Abstract
Bioactivity-guided fractionation methods were used to identify and purify active components in Tinta Cão grape pomace extract (GPE) that inhibit intestinal α-glucosidases. One active α-glucosidase inhibitor and one new natural product determined as 6-O-(p-coumaroyl)-D-glucopyranoside and methyl 6-O-(p-coumaroyl)-β-D-galactopyranoside, respectively, were isolated from GPE that were previously shown to potently inhibit α-glucosidase. Analysis of the relationship between structures and activity suggested that C1-OH of saccharide moiety in phenolic glycosides is necessary for this potent inhibition of intestinal α-glucosidases for the potential development of a novel anti-hyperglycaemic dietary supplement.
Keywords: Red wine grapes, Anti-hyperglycaemic agents, α-Glucosidase, Diabetes, 6-O-p-trans-coumaroyl-D-glucopyranoside
1. Introduction
The worldwide prevalence of diabetes has become a massive health burden significantly decreasing quality of life and increasing morbidity and mortality, all at a huge economic cost (McAdam Marx, 2013). This alarming global rise in diabetes rates has made it necessary to explore novel approaches to prevent and control the disease. Traditional anti-hyperglycaemic agents have shown limited long-term efficacy and often come with considerable side effects (Hogan et al., 2010). The huge economic costs, inability to provide durable glycaemic control as well as the development of side effects ranging from hypoglycaemia to impaired gastrointestinal function have raised concerns regarding the use of common anti-hyperglycaemic agents, namely metformin, sulphonylureas, thiazolidinediones, GLP-1 receptor agonists, and even insulin (Charbonnel, Penfornis, Varroud-Vial, Kusnik-Joinville, & Detournay, 2012; Deacon, 2011; Dormandy et al, 2005; Inzucchi et al, 2012; Kahn et al, 2006; Majumdar & Inzucchi, 2013; Molitch, 2013). Therefore, it is crucial to develop alternative therapeutic strategies that will broaden treatment options and provide a safe and affordable substitute to currently available therapies.
In the shift from the traditional management of blood glucose, treatment of postprandial hyperglycaemia has become an intriguing target to improve overall glycaemic control (Bell, 2001; Obiro, Zhang, & Jiang, 2008; van de Laar et al., 2005; Yamagishi, Nakamura, & Takeuchi, 2005; Zhang et al., 2011). Postprandial hyperglycaemia, one of the earliest signs of type-2 diabetes, is thought to aggravate the disease by inducing glucose toxicity and β-cell function deterioration, which can ultimately give rise to an irreversible state of diabetes (Bell, 2001; Jovanovic, 1999). Since it is linked to the amount of consumed starch and its rate of digestion (Zhang et al., 2011), postprandial hyperglycaemia can be managed by controlling carbohydrate digestion and absorption (Dehghan-Kooshkghazi & Mathers, 2004; Jenkins et al., 2002; Rengasamy, Aderogba, Amoo, Stirk, & Van Staden, 2013), specifically by inhibiting digestive enzymes responsible for the break-down of starch (Chiasson et al., 2002; Kawamori et al., 2009; Mata, Cristians, Escandon-Rivera, Juarez-Reyes, & Rivero-Cruz, 2013; Rengasamy et al., 2013; Sales, Souza, Simeoni, & Silveira, 2012). α-Glucosidases play a major role in controlling starch digestion and therefore postprandial blood glucose, a target for diabetes management. The inhibition of α-glucosidases is effective in both preventing and treating type-2 diabetes through reducing postprandial hyperglycaemia (Casirola & Ferraris, 2006). However, one of the most widely used inhibitors, acarbose, chemically known as O-4,6-dideoxy4-[[(1S,4R,5S,6S)-4,5, 6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino]-α-D-glucopyranosyl-(1→4)-O-α-D-glucopyranosyl-(1→4)-D-glucose, that has been used for diabetes treatment was found to exhibit a non-specific inhibition of α-amylase, resulting in excessive accumulation of undigested carbohydrate in the colon, thus generating undesirable gastrointestinal side effects (Zhang et al., 2011).
Research to identify novel inhibitors has increased in the last three decades. We have recently found that whole grape pomace (Hogan et al., 2011), grape skin extract (Zhang et al., 2011) and grape seed extract (Zhou, Hogan, Canning, & Sun, 2012) inhibit mammalian intestinal α-glucosidase activity in vitro and/or in vivo and suppress postprandial glycaemic response in streptozocin-treated mice. Low molecular weight phenolics of grape juice and winemaking inhibited oxidation of human low-density lipoprotein cholesterol and DNA strand breakage (de Camargo, Regitano-d’Arce, Biasoto, & Shahidi, 2014). However, the components responsible for these activities are unknown, to our knowledge. The current research aims to isolate and identify these component(s), using Tinta Cão GPE as the separation material, due to its observed potent α-glucosidase inhibiting property. The results may pave the way for the future development of a natural α-glucosidase inhibitor from red wine grapes, thus establishing a novel anti-diabetic strategy.
