Abstract
Over the past millennium, great strides have been made in expanding the human life span. Such gains however, have come with the cost of increasing age-related diseases such as Alzheimer’s disease. Slowing “biological” aging therefore may be a way to reduce morbidity and significantly impact the quality of life for the elderly. Enhanced angiotensin II (Ang II) signal transduction has been implicated in premature aging and evidence exists for the prolongation of life through blockade of the Ang II type-1 receptor (AT1R). In this Viewpoint article, we will discuss noteworthy similarities between Ang II pathophysiology and Alzheimer’s disease as potential intervention points to promote healthy aging.
Keywords: Senescence, Protein aggregation, Autophagy, Mitochondria
Renin angiotensin aldosterone system (RAAS) has evolved as a protective endocrine system against events such as dehydration and hemorrhage. In response to reduced renal blood flow, renin secretion is enhanced to stimulate proteolytic production of angiotensin I from angiotensinogen and subsequent conversion to the peptide hormone, angiotensin II (Ang II) by angiotensin converting enzyme (ACE). Physiologically, Ang II stimulates salt and water retention and vascular contraction to maintain blood pressure via AT1R. An alternate system to counterbalance the biological activities of the RAAS has been discovered involving angiotensin (1–7) which is generated via ACE2 1. The RAAS endocrine activity was critical for the survival of our ancestors who would engage in long periods of organized hunting which enhanced the risk of dehydration. There are interesting observations that African populations have significantly higher frequency of A(−6) promoter variant of AGT, which is associated with increased risk of essential hypertension. Less frequency of this variant found in the North Eurasian population further suggests the genetic as well as geographic selection of RAAS in humans 2.
While the RAAS activity appears to have been vital for our ancestors, the role this endocrine system plays for protection in human life may no longer be relevant in modern society. Moreover, homo sapiens have nearly doubled in longevity in less than two centuries, a period basically negligible for evolutionary adjustment. In other words, our bodies were designed to optimally function under the influence of the RAAS until reproduction age. The additional ~40 years of exposure to this endocrine activity is the very period where RAAS is considered harmful. Here, we would like to discuss the molecular mechanism by which Ang II via its main receptor, AT1R, accelerates physiological aging processes, its commonalities with neurodegenerative diseases, and future research directions to further our ability to develop novel therapeutic approaches.
AT1R promotes premature aging: what is known?
Within a population of young adults of the same chronological age, a select number actually demonstrated early signs of biological aging (declined integrity in multiple organ systems). Identification of detailed molecular mechanisms leading to biological aging will provide us with new therapeutic opportunities for these individuals to protect them from premature cardiovascular and metabolic problems and effectively reduce the associated health burden. While ACE inhibitors and AT1R blockers are clinically effective in slowing the development of age-related cardiovascular and metabolic diseases, it has been difficult to dissect the mechanism of intervention as many of the protective effects include prevention of biological aging in target organs. However, accumulating evidence obtained by both experimental and clinical studies suggests that Ang II accelerates biological aging via AT1R. At the cellular level, Ang II has been shown to induce premature senescence via AT1R such as in vascular smooth muscle cells (VSMCs), which causes senescence associated secretory phenotype (SASP) and sustained inflammation 3. Mice or rats treated with ACE inhibitors or an AT1R blocker extended life span as with genetic deletion of AT1AR in mice 4. In humans, the AT1R gene promoter variant GG rs275653 is observed significantly in centenarians and is associated with longevity and lower blood pressure 5. Several key mechanisms have been demonstrated by which AT1R mediates premature cardiovascular aging. These include enhanced oxidative stress, activation of mammalian target of rapamycin/mTOR and interference on aging-protective sirtuins, Klotho and peroxisome proliferator-activated receptor-γ coactivator-1α 1. Interestingly, AT1R blockers appear protective against dementia and Alzheimer’s disease. AT1R blockers have also been shown to improve cognitive function and reduce amyloid-β accumulation in an animal model of Alzheimer’s disease 6. While these data are somewhat debatable based on conflicting studies, recent literature suggest that there is a common molecular mechanism, which underlies Ang II/AT1R-dependent enhancement of cardiovascular aging and neurodegenerative diseases in the brain.
