Abstract
Background:
The epidemic of drug overdose deaths in the United States has led to an increase in organ donors.
Objective:
To characterize donors who died of overdose and to analyze outcomes among transplant recipients.
Design:
Prospective observational cohort study.
Setting:
Scientific Registry of Transplant Recipients, 1 January 2000 to 1 September 2017.
Participants:
138 565 deceased donors; 337 934 transplant recipients at 297 transplant centers.
Measurements:
The primary exposure was donor mechanism of death (overdose-death donor [ODD], trauma-death donor [TDD], or medical-death donor [MDD]). Patient and graft survival and organ discard (organ recovered but not transplanted) were compared using propensity score-weighted standardized risk differences (sRDs).
Results:
A total of 7313 ODDs and 19 897 ODD transplants (10 347 kidneys, 5707 livers, 2471 hearts, and 1372 lungs) were identified. Overdose-death donors accounted for 1.1% of donors in 2000 and 13.4% in 2017. They were more likely to be white (85.1%), aged 21 to 40 years (66.3%), infected with hepatitis C virus (HCV) (18.3%), and increased-infectious risk donors (IRDs) (56.4%). Standardized 5-year patient survival was similar for ODD organ recipients compared with TDD organ recipients (sRDs ranged from 3.1% lower to 3.9% higher survival) and MDD organ recipients (sRDs ranged from 2.1% to 5.2% higher survival). Standardized 5-year graft survival was similar between ODD and TDD grafts (little difference for kidneys and lungs, marginally lower [sRD, −3.2%] for livers, and marginally higher [sRD, 1.9%] for hearts). Kidney discard was higher for ODDs than TDDs (sRD, 5.2%) or MDDs (sRD, 1.5%); standardization for HCV and IRD status attenuated this difference.
Limitation:
Inability to distinguish between opioid and nonopioid overdoses.
Conclusion:
In the United States, transplantation with ODD organs has increased dramatically, with noninferior outcomes in transplant recipients. Concerns about IRD behaviors and hepatitis C among donors lead to excess discard that should be minimized given the current organ shortage.
Overdose deaths in the United States have nearly tripled over the past 15 years, with 52 404 reported in 2015 (1, 2). Younger adults are disproportionately affected, with the highest rates among those aged 25 to 55 years in the Northeast, Midwest, and South (1, 3). At the same time, the United States has a severe shortage of organ donors for transplant, with more than 120 000 patients on national waitlists but only 10 281 donors in 2017 (4). Median wait times range from 5 to 7 years, and for some candidates, the risk for death while on the waitlist is greater than the chance of receiving an organ (5, 6).
Although almost all transplants provide a survival benefit, optimal outcomes are observed with organs from young trauma-death donors (TDDs) who donate after brain death (7–9). Overdose-death donors (ODDs) often experience anoxic brain death and have few comorbidities; thus, their organs could have excellent recipient outcomes, similar to TDD organs. However, ODDs might be designated as increased- infectious risk donors (IRDs) due to behaviors that increase risk for HIV, hepatitis B virus (HBV), or hepatitis C virus (HCV) infection (10, 11). The IRD label might reduce use of ODD organs because it is associated with organ discard (surgical recovery without subsequent use for transplant) (12). Moreover, ODDs are increasingly positive for HCV antibodies (10, 11), and inferior outcomes might be expected due to HCV infection (13–16). Finally, concerns that illicit drug use compromises organ quality might exist; for example, injection drug use is associated with lung granulomatosis (17).
To inform provider and patient decision making with regard to ODD transplants, we used national registry data to examine posttransplant outcomes and organ discard associated with ODDs compared with TDDs and medical-death donors (MDDs).
Methods
Data Sources
This study used data from the Scientific Registry of Transplant Recipients (SRTR) external release (available September 2017). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, United States Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. We also used data from the Centers for Disease Control and Prevention (CDC) Multiple Cause of Death database, which contains data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program (18). This study used deidentified data and was exempted by the Johns Hopkins School of Medicine Institutional Review Board (NA_00042871).
