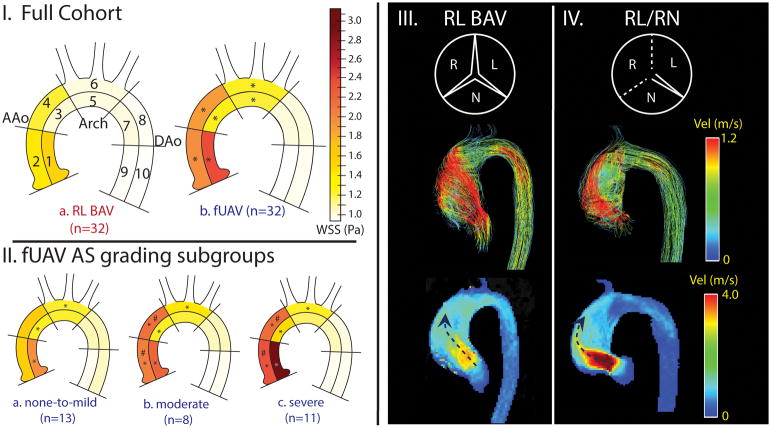
Sievers type II biscuspid aortic valve (BAV) disease is a rare functionally unicuspid variant of the congenitally abnormal BAV that has been associated with earlier onset of BAV-associated aortopathy.1 4D flow MRI studies have shown that differences in BAV valve fusion pattern (right-left (RL) vs. right-non-coronary (RN) cusp fusion) are associated with altered aortic flow and regional differences in wall shear stress (WSS), suggesting a physiologic mechanism by which valve morphology can influence aortic remodeling.2, 3 A recent 4D flow study in 571 BAV patients found that the concurrent presence of aortic stenosis (AS) can alter aortic hemodynamics and override the effects of valve phenotype.4 However, a focused analysis of aortic hemodynamics in patients with rare functionally unicuspid aortic valves (fUAV, 0.02% versus 1% overall BAV incidence) is missing from the literature. The goal of this study was to investigate if hemodynamic markers of aortic remodeling are more severely altered in patients with fUAVs than those with BAV.
4D flow MRI of the aorta was performed in 32 fUAV patients (6F/26M, age=45.9±11.8Y, mean ascending aortic (MAA) diameter =41.0±3.8mm) with four different valve-fusion patterns (three mixed-partial fusion patterns, one full fusion with RL and RN fusion). Thirty-two patients with RL-BAV matched for aorta size and age served as controls (7F/25M, age=49.2±11.9Y, MAA=41.1±3.7mm). All subjects underwent standard-of-care cardiothoracic MRI including 2D cine phase-contrast MRI to assess AS severity based on peak systolic velocity (none: <2.0m/s, mild: 2.0–2.9m/s, moderate: 3.0–3.9m/s, severe: >4.0m/s). Prospectively ECG-gated, free-breathing 4D flow scan parameters were: TE/TR=2.1–2.5/4.6–4.9ms, flip angle=15°, voxel size=(2.1–3.5)3mm3, Venc=150–450cm/s, temporal resolution=36.8–39.2ms. The study was based on retrospective inclusion of patients who underwent 4D flow MRI as part of the standard-of-care MRI protocol with Institutional Review Board of Northwestern University-approved waiver of informed consent. Deidentified data, materials and methods will be made available to other researchers for purposes of reproducing the results of replicating the procedure on reasonable request.
4D flow MRI data analysis included background phase correction, 3D segmentation of the aorta, and calculation of a systolic velocity maximum intensity projection (MIP) to visualize blood flow and quantify systolic peak velocities (PVs) in 3 regions of interest (ROIs) (Fig. 1.Ia). In addition, regional averaged maximum 2% systolic 3D wall shear stress (WSS) was calculated along the surface of the aorta in ten ROIs as described previously.5 Continuous variables were evaluated for parameter normality with a Lilliefors test, and an analysis of variance (ANOVA) or Kruskal-Wallis test was performed accordingly. In cases of an F statistic with a significance < 0.05 or comparison with only two groups, groups were compared using either a non-parametric Wilcoxon rank-sum or a two-tailed, unpaired student t-test with a Bonferroni correction.
Figure 1.
I, AAo (regions 1–4), Arch (5,6), DAo (7–10) indicate where peak velocities were quantified. Separated ROIs (1–10 indicate where WSS was quantified. I, a) RL BAV compared to the a) entire fUAV cohort; II, fUAV stenosis subgroup comparison. (*) represents significant differences compared to the RL BAV group. (#) represents subgroup differences. Note the increased WSS in the AAo and arch in all fUAV fusion and stenosis subgroups in comparison to the RL BAV patients. III,IV top: valve morphology (dotted lines represent fusion), middle: systolic streamlines, bottom: velocity MIPs. While III and IV both show eccentric valve orifices and outflow jets accompanied by complex deranged flow, the MIPs show much higher velocity outflow jets in the RL/RN fUAV patient with severe AS compared to the RL BAV patient with no AS.
