Abstract
The importance of sex as a biological variable is being recognized by more and more researchers, including those using Drosophila melanogaster as a model organism. Differences between the two sexes are not confined to well-known reproductive behaviors, but include other behaviors and physiological characteristics that are considered “common” to both sexes. It is possible to categorize sexual dimorphisms into “qualitative” and “quantitative” differences, and this review focuses on recent advances in elucidating genetic and neurophysiological basis of both qualitative and quantitative sex differences in Drosophila behavior. While sex-specific behaviors are often mediated by sexually dimorphic neural circuits, quantitative sexual dimorphism is caused by sex-specific modulation of a common neuronal substrate.
1. Introduction
The significance of sex as a biological variable in animal physiology and behavior (including humans) has been underappreciated at times (for recent reviews, see [1–3]). Recently, however, both basic and clinical researchers have widely recognized that the genetic, neuronal, and physiological mechanisms underlying sexual dimorphisms are important topics of research [4–6]. The common fruit fly Drosophila melanogaster offers an intriguing platform to study sex differences from genetic, neurobiological, and behavioral perspectives. Unlike in most vertebrates, sex determination in Drosophila does not involve hormones. Instead, it is mediated exclusively by a genetic cascade originating from the composition of the sex chromosomes [7]. Stereotypical and robust sex-specific differences in behavior provide both qualitative and quantitative readouts of the effects that genes and neurons exert on sexually dimorphic behavior. This review focuses on recent findings on the genetic and neurophysiological mechanisms underlying sex differences in Drosophila behavior. While most researchers use one of the two sexes in experiments, I attempt to focus on comparative studies that involve both sexes wherever possible.
2. Qualitative and quantitative sexual dimorphism in Drosophila
Many behavioral traits show sex differences. While such differences often result in a continuous spectrum of behavior, a dichotomy of ”qualitative” and “quantitative” differences can provide a useful working framework [8]. Here, I will first briefly describe qualitative sexual dimorphism in Drosophila behavior (including sex-specific behaviors), followed by a description of quantitative differences.
Like many animal species [8], the vast majority of qualitative behavioral differences between the two sexes in Drosophila are related to reproduction. Male courtship behavior is among the most intensely studied examples [7]. In response, females either accept or reject male courtship, and upon mating, begin ovipositing [9]. Anatomical constraints restrict some of these behaviors to one sex (e.g., oviposition to females). Other behaviors, such as generation of a courtship song [10], are not associated with such an obvious anatomical constraint, but are exclusively expressed by one sex in natural conditions. Some behaviors are expressed by both sexes in a comparable context, yet the accompanying motor programs have sex-specific patterns. This is the case for some actions during aggressive interactions [11].
The neuronal basis of Drosophila sexual behavior has been extensively reviewed in the past few years [7, 9, 12–14]. Two sex-determining transcription factors, doublesex (dsx) and fruitless (fru), play critical roles in specifying numerical and morphological sexual dimorphism of the neurons expressing them. Between 1,700 and 3,300 fru-expressing neurons [15, 16] and 400 – 700 dsx-expressing neurons [17–19] are found in the male central nervous system, whereas ~3,000 fru-expressing neurons [16] and 300 – 400 dsx-expressing neurons [18, 19] are found in the female central nervous system. These neurons control many, if not all, aspects of sex-specific behaviors. In the first section, I will review recent findings concerning neuronal populations at the intersection of dsx and fru. While gene structure and molecular function of dsx and fru are important topics themselves, I will defer discussion of these topics to other excellent reviews [7, 20, 21].
In contrast, much less attention has been paid to quantitative sexual dimorphisms in Drosophila behavior. Recently, however, intriguing findings are uncovering genetic and neural bases of behaviors that shows quantitative differences between males and females. In the latter sections of this review, I will specifically focus on two such behaviors: sleep, and chemosensory-guided behavior. While both males and females exhibit these behaviors, sexually dimorphic modulatory mechanisms quantitatively alter them in context-specific manners.
3. dsx, fru and sex-specific behaviors
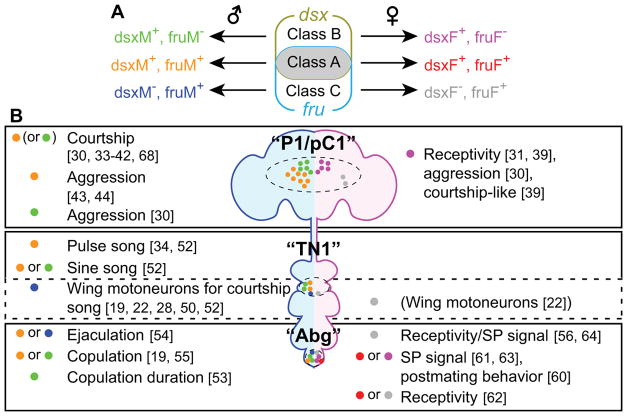
A common notion is that dsx and fru each define specific cell types, which can be further subdivided into smaller subclasses [18, 22, 23]. In particular, many classes of neurons that express sex-specific transcripts of fru are functional subunits for sexual behaviors (reviewed in [7, 9, 14]. However, one complication is that expression patterns of fru and dsx do not define mutually exclusive populations of neurons. Instead, dsx and fru create at least three distinct “cell types” (Figure 1A): (1) neurons that express both dsx and fru (called “class A” in this review), (2) neurons that express dsx only (“class B”), and (3) neurons that express fru only (“class C”). Immunohistochemistry studies have revealed only a small number of clusters of class A neurons, which are often in close proximity to class B or class C neurons [18, 24, 25]. Class A neurons are collectively necessary for male courtship behavior [26], and include three major clusters with specific functions for sexual behaviors: (1) the “P1/pC1” cluster, which is located at the mid-posterior part of the central brain, (2) the “TN1” cluster, which is located in the thoracic ganglia, and (3) the abdominal ganglion (Abg) cluster (Table 1, Figure 1B). Increasing genetic access to specific subpopulation within each cluster (Table 2) has begun to reveal that class A, class B, and class C neurons can assume distinct functions in controlling sex-specific behaviors. While most publications only refer to neurons-of-interest as “fru-expressing” or “dsx-expressing”, it would be useful to clarify which of these three types their neurons-of-interest belong to.
Figure 1. Cell types specified by dsx and fru.
A. dsx and fru expression are partially overlapping, creating three “cell types”. Since both fru and dsx produce sex-specific transcripts (fruM and fruF for fru, dsxM and dsxF for dsx), class A, class B, and class C neurons in both sexes express a unique combination of sex-specific transcripts. B. Summary of behaviors influenced by the manipulation of class A, class B, or class C neurons in “P1/pC1” (fru-expressing (P1) neurons are also referred to as pMP-e [23] or pMP4 [22])”, “TN1”, or “Abg” clusters (see text for details). Colors of dots correspond to cell types in A. Motoneurons near the TN1 cluster do not express dsx (see text for details), but are shown here in a broken line box as relevant neurons. Neurotransmitter and/or neuromodulator expression is omitted for clarity, but these details often provide further information concerning the identity of neurons.
Table 1.