Crossroads between Alzheimer’s and Ang II pathology: an aggregate gridlock?
Alzheimer’s disease is the most common neurodegenerative disease associated with dementia in the elderly. Various mechanisms have been implicated in the neurodegenerative pathogenesis which include DNA damage, lysosomal dysfunction, epigenetic modulation and immune dysregulation. Importantly, in Alzheimer’s diseases and other neurodegenerative diseases, the homeostasis among protein synthesis, folding, and clearance of unfolded proteins, termed proteostasis, is disturbed. This leads to an accumulation of oligomerized and aggregated proteins (extracellular amyloid-β, intracellular Tau as well as a large set of protein aggregates) that ultimately induce protein toxicity (proteotoxicity). Chronic adaptive as well as maladaptive inflammatory responses against proteotoxicity (proteinopathy) will lead to sustained synaptic degeneration. One of the cell’s defense mechanisms to protect against this proteotoxicity is a clearance system called “aggrephagy.” This term was introduced to explain the process of selective sequestration of the aggregates by the autophagy machinery through aggresome formation. Another defense mechanism is the ER’s signal transduction pathway called the unfolded protein response (UPR/UPRER). This evolutionarily conserved response is activated by the accumulating misfolded proteins causing ER stress and functions to restore cellular homeostasis. In aging, proteolytic autophagy is impaired, which activates signaling pathways to induce cellular senescence 7. Indeed, senescence and SASP phenotypes are observed in several cell types in Alzheimer’s brains, which is associated with mitochondrial dysfunction, oxidative stress and inflammation 8. In Alzheimer’s, decreased mitochondrial bioenergetic function is also associated with disturbed mitochondrial fission/fusion dynamics 9.
As expected, enhancement of ER stress is commonly observed in several neurodegenerative diseases including Alzheimer’s. In Alzheimer’s disease, the two major cellular defense mechanisms to reduce protein aggregates before and after their formation, the UPRER and aggrephagy, respectively, appear insufficient. Moreover, when mitochondrial proteostasis is further impaired, it initiates the “mitochondrial” UPR (UPRmt) which signals for a global decrease in translation to prevent an influx of newly synthesized proteins from overwhelming the mitochondrion. Likewise, mitochondrial chaperone and quality control protease genes are induced to facilitate de novo protein folding and degrade misfolded proteins. While prolonged UPRmt activity may have negative repercussions such as propagation of dangerous mitochondrial DNAs (intracellular accumulation of mtDNA-deletion mutations), UPRmt activity decreases with age and multiple studies suggest activation of UPRmt may actually enhance longevity 10. Evidence of the enhanced UPRmt has been seen in Alzheimer’s, and pharmacological as well as genetic promoters of UPRmt reduce proteotoxicity in a mouse model of Alzheimer’s 11. Mitochondria appear to be a central player for aging-associated protein aggregation as mitochondrial translational efficiency maintains proteostasis and extends life span 12.