Study Population
We identified 337 934 adult patients from 297 transplant centers who received a transplant from a deceased donor between 1 January 2000 and 1 September 2017 (177 522 kidneys, 97 670 livers, 35 710 hearts, and 27 032 lungs). Recipients missing information on donor mechanism of death (0.01% [n = 36]) were excluded.
Categorization of Donor Type
The primary exposure was donor mechanism of death, categorized as overdose (mechanism reported as drug intoxication), trauma (mechanism reported as blunt injury, drowning, gunshot, stab wound, asphyxiation, seizure, electric shock, or sudden infant death syndrome), or medical (mechanism reported as intracranial hemorrhage, stroke, myocardial infarction, natural causes, or other).
Ascertainment of Overdose Death Rates
To compare state overdose death rates with donation rates of ODDs, we compiled annual age-adjusted rates of overdose death from CDC Multiple Cause of Death data from 2000 to 2016. Overdose deaths were identified using CDC cause-of-death codes (X40 to X44, X60 to X64, X85, and Y10 to Y14) (1).
Characterization of ODDs
To characterize ODDs, we identified 138 565 deceased donors with at least 1 organ recovered between 1 January 2000 and 1 September 2017. We report characteristics by donor type (ODD, TDD, or MDD). To determine state and census region of donors, we used donor hospital and residential ZIP codes; these were unavailable for 2739 (1.98%) donors.
Statistical Analysis
Model Specification for Analyses of Posttransplant Outcomes
To compare posttransplant outcomes among recipients of ODD, TDD, and MDD organs, we used a 2-step propensity score-weighted approach to estimate standardized differences in 5-year outcomes (19). Time- to-event outcomes were death-censored graft failure (defined as retransplant, dialysis [for kidney transplants], or graft failure, with censoring for mortality) and mortality (without censoring for graft failure). Five-year death-censored graft survival and patient survival are reported. Mortality data were obtained via linkage to the Social Security Death Master File, which has a reporting delay; thus, recipients were administratively censored on 28 February 2017.
First, ODD, TDD, or MDD organ recipients were standardized across potential confounders by using multinomial logistic regression models, with donor type as the dependent variable and recipient and donor factors as independent variables. Variable selection was based on SRTR risk adjustment models (20). From the regression results, we derived the probability of having received an ODD organ and calculated inverse probability weights (IPWs) based on the recipient’s vector of covariates. Separate models were built for kidney, liver, heart, and lung transplants. Model variables and missingness are shown in Appendix Table 1 (available at Annals.org). For variables missing fewer than 1% of values, complete- case adjustment was used, and for those missing more than 1%, a missing indicator was included in the initial step of the IPW standardization. Thus, ODD, TDD, and MDD organ recipients were balanced across known and unknown values before outcome measurement (21). Second, we estimated weighted (standardized) 5-year patient and graft survival and standardized risk differences (sRDs) (22, 23). The 95% CIs around the standardized survival rates and risk differences were empirically derived using bootstrap methods (200 iterations per organ). Recipients of multiple organs (n = 16 452) were included in initial counts of ODD, TDD, and MDD transplants but were excluded from posttransplant analyses because they are clinically distinct from recipients of single organs.
