Attentional orienting towards emotion: P2 and N400 ERP effects. Neuropsychologia, 49(11), 3121–3129. [DOI] [PubMed] [Google Scholar]
- Kappenman ES & Luck SJ (2012). ERP Components: The ups and downs of brain wave recordings In Luck SJ & Kappenman ES (Eds). The Oxford Handbook of Event-Related Potential Components. Oxford University Press; Oxford, United Kingdom. [Google Scholar]
- Kim S, Kim JS, Jin MJ, Im CH, & Lee SH (2017). Dysfunctional frontal lobe activity during inhibitory tasks in individuals with childhood trauma: An event-related potential study. NeuroImage: Clinical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, & Knight RT (2009). Socioeconomic disparities affect prefrontal function in children.Journal of cognitive neuroscience, 21(6), 1106–1115. [DOI] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, McDermott JM, & Fox NA (2014). Cognitive control moderates early childhood temperament in predicting social behavior in 7‐year‐old children: an ERP study. Developmental science, 17(5), 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, White LK, McDermott JM, & Fox NA (2012). Neural activation underlying cognitive control in the context of neutral and affectively charged pictures in children. Brain and cognition, 79(3), 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli DA, & Forstmeier S (2004). When ‘go’and ‘nogo’are equally frequent: ERP components and cortical tomography. European Journal of Neuroscience, 20(9), 2483–2488. [DOI] [PubMed] [Google Scholar]
- Little R & Yau L (1996). Intent-to-treat analysis for longitudinal studies with drop-outs. Biometrics, 52, 1324–1333. [PubMed] [Google Scholar]
- Loman MM, Johnson AE, Westerlund A, Pollak SD, Nelson CA, & Gunnar MR (2013). The effect of early deprivation on executive attention in middle childhood. Journal of Child Psychology and Psychiatry,54(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Measelle JR, Ablow JC, Essex MJ, Dierker L, … & Kupfer DJ (2002). Differential performance of the MacArthur HBQ and DISC-IV in identifying DSM-IV internalizing psychopathology in young children. Journal of the American Academy of Child & Adolescent Psychiatry, 41(4), 458–466. [DOI] [PubMed] [Google Scholar]
- Maeno T, Gjini K, Iramina K, Eto F, & Ueno S (2004, August). Event-related potential P2 derived from visual attention to the hemi-space. Source localization with LORETA In International Congress Series (Vol. 1270, pp. 262–265). Elsevier. [Google Scholar]
- Mathalon DH, Whitfield SL, & Ford JM (2003). Anatomy of an error: ERP and fMRI. Biological psychology, 64(1), 119–141. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Troller-Renfree S, Vanderwert R, Nelson CA, Zeanah CH, & Fox NA (2013). Psychosocial deprivation, executive functions, and the emergence of socio-emotional behavior problems. Frontiers in Human Neuroscience, 7, 1–11. doi: 10.3389/fnhum.2013.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Westerlund A, Zeanah CH, Nelson CA, & Fox NA (2012). Early adversity and neural correlates of executive function: Implications for academic adjustment. Developmental Cognitive Neuroscience, 2S, S59–S66. doi: 10.1016/j.dcn.2011.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane A, Clark CR, Bryant RA, Williams LM, Niaura R, Paul RH, … & Gordon E (2005). The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects. Journal of integrative neuroscience,4(01), 27–40. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2014). Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to ADHD. Biological Psychiatry, 76, 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC & McCall RB (2010). Behavior problems in children adopted from psychosocially depriving institutions. Journal of Abnormal Child Psychology, 38, 459–470. doi: 10.1007/s10802-009-9383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54, 114–122. [DOI] [PubMed] [Google Scholar]
- Miller FG (2009). The randomized controlled trial as a demonstration project: An ethical perspective. American Journal of Psychiatry, 166, 743–745. [DOI] [PubMed] [Google Scholar]
- Millum J, & Emanuel EJ (2007). The ethics of international research with abandoned children. Science (New York, NY), 318(5858), 1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Fox NA, & Zeanah CH (2014). Romania’s abandoned children: deprivation, brain development, and the struggle for recovery. Harvard University Press; Cambridge, MA. [Google Scholar]