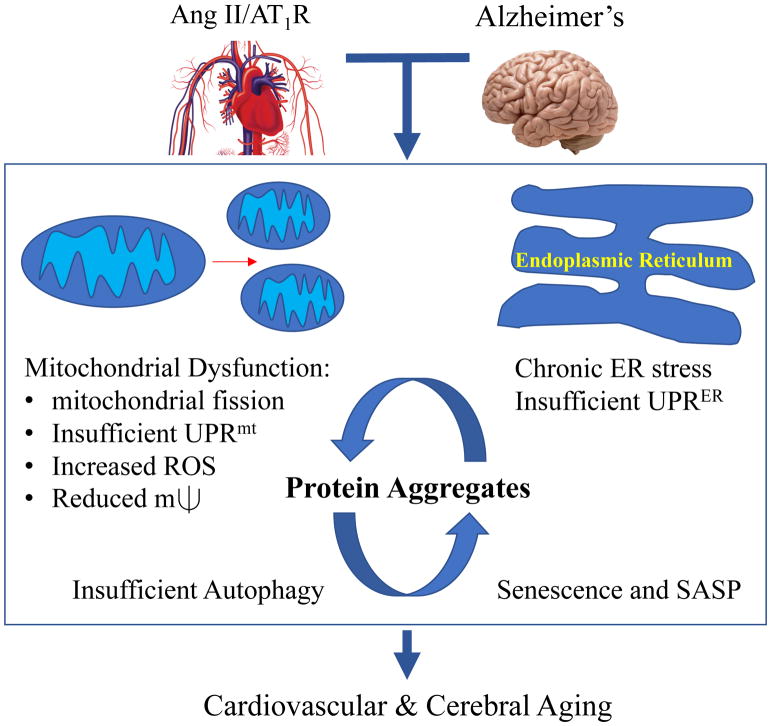
Ang II has been shown to induce mitochondrial dysfunction via AT1R in several target cells. Mechanistically, Ang II increases mitochondrial ROS, induces their fission and decreases mitochondrial membrane potential 1, 13. Ang II causes ER stress in VSMCs which is involved in many age-related cardiovascular pathologies including endothelial dysfunction, arterial stiffness and cardiovascular fibrosis 1. Given that aging-related molecular mechanisms are shared by Ang II-associated cardiovascular disease and the Alzheimer’s brain, it is not surprising that common insoluble protein aggregates are increased in both the aging heart and chronically Ang II infused heart. Moreover, these aggregates share much in common with those found in neurodegenerative diseases as well as senescent myofibroblasts 14. Despite the enhancement of protein aggregates induced by Ang II or aldosterone infusion in rat renal proximal tubules, neither formation of aggresomes nor autophagy was increased 15. However, Ang II stimulation of autophagy has also been reported in other cell/tissue types and mediates either protective or deleterious effects dependent on the experimental conditions 1. Taken together, maladaptation against intracellular protein aggregates could be a common mechanism by which premature aging is accelerated in Alzheimer’s disease as well as in the cardiovascular system associated with enhanced RAAS activity (Figure).
Figure.
Ang II associated cardiovascular disease and Alzheimer’s share aging-related molecular mechanisms including mitochondrial dysfunction and insufficient ER response to stress. These contribute to accumulating insoluble protein aggregates which are enhanced by insufficient autophagy clearance and propagation of cell senescence, and senescence associated secretory phenotype (SASP).
Research directions to prevent “Ang”heimer
Accumulating findings discussed above suggest that interventions against misfolded protein aggregates may potentially prevent age-associated cardiovascular diseases as well as neurodegenerative diseases. Multiple therapeutic points should be considered in the pathways, where specific protein synthesis is enhanced and proteostasis is impaired. For example, research in this area can focus on preventing early protein misfolding, clearing the accumulated aggregates, or enhancing the cell defense mechanisms. Potential targets may also include interventions on mitochondrial quality controls. Interventions on senescence and more realistically toward SASP could also be effective. However, many controversies and fundamental questions remain unresolved in the RAAS/cardiovascular systems. Induction of protein synthesis and hypertrophic/fibrotic responses induced by Ang II are considered highly demanding for mitochondrial bioenergetics pointing to a discrepancy in the current literature. Moreover, as seen in VSMCs and rodents treated with Ang II, protective feed-back mechanisms including UPRER, UPRmt, and autophagy appear to be limited to levels which will not interfere with protein synthesis so that hypertrophic adaptations to Ang II stimulation can be achieved. It is possible that cells maintain their UPR and autophagy at higher levels than in their quiescent condition by accumulating protein aggregates as an internal detoxification mechanism. In turn, this may help to establish proper enhancement in protein synthesis and tissue remodeling. Of course, further research is needed regarding the molecular, organellar and cellular regulation of protein aggregates enhanced by Ang II and their relations to age-related cardiovascular diseases. Ultimately, this research direction is not only significant in cardiovascular diseases but has the potential to impact therapeutic interventions across a number of aging-related diseases.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health grants HL128324 (S.E. and V.R.), HL133248 (S.E.), DK111042 (R.S. and S.E.), and American Heart Association grants 16GRNT30410007 (S.E.), 16GRNT30130013 (V.R.).