Summary of cell numbers in clusters that contain class A neurons.
| “P1/pC1” | “TN1” | “Abg” | ||||
|---|---|---|---|---|---|---|
| Sex | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ |
| Overlap of dsx and fru (class A) | 18*1 [25], 34 [28] | 0*2 [31] | 9 [25], 17 [28] | N.D. | 112*3, 6 [24] | N.D. |
| dsx-expressing (class A+B) | 30–50 [17], 53 [19], 57 [18], 65 [27], 83*1 [25] | 5 [27], 8 [19], 9 [18], 16*1 [25] | 15 [25], 18–24 [17], 22 [18], 23 [19] | 0 [25], 0 [18], 0 [19] | 200–300*6 [17], 231*3, 6 [24], 254*6 [18], 275*6 [19] | 300*6 [18], 315*6 [19] |
| fru-expressing (class A+C) | 21 [29], 38 [23], 48 [27], 73 [15], 97, 177*4 [16] | 7 [27], 9 [23], 163*4 [16] | 83 [15], 105, 136 [28] 235, 441*5 [16] | 112 [28] 395*5 [16] | 91 [15], 112*3, 6 [24], 244, 504*6 [16] | 516*6 [16] |
Cell numbers (bold letters) are based on either immunohistrochemistry or genetic reagents targeting fru- or dsx-expressing neurons (such as knock-in alleles for either gene). Numbers are rounded to the closest integer. Unless otherwise noted, cell numbers are per hemibrain and counted at the adult stage. N.D., no data.
Cells include nearby “pC2” cluster.
Inferred by the absence of overlap between fru and dsx gene products.
Cells counted at two-day old pupal stage.
Cells include other neurons positioned posteriorly.
Cells include other neurons positions in pro- and meso-thoracic ganglia.
Cell numbers represent the total number from both hemibrains.
Table 2.
List of genetic reagents used to characterize functions of P1/pC1, TN1, and Abg neuronal clusters on Drosophila sexual behaviors.
| Neuronal cluster | Sex | dsx | fru | Function | Genetic reagent | Ref. | Note |
|---|---|---|---|---|---|---|---|
| (1) P1/pC1 | Male | (+) | + | Courtship | fruNP21 clone | [35] | Called P1 |
| Male | + | (+/−) | Courtship | dsxGAL4 clone | [37, 38] | Called pC1 | |
| Male | (+) | + | Courtship | NP2361 ∪ fruFLP | [30, 34, 41] | Called P1 | |
| Male | + | (+/−) | Courtship | GMR71G01-LexA ∩ dsxGAL4 | [36, 39, 40, 68] | Called P1/pC1 | |
| Male | (+) | + | Courtship | GMR71G01-LexA ∪ fruGAL4 | [36] | Called P1 | |
| Male | (+) | +/− | Courtship / aggression | P1a-GAL4 (GMR15A01AD ∪ GMR71G01DBD) | [33, 42–44, 68] | Called P1/ P1a | |
| Male | (+) | + | Aggression | GMR71G01-GAL4 ∪ fruFLP | [43, 44] | Called P1 | |
| Male | (+) | + | Aggression | GMR15A01-GAL4 ∪ fruFLP | [43] | Called P1 | |
| Male | + | +/− | Courtship / aggression | NP2361 ∪ dsxFLP | [30] | Called pC1 | |
| Male | + | − | Aggression | NP2361 ∪ dsxFLP – fruP1.LexA | [30] | Called fru-pC1 | |
| Female | + | (−) | Receptivity | GMR71G01-LexA ∪ dsxGAL4 | [31, 39] | Called pC1 | |
| Female | + | (−) | Aggression | NP2361 ∪ dsxFLP | [30] | Called pC1 | |
| Female | + | (−) | Courtship | GMR71G01-LexA ∪ dsxGAL4 | [39] | Called pC1 | |
| (2) TN1 | Male | + | + | Courtship pulse song, inter-pulse interval | [VT17258, VT5534, VT19579, VT57239, VT46099] ∪ fruFLP | [34] | vPR6 |
| Male | + | N.D. | Courtship pulse song | GMR24B02-LexA ∪ GMR41A01AD ∪ dsxDBD | [52] | TN1C: possibly the same as vPR6 | |
| Male | N.D. | + | Courtship pulse song | VT43702 ∪ fruFLP | [34] | vMS11 | |
| Male | − | + | Courtship pulse song | GMR14B02-LexA ∪ GMR21A01-GAL4 | [50] | Hg1 (motoneuron) | |
| Male | − | + | Courtship pulse song | GMR52E01-LexA | [52] | Hg1 (motoneuron) | |
| Male | + | N.D. | Courtship sine song | GMR24B02-LexA ∪ VT017258AD ∪ dsxDBD | [52] | TN1A | |
| (3) Abg | Male | N.D. | + | Ejaculation | crz-GAL4 | [54] | Cholinergic |
| Male | N.D. | (+) | Ejaculation | Tph-GAL4 | [54] | Serotonergic | |
| Male | + | − | Copulation | NP2719 | [53] | GABAergic | |
| Male | + | N.D. | Copulation | vGlutAD ∪ dsxDBD | [19] | Glutamatergic | |
| Male | + | N.D. | Copulation | GadAD ∪ dsxDBD | [19] | GABAergic | |
| Male | + | N.D. | Copulation | Trh-GAL4 ∪ dsxFLP | [55] | Serotonergic | |
| Female | N.D. | + | Copulation / receptivity | Abd-B-GAL4 ∪ fruFLP | [62] | ||
| Female | + | − | Copulation / receptivity | SAG-1-GAL4 (VT50405AD ∪ VT7068DBD), SAG-2-GAL4 (VT50405AD ∪ VT45154DBD) | [56, 64] | ||
| Female | + | N.D. | Copulation / receptivity | MIP-GAL4 ∪ dsxFLP | [60] |
3.1 “P1/pC1” cluster: a “center for sexual behaviors” with multiple functions
Within the dorsal posterior part of the fly brain is a cluster of fru-expressing neurons called “P1” neurons, and a cluster of dsx-expressing neurons called “pC1” neurons [15, 17, 27]. In the male brain, the P1 and pC1 populations of neurons overlap (i.e., some P1/pC1 neurons express both dsx and fru) [25, 28] (Table 1). In flies that lack the male-specific isoform of dsx, at least a subset of male P1 neurons is absent [29]. This implies that male P1 neurons are class A, although this has not been determined. In contrast, male pC1 neurons include class B neurons [30]. The class A P1/pC1 cluster is absent in females [29, 31]. However, in females, the neuronal lineage that generates the male P1/pC1 cluster gives rise to fru-expressing neurons that appear morphologically distinct from pC1 neurons [23, 27], suggesting that class C P1/pC1 neurons exist in females. As was discussed elsewhere [7, 9, 14, 32], this sexually dimorphic cluster plays major roles in sex-specific behaviors, both in males and females. Recent studies have uncovered a surprising degree of multifunctionality associated with this cluster.
In males, artificial activation of class A and/or class B P1/pC1 neurons consistently induces courtship behavior [33–42]. When another male is present, however, activation of a subset of presumably class A P1/pC1 neurons induces aggressive behavior [43, 44]. Activation of class B P1/pC1 neurons also induces aggression toward males without courtship behavior [30]. Curiously, Koganezawa et al. reported that presumably class A P1/pC1 neurons induce very little aggressive behavior [30]. One possible explanation for this discrepancy is that the GAL4 drivers used by the different groups label functionally different subclasses of class A P1/pC1 neurons. In fact, male class A P1/pC1 neurons can be morphologically classified into at least 10 subclasses [45].
