Abstract
Purpose:
Coefficients of determination (R2) for continuous longitudinal data are typically reported as time-constant, if they are reported at all. The widely used mixed model with random intercepts and slopes yields the total outcome variance as a time-varying function. We propose a generalized and intuitive approach based on this variance function to estimate the time-varying predictive power (R2) of a variable on outcome levels and changes.
Methods:
Using longitudinal estimated glomerular filtration rate (eGFR) from the Chronic Kidney Disease in Children Study, linear mixed models characterized the R2 for two chronic kidney disease (CKD) risk factors measured at baseline: a traditional marker (proteinuria) and a novel marker (fibroblast growth factor 23; FGF23).
Results:
Time-varying R2 divulged different disease processes by risk factor and diagnoses. Among children with glomerular CKD, time-varying R2 for proteinuria had significant upward trends, suggesting increasing power to predict eGFR change, but crossed with FGF23, which was higher up to 2.5 years from baseline. In contrast, among those with non-glomerular CKD, proteinuria explained more than FGF23 at all times, and time-varying R2 for each risk factor was not substantially different from time-constant estimates.
Conclusions:
Proteinuria and FGF23 explained substantial eGFR variability over time. Time-varying R2 can characterize predictive roles of risk factors on disease progression, overcome limitations of time-constant estimates and are easily derived from mixed effects models.
Keywords: epidemiologic methods, coefficient of determination, chronic kidney disease, pediatrics, glomerular filtration rate, proteinuria, fibroblast growth factor
BACKGROUND
Clinical epidemiologists are often interested in the prospective predictive power of biomarkers. Determining how much variability of an outcome is explained by the level of a predictive biomarker over time provides an important epidemiological characterization of disease progression. Several methods have been proposed that summarize predictive power by the coefficient of determination (referred to as R2) in the setting of longitudinal data: typically this is a single fixed value that describes the proportion of variability of the outcome of interest over time explained by an exposure [1–8]. However, there is a reasonable biological expectation that in some settings, the predictive power of a biomarker on an outcome is time-varying and the assumption that R2 is constant over time may not be appropriate. To our knowledge, few studies have quantified this type of dynamic relationship.
Linear mixed effects models are commonly used as conventional tools to parametrically characterize longitudinal changes of a continuous outcome and how these are modified by an exposure [9–14]. In addition to providing estimates of outcome levels and changes, the variance components serve to characterize the behavior of the outcome variance over time, enabling the calculation of R2. For the simpler case of random intercepts only, it is well known that the variance of the outcome is simply the sum of the between individuals’ variance and the within individuals’ variance. Hence, the associated R2 is time-constant [2,5,7]. In contrast, in the more common case of allowing random intercepts and slopes, the variance of the outcome is time-varying and so is the associated R2.
The purpose of the present study was to construct and compare time-fixed and time-varying R2 values in the context of pediatric chronic kidney disease (CKD) progression. Methodologically, we show cases where the time-constant R2 oversimplifies the behavior of the data and discuss the need to enrich models to ensure that the proposed model of random intercepts and slopes suffice. Epidemiologically, we aimed to compare separately and combined the baseline levels of urine protein (a marker of kidney injury [15–20]), and baseline levels of fibroblast growth factor 23 (FGF23; a bone-derived hormone and a novel CKD risk factor associated with mineral metabolism [21–23]). Urine protein is a ubiquitous clinical measurement; in contrast, FGF23 is currently not a clinical biomarker. Lastly, we sought to demonstrate how heterogeneity of effects by underlying CKD diagnoses can improve epidemiologic understanding of these conditions. This applied example seeks to highlight the utility of time-varying explained variability, the parameters of which are easily obtained in widely used linear mixed models.
METHODS
Study population
The Chronic Kidney Disease in Children (CKiD) Cohort
The CKiD study is an ongoing pediatric cohort study of CKD who were enrolled between 1 to 16 years of age at 54 pediatric nephrology centers from the United States and Canada. Eligibility included a diagnosis of CKD and an estimated glomerular filtration rate (eGFR) between 30 and 90 ml/min|1.73m2 [24] with 891 children were enrolled prior to April 2014. Briefly, clinical and demographic information, including biological specimens for immediate analysis and for repository storage, were collected at annual study visits. All biological specimens were analyzed at the CKiD central laboratory, with the exception of plasma C-terminal FGF23, which was measured using repository specimens at the University of California, San Francisco [23]. Full details of the study protocol have been previously published [25].
Outcome
At annual study visits, the primary outcome was eGFR based on the 2012 CKiD equation which utilized serum creatinine, cystatin c, blood urea nitrogen, as well as sex and height data [24]. Participants who contributed at least two visits with eGFR data were included.