Footnotes
Disclosures
None
References
- 1.Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakajima T, Wooding S, Sakagami T, Emi M, Tokunaga K, Tamiya G, Ishigami T, Umemura S, Munkhbat B, Jin F, Guan-Jun J, Hayasaka I, Ishida T, Saitou N, Pavelka K, Lalouel JM, Jorde LB, Inoue I. Natural selection and population history in the human angiotensinogen gene (AGT): 736 complete AGT sequences in chromosomes from around the world. Am J Hum Genet. 2004;74:898–916. doi: 10.1086/420793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Monticone RE, Lakatta EG. Proinflammation of aging central arteries: a mini-review. Gerontology. 2014;60:519–529. doi: 10.1159/000362548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: how its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. Am J Physiol Heart Circ Physiol. 2015;309:H15–44. doi: 10.1152/ajpheart.00459.2014. [DOI] [PubMed] [Google Scholar]
- 5.Benigni A, Orisio S, Noris M, Iatropoulos P, Castaldi D, Kamide K, Rakugi H, Arai Y, Todeschini M, Ogliari G, Imai E, Gondo Y, Hirose N, Mari D, Remuzzi G. Variations of the angiotensin II type 1 receptor gene are associated with extreme human longevity. Age (Dordr) 2013;35:993–1005. doi: 10.1007/s11357-012-9408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saavedra JM. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin Sci (Lond) 2012;123:567–590. doi: 10.1042/CS20120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno-Blas D, Gorostieta-Salas E, Castro-Obregon S. Connecting chaperone-mediated autophagy dysfunction to cellular senescence. Ageing Res Rev. 2018;41:34–41. doi: 10.1016/j.arr.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Ovadya Y, Krizhanovsky V. Senescent cells: SASPected drivers of age-related pathologies. Biogerontology. 2014;15:627–642. doi: 10.1007/s10522-014-9529-9. [DOI] [PubMed] [Google Scholar]
- 9.Cadonic C, Sabbir MG, Albensi BC. Mechanisms of Mitochondrial Dysfunction in Alzheimer’s Disease. Mol Neurobiol. 2016;53:6078–6090. doi: 10.1007/s12035-015-9515-5. [DOI] [PubMed] [Google Scholar]
- 10.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, Moullan N, Potenza F, Schmid AW, Rietsch S, Counts SE, Auwerx J. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature. 2017;552:187–193. doi: 10.1038/nature25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suhm T, Kaimal JM, Dawitz H, Peselj C, Masser AE, Hanzen S, Ambrozic M, Smialowska A, Bjorck ML, Brzezinski P, Nystrom T, Buttner S, Andreasson C, Ott M. Mitochondrial Translation Efficiency Controls Cytoplasmic Protein Homeostasis. Cell Metab. 2018;27:1309–1322 e1306. doi: 10.1016/j.cmet.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi S, Kawai T, Scalia R, Rizzo V. Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension. 2018;71:804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayyadevara S, Mercanti F, Wang X, Mackintosh SG, Tackett AJ, Prayaga SV, Romeo F, Shmookler Reis RJ, Mehta JL. Age- and Hypertension-Associated Protein Aggregates in Mouse Heart Have Similar Proteomic Profiles. Hypertension. 2016;67:1006–1013. doi: 10.1161/HYPERTENSIONAHA.115.06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheema MU, Poulsen ET, Enghild JJ, Hoorn EJ, Fenton RA, Praetorius J. Aldosterone and angiotensin II induce protein aggregation in renal proximal tubules. Physiol Rep. 2013;1:e00064. doi: 10.1002/phy2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]