2. Materials and methods
2.1. General
Analytical grade organic solvents were utilized for grape pomace extraction and open column chromatography, while HPLC grade methanol (Fisher Scientific, Atlanta, GA, USA) and de-ionized water from Milli-Q Integral Water Purification System (EMD Millipore, Gibbstown, NJ, USA) were used for HPLC analysis. Intestinal acetone powders from rat and 4-nitrophenyl-α-D-glucopyranoside (pNPG) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acarbose was obtained from LKT Laboratories, Inc. (St. Paul, MN, USA). HP-20 Diaion Resin Styrenic Adsorbent and silica gel 40–60 µm were purchased from Sorbent Technologies (Atlanta, GA, USA) and Acros Organics (Morris Plains, NJ, USA), respectively. TLC Silica Gel 60 F254 was acquired from EMD Millipore.
Optical rotations were recorded on a Perkin-Elmer 341 polarimeter (Perkin Elmer, Norwalk, CT, USA). Digilab Excalibur Series FTS 3000MX spectrophotometer (Digilab, Inc., Marlborough, MA, USA) was used for the measurement of IR spectrum. NMR spectra were recorded on a Varian Mercury 400 MHz instrument (Varian, Inc., Palo Alto, CA, USA). Chemical shifts are presented in δ values. J values are presented in Hz. HRESIMS of the compounds was analysed on a Waters LCT Premier High Resolution Exact Mass Spectrometer (Waters Corp., Milford, MA, USA), while EIMS analysis took place using a VG 70-250-S Mass Spectrometer (Micromass Corp., Manchester, UK).
2.2. Plant material
Fresh Tinta Cão (Vitis vinifera) grape pomace was kindly provided by Chrysalis Vineyards (Middleburg, VA, USA) via the Agricultural Research Station at Virginia State University (Petersburg, VA, USA).
2.3. Extraction and separation
Fresh grape pomace was dried in a food dehydrator at 35 °C for 28 h then ground to a powder. Two kilograms of grape pomace powder were soaked and stirred overnight 8 times (w/v) in 50% acetone and supernatants were spun then filtered via vacuum filtration using 20 µm Whatman filter paper. The filtrate was then concentrated in vacuo. The GPE aqueous concentrate was extracted with an equal volume of ethyl acetate three times with a separatory funnel, followed by extraction with an equal volume of n-butanol three times. Water, ethyl acetate and n-butanol soluble fractions were concentrated in vacuo and tested for enzyme inhibition. Column chromatography was then employed for fractionation of the active extract.
Solid phase extraction was used for scale preparation and open glass columns were packed with silica gel (normal phase), C18 (reversed-phase), Sephadex LH-20 (molecular sizing), Cyano absorbent (universal phase), Diaion HP-20 (absorbent resin), and Dowex 50 × 4–400 (ion exchange resin). These stationary phases were examined for their capacity for separation using water, acetone, methanol, ethanol, ethyl acetate and methylene chloride as eluents. The method yielding sub-fraction(s) with highest enzyme inhibition potency and potential for reproducibility was selected as the optimal fractionation method. After extensive evaluation and comparison, Diaion HP-20 open column was selected, and eluted with H2O, 30% ethanol, 50% ethanol, 70% ethanol, and 100% ethanol as eluents. Fractions were monitored on thin layer chromatography (TLC) and visualized under UV (254 nm and 365 nm) and 10% H2SO4 (in EtOH). Following enzyme inhibition screening, HPLC was used for detection and purification.