In females, class B P1/pC1 neurons are involved in controlling receptivity to male courtship [31, 39]. Surprisingly, strong activation of these neurons in females induces male courtship-like behavior [39]. Moreover, activation of class B P1/pC1 neurons via a different GAL4 driver induces female aggressive behavior [30]. There are only ~10 class B P1/pC1 neurons in females (Table 1), and it is currently unclear whether these different types of behavioral effects are caused by specific subpopulations of these neurons. Lastly, chromosomally female flies (e.g. those carrying two copies of the X chromosome) that express a male-specific isoform of fru perform at least some form of male courtship behaviors [28, 46], even though they apparently lack class A P1/pC1 neurons [29]. It remains unclear which neurons within the central brain mediate courtship behavior in this genotype.
Although the “P1” and “pC1” nomenclatures have been used to describe neurons labeled by a variety of genetic reagents (Table 2), comparison of cell numbers (Table 1) suggest that each reagent likely labels only a subset of P1/pC1 neurons. As stated above, these subsets may define functionally distinct subpopulations that may play specific roles in sexual behaviors, or may be involved in processing specific sensory modalities. Such findings may clarify whether the P1/pC1 cluster controls multiple types of behaviors through a population coding mechanism, or through cell-type specific division of functions within this population [32]. In either case, comparison of data using different genetic reagents requires caution, even if the same terminology (such as “P1” or “pC1) is used to refer to the neurons of interest. This point also applies to other neuronal populations below.
3.2 “TN1” neurons: generating the male-specific courtship song
Male flies generate courtship song by vibrating a wing. The courtship song consists of two sound components: pulse song and sine song [7, 10]. Stimulation of dsx- and fru-expressing neurons drives the generation of courtship song even in a headless fly [47, 48]. This suggests that the neural components necessary to generate courtship song, namely motoneurons that control wing motions [49, 50] and a so-called “pattern generator” [47], reside in the ventral nerve chord.
A male-specific cluster of dsx-expressing neurons located between the pro- and meso-thoracic ganglia is named TN1 [17, 18, 25, 28]. Many fru-expressing neurons are also found in this region [15, 16, 22, 23, 51], and fru expression partially overlaps with dsx [25, 28]. This suggests that class A, class B, and class C neurons all exist in this area. Interestingly, class C neurons include motoneurons that innervate muscles involved in controlling the wings [19, 22, 28, 50]. Although the name “TN1” was originally given to dsx-expressing (class A or B) neurons, I will extend discussion to the class C neurons near the TN1 cluster in the following section.
A subset of class A TN1 neurons, named vPR6 [22], is primarily involved in producing pulse song [34, 52], whereas a morphologically distinct subset, called TN1A, primarily controls production of sine song [52]. Although the expression of fru in TN1A has not been directly determined, wiring of these neurons to the downstream motoneuron, hg1 (which controls sine song [50]), requires dsx [25, 52]. Thus, as with the P1/pC1 cluster, different subpopulations of the TN1 cluster are involved in specific aspects of a sexual behavior. Since the wing-control muscle ps1 primarily controls pulse song [50], vPR6 may synapse onto another fru-expressing motoneuron that innervates ps1 [50]. Functions of other types of TN1 neurons [28, 52] are currently unknown, although fru-expressing (class A or C) interneurons, called vMS11 (whose cell bodies reside near the TN1 cluster), influence pulse song production [34].
How females [39, 47] and chromosomally female individuals that express the male-specific isoform of fru [28, 46, 47] are capable of producing courtship song remains an open question. Because these individuals lack TN1 (Table 1), their production of male courtship song must depend on as yet uncharacterized neurons.
3.3 Abdominal ganglion (Abg) neurons: controlling copulation in both sexes
The Abg contains anatomically and functionally heterogeneous neuronal populations that are involved in coordinating copulation-related behaviors in both males and females. Both dsx and fru are expressed in numerous Abg neurons in both sexes [15–18, 22–24, 28, 51]. Although relatively poorly characterized, all class A, class B, and class C neurons seem to be present in Abg, at least in males [18, 24, 28].
In males, ~80 glutamatergic, dsx-expressing motoneurons serve to maintain copulation, whereas ~150 GABAergic, dsx-expressing neurons locally inhibit glutamatergic neurons, thereby promoting the termination of copulation [19]. Among them, ~12 GABAergic class B neurons specifically control the duration of copulation [53]. Four fru-expressing (class A or C), corazonin (crz)-expressing neurons promote ejaculation [54]. These crz+ neurons (also cholinergic) are likely upstream of a subset of ~20 male-specific, fru-expressing serotonergic neurons (called SAbg) that innervate male internal reproductive organs [24, 54]. Approximately 8 dsx-expressing, serotonergic neurons (class A or B) in this region are also necessary for successful copulation [55]. Whether these neurons overlap with the fru-expressing SAbg neurons downstream of the above-mentioned crz-expressing neurons is unclear.
Functionally diverse Abg neurons are also present in females. First, female-specific class C ascending neurons, called SAG neurons, receive input from sex peptide sensory neurons (SPSNs) located in the uterus [56]. SPSNs receive sex peptide (SP) from a male during copulation [57–59], and induce post-mating behavioral changes, including rejection of male courtship. It has been proposed that binding of SP by SPSNs reduces neuronal transmission from these neurons to the upstream SAG neurons, and that this reduced SAG neuronal activity causes post-mating behavioral changes [56]. Signaling from SPSNs to SAGs is gated by other female-specific, dsx-expressing (class A or B), local interneurons in Abg that co-express myoinhibitory peptide (MIP) [60]. dsx-expressing ascending neurons in Abg may include other types of neurons involved in female receptivity [61], but the functions of these neurons are currently uncharacterized. Lastly, fru-expressing (class A or C) Abg local interneurons labeled by Abd-B are necessary for female copulation [62], whereas dsx-expressing, octopaminergic neurons promote post-mating behavior [63]. Relationships between these neurons in a copulation-controlling circuit remains to be resolved. Further characterization of Abg neurons in both males and females will be necessary to integrate the above findings, and to assign precise functions to Abg neurons.
4. Regulation of sleep: sex-invariant behavior regulated via sex-specific mechanisms
Recent studies have begun to illuminate sex-specific modulatory mechanisms that alter the amount of sleep in both males and females (Figure 2). Well-characterized genetic and neuronal sleep mechanisms make Drosophila sleep a powerful model system for understanding the origins of this quantitative sexual dimorphism in behavior.
Figure 2. Sex-specific modulations of sleep.