Exposures
The time origin for this analysis was the first visit when the two predictors of interest were measured: proteinuria (i.e., urine protein to creatinine ratio, mgP/mgCr) and plasma FGF23 (RU/ml). This first visit was considered the baseline, or index visit and the primary objective was to determine how these two markers explained the variability of eGFR at the index visit and variability of trajectories. The inter- and-intra assay coefficients of variation (CVs) for creatinine was 2.1% and 0.8%, respectively; and for proteinuria was 3.8% and 5.2% (Roche Diagnostics, Indianapolis, IN). For FGF23, the inter- and intra-assay CVs were 11.5% and 5.7%, respectively (Immutopics Int., San Clemente, CA).
All analyses were stratified by underlying CKD diagnosis, classified as either non-glomerular (largely congenital kidney abnormalities) or glomerular (mostly non-congenital diseases) [26]. Previous studies have described the heterogeneity of disease progression by the glomerular and non-glomerular classifications [11,14,19,27–29]. In addition, those with glomerular diagnoses were enrolled with higher eGFR than children with non-glomerular CKD.
Statistical methods
Four linear mixed models (random intercepts and slopes) were fit: the null (or reference) model included only time as the independent variable; the second model included baseline proteinuria as the independent variable; the third model included baseline FGF23 as the independent variable; and the fourth model included both baseline proteinuria and FGF23 as independent variables, allowing both to modify the intercepts and the effects of time. To make the outcome closer to a Gaussian distribution, eGFR was converted to the natural log scale while proteinuria and FGF23 were log2 transformed so that the regression coefficients could be interpreted as the effect of doubling the levels of each of the predictors.
At the jth visit of the ith participant occurring tij years from baseline, the mixed models were of the form:
| [1] |
where Z = [1], [1, log2(proteinuriai0)], Z = [1, log2(FGF23i0)] , and Z = [1, log2(protenuriai0), log(FGF23i0)] for models 1 to 4, respectively, and the number of α and β coefficients for each corresponding model were 2, 4, 4 and 6.
The between-subject deviations from the population intercept and slope were distributed as:
The within-subject deviations were assumed to be independent from the between-subject deviations and distributed as:
Therefore, the total variance of eGFR (in the log scale) at t years from baseline is determined by:
| [2] |
Biomarkers as independent variables may explain (reduce) any of the components of the total variance (from the between individual variability of initial values, , and slopes, , to the within individual variance, σ2). Separate comparisons of the three components of variance may yield the inadmissible result of negative R2 and fails to incorporate the expected covariance between the random intercepts and slopes (σ12). Combining the components to describe the total variance of the outcome over time overcomes these limitations.
The R2 corresponds to the ratios of total variance from models 2 to 4 to that of model 1 (i.e., null). In particular, for the fourth model with the two biomarkers, this is formally expressed as:
| [3] |
The R2 values are the proportions of total eGFR variability explained by a risk factor(s) that is not explained by passage of time alone.
For biomarkers that only slightly modify and σ12, the R2 may be described by a constant value and conforms to the R2 from a linear mixed model with random intercepts only [5,7] (i.e., and σ12 = 0). Indeed, a mixed model with random intercepts only yields the total variance as simply . The time-constant R2 is simply one minus the ratio of the constant variances from the explanatory and null models. To determine whether the 95% confidence interval for the time-constant R2 contained the dynamic nature of the time-varying R2, bootstrap methods were used.
All analyses were performed using PROC MIXED in SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina). Graphical figures were conducted in S-PLUS version 8.2. All statistical significance was evaluated at the 5% level.
RESULTS
A total of 679 children contributed an index study visit with measurements of proteinuria and FGF23, and at least one measurement of eGFR after the index visit. Table 1 displays clinical and demographic characteristics of these 679 children stratified by glomerular (n= 186) and non-glomerular (n= 493) diagnoses. Duration of CKD at the time of study entry was much longer among those with a non-glomerular diagnosis, consistent with kidney diseases primarily caused by congenital abnormalities. Table 2 summarizes the dates of enrollment and the longitudinal eGFR data used in the analysis. Based on the study recruitment design, children with a non-glomerular diagnosis were enrolled earlier and thus, were followed longer than those with a glomerular diagnosis, who were enrolled later.
Table 1.
Baseline Characteristics of Children with Underlying Glomerular (n= 186) and Non-Glomerular (n= 493) Chronic Kidney Disease. % (N) or Median [25th percentile, 75th percentile]
| Glomerular CKD n= 186 (27%) |
Non-glomerular CKD n= 493 (73%) |
|
|---|---|---|
| Age, years | 15 [12, 17] | 11 [8, 15] |
| Female sex | 48.4% (90) | 35.1% (173) |
| Race | ||
| Caucasian | 55.9% (104) | 73.2% (361) |
| Black | 28.5% ( 53) | 16.6% ( 82) |
| Other | 15.6% ( 29) | 10.1% ( 50) |
| Hispanic ethnicity | 15.8% ( 29) | 13.9% ( 68) |
| Duration of CKD, years | 4.5 [2.5, 8.8] | 10.4 [7.2, 14.2] |
| SBP z-score | ||
| Proteinuria, mg/mgCr | 0.45 [0.20, 1.17] | 0.25 [0.10, 0.61] |
| FGF23, RU/mL | 106 [76, 169] | 112 [78, 171] |
| eGFR level, ml/min|1.73m2 | 63 [48, 82] | 52 [38, 64] |
Table 2.