2.4. Semi-preparative HPLC purification
Potential active sub-fractions were dissolved in methanol and filtered using a 0.45 micron, 3 mm syringe filter. Reversed-phase HPLC was employed to determine purity and separate components, using a Hitachi HPLC system (Model L-2200 Autosampler, Model L-2100/2130 Pump) from Hitachi High-Tech Technologies (Tokyo, Japan). Phalanx C18, 5 µm column (4.6 × 150 mm) (Higgins Analytical, Inc. CA, USA) and VP 250/10 Nucleodur C18 Gravity, 5 µm column (Macherey-Nagel, Düren, Germany) were used for analytical separation and semi-preparative purification, respectively. Methanol and H2O were utilized as mobile phase solvents A and B, respectively. Gradient systems were used as follows: 0–3 min, 0–35% A; 3–10 min, 35% A; 10–13 min, 35–53% A; 13–16 min, 53% A; 16–20 min, 53–100% A; 20–22 min, 100% A; 22–25 min, 100–35% A; 25–28 min, 35% A. Flow rate was set at 2.5 mL/min. UV–VIS spectrum was recorded in methanol/H2O using a Hitachi DAD L-2455 Diode Array Detector. HPLC fractions were manually collected and tested for enzyme inhibition, and those corresponding to an active pure compound later underwent NMR and MS analysis for identification.
2.5. Compound characteristics
2.5.1. 6-O-p-trans-coumaroyl-D-glucopyranoside
Amorphous white powder, UV λmax 308, 275, 236, 219 nm (in MeOH/H2O); ESIMS m/z: 327 [M + H]+, 349 [M + Na]+. 1H NMR (500 MHz, CD3OD), p-coumaric acid moiety: δ7.34 (2H, d, J = 8.0 Hz, H-2, H-6), 6.81 (2H, d, J = 8.0 Hz, H-3, H-5), 7.51 (1 H, d, J = 16.0 Hz, H-7), 6.22 (1 H, dd, J = 16.0, 1.5 Hz, H-8). Glucose moiety: δ 4.98 (d, J = 3.5 Hz, H-l′α), 4.39 (d, H-1′β), 3.07–4.35 (overlapping, other sugar protons). 13C NMR (126 MHz, CD3OD), p-coumaroyl moiety: δ 169.13 (C-9, α-D), 169.23 (C-9, β-D), 161.26 (C-4), 146.75 (C-7), 131.16 (C-2); 127.09 (C-1), 116.83 (C-3); 114.95 (C-8, α-D), 114.88 (C-9, β-D); glucose moiety: 98.22 (C-1′, β-D); 74.74 (C-2′, β-D); 77.89 (C-3′, β-D); 71.72 (C-4′, β-D), 64.82 (C-6′, β-D); 76.17 (C-5′, β-D), 64.89 ((C-6′, β-D); 93.97 (C-1′, α-D); 75.42 (C-2′, α-D); 73.74 (C-3′, α-D); 70.76 (C-4′, α-D); 71.96 (C-5′, α-D); 64.69(C-6′, α-D).
2.5.2. Methyl 6-O-(p-coumaroyl)-β-D-galactopyranoside
Amorphous white powder, UV λmax 308, 275, 236, 219 nm (in MeOH/H2O); ESIMS m/z: 341 [M + H]+, 363 [M + Na]+. 1H NMR (500 MHz, CD3OD), p-coumaric acid moiety: δ7.46 (2H, d, J = 8.5 Hz, H-2, H-6), 6.81 (2H, d, J = 8.5 Hz, H-3, H-5), 7.63 (1 H, d, J = 16.0 Hz, H-7), 6.35 (1 H, dd, J = 16.0, 1.5 Hz, H-8); galactose moiety: δ 4.47 (2H, dd, J gem = 12.0, 2.0 Hz, H-6′a), 4.30 (1H, dd, J = 7.9, 6.5 Hz, H-1), 3.30–3.85 (overlapping, other sugar protons), 3.41 (3H, s, —OCH3). 13C NMR (126 MHz, CD3OD), p-coumaroyl moiety: δ 169.17 (C-9), 161.286 (C-4), 146.82 (C-7), 131.19(C-2, 6); 127.05 (C-1), 116.83 (C-3, 5); 114.83 (C-8); Galactose moiety: δ101.2(C-1′), 71.8(C-2′), 75.0(C-3′), 71.1(C-4′), 73.4(C-5′), 64.7(C-6′), and 55.59 (—OCH3).
2.6. Preparation of rat α-glucosidases
Intestinal acetone powders from rat were extracted with 0.05 M phosphate buffer (PB) pH 6.8 at a concentration of 25 mg/mL. The solution was soaked and stirred overnight at 450 rpm and supernatants were isolated and spun at 200 × g for 5 minutes, and vacuum filtered through a 20 µm Whatman filter paper. The filtered solution was frozen at −80 °C, lyophilized and reconstituted with 0.05 M PB pH 6.8 to a concentration of 25 mg/mL. Ready-to-use aliquots of this concentration were stored at −20 °C.