Discard of Organs From ODDs
To characterize discard of ODD organs (surgical recovery of the organ without transplant), we identified 187 276 kidneys, 87 947 livers, 32 144 hearts, and 24 598 lungs recovered from donors between 1 January 2005 (when key donor characteristics became available) and 1 September 2017. To address confounding, we used a 2-step IPW approach as described earlier. We built multinomial logistic regression models with donor type as the dependent variable and donor factors (age, sex, race, body mass index, diabetes, hypertension, donation after circulatory death, creatinine level >133 μmol/L [>1.5 mg/dL], and calendar year of organ recovery) as independent variables. We derived the probability of being an ODD and calculated IPWs based on the donor’s vector of covariates. Weights were applied to logistic regression models, with discard as the dependent variable and donor type as the independent variable. Standardized mean differences in covariates were visually assessed, and covariates that remained unbalanced were included in the final model. From logistic regression models, we derived standardized risk and sRDs in discard associated with ODD organs versus both TDD and MDD organs. Logistic regression models included SE adjustment for donation service areas (58 geographic regions used for organ allocation) to account for correlation among donors from the same service area.
We estimated discard risk after additional standardization by donor HCV and IRD status (see Appendix Table 2, available at Annals.org). Kidney and liver models were run with standardization for HCV and IRD status, and heart and lung models were run with standardization for IRD status only (<1% of hearts and lungs were from HCV-positive donors). Donors with missing values for diabetes (0.5%), hypertension (0.4%), HCV status (0.1%), IRD status (0.2%), or body mass index (0.3%) were excluded.
Sensitivity Analyses
To address confounding by variation across transplant centers, we performed a sensitivity analysis in which we estimated the standardized hazard ratio of mortality or graft loss associated with ODD organs, using Cox regression with and without stratification by center. To characterize potential unmeasured confounding needed to explain away our observed results, we calculated E-values for each standardized hazard ratio (24). Details are provided in the Appendix (available at Annals.org).
All statistical analyses were performed using the functions mlogit and logit and the survival package stcox in Stata/SE, version 14 (StataCorp). We used a 2-sided α level of 0.05 to indicate a statistically significant difference. E-values were calculated using the E-value package in R statistical software (24, 25).
Role of the Funding Source
This work was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (grants K23CA177321–01A1 and K24DA034621), and by the National Institute of Diabetes and Digestive and Kidney Diseases (grants K24DK101828, R01AI120938, F30DK095545, R01DK111966, R01AG042504, D01DK096008, and K23DK101677). The funding sources had no role in the design, conduct, or reporting of the study or the decision to publish the manuscript.
Results
Increase in ODDs and Transplants
We identified 7313 ODDs with at least 1 organ recovered during the study. The number of ODDs increased by 17% per year, from 66 in 2000 to 1263 in 2016 (1.1% and 12.7% of the national pool, respectively) (Figure 1 [top]). The number of TDDs increased by 1.6% per year, from 2598 to 3511, and the number of MDDs increased by 2.3% per year, from 3320 to 5202. Overdose deaths increased from 17 415 in 2000 to 63 632 in 2016 (Figure 1 [top]). Organs from ODDs were used in 19 897 transplants (10 347 kidneys, 5707 livers, 2471 hearts, and 1372 lungs). The number of ODD transplants increased from 149 in 2000 to 3533 in 2016 (1804 kidneys, 1013 livers, 454 hearts, and 262 lungs) (Figure 1 [bottom]). During the study, 274 transplant programs performed ODD transplants; the median number of ODD transplants per program increased from 2 (interquartile range, 1 to 2) in 2000 to 10 (interquartile range, 3 to 25) in 2016.
Figure 1. Overdose deaths and ODD donors with organs recovered (top) and transplants performed using ODD organs (stratified by organ) (bottom) between 2000 and 2016.
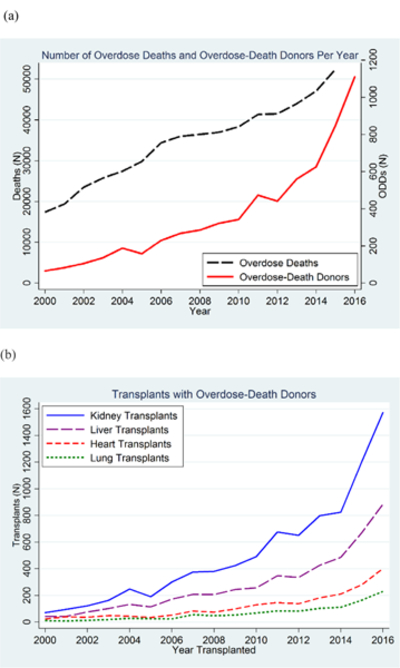
The number of donors with ODD organs recovered increased by 17% per year, from 66 in 2000 to 1263 in 2016. A total of 3533 transplants were performed using ODD organs in 2016, compared with 149 in 2000. ODD = overdose-death donor.