Description of longitudinal GFR data available. Median [interquartile range] and % (n).
| Glomerular CKD n= 186 (27%) |
Non-glomerular CKD n= 493 (73%) |
|
|---|---|---|
| Year of study entry | September 2012 [July 2008, January 2014] |
August 2008 [August 2007, June 2012] |
| Number of eGFR measurements | ||
| 2 to 3 | 56.5% (105) | 29.8% (147) |
| 4 to 5 | 27.4% ( 51) | 34.9% (172) |
| 6 to 7 | 8.1% ( 15) | 17.4% ( 86) |
| 8 or more | 8.1% ( 15) | 17.8% ( 88) |
| Years of follow-up | 2.2 [1.2, 3.3] | 3.5 [2.2, 6.0] |
Table 3 presents the results from the four models (Table 3a for children with glomerular CKD and Table 3b for children with non-glomerular CKD). The parameter estimates for the coefficients for the fixed effects are presented in the upper portion of the table; in the lower portion of the table, the variance parameters corresponding to the random effects are presented as standard deviations and correlations.
Table 3a.
Results from Linear Mixed Effects Models for Log-Transformed eGFR with Random Intercepts and Slopes among Children with a Glomerular CKD Diagnosis.
| Model | ||||
|---|---|---|---|---|
| Parameter | Null | Proteinuria only | FGF23 only | Proteinuria and FGF23 |
| Fixed effect level | ||||
| Constant (Proteinuria= 0.45, FGF23= 110) | 4.113 ± 0.029 | 4.117 ± 0.027 | 4.14 ± 0.025 | 4.138 ± 0.024 |
| log2(Proteinuria/0.45) | 0 | −0.074 ± 0.014 | 0 | −0.039 ± 0.013 |
| log2(FGF23 / 110) | 0 | 0 | −0.219 ± 0.026 | −0.187 ± 0.027 |
| Fixed effect slope (years from baseline) | ||||
| Constant (Proteinuria= 0.45, FGF23= 110) | −0.087 ± 0.010 | −0.082 ± 0.009 | −0.082 ± 0.010 | −0.080 ± 0.009 |
| log2(Proteinuria/0.45) | 0 | −0.033 ± 0.005 | 0 | −0.029 ± 0.005 |
| log2(FGF23 / 110) | 0 | 0 | −0.044 ± 0.011 | −0.022 ± 0.010 |
| Random effects | ||||
| S.D. (level) [σ1] | 0.379 | 0.349 | 0.316 | 0.308 |
| S.D. (slope) [σ2] | 0.111 | 0.084 | 0.103 | 0.082 |
| Correlation [] | 0.377 | 0.262 | 0.221 | 0.173 |
| S.D. residual [σ] | 0.139 | 0.141 | 0.139 | 0.141 |
| AIC | 91.109 | 39.553 | 32.108 | 5.383 |
Bold indicates p<0.05 for fixed effects. Estimates ± Standard errors. Corresponding 95% confidence intervals may be calculated as ± 1.96 × Standard errors.
Abbreviations: S.D., standard deviation; SBP z-score, systolic blood pressure z-scores based on the normal population adjusted for age, gender and height; FGF23, fibroblast growth factor 23; AIC, Akaike’s information criterion
The coefficient parameters are presented in the log scale and can be re-expressed on the natural scale by taking antilogs of the parameter estimates for a more meaningful interpretation. For example, in Table 3a, the mean eGFR level at entry (approximately 4.5 years after disease onset) was 61.1 ml/min|1.73m2 (= exp(4.113)). For the slope in this model, the average change per year can be expressed as −8.3% (= (exp(−0.087))-1 × 100). The parameters for log2(Proteinuria/0.45) and log2(FGF23/110) refer to the difference in GFR levels or slope (in the log scale) corresponding to a doubling of the level of the biomarker. In Table 3a, for the proteinuria only model, children with twice the level of proteinuria as a reference group (e.g., 0.90 vs. 0.45 mgP/mgCr) would have a 7.1% lower GFR at study entry (= [exp(−0.074) – 1] × 100). Likewise, the average GFR decline for children with proteinuria levels of 0.90 mgP/mgCr is estimated as −10.8% per year (= [exp(−0.082 – 0.033) – 1] × 100) compared to children with a level of 0.45 mgP/mgCr whose decline is estimated as −7.9% (= [exp(−0.082) – 1] × 100).
Among those with glomerular CKD (Table 3a) and relative to the reference model, for the proteinuria only model, the standard deviations of levels and slope and the correlation decreased. For the FGF23 only model, the standard deviation of level decreased more than the proteinuria only model, while the standard deviation of slopes was higher than the proteinuria only model. Lastly, when both proteinuria and FGF23 were included in the model, the standard deviations were lowest for both the level and slope and the correlation was also diminished. The AIC of the model including both baseline proteinuria and FGF23 was substantially lower than each of the two models containing one predictor.