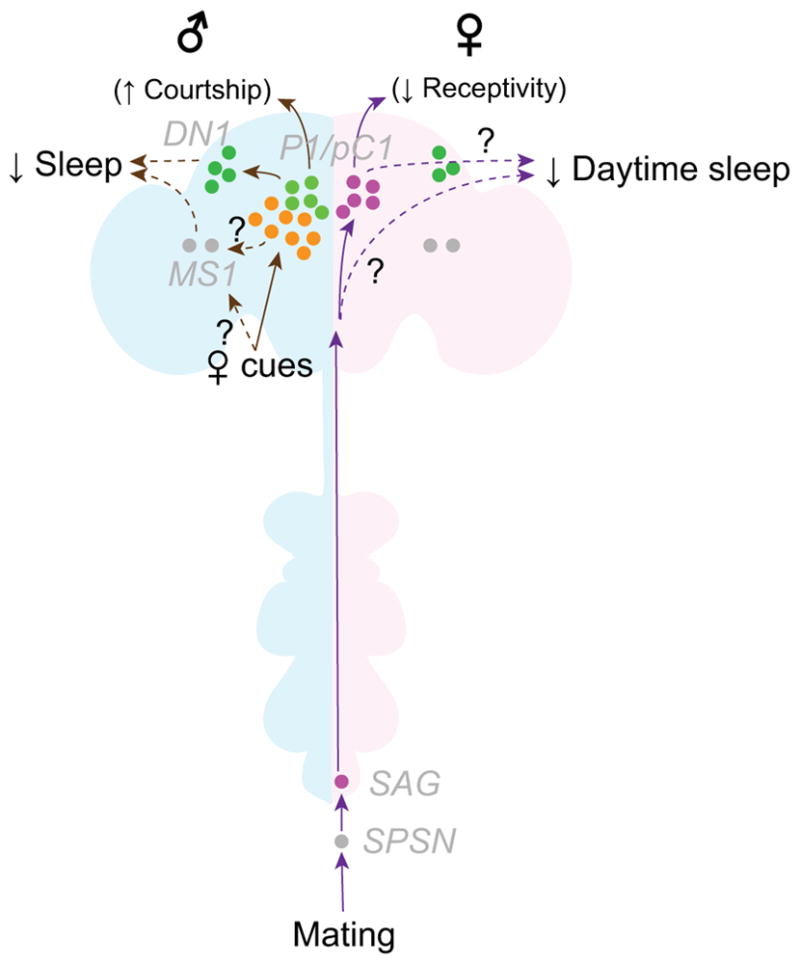
Purple arrows indicate female-specific, mating-dependent modulations of sleep, and brown arrows indicate male-specific sleep modulations in response to the presence of female cues. Gray italic letters indicate neuronal names. Solid arrows represent established neuronal connections (but do not necessarily represent monosynaptic connections); dotted arrows represent uncharacterized but potential neuronal connections. Data sources: [31, 56, 64, 65, 68, 71, 72].
While virgin and mated males sleep similar amounts, mating reduces daytime sleep in females [64–66], likely to meet demands of feeding and oviposition [66]. This may account for the longstanding observation that females are more active than males during the day [67]. Like receptivity changes described above [56], this post-mating reduction in sleep is largely mediated by the SPSN-SAG axis [64–66]. How signal from SAGs modulate the core sleep-controlling circuit is an intriguing question. One potential link is the class B pC1 neurons in females, which promote receptivity to male courtship [31]. Activation of this cluster modestly increases sleep, partially mimicking the high levels of sleep exhibited by virgin females [68].
While males do not change the amount of sleep after mating [64], males housed with females sleep significantly less than those housed with other males [69–72], and exhibit increased courtship behavior [71, 72]. Sexually satiated males sleep more than sexually inexperienced males, even in the presence of females [72]. These data suggest that male sexual arousal and sleep drive are inversely correlated. In fact, activation of the class A P1/pC1 neurons greatly reduces male sleep [68, 71, 72], even at low levels of activation that do not induce overt courtship behavior [68]. P1/pC1 neurons make mutually excitatory connections with fru-expressing circadian clock neurons, the DN1s [68, 70, 73]. DN1 neurons are sexually dimorphic in terms of numbers (males have more DN1 neurons than females) [70] and daily activity patterns [74]. Also, a group of octopaminergic neurons, called MS1 neurons, promote wakefulness in males, presumably by influencing the activity of fru-expressing neurons [72]. Thus, DN1 and MS1 neurons are the neural substrates that modulate sleep drive by affecting male sexual arousal. In particular, interactions between P1/pC1 and DN1 neurons may account for a sexually dimorphic sleep-regulating mechanism.
5. Regulation of chemosensory behavior: ethologically relevant modulations in both sexes
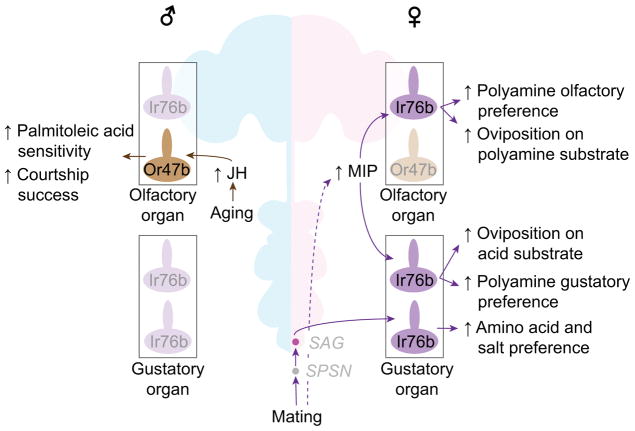
Many Drosophila pheromone receptors have been identified [14, 75, 76]. While some pheromone compounds may be differentially represented in each sex via sexually dimorphic neuronal wiring [77–81], sex-specific modulation of chemosensory inputs (pheromones or other chemicals) has generally not been investigated. In this section, I will specifically focus on recent findings that illuminate sex-specific modulation of chemosensory systems, and the resulting quantitative sexual dimorphisms in neurophysiology and behavior (Figure 3).
Figure 3. Sex-specific modulations of chemosensory-guided behaviors.
Purple arrows indicate female-specific, mating-dependent modulations of chemosensory-guided behaviors, and brown arrows indicate male-specific, age-dependent modulations. Gray italic letters indicate neuronal names. Ir76b subclasses can be further divided by the co-expression of receptors, which are omitted in this panel for clarify. Data sources: [85, 87, 88, 95]. JH: juvenile hormone.
Mating increases feeding quantity specifically in females [64, 82]. Mated females show elevated gustatory preference, relative to both virgin females and males, to amino acid-rich substrates [83–86], salt [87], and polyamines [88]. Olfactory preference to polyamines is also elevated in females after mating, to a level comparable to that shown by males [88]. All these substrates are sensed by chemosensory neurons that express the ionotropic receptor, Ir76b [89], but amino acid-sensing taste neurons, salt-sensing taste neurons, and polyamine-sensing olfactory neurons are distinct from each other [85, 89, 90]. A mutation of the sex peptide receptor (SPR) abolishes these mating-dependent changes in gustatory and olfactory preference [83, 88]. Once again, post-mating increases in salt and amino acid appetites depend on the SPSN-SAG axis [82, 87]. In contrast, post-mating changes in olfactory and gustatory preference to polyamines are mediated by SPR in the sensory neurons themselves [88]. There, MIP (the ancestral SPR ligand) serves as an autocrine signal to alter the response of Ir76b-expressing neurons to polyamines in a female-specific manner [88]. Thus, the SP system modulates multiple chemosensory-guided behaviors using distinct molecular and neuronal mechanisms.