ODDs and State-Level Overdose Death Rates
In 2000, ODDs accounted for 0% of donors in 15 states and fewer than 6% of donors in all states (Figure 2 [top]). The states with the highest percentages were Maine (5.3%), Rhode Island (5.3%), Massachusetts (4.1%), Delaware (3.4%), and Maryland (2.7%). In 2016, ODDs accounted for at least 10% of donors in 29 states (Figure 2 [top]), with the highest percentages in Massachusetts (35.6%), New Hampshire (32.4%), New Jersey (25.7%), New York (23.1%), and Maryland (22.7%).
Figure 2. Percentage of ODDs in statewide donor pool (top) and age-adjusted overdose death rates per 100 000 persons (bottom) in 2000 and 2016. ODD=overdose-death donor.
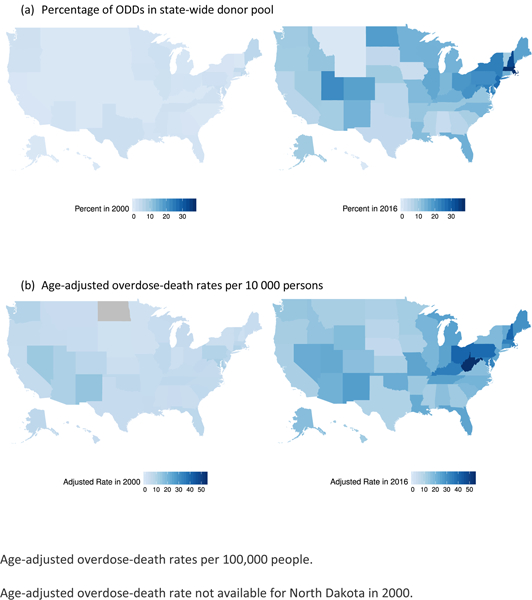
(a) Percentage of ODDs in state-wide donor pool (b) Age-adjusted overdose-death rates per 10 000 persons Age-adjusted overdose-death rates per 100,000 people. Age-adjusted overdose-death rate not available for North Dakota in 2000.
Age-adjusted overdose death rates also increased (Figure 2 [bottom]). In 2000, 6 states and the District of Columbia reported rates greater than 10 per 100 000 persons, with the highest rates in New Mexico (15.1 per 100 000 persons), Nevada (13.7 per 100 000 persons), the District of Columbia (13.4 per 100 000 persons), Maryland (11.3 per 100 000 persons), and Arizona (10.6 per 100 000 persons). In 2016, 48 states and the District of Columbia reported rates greater than 10 per 100 000 persons, with the highest rates in West Virginia (52.0 per 100 000 persons), Ohio (39.1 per 100 000 persons), New Hampshire (39.0 per 100 000 persons), the District of Columbia (38.8 per 100 000 persons), and Pennsylvania (37.9 per 100 000 persons).