For children with non-glomerular CKD (Table 3b) and relative to those with glomerular disease, there was less variability in the random slopes, suggesting a more homogeneous behavior of disease progression. While including both biomarkers as predictors offered the best model fit by AIC; in univariate models, baseline proteinuria had more predictive value than FGF23, in contrast to those with a glomerular diagnosis.
Table 4 presents the variance parameters from random intercepts only models. The residual (or error) term variability was consistent across the null, proteinuria only, FGF23 only and proteinuria and FGF23 combined models. The standard deviations of the random intercepts decreased with the inclusion of the biomarkers for both children with glomerular and non-glomerular CKD diagnoses. For children with glomerular CKD, the FGF23 only model had lower total variance (which is equal to the sum of the between and within individuals variances; Equation 2) compared to the proteinuria only model (0.153 vs. 0.172). In contrast, for children with non-glomerular CKD, the proteinuria only model had lower total variance compared with the FGF23 only model (0.138 vs. 0.153).
Table 3b.
Results from Linear Mixed Effects Models for Log-Transformed eGFR with Random Intercepts and Slopes among Children with a Non-Glomerular CKD diagnosis.
| Model | ||||
|---|---|---|---|---|
| Parameter | Null | Proteinuria only | FGF23 only | Proteinuria and FGF23 |
| Level | ||||
| Constant (Proteinuria= 0.45, FGF23= 110) | 3.892 ± 0.017 | 3.906 ± 0.014 | 3.920 ± 0.015 | 3.926 ± 0.013 |
| log2(Proteinuria/0.45) | 0 | −0.113 ± 0.008 | 0 | −0.093 ± 0.008 |
| log2(FGF23 / 110) | 0 | 0 | −0.188 ± 0.015 | −0.149 ± 0.013 |
| Slope (years from baseline) | ||||
| Constant (Proteinuria= 0.45, FGF23= 110) | −0.051 ± 0.003 | −0.048 ± 0.003 | −0.049 ± 0.003 | −0.048 ± 0.003 |
| log2(Proteinuria/0.45) | 0 | −0.016 ± 0.002 | 0 | −0.015 ± 0.002 |
| log2(FGF23 / 110) | 0 | 0 | −0.015 ± 0.004 | −0.008 ± 0.003 |
| Random effects | ||||
| S.D. (level) [σ1] | 0.368 | 0.310 | 0.317 | 0.274 |
| S.D. (slope) [σ2] | 0.061 | 0.054 | 0.060 | 0.054 |
| Correlation [] | 0.477 | 0.310 | 0.427 | 0.283 |
| S.D. residual [σ] | 0.111 | 0.111 | 0.111 | 0.111 |
| AIC | −1239.604 | −1418.868 | −1363.118 | −1513.796 |
Bold indicates p<0.05 for fixed effects. Estimates ± Standard errors. Corresponding 95% confidence intervals may be calculated as ±1.96 × Standard errors.
Abbreviations: S.D., standard deviation; FGF23, fibroblast growth factor 23; AIC, Akaike’s information criterion.
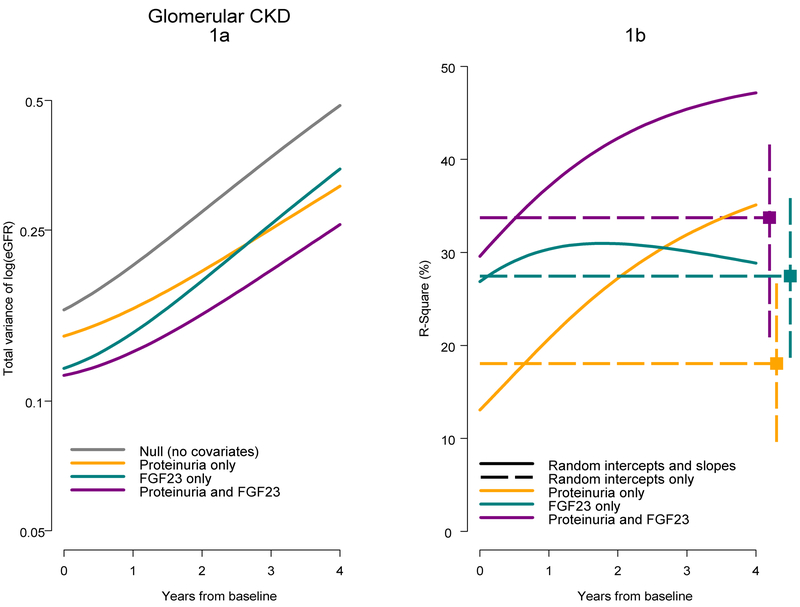
For children with glomerular CKD diagnoses and using Equation 2 (Methods), Figure 1a presents the total variance of eGFR (in the log scale) as a function of the model parameters of variability and correlation in Table 2a with time 0 corresponding to about 4.5 years after disease onset. When comparing the proteinuria only and FGF23 only models, the lines cross at about 2.5 years. In the combined model, the estimated total variance was lowest and incorporated the short-term explanatory power of FGF23 with the longer-term lower variability related to proteinuria. Figure 1b presents the R2 over time based on the estimates of variance in Figure 1a and Equation 3. The R2 for the FGF23 only model was relatively stable over time, while the R2 for the proteinuria only model increased from about 13% at baseline to 35% by 4 years after baseline. The R2 for the proteinuria only model surpassed the R2 for the FGF23 only model at about 2.7 years. This underscores the influence of baseline proteinuria on longer term levels of eGFR compared to the stronger short-term influences of FGF23. For the combined model, the baseline R2 was close to the R2 from the FGF23 only model (30% vs. 27%) and the shape of this function was more similar to the R2 from the proteinuria only model. At 4 years, the R2 from this combined model was 47%.