In addition, mated females oviposit preferentially on acid- and polyamine-containing sweet substrates (more so than is seen with virgin females) [88, 91–93]. Two separate mechanisms can account for the attraction to acid-containing substrates: (1) acid- and salt-sensing Ir76b-expressing tarsal neurons [93], and (2) mechanosensory stimuli from the oviduct (caused by the physical presence of eggs) [94]. Attraction to amine-rich substrates is mediated by Ir76b-expressing olfactory sensory neurons [90]. In summary, Ir76b-expressing sensory neurons detect multiple known chemical compounds that are relevant for chemosensory-guided behaviors, and their activity is modulated in a female-specific, mating-dependent manner.
In males, age-dependent sensitization of pheromone-sensing olfactory sensory neurons elevates mating success. Seven-day old males have higher courtship success than 2-day old males, and this difference depends on the odorant receptor, Or47b [95]. Concomitantly, Or47b-expressing olfactory neurons become more sensitive to the courtship-promoting pheromone, palmitoleic acid, as male flies age [95]. This male-specific modulation is mediated by juvenile hormone, which acts directly on Or47b-expressing neurons through its receptor, methoprene-tolerant (Met) [95]. Or47b-expressing olfactory neurons project to one of a few sexually dimorphic glomeruli [16, 96]. It will be interesting to determine whether other chemosensory systems that exhibit morphological sexual dimorphism (which are often involved in detecting sex-specific compounds) [77–80] are modulated by a male-specific mechanism, and how juvenile hormone can exert such a sex-specific effect.
6. Conclusion
It appears that qualitative dimorphism in behavior is primarily controlled by fru- or dsx-expressing neurons, whereas quantitative dimorphism involves sexually dimorphic modulation of neural components that are common to both sexes. Males and females experience different types of physiological changes during life, and they must have evolved to adjust their behaviors using sexually dimorphic mechanisms.
Recent advances in our understanding of the neural circuits controlling sex-specific behaviors have been driven by a growing variety of genetic reagents (Table 2). Commonly used nomenclatures (such as “P1” or “pC1”) may not provide sufficient resolution to classify functionally relevant neuronal groups. Therefore, a comprehensive and well-defined cell type classification system that incorporates neuronal lineages [23, 27, 97, 98], morphologies [22, 23, 45, 99], connectivity maps [100–102], and gene expression profiles [103–105] across sexes will be necessary. Implementing the class A, B, and C system proposed here could be a small step toward facilitating this process. It is also important to recognize that behavioral phenotypes may become apparent only in a specific condition, and therefore, the reported behavioral phenotype may not exclude involvement of the neurons-of-interest in other types of behavior.
Quantitative sexual dimorphisms in behavior covered in this review are often modulated by sex-specific neuronal mechanisms, such as courtship-promoting P1/pC1 neurons in males, or the SPSN-SAG axis in females. It is therefore tempting to speculate that roles of currently uncharacterized sexually dimorphic neurons may include modulation of behaviors common to both sexes in a sexually dimorphic manner. At the same time, it is important to remember that sexually dimorphic, non-neuronal physiological or genetic factors (such as maternally inherited mitochondrial genes) can contribute to the expression of behaviors [106–109]. Like in studies using vertebrates, most Drosophila studies use only one sex. Comparative approaches that use both sexes, common genetic reagents, and standardized behavioral paradigms will greatly facilitate our understanding of the genetic, physiological, and neural mechanisms that create sexual dimorphism in Drosophila behavior.
Highlights.
Drosophila exhibits qualitative and quantitative sexual dimorphisms in behavior.
A class of sexually dimorphic neurons may contain multiple subclasses.
Sex differences can be generated by sex-specific neuronal modulations.
Comparative studies on physiology and behavior across sexes are informative.
Acknowledgments
The author apologizes for not being able to cite all the relevant publications due to space limit. The author thank Drs. Richard Benton, Jeff Donlea, Ilona Grunwald Kadow, and Nilay Yapici for their critical comments on the manuscript, and Dr. David O’Keefe for scientific editing of the manuscript. This work is supported by NIH grants NIGMS R35 (GM119844-01) and NIDCD R01 (DC015577-01A1) to K.A.
Abbreviation
- fru
fruitless
- dsx
doublesex
- SP
sex peptide
- SPR
sex peptide receptor
- SPSN
sex peptide sensory neuron
- crz
corazonin
- MIP
myoinhibitory peptide
Footnotes
Declaration of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jazin E, Cahill L. Sex differences in molecular neuroscience: from fruit flies to humans. Nat Rev Neurosci. 2010;11:9–17. doi: 10.1038/nrn2754. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manoli DS, Fan P, Fraser EJ, Shah NM. Neural control of sexually dimorphic behaviors. Curr Opin Neurobiol. 2013;23:330–338. doi: 10.1016/j.conb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, et al. Opinion: Sex inclusion in basic research drives discovery. Proc Natl Acad Sci U S A. 2015;112:5257–5258. doi: 10.1073/pnas.1502843112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy MM, Woolley CS, Arnold AP. Incorporating sex as a biological variable in neuroscience: what do we gain? Nat Rev Neurosci. 2017;18:707–708. doi: 10.1038/nrn.2017.137. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto D, Koganezawa M. Genes and circuits of courtship behaviour in Drosophila males. Nat Rev Neurosci. 2013;14:681–692. doi: 10.1038/nrn3567. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellendersen BE, von Philipsborn AC. Neuronal modulation of D. melanogaster sexual behaviour. Curr Opin Insect Sci. 2017;24:21–28. doi: 10.1016/j.cois.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Murthy M. Unraveling the auditory system of Drosophila. Curr Opin Neurobiol. 2010;20:281–287. doi: 10.1016/j.conb.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- 13.Pavlou HJ, Goodwin SF. Courtship behavior in Drosophila melanogaster: towards a ‘courtship connectome’. Curr Opin Neurobiol. 2013;23:76–83. doi: 10.1016/j.conb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auer TO, Benton R. Sexual circuitry in Drosophila. Curr Opin Neurobiol. 2016;38:18–26. doi: 10.1016/j.conb.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, Hall JC. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. Journal of neurobiology. 2000;43:404–426. doi: 10.1002/1097-4695(20000615)43:4<404::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Lee G, Hall JC, Park JH. Doublesex gene expression in the central nervous system of Drosophila melanogaster. J Neurogenet. 2002;16:229–248. doi: 10.1080/01677060216292. [DOI] [PubMed] [Google Scholar]