2.7. α-Glucosidase inhibition assay
Four millimolars of 4-nitrophenyl-α-D-glucopyranoside (pNPG) was used as a substrate while 50 µg/mL Acarbose served as a positive control. Ninety six-well bioassay microplates were prepared to contain 115 µL of GPE fraction/sub-fraction or control, 90 µL of enzyme solution and 45 µL of substrate solution per well. Absorbance was obtained at a 405 nm wavelength at the start of the reaction and following 30 minutes of incubation at 37 °C, using a Perkin Elmer HTS 7000 Bio Assay Reader and software. Per cent inhibition by tested samples was calculated using the following formula:
| (1) |
2.8. Statistical analysis
Results were analysed using one-way analysis of variance (ANOVA) and Tukey’s HSD post-hoc analyses, comparing outcomes with P < 0.05 indicating statistical significance. SPSS 22.0 for Windows (IBM Corp., Armonk, NY, USA) was utilized to perform these tests. Data for each dependent variable are reported as mean ± SEM.
3. Results and discussion
Research investigating the biological activity of plant-derived components commonly requires the isolation and characterization of bioactive compounds prior to proceeding to further evaluation (Azmir et al., 2013; Pieters & Vlietinck, 2005; Sasidharan, Chen, Saravanan, Sundram, & Yoga Latha, 2011). Grape pomace contains diverse groups of bioactive compounds, some with similar chemical properties, hence posing a challenge in our method development for separation and purification of active α-glucosidase inhibitors. The details about fractionation and isolation steps are discussed below.
3.1. Activity of GPE fractions
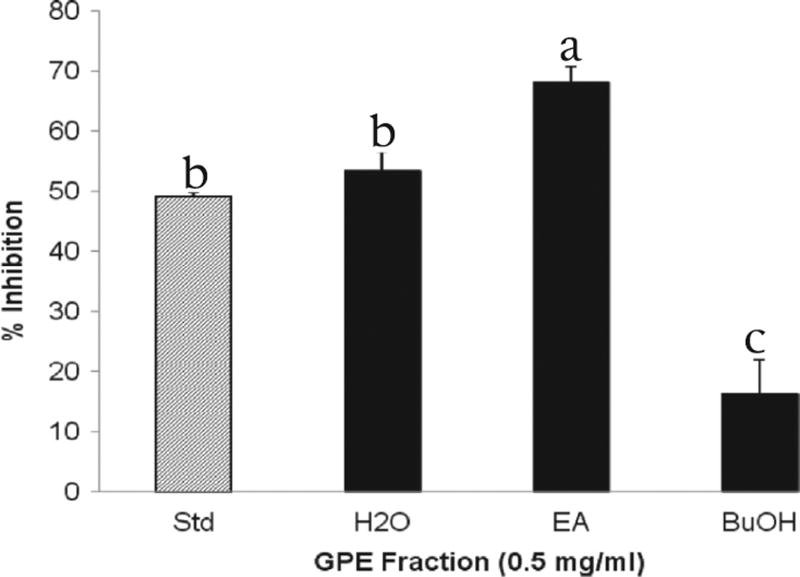
Two of the liquid–liquid extracted GPE fractions significantly suppressed rat intestinal α-glucosidase enzyme activity. Per cent enzyme inhibition by GPE fractions is presented in Fig. 1. At a concentration of 0.5 mg/mL, the ethyl acetate-soluble fraction of Tinta Cão GPE exerted the strongest inhibition of intestinal α-glucosidases, measured as 68% (P < 0.05), the water-soluble fraction exhibited a lesser yet remarkable inhibitory effect (53.4%). The ethyl acetate fraction had the highest α-glucosidase inhibitory activity but was a mixture of many compounds. However, there still were large diverse metabolites in this fraction.
Fig. 1.
Per cent α-glucosidase inhibition per GPE fraction. Enzyme activity was determined by measuring p-nitrophenol release from pNPG at 405 nm. Acarbose (50 µg/mL) is the standard and denoted as Std. H2O, water fraction. EA, ethyl acetate fraction. BuOH, butanol fraction. Bars marked with different superscripts are significantly different (p < 0.05).