The majority of fUAV patients had AS (11/32 mild, 8/32 moderate, and 11/32 severe) while most RL-BAV patients presented with normal valve function (6/32 mild, 4/32 moderate, 1 severe). WSS was significantly elevated throughout the AAo and arch in fUAV patients compared to the RL-BAV cohort (regions 1–6, 23–56% increase, p<0.01, Fig. 1.I). In addition, PV was significantly increased throughout the entire aorta in fUAV patients (AAo: 3.92±1.0m/s vs. 2.31±1.06m/s; Arch: 1.79±0.52m/s vs. 1.31±0.40m/s; DAo: 1.31±0.32m/s vs. 1.16±0.23m/s, p<0.001). Subgroup analysis of fUAV patients based on AS grading (Fig 1.II) showed significantly elevated AAo WSS and PV for moderate/severe AS compared to none-to-mild AS (WSS: region 3,4: 31–34% increase, p<0.006; PV: none-to-mild: 3.05±0.55m/s, moderate: 4.21±0.57m/s, severe: 4.72±0.88m/s, p<0.01).
fUAV patients as a whole expressed more severe markers of aortic hemodynamics than RL-BAV patients, who have already been found to have significantly increased AAo WSS and PVs compared to healthy controls.2 Our findings suggest that the more severe aortic remodeling and BAV-associated disease seen in fUAV patients may be due to the inherently more stenotic fUAV valve phenotype.
A subgroup analysis based on the four valve fusion patterns revealed that RL/RN (Fig 1.IV) was the only fusion subgroup with significantly higher WSS in the entire AAo compared to the RL-BAV group. The RL/RN subgroup was also significantly more stenotic than 2/3 other fusion subgroups, as well as the RL-BAV group (p<0.01). Within our cohort, the RL/RN fusion pattern could be considered the most pathological and inherently stenotic valve type, because it involves entire fusion of the right cusp with its neighboring cusps. Moreover, increased WSS and PV for fUAV with moderate/severe AS support prior studies in suggesting that the presence of AS can significantly alter hemodynamic markers of remodeling (WSS and PV) and mask the underlying effects of valve fusion patterns.4
The main limitation of this study was the small sample size relative to many presentations. While we controlled for aortic dimensions, AS, aortic regurgitation (AR) and fusion pattern are also intertwined in their effects on hemodynamics and on each other—only 4 fUAV patients with AR did not have concurrent AS, and small fusion subgroups made it impossible to separate these effects. Nonetheless, this 4D flow study is the first to investigate changes in aortic 3D hemodynamics in a cohort of patients with rare fUAV. The mechanism behind the early development of symptomatic valvular dysfunction and aortopathy in fUAV patients is still subject to debate. Competing mechanisms include small fUAV valve opening areas and associated AS-like symptoms at or before birth, fUAV morphology being more prone to valve calcification, fUAV morphology altering hemodynamics independently of stenosis. The true mechanism is likely an interplay of these processes. In conclusion, this study provides a starting point for evaluation of the rare Sievers type II BAV, and further supports the need to consider AS status when investigating valve-mediated aortic remodeling in all BAV subtypes.
Supplementary Material
Acknowledgments
Sources of Funding
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the NIH under award nos. F30HL137279, R01HL133504, K25HL119608, R01HL115828, and by the American Heart Association under awards 16SDG30420005 and 18POST33990451.
The authors thank Dr. Ozair Rahman from Northwestern University for providing insight and support in data analysis.
Footnotes
Disclosures
None
References
- 1.Mookadam F, Thota VR, Garcia-Lopez AM, Emani UR, Alharthi MS, Zamorano J, Khandheria BK. Unicuspid aortic valve in adults: a systematic review. The Journal of heart valve disease. 2010;19:79–85. [PubMed] [Google Scholar]
- 2.Barker AJ, Markl M, Bürk J, Lorenz R, Bock J, Bauer S, Schulz-Menger J, von Knobelsdorff-Brenkenhoff F. Bicuspid Aortic Valve Is Associated With Altered Wall Shear Stress in the Ascending Aorta. Circulation: Cardiovascular Imaging. 2012;5:457–466. doi: 10.1161/CIRCIMAGING.112.973370. [DOI] [PubMed] [Google Scholar]
- 3.Hope MD, Hope TA, Crook SE, Ordovas KG, Urbania TH, Alley MT, Higgins CB. 4D flow CMR in assessment of valve-related ascending aortic disease. JACC: Cardiovascular Imaging. 2011;4:781–787. doi: 10.1016/j.jcmg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 4.van Ooij P, Markl M, Collins JD, Carr JC, Rigsby C, Bonow RO, Malaisrie SC, McCarthy PM, Fedak PW, Barker AJ. Aortic Valve Stenosis Alters Expression of Regional Aortic Wall Shear Stress: New Insights From a 4-Dimensional Flow Magnetic Resonance Imaging Study of 571 Subjects. Journal of the American Heart Association. 2017;6:e005959. doi: 10.1161/JAHA.117.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potters WV, Ooij P, Marquering H, vanBavel E, Nederveen AJ. Volumetric arterial wall shear stress calculation based on cine phase contrast MRI. Journal of Magnetic Resonance Imaging. 2015;41:505–516. doi: 10.1002/jmri.24560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.