- 18.Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat Neurosci. 2010;13:458–466. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Pavlou HJ, Lin AC, Neville MC, Nojima T, Diao F, Chen BE, White BH, Goodwin SF. Neural circuitry coordinating male copulation. Elife. 2016;5:e20713. doi: 10.7554/eLife.20713. Pavlou et al. created a novel knock-in allele of dsx to systematically study dsx-expressing neurons based on neurotransmitter identities. They found that dsx-expressing glutamatergic and GABAergic neurons in the male abdominal ganglion have distinct functions: GABAergic neurons locally inhibit glutamatergic motoneurons that innervate the reproductive organ, regulating the duration of copulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billeter JC, Rideout EJ, Dornan AJ, Goodwin SF. Control of male sexual behavior in Drosophila by the sex determination pathway. Curr Biol. 2006;16:R766–776. doi: 10.1016/j.cub.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Siwicki KK, Kravitz EA. Fruitless, doublesex and the genetics of social behavior in Drosophila melanogaster. Curr Opin Neurobiol. 2009;19:200–206. doi: 10.1016/j.conb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GS. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billeter JC, Villella A, Allendorfer JB, Dornan AJ, Richardson M, Gailey DA, Goodwin SF. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr Biol. 2006;16:1063–1076. doi: 10.1016/j.cub.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Sanders LE, Arbeitman MN. Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev Biol. 2008;320:378–390. doi: 10.1016/j.ydbio.2008.05.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y, Baker BS. Genetic identification and separation of innate and experience-dependent courtship behaviors in Drosophila. Cell. 2014;156:236–248. doi: 10.1016/j.cell.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Ren Q, Awasaki T, Huang YF, Liu Z, Lee T. Cell class-lneage analysis reveals sexually dimorphic lineage compositions in the Drosophila brain. Curr Biol. 2016;26:2583–2593. doi: 10.1016/j.cub.2016.07.086. Ren et al. developed a novel genetic approach, cell class-lineage intersection (CLIn), to label specific GAL4-expressing neurons derived from each of 8 type II neural lineage. Using this approach, they demonstrated that sexual dimorphisms in the number of neurons generated, and in the number of neurons that undergo apoptosis, accounts for the sexually dimorphic number of P1/pC1 neurons. [DOI] [PubMed] [Google Scholar]
- 28.Rideout EJ, Billeter JC, Goodwin SF. The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Curr Biol. 2007;17:1473–1478. doi: 10.1016/j.cub.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and Doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 30**.Koganezawa M, Kimura K, Yamamoto D. The neural circuitry that functions as a switch for courtship versus aggression in Drosophila males. Curr Biol. 2016;26:1395–1403. doi: 10.1016/j.cub.2016.04.017. In this study, Koganezawa et al. characterized a neuronal circuit that produces aggressive and courtship behavior through double inhibitory neuronal layers, which ultimately activates either aggression- or courtship-promoting neurons. In contrast to Hoopfer et al. [43], they characterized the fru-expressing P1/pC1 cluster as exclusively courtship promoting, and class B P1/pC1 neurons as exclusively aggression promoting. Based on these data, they claim that aggression and courtship are ultimately executed by mutually exclusive populations within P1/pC1 neurons. [DOI] [PubMed] [Google Scholar]
- 31.Zhou C, Pan Y, Robinett CC, Meissner GW, Baker BS. Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron. 2014;83:149–163. doi: 10.1016/j.neuron.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 32.Anderson DJ. Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci. 2016;17:692–704. doi: 10.1038/nrn.2016.125. [DOI] [PubMed] [Google Scholar]
- 33.Inagaki HK, Jung Y, Hoopfer ED, Wong AM, Mishra N, Lin JY, Tsien RY, Anderson DJ. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat Methods. 2014;11:325–332. doi: 10.1038/nmeth.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Pan Y, Meissner GW, Baker BS. Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc Natl Acad Sci U S A. 2012;109:10065–10070. doi: 10.1073/pnas.1207107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohatsu S, Yamamoto D. Visually induced initiation of Drosophila innate courtship-like following pursuit is mediated by central excitatory state. Nat Commun. 2015;6:6457. doi: 10.1038/ncomms7457. [DOI] [PubMed] [Google Scholar]
- 38.Kimura K, Sato C, Koganezawa M, Yamamoto D. Drosophila ovipositor extension in mating behavior and egg deposition involves distinct sets of brain interneurons. PLoS One. 2015;10:e0126445. doi: 10.1371/journal.pone.0126445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Rezaval C, Pattnaik S, Pavlou HJ, Nojima T, Bruggemeier B, D’Souza LAD, Dweck HKM, Goodwin SF. Activation of latent courtship crcuitry in the brain of Drosophila females induces male-like behaviors. Curr Biol. 2016;26:2508–2515. doi: 10.1016/j.cub.2016.07.021. In this study, Rezaval et al. found that class B P1/pC1 neurons can induce male courtship-like behavior in females. This surprising phenotype, together with a previous finding that the female thoracic ganglia contains fru-expressing neurons capable of producing courtship song [47], suggests that neural circuits that mediate sex-specific behaviors may exist in both sexes, but are recruited in a sex-specific manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou C, Franconville R, Vaughan AG, Robinett CC, Jayaraman V, Baker BS. Central neural circuitry mediating courtship song perception in male Drosophila. Elife. 2015;4:e08477. doi: 10.7554/eLife.08477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bath DE, Stowers JR, Hormann D, Poehlmann A, Dickson BJ, Straw AD. FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nat Methods. 2014;11:756–762. doi: 10.1038/nmeth.2973. [DOI] [PubMed] [Google Scholar]
- 42.Kallman BR, Kim H, Scott K. Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. Elife. 2015;4:e11188. doi: 10.7554/eLife.11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Hoopfer ED, Jung Y, Inagaki HK, Rubin GM, Anderson DJ. P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. Elife. 2015;4:e11346. doi: 10.7554/eLife.11346. In this study, Hoopfer et al. conducted a behavioral screening of 3,038 GAL4 lines, and characterized a group of 8–10 P1/pC1 cluster neurons that promote aggressive and courtship behavior with distinct temporal dynamics. Based on the behavioral data from optogenetic activation of this population of neurons, they proposed a model in which different activity levels, coupled with the presence of either male or female, influence the choice of social behavior (aggression and courtship) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe K, Chiu H, Pfeiffer BD, Wong AM, Hoopfer ED, Rubin GM, Anderson DJ. A circuit node that integrates convergent input from nuromodulatory and social behavior-promoting neurons to control aggression in Drosophila. Neuron. 2017;95:1112–1128e1117. doi: 10.1016/j.neuron.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Costa M, Manton JD, Ostrovsky AD, Prohaska S, Jefferis GS. NBLAST: rapid, sensitive comparison of neuronal structure and construction of neuron family databases. Neuron. 2016;91:293–311. doi: 10.1016/j.neuron.2016.06.012. This study presents NBLAST, a novel computational approach that identifies a neuron-of-interest in the Drosophila central brain by using its neuronal morphology as a cue to search through the database of single neurons registered into a standard brain model. Among several intriguing findings presented in this article is that class A P1/pC1 neurons in the male brain can be classified into at least 10 anatomically distinct subclasses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Clyne JD, Miesenbock G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 48.Pan Y, Robinett CC, Baker BS. Turning males on: activation of male courtship behavior in Drosophila melanogaster. PLoS One. 2011;6:e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ewing AW. Neuromuscular basis of courtship song in Drosophila - role of the direct and axillary wing muscles. Journal of Comparative Physiology. 1979;130:87–93. [Google Scholar]