Figure 1.
Total variance and R-square as a function of time for mixed effects models for children with a glomerular CKD diagnosis. Figure 1a presents estimates of total eGFR variance for four mixed models in Table 2a. Figure 1b presents time-varying R-square values for mixed models (random intercepts and slopes; Table 2a) and time-constant R-square values based on mixed models with random intercepts only in Table 3.
The points on the right-most part of Figure 1b, depict the R2 derived from the parameters described in Table 3 based on the models with random intercepts only. Dashed horizontal lines reflect the assumption of constant R2 over time. Bootstrapped 95% confidence intervals are depicted by the vertical bounds. For FGF23, the estimated time-constant R2 was very similar to the time-varying R2 for the same model. However, for the proteinuria only and combined models, the time-constant R2 values were substantially different from the time-varying R2 values.
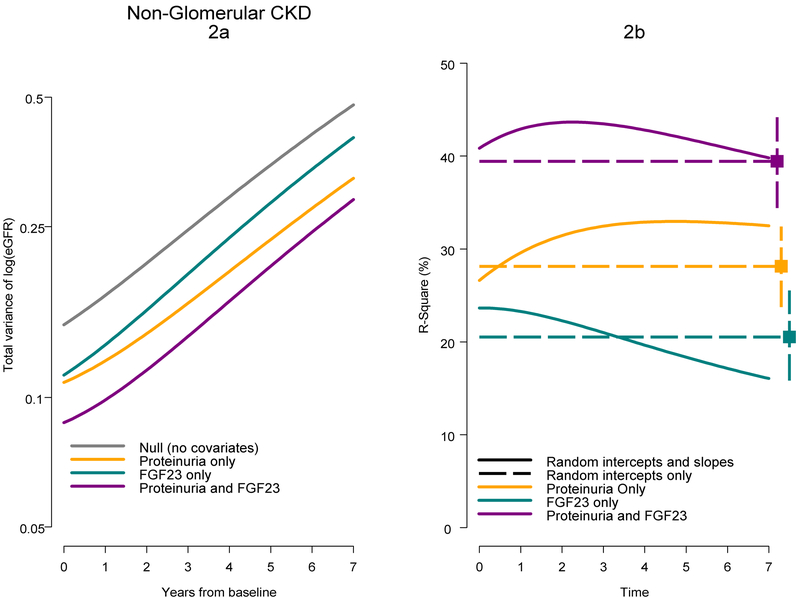
Figure 2 presents the corresponding total variance and R2 over time for children with a non-glomerular CKD, and based on parameters presented in Tables 2b and bottom of Table 3, with time 0 corresponding to approximately 10.5 years after disease onset. The R2 for the FGF23 only model diminished over time, while the R2 for the proteinuria only model increased slightly over 3 years and then plateaued. The combined model offered the highest R2 value and increased from 41% at baseline to 44% about 2.5 years after baseline and diminished thereafter (40% at 7 years). All curves in this group were reasonably summarized by the time-constant R2 values from models based on random intercepts only and were contained in the bootstrapped 95% confidence intervals.
Figure 2.
Total variance and R-square as a function of time for mixed effects models for children with a non-glomerular CKD diagnosis. Figure 2a presents estimates of total eGFR variance for four mixed models in Table 3a. Figure 2b presents time-varying R-square values for mixed models (random intercepts and slopes; Table 2b) and time-constant R-square values based on mixed models with random intercepts only in Table 3.
As an additional analysis, SBP z-score (based on the normal population) was investigated as another predictive biomarker. The main effects were minimal and this was consistent with previous publications [30]; the variance components were not different than the null model and it offered no additional predictive power (results not shown). This was likely explained by the close relationship between blood pressure and proteinuria.
DISCUSSION
Linear mixed effect models are commonly used in epidemiologic studies to quantify the effect of an exposure on a continuous outcome over time. While these models offer an appropriate approach to account for within-person repeated measurements to validly estimate population levels and changes, the variance and covariance parameters are likely overlooked in favor of reporting only coefficient estimates of fixed effects.