3.2. Activity of GPE sub-fractions
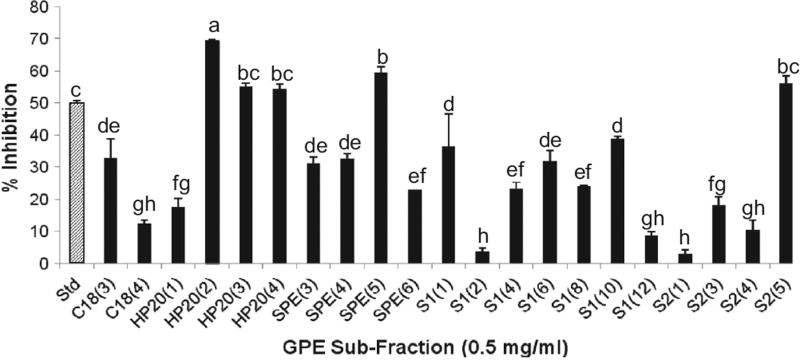
Column chromatography with HP20, C18, and silica gel, was employed to further fractionate the ethyl acetate-soluble fraction of Tinta Cão GPE. Following TLC-assisted elimination of redundant sub-fractions obtained from column separation, it was determined that 5 sub-fractions (at 0.5 mg/mL) outweighed the standard in enzyme inhibition. Fraction 2 of the HP-20 open column, eluted with 30% (v/v) ethanol, exhibited 69.82% inhibition. It was selected for further fractionation since it was significantly more active than all tested sub-fractions and the standard (P < 0.05), and it appeared more reproducible than the others. Activity of sub-fractions is summarized in Fig. 2. It is important to note that column chromatography separation may have been affected by a variety of factors namely amount of sample loaded in the column, storage time and processing time. The active sub-fractions retained were then analysed and purified by semi-preparative HPLC.
Fig. 2.
– Per cent α-glucosidase inhibition per GPE sub-fraction. Enzyme activity was determined by measuring p-nitrophenol release from pNPG at 405 nm. Acarbose (50 µg/mL) is the standard and denoted as Std. C18, HP20, SPE, S1, and S2 stand for reversed-phase C18 column, Diaion resin HP-20 column, solid phase extraction column, silica gel column, and smaller silica gel column, respectively. Bars marked with different superscripts are significantly different (p < 0.05).
3.3. Activity of pure compounds isolated from GPE
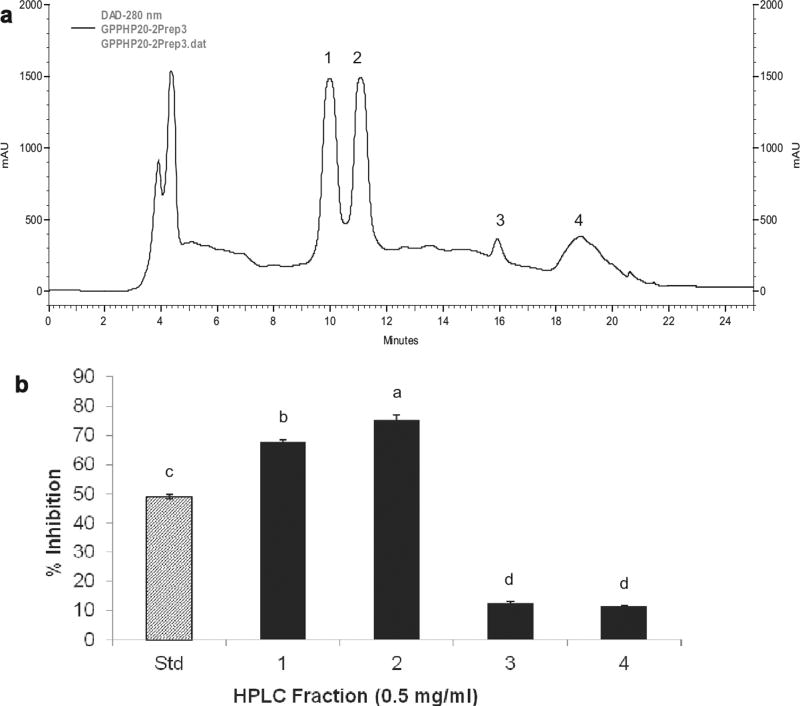
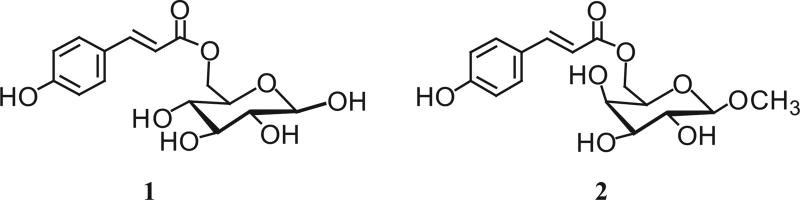
The selected active sub-fraction underwent HPLC purification revealing that it is a mixture of a small number of compounds, as shown in Fig. 3a. The numbered peaks correspond to HPLC fractions that were manually collected, bioassayed and analysed. As portrayed in Fig. 3b, upon α-glucosidase inhibition screening, it appeared that HPLC fractions 1 and 2 possessed the inhibitory activity under question, with 68 and 75% inhibition, respectively. On the other hand, no remarkable activity was observed with fractions 3 and 4. Peaks 1 and 2, isolated as purified compounds, were hence selected for chemical characterization and later identified as 6-O-p-trans-coumaroyl-D-glucopyranoside stereoisomers (Fig. 4). The exact mechanism whereby this compound inhibits α-glucosidase requires further investigation. To our knowledge, this compound was not previously reported in grapes, nor associated with the functions described in this work. The remaining active sub-fractions collected from column chromatography were also purified by semi-preparative HPLC; we have identified a number of phenolic compounds including resveratrol, catechin, ellagic acid, ferulic acid, caffeic acid, gallic acid, p-coumaric acid, cyanidin-3-glucoside, petunidin-3-glucoside, and peonidin-3-glucoside, among others, but no active compounds were isolated, likely due to the loss of activity in the process or the possibility of synergistic effects, which is also a subject of further study.