- 50.Shirangi TR, Stern DL, Truman JW. Motor control of Drosophila courtship song. Cell Rep. 2013;5:678–686. doi: 10.1016/j.celrep.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 52**.Shirangi TR, Wong AM, Truman JW, Stern DL. Doublesex Regulates the Connectivity of a Neural Circuit Controlling Drosophila Male Courtship Song. Dev Cell. 2016;37:533–544. doi: 10.1016/j.devcel.2016.05.012. Using high-resolution sound analysis of courtship song and sophisticated genetic reagents, they dissected TN1 neuronal cluster into anatomically and functionally distinct populations of neurons. TN1A mainly modulates sine song production, whereas TN1C modulates pulse song. TN1A synapse onto a class of motorneuron, hg1, which activates flight muscles critical for the generation of sine song. dsx is required to form a proper functional connection with hg1, demonstrating the importance of a sex-determining gene on the formation of a circuit that supports a sex-specific behavior. [DOI] [PubMed] [Google Scholar]
- 53.Crickmore MA, Vosshall LB. Opposing dopaminergic and GABAergic neurons control the duration and persistence of copulation in Drosophila. Cell. 2013;155:881–893. doi: 10.1016/j.cell.2013.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tayler TD, Pacheco DA, Hergarden AC, Murthy M, Anderson DJ. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc Natl Acad Sci U S A. 2012;109:20697–20702. doi: 10.1073/pnas.1218246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yilmazer YB, Koganezawa M, Sato K, Xu J, Yamamoto D. Serotonergic neuronal death and concomitant serotonin deficiency curb copulation ability of Drosophila platonic mutants. Nat Commun. 2016;7:13792. doi: 10.1038/ncomms13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng K, Palfreyman MT, Hasemeyer M, Talsma A, Dickson BJ. Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron. 2014;83:135–148. doi: 10.1016/j.neuron.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 58.Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–518. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61:519–526. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jang YH, Chae HS, Kim YJ. Female-specific myoinhibitory peptide neurons regulate mating receptivity in Drosophila melanogaster. Nat Commun. 2017;8:1630. doi: 10.1038/s41467-017-01794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rezaval C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, Goodwin SF. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr Biol. 2012;22:1155–1165. doi: 10.1016/j.cub.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bussell JJ, Yapici N, Zhang SX, Dickson BJ, Vosshall LB. Abdominal-B neurons control Drosophila virgin female receptivity. Curr Biol. 2014;24:1584–1595. doi: 10.1016/j.cub.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rezaval C, Nojima T, Neville MC, Lin AC, Goodwin SF. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr Biol. 2014;24:725–730. doi: 10.1016/j.cub.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Garbe DS, Vigderman AS, Moscato E, Dove AE, Vecsey CG, Kayser MS, Sehgal A. Changes in female Drosophila sleep following mating are mediated by SPSN-SAG neurons. J Biol Rhythms. 2016;31:551–567. doi: 10.1177/0748730416668048. Mating decreases sleep in females through the activity of SP. Garbe et al. determined that this effect is mediated by the SPSN-SAG axis by showing that both chronic and acute silencing of either neuron abolishes mating-dependent changes in sleep. This finding extends the role of the SPSN-SAG axis to sleep regulation. [DOI] [PubMed] [Google Scholar]
- 65.Dove AE, Cook BL, Irgebay Z, Vecsey CG. Mechanisms of sleep plasticity due to sexual experience in Drosophila melanogaster. Physiol Behav. 2017;180:146–158. doi: 10.1016/j.physbeh.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 66.Isaac RE, Li C, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc Biol Sci. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Helfrich-Forster C. Differential control of morning and evening components in the activity rhythm of Drosophila melanogaster - sex-specific differences suggest a different quality of activity. J Biol Rhythms. 2000;15:135–154. doi: 10.1177/074873040001500208. [DOI] [PubMed] [Google Scholar]
- 68*.Chen D, Sitaraman D, Chen N, Jin X, Han C, Chen J, Sun M, Baker BS, Nitabach MN, Pan Y. Genetic and neuronal mechanisms governing the sex-specific interaction between sleep and sexual behaviors in Drosophila. Nat Commun. 2017;8:154. doi: 10.1038/s41467-017-00087-5. In this study, Chen et al. showed that activation of class A P1/pC1 neurons decreases sleep in males but increases sleep in females. In males, P1/pC1 neurons and the DN1 circadian clock neurons form mutually excitatory connections, linking the levels of courtship arousal and sleep drive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujii S, Krishnan P, Hardin P, Amrein H. Nocturnal male sex drive in Drosophila. Curr Biol. 2007;17:244–251. doi: 10.1016/j.cub.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanafusa S, Kawaguchi T, Umezaki Y, Tomioka K, Yoshii T. Sexual interactions influence the molecular oscillations in DN1 pacemaker neurons in Drosophila melanogaster. PLoS One. 2013;8:e84495. doi: 10.1371/journal.pone.0084495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beckwith EJ, Geissmann Q, French AS, Gilestro GF. Regulation of sleep homeostasis by sexual arousal. Elife. 2017;6:e27445. doi: 10.7554/eLife.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Machado DR, Afonso DJ, Kenny AR, Oztu Rk-Colak A, Moscato EH, Mainwaring B, Kayser M, Koh K. Identification of octopaminergic neurons that modulate sleep suppression by male sex drive. Elife. 2017;6:e23130. doi: 10.7554/eLife.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujii S, Amrein H. Ventral lateral and DN1 clock neurons mediate distinct properties of male sex drive rhythm in Drosophila. Proc Natl Acad Sci U S A. 2010;107:10590–10595. doi: 10.1073/pnas.0912457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74**.Guo F, Yu J, Jung HJ, Abruzzi KC, Luo W, Griffith LC, Rosbash M. Circadian neuron feedback controls the Drosophila sleep--activity profile. Nature. 2016;536:292–297. doi: 10.1038/nature19097. In this study, Guo et al. characterized a sleep-promoting role of the DN1 clock neurons using functional imaging, genetics, and optogenetic manipulations. DN1s release glutamate and inhibit one class of core pacemaker cells, the E cells. Interestingly, direct measurement of DN1 activity using a genetically encoded luciferase-based reagent (CaLexA-LUC) revealed striking sexual dimorphism in the daily activity pattern of DN1 neurons, possibly accounting for sexually dimorphic sleep/activity patterns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yew JY, Chung H. Drosophila as a holistic model for insect pheromone signaling and processing. Curr Opin Insect Sci. 2017;24:15–20. doi: 10.1016/j.cois.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 76.Kohl J, Huoviala P, Jefferis GS. Pheromone processing in Drosophila. Curr Opin Neurobiol. 2015;34:149–157. doi: 10.1016/j.conb.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Starostina E, Liu T, Vijayan V, Zheng Z, Siwicki KK, Pikielny CW. A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:4665–4674. doi: 10.1523/JNEUROSCI.6178-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu B, LaMora A, Sun Y, Welsh MJ, Ben-Shahar Y. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 2012;8:e1002587. doi: 10.1371/journal.pgen.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koh TW, He Z, Gorur-Shandilya S, Menuz K, Larter NK, Stewart S, Carlson JR. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron. 2014;83:850–865. doi: 10.1016/j.neuron.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kohl J, Ostrovsky AD, Frechter S, Jefferis GS. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell. 2013;155:1610–1623. doi: 10.1016/j.cell.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the Sex Peptide of Drosophila. Curr Biol. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol. 2010;20:1000–1005. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 84.Toshima N, Tanimura T. Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. J Exp Biol. 2012;215:2827–2832. doi: 10.1242/jeb.069146. [DOI] [PubMed] [Google Scholar]