Demographic and Clinical Characteristics of ODDs
Overdose-death donors were more likely than TDDs and MDDs to be identified in the Northeast and Midwest, aged 21 to 40 years (66.3% vs. 40.8% and 19.6%, respectively), and white (85.1% vs. 67.7% and 66.3%, respectively) (Table 1). Compared with TDDs, ODDs were more likely to have hypertension (18.0% vs. 11.1%), diabetes (4.6% vs. 3.7%), or previous myocardial infarction (1.6% vs. 1.3%). Compared with MDDs, ODDs were less likely to have hypertension (18.0% vs. 49.1%), diabetes (4.6% vs. 15.8%), or previous myocardial infarction (1.6% vs. 5.7%). Overdose-death donors had slightly higher creatinine levels than TDDs and MDDs (median 1.2 mg/dL vs. 0.9 and 1.0, respectively) and were more likely to donate after circulatory death (13.2% vs. 11.4% and 10.1%, respectively). Kidneys from ODDs were more likely than those from TDDs to undergo biopsy but had similar macrovesicular fat content. Cold ischemic time of transplanted kidneys was similar across donor types.
Table 1.
Characteristics of ODDs, TDDs, and MDDs With Organs Recovered Between 1 November 2000 and 1 September 2017 (n = 138 565)
| Characteristic | ODDs (n = 7313)* | TDDs (n = 53 698)† | MDDs (n = 77 554)‡ | |||
|---|---|---|---|---|---|---|
| Census region,% | ||||||
| Northeast | 23.4 | 13.2 | 19.5 | |||
| Midwest | 26.4 | 23.9 | 22.8 | |||
| South | 33.8 | 41.7 | 39.0 | |||
| West | 16.4 | 21.2 | 18.7 | |||
| Median donor age (IQR), y | 31.0 (24.0-39.0) | 26.0 (19.0-42.0) | 50.0 (39.0-58.0) | |||
| Donor age category, % | ||||||
| 0-20 y | 10.6 | 32.7 | 7.8 | |||
| 21-40 y | 66.3 | 40.8 | 19.6 | |||
| 41-60 y | 22.4 | 22.1 | 52.3 | |||
| >60 y | 0.7 | 4.4 | 20.3 | |||
| Female,% | 43.8 | 26.5 | 50.3 | |||
| Race/ethnicity,% | ||||||
| White | 85.1 | 67.7 | 66.3 | |||
| African American | 5.9 | 14.6 | 17.5 | |||
| Other | 9.0 | 17.7 | 16.2 | |||
| Median BMI (IQR), kg/m2 | 26.5 (23.2-30.8) | 24.6 (21.5-28.4) | 26.9 (23.1-31.7) | |||
| Positive for HCV antibodies,% | 18.3 | 3.2 | 4.1 | |||
| Positive for HBV core antibodies,% | 5.1 | 3.0 | 6.9 | |||
| Increased infectious risk,%§ | 56.4 | 14.3 | 8.8 | |||
| History of hypertension,% | 18.0 | 11.1 | 49.1 | |||
| History of diabetes,% | 4.6 | 3.7 | 15.8 | |||
| History of cancer,% | 1.6 | 1.4 | 4.7 | |||
| Previous myocardial infarction,% | 1.6 | 1.3 | 5.7 | |||
| Donation after circulatory death,% | 13.2 | 11.4 | 10.1 | |||
| Median serum creatinine level (IQR) | ||||||
| μmol/L | 106 (71-177) | 80 (62-115) | 88 (62-141) | |||
| μmg/dL | 1.2 (0.8-2.0) | 0.9 (0.7-1.3) | 1.0 (0.7-1.6) | |||
| Kidney biopsy performed,% | 38.2 | 24.2 | 53.5 | |||
| Median kidney macrovesicular fat content (IQR),% | 5.0 (0.0-10.0) | 5.0 (0.0-15.0) | 5.0 (0.0-15.0) | |||
| Median cold ischemic time (IQR), h∥ | 16 (11-22) | 16 (11-22) | 17 (12-23) | |||
BMI = body mass index; HBV = hepatitis B virus; HCV = hepatitis C virus; IQR = interquartile range; MDD = medical-death donor; ODD = overdose-death donor; TDD = trauma-death donor.
Mechanism of death reported as drug intoxication.
Mechanism of death reported as blunt injury, drowning, gunshot, stab wound, asphyxiation, seizure, electric shock, or sudden infant death syndrome.