Characterizing time-varying variance has utility in describing different disease processes related to different risk factors. In our application, for children with glomerular CKD, baseline levels of FGF23 were more strongly associated with short-term variability (i.e., within 2 years of index visit) in eGFR compared to proteinuria. However, proteinuria measured at the same time was more strongly related to longer term outcomes (i.e., after 3 years) than FGF23. There is biological support for these findings as proteinuria is a marker of and contributor to kidney injury. FGF23, a novel marker of CKD risk and kidney function, reflects dysregulation of phosphorus and vitamin D metabolism that is more related to concurrent CKD severity and eGFR level. Among those with a non-glomerular CKD diagnosis, proteinuria was more predictive of short-term and long-term eGFR outcomes than FGF23, but there was substantial benefit to including both in the model.
Clinically, these results underscore the importance of proteinuria and FGF23 as therapeutic targets for both diagnosis groups. The use of an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker is an effective intervention to reduce proteinuria [31] and a standard approach to pediatric CKD management. There is currently no therapy approved for FGF23 management in pediatric CKD; thus, there is a need for clinical trials to investigate potential interventions targeting dietary phosphorus management [32] or bone disorders or growth retardation [33]. Interestingly, the effect of FGF23 on eGFR level at entry and change was much stronger among those with glomerular diagnoses compared to the non-glomerular group (−0.219 vs. −0.049 for level, respectively; −0.044 vs. −0.016 for change, respectively; Tables 3a and 3b). These results suggest that children with glomerular CKD are an important target population for exploring FGF23 therapies.
Zheng et al. [1] proposed a simple and intuitive method to calculate R2 values from generalized linear mixed models (with random intercepts and slopes) as the square of the correlation of observed and predicted outcomes. This model assumes a time-constant R2 and the results were inferentially similar to the R2 values determined by the random intercept only models. Specifically, among the children with glomerular CKD, the proteinuria only, FGF23 only and combined model R2 values were 16%, 28% and 32%. Among the children with non-glomerular CKD, these R2 values were 23%, 16% and 31%, respectively. While this method offered consistent inferences and an appealing intuitive approach, it lacks a depiction of the dynamic nature of the exposure-outcome relationship.
There are limitations in deriving time-varying R2 from these models. First, a characterization of total variance and R2 values (time-varying or time-constant) is not an indicator of correct model specification or model fit. Indeed, these parameters assume correct specification and have the same assumption when linear mixed models are used to summarize longitudinal data. Second, the estimate of time-varying total variance is influenced by non-sparse data. It is common for longitudinal cohort studies to have more data in the early years of the study, with less later on due to delayed enrollment or drop out, and confidence in the estimated total variance should be commensurate to the presence of data (i.e., the confidence intervals of the time-varying R2 will increase over time, in general). Third, longitudinal measurement of predictive biomarkers are ideal for characterizing disease progression, however, longitudinal FGF23 was not available. Therefore, these models may be interpreted from a clinically meaningful perspective as the predictive power after biomarker measurements.
It should be noted that in four of the six instances in our application, time-varying R2 values (from random intercepts and slopes) were reasonably similar to their corresponding time-constant R2 (from random intercepts only). While the best model fit by AIC in all cases included random intercepts and slopes (compared to random intercepts only), these results show that for coefficients of determination, there may not be substantial departures from the time-constant R2 of the random intercepts only model. The time-constant R2 assumption was sufficient in four instances, but there was important information obtained in the other two when allowing the R2 to vary by time.
It is also possible that the relationship between the outcome and time is not linear (as was assumed in the present analysis). Indeed, eGFR decline in CKD populations, particularly those with glomerular CKD, is often not linear. If so, then time-varying R2 as based on a quadratic polynomial of the total variance may oversimplify the heterogeneity over time. To address this, we explored a quadratic random effect of time as a sensitivity analysis. Using the same general approach, the total variance for this model is a quartic polynomial. In our application, for the two instances in which time-varying R2 was identified, we fit models with a quadratic random effect of time. For the quadratic term in each model, the fixed effect was not significant, the variance of the random effect was negligible (i.e., no randomness) and the model AIC values were higher than the models specifying a linear effect of time. While we did not find evidence of non-linear effects of time, we recommend investigating more complex models of the outcome over time for a complete assessment of different potential shapes of time-varying R2 functions with consideration of model fit parameters (e.g., AIC values).
The correlation between the intercept and the slope was positive indicating that lower eGFR at baseline was associated with faster eGFR decline (Tables 3a and 3b). The square of this correlation provided an estimate of the proportion of variability in the random (individual) slopes that was explained by the baseline eGFR level (random intercept). For the children with glomerular CKD, these R2 values ranged from 14% in the null model to 3% in the model including proteinuria and FGF23. For the non-glomerular group, this ranged from 22% and 11%, respectively. It is intuitive that the predictive power of baseline eGFR on changes over time diminishes as biomarkers of risk are included in the model. However, within the non-glomerular group, there remained substantial explanatory power of baseline eGFR on slopes (11%) in the presence of both proteinuria and FGF23.