- 85.Ganguly A, Pang L, Duong VK, Lee A, Schoniger H, Varady E, Dahanukar A. A molecular and cellular context-dependent role for Ir76b in detection of amino acid taste. Cell Rep. 2017;18:737–750. doi: 10.1016/j.celrep.2016.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uchizono S, Tabuki Y, Kawaguchi N, Tanimura T, Itoh TQ. Mated Drosophila melanogaster females consume more amino acids during the dark phase. PLoS One. 2017;12:e0172886. doi: 10.1371/journal.pone.0172886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87*.Walker SJ, Corrales-Carvajal VM, Ribeiro C. Postmating circuitry modulates salt taste processing to increase reproductive output in Drosophila. Curr Biol. 2015;25:2621–2630. doi: 10.1016/j.cub.2015.08.043. In this study, Walker et al. showed that mating increases the consumption of a concentrated salt solution. This behavior change is mediated by Ir76b-expressing sensory neurons and SP. Unlike polyamine-guided behavior, female-specific changes in salt preference is mediated by the SPSN-SAG axis, providing multiple mechanisms by which females alter chemosensory-guided behaviors after mating. [DOI] [PubMed] [Google Scholar]
- 88**.Hussain A, Ucpunar HK, Zhang M, Loschek LF, Kadow ICG. Neuropeptides modulate female chemosensory processing upon mating in Drosophila. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.1002455. In this study, Hussain et al. characterized the genetic mechanism by which polyamine-guided behavior is modulated by mating specifically in females. Together with their accompanying paper [90], they showed that Ir76b-expressing neurons include polyamine-sensitive olfactory neurons that co-express Ir41a, and gustatory sensory neurons. Sensitivity to polyamine is modulated by MIP that is released from the sensory neurons themselves, through its receptor SPR. Interestingly, SPR signal increases the sensitivity of gustatory neurons to polyamine, while decreasing the sensitivity of olfactory neurons. Distinct intracellular signaling mechanism may account for the different neurophysiological effects of SPR activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang YV, Ni J, Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013;340:1334–1338. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hussain A, Zhang M, Ucpunar HK, Svensson T, Quillery E, Gompel N, Ignell R, Grunwald Kadow IC. Ionotropic Chemosensory Receptors Mediate the Taste and Smell of Polyamines. PLoS Biol. 2016;14:e1002454. doi: 10.1371/journal.pbio.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319:1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Joseph RM, Devineni AV, King IF, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc Natl Acad Sci U S A. 2009;106:11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen Y, Amrein H. Ionotropic Receptors Mediate Drosophila Oviposition Preference through Sour Gustatory Receptor Neurons. Curr Biol. 2017;27:2741–2750e2744. doi: 10.1016/j.cub.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gou B, Liu Y, Guntur AR, Stern U, Yang CH. Mechanosensitive neurons on the internal reproductive tract contribute to egg-laying-induced acetic acid attraction in Drosophila. Cell Rep. 2014;9:522–530. doi: 10.1016/j.celrep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95**.Lin HH, Cao DS, Sethi S, Zeng Z, Chin JS, Chakraborty TS, Shepherd AK, Nguyen CA, Yew JY, Su CY, et al. Hormonal modulation of pheromone detection enhances male courtship success. Neuron. 2016;90:1272–1285. doi: 10.1016/j.neuron.2016.05.004. Seven-day old male flies are more effective than 2-day old flies in courting and mating with female flies. Lin et al. demonstrated that this age-dependent courtship advantage is mediated by male-specific increase in sensitivity of Or47b-expressing olfactory sensory neurons, which detect the female pheromone palmitoleic acid, in aged males. This process is mediated by juvenile hormone, and the juvenile hormone receptor Met is necessary in Or47b-expressing neurons for this increase in sensitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kondoh Y, Kaneshiro KY, Kimura K, Yamamoto D. Evolution of sexual dimorphism in the olfactory brain of Hawaiian Drosophila. Proc Biol Sci. 2003;270:1005–1013. doi: 10.1098/rspb.2003.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu HH, Awasaki T, Schroeder MD, Long F, Yang JS, He Y, Ding P, Kao JC, Wu GY, Peng H, et al. Clonal development and organization of the adult Drosophila central brain. Curr Biol. 2013;23:633–643. doi: 10.1016/j.cub.2013.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ito M, Masuda N, Shinomiya K, Endo K, Ito K. Systematic analysis of neural projections reveals clonal composition of the Drosophila brain. Curr Biol. 2013;23:644–655. doi: 10.1016/j.cub.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 99.Chiang AS, Lin CY, Chuang CC, Chang HM, Hsieh CH, Yeh CW, Shih CT, Wu JJ, Wang GT, Chen YC, et al. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr Biol. 2011;21:1–11. doi: 10.1016/j.cub.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 100.Shih CT, Sporns O, Yuan SL, Su TS, Lin YJ, Chuang CC, Wang TY, Lo CC, Greenspan RJ, Chiang AS. Connectomics-based analysis of information flow in the Drosophila brain. Curr Biol. 2015;25:1249–1258. doi: 10.1016/j.cub.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 101.Schneider-Mizell CM, Gerhard S, Longair M, Kazimiers T, Li F, Zwart MF, Champion A, Midgley FM, Fetter RD, Saalfeld S, et al. Quantitative neuroanatomy for connectomics in Drosophila. Elife. 2016;5:e12059. doi: 10.7554/eLife.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, Milkie D, Torrens O, Price J, Fisher CB, Sharifi N, et al. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. bioRxiv. 2017 doi: 10.1101/140905. Freely accessible. [DOI] [PMC free article] [PubMed]
- 103.Li H, Horns F, Wu B, Xie Q, Li J, Li T, Luginbuhl DJ, Quake SR, Luo L. Classifying Drosophila olfactory projection neuron subtypes by single-cell RNA sequencing. Cell. 2017;171:1206–1220. doi: 10.1016/j.cell.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Croset V, Treiber CD, Waddell S. Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. bioRxiv. 2017 doi: 10.1101/237818. Freely accessible. [DOI] [PMC free article] [PubMed]
- 105.Konstantinides N, Kapuralin K, Fadil C, Barboza L, Satija R, Desplan C. Phenotypic convergence in the brain: distinct transcription factors regulate common terminal neuronal characters. bioRxiv. 2018 doi: 10.1101/243113. Freely accessible. [DOI] [PMC free article] [PubMed]
- 106.Regan JC, Khericha M, Dobson AJ, Bolukbasi E, Rattanavirotkul N, Partridge L. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. Elife. 2016;5:e10956. doi: 10.7554/eLife.10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel MR, Miriyala GK, Littleton AJ, Yang H, Trinh K, Young JM, Kennedy SR, Yamashita YM, Pallanck LJ, Malik HS. A mitochondrial DNA hypomorph of cytochrome oxidase specifically impairs male fertility in Drosophila melanogaster. Elife. 2016;5:e16923. doi: 10.7554/eLife.16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Camus MF, Wolf JB, Morrow EH, Dowling DK. Single nucleotides in the mtDNA sequence modify mitochondrial molecular function and are associated with sex-specific effects on fertility and aging. Curr Biol. 2015;25:2717–2722. doi: 10.1016/j.cub.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 109.Aw WC, Garvin MR, Melvin RG, Ballard JWO. Sex-specific influences of mtDNA mitotype and diet on mitochondrial functions and physiological traits in Drosophila melanogaster. PLoS One. 2017;12:e0187554. doi: 10.1371/journal.pone.0187554. [DOI] [PMC free article] [PubMed] [Google Scholar]