Mechanism of death reported as intracranial hemorrhage, stroke, myocardial infarction, natural causes, or other.
Calculated among donors with organs recovered after 2005, when information became available.
Among kidneys that were used for transplant.
HCV and IRD Status of ODDs
A higher percentage of ODDs were HCV-positive (18.3% vs. 3.2% for TDDs and 4.1% for MDDs). Prevalence of HCV infection increased over time among ODDs (7.8% in 2000, 24.2% in 2016, and 30.0% in 2017) but changed minimally among TDDs and MDDs (Figure 3 [top]). A higher percentage of ODDs (56.4%) were labeled as IRDs compared with TDDs (14.3%) and MDDs (8.8%) (Table 1). Among ODDs, the percentage of IRDs increased from 31.4% in 2005 (when data were first available) to 71.8% in 2017. The percentage increased from 9.6% to 22.7% among TDDs and from 5.7% to 15.6% among MDDs over the same period (Figure 3 [bottom]).
Figure 3. Percentage of donors who were HCV-positive (top) and who were designated as IRDs according to U.S. Public Health Service behavioral criteria (bottom).
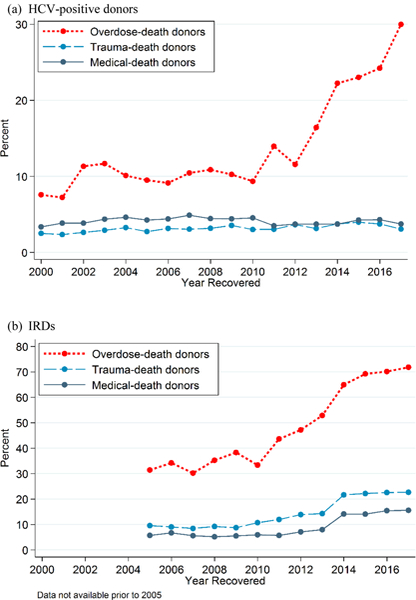
Percentages are stratified by mechanism of death. Information on IRD status was not available before 2005. HCV = hepatitis C virus; IRD = increased-infectious risk donor; MDD = medical-death donor; ODD = overdose-death donor; TDD = trauma-death donor.
Patient Survival With ODD Transplants
Unadjusted 5-year patient survival was similar for recipients of ODD and TDD kidneys (86.3% vs. 86.2%) and was higher for ODD than MDD transplants (86.3% vs. 80.7%) (Table 2). After standardization, 5-year survival rates were 83.1%, 86.2%, and 81.0% for ODD, TDD, and MDD transplants, respectively. For kidney recipients, sRDs were −3.1% (95% CI, −8.0% to 0.02%) for ODD versus TDD transplants and 2.1% (CI, −2.8% to 5.3%) for ODD versus MDD transplants (Table 2). A negative sRD indicates lower survival for ODDs, and a positive sRD indicates higher survival.
Table 2.