- New AS, Buchsbaum MS, Hazlett EA, Goodman M, Koenigsberg HW, Lo J, … & Siever LJ (2004). Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology, 176(3–4), 451–458. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Holroyd CB, Schurger A, & Cohen JD (2004). Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cerebral Cortex, 14(7), 741–747. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, & Ridderinkhof KR (2003). Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience, 3(1), 17–26. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Tang Y, Chorlian DB, Roopesh BN, Manz N, … & Porjesz B (2012). Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biological psychology, 89(1), 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, …Gunnar MR (2010). Neurodevelopmental effects of early deprivation in post-institutionalized children. Child Development, 81, 224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J (2012). Neuropsychology of P300. Oxford handbook of event-related potential components, 159–188. [Google Scholar]
- Rid A (2012). When is research socially valuable? Lessons from the Bucharest Early Intervention Project. The Journal of Nervous and Mental Disease, 200, 248–249. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, & Carter CS (2004). Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56, 129–140. doi: 10.1016/j.bandc.2004.09.016 [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, & Brammer MJ (2009). Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. American Journal of Psychiatry, 166(1), 83–94. [DOI] [PubMed] [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, & Klim P (1999). Confirmation of an inhibitory control deficit in attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology, 28 (3), 227–235. [DOI] [PubMed] [Google Scholar]
- Schachar R, Tannock R, Marriott M, & Logan G (1995). Deficient inhibitory control in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology, 23 (4), 411–437. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, & Zuccolotto A (2002). E-Prime: User’s guide. Psychology Software Incorporated. [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 28–38. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, & Nelson CA (2012). Variation in neural development as a result of exposure to institutionalization early n childhood. PNAS, 109, 12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SE, Sonuga-Barke EJS, Kreppner JM, Beckett C, Castle J, Colvert E, … Rutter M (2008). Inattention/overactivity following early severe institutional deprivation: Presentation and associations in early adolescence. Journal of Abnormal Child Psychology, 36, 385–398. doi: 10.1007/s10802-007-9185-5 [DOI] [PubMed] [Google Scholar]
- Tibu F, Sheridan MA, McLaughlin KA, Nelson CA, Fox NA, & Zeanah CH (2016). Disruptions of working memory and inhibition mediate the association between exposure to institutionalization and symptoms of attention deficit hyperactivity disorder. Psychological Medicine, 46, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Veit R, Anders S, & Birbaumer N (2007). Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: An interactive fMRI study. Neuroimage, 34(1), 470–478. [DOI] [PubMed] [Google Scholar]
- Troller-Renfree S, Nelson CA, Zeanah CH, & Fox NA (2016). Deficits in error monitoring are associated with externalizing but not internalizing symptoms amongst children with a history of institutionalization. Journal of Child Psychology and Psychiatry, 57, 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller–Renfree S, Zeanah CH, Nelson CA, & Fox NA (2017). Neural and Cognitive Factors Influencing the Emergence of Psychopathology: Insights From the Bucharest Early Intervention Project. Child Development Perspectives. DOI: 10.1111/cdep.12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen V, & Carter CS (2002). The timing of action-monitoring processes in the anterior cingulate cortex. Journal of cognitive neuroscience,14(4), 593–602. [DOI] [PubMed] [Google Scholar]
- Van Voorhis S, & Hillyard SA (1977). Visual evoked potentials and selective attention to points in space. Perception & Psychophysics, 22(1), 54–62. [Google Scholar]
- Wiik KL, Loman MM, Van Ryzin MJ, Armstrong JM, Essex MJ, Pollak SD, & Gunnar MR (2011). Behavioral and emotional symptoms of post-institutionalized children in middle childhood. Journal of Child Psychology and Psychiatry, 52, 56–63. doi: 10.1111/j.1469-7610.2010.02294.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, & Guthrie D (2009). Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry, 166, 777–785. doi: 10.1176/appi.ajp.2009.08091438 [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Koga SF, Simion B, Stanescu A, Tabacaru CL, Fox NA, & Nelson CA (2006). Ethical considerations in international research collaboration: the Bucharest Early Intervention Project. Infant Mental Health Journal, 27(6), 559–576. [DOI] [PubMed] [Google Scholar]
- Zeanah C, Koga S, Simion B, Stanescu A, Tabacaru C, Fox N, & Nelson CA (2006). Ethical dimensions of the BEIP: Response to commentary. Infant Mental Health J, 27, 581–583. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, & Koga S (2003). Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest early intervention project. Development and Psychopathology, 15, 885–907. doi: 10.1017/S0954579403000452 [DOI] [PubMed] [Google Scholar]