In the present analysis, children with glomerular CKD had shorter follow-up time than children with non-glomerular CKD. These differences in observed study time were largely due to the recruitment design of CKiD. It is possible that with longer duration of follow-up time among the glomerular CKD group, the disposition of the time-varying R2 may differ. If differential drop-out were an issue, implementation of joint longitudinal and survival models with random intercepts and slopes is one method to control for this bias [34]. Furthermore, the variance-covariance estimates of the random effects in a joint model can determine the R2 measure proposed here. Indeed, our approach can be readily applied to this setting as well.
In summary, linear mixed models are commonly used in epidemiologic analyses to describe the effect of an exposure on an outcome in a longitudinal setting. While interpretation of fixed effect coefficients from these models are likely to provide primary inferences, we believe that investigation of variance parameters can provide important epidemiologic insight into disease processes or novel risk factors. Importantly, these parameters and the assumptions of time-varying variance are explicitly embedded within this commonly used model structure. This approach extends these assumptions to calculate R2 values that can demonstrate heterogeneity over time. Deriving these estimates is simple and accessible and uses parameters that are standard output in statistical software. These results should encourage epidemiologists to explore time-varying coefficients of determination in these settings as these may yield important insights.
Table 3.
Random Effects Parameters from Linear Mixed Effects Models with Random Intercepts Only Stratified by Glomerular and Non-Glomerular CKD Diagnoses.
| Among children with glomerular CKD | ||||
|---|---|---|---|---|
| Parameter | Null | Proteinuria only | FGF23 only | Proteinuria and FGF23 |
| S.D. (level) [σ1] | 0.417 | 0.374 | 0.343 | 0.328 |
| S.D. (slope) [σ2] | 0 | 0 | 0 | 0 |
| Correlation [] | NA | NA | NA | NA |
| S.D. residual [σ] | 0.192 | 0.180 | 0.188 | 0.179 |
| AIC | 221.760 | 132.725 | 145.526 | 90.566 |
| Among children with non-glomerular CKD | ||||
| Null | Proteinuria only | FGF23 only | Proteinuria and FGF23 |
|
| S.D. (level) [σ1] | 0.412 | 0.342 | 0.361 | 0.308 |
| S.D. (slope) [σ2] | 0 | 0 | 0 | 0 |
| Correlation [] | NA | NA | NA | NA |
| S.D. residual [σ] | 0.150 | 0.146 | 0.150 | 0.146 |
| AIC | −589.712 | −854.061 | −710.660 | −936.368 |
Abbreviations: S.D., standard deviation; AIC, Akaike’s information criterion.
Acknowledgements
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri - Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-082194, U01-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid.
References
- [1].Zheng B, Agresti A. Summarizing the predictive power of a generalized linear model. Stat Med 2000;19:1771–81. [DOI] [PubMed] [Google Scholar]
- [2].Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. SAGE; 2002. [Google Scholar]
- [3].Xu R Measuring explained variation in linear mixed effects models. Stat Med 2003;22:3527–41. doi: 10.1002/sim.1572. [DOI] [PubMed] [Google Scholar]
- [4].Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O. An R2 Statistic for Fixed Effects in the Linear Mixed Model. Stat Med 2008;27:6137–57. doi: 10.1002/sim.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. SAGE; 2011. [Google Scholar]
- [6].Demidenko E, Sargent J, Onega T. Random effects coefficient of determination for mixed and meta-analysis models. Commun Stat Theory Methods 2012;41:953–69. doi: 10.1080/03610926.2010.535631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata, Volume I: Continuous Responses, Third Edition. Stata Press; 2012. [Google Scholar]
- [8].Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 2013;4:133–42. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- [9].Ettinger AS, Téllez-Rojo MM, Amarasiriwardena C, Peterson KE, Schwartz J, Aro A, et al. Influence of Maternal Bone Lead Burden and Calcium Intake on Levels of Lead in Breast Milk over the Course of Lactation. Am J Epidemiol 2006;163:48–56. doi: 10.1093/aje/kwj010. [DOI] [PubMed] [Google Scholar]
- [10].Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of Cognitive Function in Late Life in the United States: Demographic and Socioeconomic Predictors. Am J Epidemiol 2009;170:331–42. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pierce CB, Cox C, Saland JM, Furth SL, Muñoz A. Methods for characterizing differences in longitudinal glomerular filtration rate changes between children with glomerular chronic kidney disease and those with nonglomerular chronic kidney disease. Am J Epidemiol 2011;174:604–12. doi: 10.1093/aje/kwr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hidalgo G, Ng DK, Moxey-Mims M, Minnick ML, Blydt-Hansen T, Warady BA, et al. Association of income level with kidney disease severity and progression among children and adolescents with CKD: a report from the Chronic Kidney Disease in Children (CKiD) Study. Am J Kidney Dis 2013;62:1087–94. doi: 10.1053/j.ajkd.