Fig. 3.
HPLC analysis and purification of GPE-derived active sub-fraction. (a) Semi-preparative HPLC chromatogram of GPE-derived active sub-fraction. (b) α-Glucosidase inhibitory activity of GPE-derived HPLC fractions. Enzyme activity was determined by measuring p-nitrophenol release from pNPG at 405 nm. Acarbose (50 µg/mL) is the standard and denoted as Std. 1, compound 1; 2, compound 2; 3, compound 3; 4, HPLC fraction 4. Bars marked with different superscripts are significantly different (p < 0.05).
Fig. 4.
Structures of 6-O-(p-coumaroyl)-D-glucopyranoside (1) and methyl 6-O-(p-coumaroyl)-β-D-galactopyranoside (2) from grape pomace.
3.4. Chemical structure elucidation
The isolated compound appeared as two peaks (1 and 2) with different retention times in the HPLC chromatogram (Fig. 3a). However, the corresponding fractions displayed identical NMR spectra, one set of signals of p-coumaric acid moiety and two sets of signals of glucose moiety in their 1H NMR and 13C NMR spectra, and the same ESIMS spectra, m/z 327 [M + H]+, and 349 [M + Na]+. The spectral data demonstrated that HPLC peaks 1 and 2 represent a stereoisomer pair of 6-O-p-trans-coumaroyl-D-glucopyranoside, with a minor difference of the ratio of α and β-configurations between peak 1 and peak 2: 1.00:1.80 and 1.00:1.04 as quantified by 13C NMR spectra, respectively. The presented chromatographic pattern and preparative results have previously represented this compound, when extracted from other plant materials such as Prunus buergeriana (Shimomura, Sashida, & Adachi, 1988), Picrorhiza scrophulariiflora (Huang, Liao, Nie, Ding, & Peng, 2004) Flacourtia indica (Amarasinghe, Jayasinghe, Hara, & Fujimoto, 2007), and Petrorhagia velutina (D’Abrosca et al., 2010). So far, no optical purity could be obtained. The review of the literature also indicates that this compound has not been previously investigated for bioactivity, particularly α-glucosidase inhibition and antioxidant capacity. A natural, food-derived compound possessing the potential for the development of an anti-hyperglycaemic supplement is a very promising future anti-diabetic strategy.
The NMR spectra of peak 3 compared to peaks 1 and 2 showed the presence of an additional —OCH3 signal, beside one set of p-coumaroyl and monosaccharide signals. 13C NMR spectra showed 101.2, 71.8, 75.0, 71.1, 73.4, and 64.7 were associated to β-D galactopyranosyl moiety. ESIMS spectra showed m/z 341 [M + H]+ and 363 [M + Na]+. All data of compound 2 correspond to the synthetic compound, methyl 6-O-(p-coumaroyl)-β-D-galactopyranoside (Helm, Ralph, & Hatfield, 1992), which is isolated from nature for the first time.
3.5. Analysis of chemical structure and inhibitory activity
The inhibition of compound 2 on α-glucosidase was significantly lower than that of compound 1, their difference contributed to saccharide moiety. Other preparative HPLC fractions and commercially available phenolic compounds reported in grapes were analysed for bioactivity, but these preparations did not show bioactivity. Acarbose’s metabolites possessed a C1-OH free also showed α-glucosidase inhibition (Chung et al., 2006; Kim et al., 1999; Wang et al., 2013), and the well-known reaction of saccharide often favours formation of the glycosidic bond with C1-OH. Comparing the structure of compound 1 to other glucosides reported, we supposed that it is necessary for α-glucosidase inhibition of phenolic glucosides to keep a C1-OH free at saccharide moiety.