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Appelhans BM, Segawa E, Janssen I, Kazlauskaite R, Thurston RC, Lewis TT, et al. Employment status, depressive symptoms, and waist circumference change in midlife women: the Study of Women’s Health Across the Nation (SWAN). Ann Epidemiol 2014;24:187–92. doi: 10.1016/j.annepidem.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ng DK, Robertson CC, Woroniecki RP, Limou S, Gillies CE, Reidy KJ, et al. APOL1-associated glomerular disease among African-American children: a collaboration of the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE) cohorts. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 2016. doi: 10.1093/ndt/gfw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wingen AM, Fabian-Bach C, Schaefer F, Mehls O. Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet Lond Engl 1997;349:1117–23. [DOI] [PubMed] [Google Scholar]
- [16].Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47. [DOI] [PubMed] [Google Scholar]
- [17].Ardissino G, Testa S, Daccò V, Viganò S, Taioli E, Claris-Appiani A, et al. Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatr Nephrol Berl Ger 2004;19:172–7. doi: 10.1007/s00467-003-1268-0. [DOI] [PubMed] [Google Scholar]
- [18].Litwin M Risk factors for renal failure in children with non-glomerular nephropathies. Pediatr Nephrol Berl Ger 2004;19:178–86. doi: 10.1007/s00467-003-1329-4. [DOI] [PubMed] [Google Scholar]
- [19].Wong CS, Pierce CB, Cole SR, Warady BA, Mak RHK, Benador NM, et al. Association of Proteinuria with Race, Cause of Chronic Kidney Disease, and Glomerular Filtration Rate in the Chronic Kidney Disease in Children Study. Clin J Am Soc Nephrol 2009;4:812–9. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nagata M, Ninomiya T, Kiyohara Y, Murakami Y, Irie F, Sairenchi T, et al. Prediction of Cardiovascular Disease Mortality by Proteinuria and Reduced Kidney Function: Pooled Analysis of 39,000 Individuals From 7 Cohort Studies in Japan. Am J Epidemiol 2013;178:1–11. doi: 10.1093/aje/kws447. [DOI] [PubMed] [Google Scholar]
- [21].Magnusson P, Hansson S, Swolin-Eide D. A prospective study of fibroblast growth factor-23 in children with chronic kidney disease. Scand J Clin Lab Invest 2010;70:15–20. doi: 10.3109/00365510903359245. [DOI] [PubMed] [Google Scholar]
- [22].Siomou E, Stefanidis CJ. FGF-23 in children with CKD: a new player in the development of CKD-mineral and bone disorder. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 2012;27:4259–62. doi: 10.1093/ndt/gfs315. [DOI] [PubMed] [Google Scholar]
- [23].Portale AA, Wolf MS, Messinger S, Perwad F, Jüppner H, Warady BA, et al. Fibroblast Growth Factor 23 and Risk of CKD Progression in Children. Clin J Am Soc Nephrol CJASN 2016;11:1989–98. doi: 10.2215/CJN.02110216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 2012;82:445–53. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol CJASN 2006;1:1006–15. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension 2008;52:631–7. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, et al. Progression of Pediatric CKD of Nonglomerular Origin in the CKiD Cohort. Clin J Am Soc Nephrol 2015;10:571–7. doi: 10.2215/CJN.07480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, et al. Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. Am J Kidney Dis 2015;65:878–88. doi: 10.1053/j.ajkd.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ng DK, Moxey-Mims M, Warady BA, Furth SL, Muñoz A. Racial differences in renal replacement therapy initiation among children with a nonglomerular cause of chronic kidney disease. Ann Epidemiol 2016;26:780–787. e1. doi: 10.1016/j.annepidem.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kogon AJ, Pierce CB, Cox C, Brady TM, Mitsnefes MM, Warady BA, et al. Nephrotic-range proteinuria is strongly associated with poor blood pressure control in pediatric chronic kidney disease. Kidney Int 2014;85:938–44. doi: 10.1038/ki.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abraham AG, Betoko A, Fadrowski JJ, Pierce C, Furth SL, Warady BA, et al. Renin-angiotensin II-aldosterone system blockers and time to renal replacement therapy in children with CKD. Pediatr Nephrol Berl Ger 2016. doi: 10.1007/s00467-016-3512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Perwad F, Portale AA. Vitamin D metabolism in the kidney: regulation by phosphorus and fibroblast growth factor 23. Mol Cell Endocrinol 2011;347:17–24. doi: 10.1016/j.mce.2011.08.030. [DOI] [PubMed] [Google Scholar]
- [33].Sgambat K, Moudgil A. Optimization of Bone Health in Children before and after Renal Transplantation: Current Perspectives and Future Directions. Front Pediatr 2014;2:13. doi: 10.3389/fped.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Elashoff R, li G, Li N. Joint Modeling of Longitudinal and Time-to-Event Data 1 edition Boca Raton: Chapman and Hall/CRC; 2016. [Google Scholar]