4. Conclusions
The compound 1,6-O-(p-coumaroyl)-D-glucopyranoside was identified and its bioactivity assessed for the first time, particularly for its α-glucosidase inhibition. A natural, food-derived compound possessing the potential for the development of an anti-hyperglycaemic supplement is very promising as an anti-diabetic agent. Thus, Tinta Cão grape pomace is a biomass that possesses a remarkable ability to inhibit mammalian α-glucosidases. This property that appears to be derived from at least one compound, 6-O-p-trans-coumaroyl-D-glucopyranoside, isolated from the pomace of this grape variety, is an important finding.
Acknowledgments
Research reported in this publication was supported by the National Center for Complementary and Integrative Health (NCCIH, formerly the National Center for Complementary and Alternative Medicine [NCCAM]) of the National Institutes of Health under Award Number R01AT007566. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Amarasinghe NR, Jayasinghe L, Hara N, Fujimoto Y. Flacourside, a new 4-oxo-2-cyclopentenylmethyl glucoside from the fruit juice of Flacourtia indica. Food Chemistry. 2007;102(1):95–97. [Google Scholar]
- Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NA, Omar AKM. Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering. 2013;117(4):426–436. [Google Scholar]
- Bell DS. Importance of postprandial glucose control. Southern Medical Journal. 2001;94(8):804–809. [PubMed] [Google Scholar]
- Casirola DM, Ferraris RP. α-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism: Clinical and Experimental. 2006;55(6):832–841. doi: 10.1016/j.metabol.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Charbonnel B, Penfornis A, Varroud-Vial M, Kusnik-Joinville O, Detournay B. Insulin therapy for diabetes mellitus: Treatment regimens and associated costs. Diabetes & Metabolism. 2012;38(2):156–163. doi: 10.1016/j.diabet.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- Chung MJ, Lee Y-S, Kim B-C, Lee S-B, Moon T-H, Lee S-J, Park K-H. The hypoglycemic effects of acarviosine-glucose modulate hepatic and intestinal glucose transporters in vivo. Food Science and Biotechnology. 2006;15(6):851–855. [Google Scholar]
- de Camargo AC, Regitano-d’Arce MA, Biasoto AC, Shahidi F. Low molecular weight phenolics of grape juice and winemaking byproducts: Antioxidant activities and inhibition of oxidation of human low-density lipoprotein cholesterol and DNA strand breakage. Journal of Agricultural and Food Chemistry. 2014;62(50):12159–12171. doi: 10.1021/jf504185s. [DOI] [PubMed] [Google Scholar]
- D’Abrosca B, Fiorentino A, Ricci A, Scognamiglio M, Pacifico S, Piccolella S, Monaco P. Structural characterization and radical scavenging activity of monomeric and dimeric cinnamoyl glucose esters from Petrorhagia velutina leaves. Phytochemistry Letters. 2010;3(1):38–44. [Google Scholar]
- Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: A comparative review. Diabetes, Obesity and Metabolism. 2011;13(1):7–18. doi: 10.1111/j.1463-1326.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- Dehghan-Kooshkghazi M, Mathers JC. Starch digestion, large-bowel fermentation and intestinal mucosal cell proliferation in rats treated with the alpha-glucosidase inhibitor acarbose. The British Journal of Nutrition. 2004;91(3):357–365. doi: 10.1079/BJN20031063. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- Helm RF, Ralph J, Hatfield RD. Synthesis of feruloylated and p-coumaroylated methyl glycosides. Carbohydrate Research. 1992;229(1):183–194. [Google Scholar]
- Hogan S, Canning C, Sun S, Sun X, Kadouh H, Zhou K. Dietary supplementation of grape skin extract improves glycemia and inflammation in diet-induced obese mice fed a Western high fat diet. Journal of Agricultural and Food Chemistry. 2011;59(7):3035–3041. doi: 10.1021/jf1042773. [DOI] [PubMed] [Google Scholar]
- Hogan S, Zhang L, Li J, Sun S, Canning C, Zhou K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutrition and Metabolism. 2010;7:71. doi: 10.1186/1743-7075-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-X, Liao X, Nie Q-J, Ding L-S, Peng S-L. Phenyl and phenylethyl glycosides from Picrorhiza scrophulariiflora. Helvetica Chimica Acta. 2004;87(3):598–604. [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: A patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, Jenkins AL, Axelsen M. Glycemic index: Overview of implications in health and disease. The American Journal of Clinical Nutrition. 2002;76(1):266S–273S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- Jovanovic L. Rationale for prevention and treatment of postprandial glucose-mediated toxicity. The Endocrinologist. 1999;9(2):87–92. [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. New England Journal of Medicine. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K. Voglibose for prevention of type 2 diabetes mellitus: A randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009;373(9675):1607–1614. doi: 10.1016/S0140-6736(09)60222-1. [DOI] [PubMed] [Google Scholar]
- Kim M-J, Lee S-B, Lee H-S, Lee S-Y, Baek J-S, Kim D, Moon TW, Robyt JF, Park K-H. Comparative study of the inhibition of α-glucosidase, α-amylase, and cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose, and acarviosine-glucose. Archives of Biochemistry and Biophysics. 1999;371(2):277–283. doi: 10.1006/abbi.1999.1423. [DOI] [PubMed] [Google Scholar]
- Majumdar SK, Inzucchi SE. Investigational anti-hyperglycemic agents: The future of type 2 diabetes therapy? Endocrine. 2013;44(1):47–58. doi: 10.1007/s12020-013-9884-3. [DOI] [PubMed] [Google Scholar]
- Mata R, Cristians S, Escandon-Rivera S, Juarez-Reyes K, Rivero-Cruz I. Mexican antidiabetic herbs: Valuable sources of inhibitors of alpha-glucosidases. Journal of Natural Products. 2013;76(3):468–483. doi: 10.1021/np300869g. [DOI] [PubMed] [Google Scholar]
- McAdam Marx C. Economic implications of type 2 diabetes management. The American Journal of Managed Care. 2013;19(Suppl. 8):s143–s148. [PubMed] [Google Scholar]
- Molitch ME. Current state of type 2 diabetes management. The American Journal of Managed Care. 2013;19(Suppl. 8):s136–s142. [PubMed] [Google Scholar]
- Obiro WC, Zhang T, Jiang B. The nutraceutical role of the Phaseolus vulgaris alpha-amylase inhibitor. The British Journal of Nutrition. 2008;100(1):1–12. doi: 10.1017/S0007114508879135. [DOI] [PubMed] [Google Scholar]
- Pieters L, Vlietinck AJ. Bioguided isolation of pharmacologically active plant components, still a valuable strategy for the finding of new lead compounds? Journal of Ethnopharmacology. 2005;100(1–2):57–60. doi: 10.1016/j.jep.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Rengasamy KRR, Aderogba MA, Amoo SO, Stirk WA, Van Staden J. Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss. Food Chemistry. 2013;141(2):1412–1415. doi: 10.1016/j.foodchem.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Sales PM, Souza PM, Simeoni LA, Silveira D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. Journal of Pharmacy and Pharmaceutical Sciences: A Publication of the Canadian Society for Pharmaceutical Sciences, Societe Canadienne Des Sciences Pharmaceutiques. 2012;15(1):141–183. doi: 10.18433/j35s3k. [DOI] [PubMed] [Google Scholar]
- Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. African journal of traditional, complementary, and alternative medicines: AJTCAM. 2011;8(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Shimomura H, Sashida Y, Adachi T. Phenylpropanoid glucose esters from Prunus buergeriana. Phytochemistry. 1988;27(2):641–644. [Google Scholar]
- van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: Results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28(1):154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- Wang L, Cui Q, Hou Y, Bai F, Sun J, Cao X, Liu P, Jiang M, Bai G. An integrated strategy of ultra-high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry and virtual screening for the identification of α-glucosidase inhibitors in acarviostatin-containing complex. Journal of Chromatography. A. 2013;1319:88–96. doi: 10.1016/j.chroma.2013.10.035. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Nakamura K, Takeuchi M. Inhibition of postprandial hyperglycemia by acarbose is a promising therapeutic strategy for the treatment of patients with the metabolic syndrome. Medical Hypotheses. 2005;65(1):152–154. doi: 10.1016/j.mehy.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hogan S, Li JR, Sun S, Canning C, Zheng SJ, Zhou KQ. Grape skin extract inhibits mammalian intestinal alpha-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mice. Food Chemistry. 2011;126(2):466–471. [Google Scholar]
- Zhou K, Hogan S, Canning C, Sun S. Inhibition of intestinal α-glucosidases and anti-postprandial hyperglycemic effect of grape seed extract. ACS Symposium Series. 2012;1093:431–441. Emerging Trends in Dietary Components for Preventing and Combating Disease. [Google Scholar]