Patient and Death-Censored Graft Survival at 5 Years*
| Variable | 5-Year Survival |
Standardized Absolute Risk Difference in Survival at 5 Years After Transplant | |
|---|---|---|---|
| Unadjusted | Standardized† | ||
| Patient survival | |||
| Kidney recipients | |||
| ODD | 86.3 (85.3 to 87.2) | 83.1 (78.2 to 86.2) | − |
| TDD | 86.2 (85.9 to 86.4) | 86.2 (86.0 to 86.5) | −3.1 (−8.0 to 0.02) |
| MDD | 80.7 (80.4 to 81.0) | 81.0 (80.8 to 81.3) | 2.1 (−2.8 to 5.3) |
| Liver recipients | |||
| ODD | 76.8 (75.2 to 78.3) | 74.8 (71.4 to 78.6) | − |
| TDD | 76.4 (75.9 to 76.9) | 76.6 (76.2 to 77.0) | −1.8 (−5.3 to 2.1) |
| MDD | 71.9 (71.5 to 72.3) | 72.0 (71.5 to 72.5) | 2.8 (−0.6 to 6.5) |
| Heart recipients | |||
| ODD | 79.2 (76.9 to 81.4) | 78.4 (74.5 to 81.9) | − |
| TDD | 77.5 (76.8 to 78.1) | 77.7 (77.0 to 78.3) | 0.8 (−3.4 to 4.3) |
| MDD | 74.4 (73.5 to 75.3) | 74.5 (73.7 to 75.5) | 3.9 (−0.3 to 7.5) |
| Lung recipients ODD | |||
| ODD | 56.5 (52.5 to 60.2) | 59.9 (50.4 to 64.6) | − |
| TDD | 55.7 (54.7 to 56.7) | 56.0 (54.8 to 57.1) | 3.9 (−5.3 to 8.5) |
| MDD | 54.4 (53.4 to 55.4) | 54.8 (53.6 to 55.8) | 5.2 (−4.5 to 9.8) |
| Death-censored graft survival | |||
| Kidney recipients | |||
| ODD | 88.8 (87.8 to 89.6) | 87.1 (85.3 to 88.9) | − |
| TDD | 86.8 (86.5 to 87.0) | 86.9 (86.6 to 87.2) | 0.2 (−1.6 to 1.9) |
| MDD | 81.8 (81.5 to 82.1) | 82.2 (81.9 to 82.5) | 4.9 (2.9 to 6.5) |
| Liver recipients | |||
| ODD | 94.8 (93.9 to 95.5) | 90.9 (87.7 to 93.6) | − |
| TDD | 93.9 (93.6 to 94.2) | 94.0 (93.8 to 94.4) | −3.2 (−6.2 to -0.4) |
| MDD | 91.8 (91.5 to 92.1) | 91.9 (91.6 to 92.2) | −1.0 (−4.2 to 1.7) |
| Heart recipients | |||
| ODD | 95.4 (94.0 to 96.5) | 95.6 (94.1 to 96.8) | − |
| TDD | 93.7 (93.3 to 94.1) | 93.8 (93.4 to 94.1) | 1.9 (0.3 to 3.2) |
| MDD | 93.0 (92.4 to 93.5) | 93.1 (92.4 to 93.6) | 2.6 (1.1 to 4.0) |
| Lung recipients | |||
| ODD | 82.1 (78.4 to 85.2) | 85.6 (78.8 to 90.3) | − |
| TDD | 83.2 (82.4 to 84.0) | 83.4 (82.6 to 84.2) | 2.2 (−4.5 to 6.5) |
| MDD | 82.2 (81.3 to 83.1) | 82.4 (81.3 to 83.3) | 3.2 (−3.4 to 7.6) |
MDD = medical-death donor; ODD = overdose-death donor; TDD = trauma-death donor.
Mechanism of death reported as drug intoxication in ODDs; blunt injury, drowning, gunshot, stab wound, asphyxiation, seizure, electric shock, or sudden infant death syndrome in TDDs; and intracranial hemorrhage, stroke, myocardial infarction, natural causes, or other in MDDs. Values are percentages (95% CIs).
Estimated using propensity score-weighted survival analysis to account for potential confounding. Propensity weights were derived from multinomial logistic regression models that balanced recipients of ODD, TDD, and MDD organs on recipient and donor factors specific to the transplanted organ. Standardized CIs were estimated using bootstrap methods with replacement.
For liver recipients, unadjusted 5-year patient survival rates were 76.8%, 76.4%, and 71.9% for ODD, TDD, and MDD transplants, respectively. Standardized 5-year survival was 74.8% for ODD transplants; sRDs were −1.8% (CI, −5.3% to 2.1%) for ODD versus TDD transplants and 2.8% (CI, −0.6% to 6.5%) for ODD versus MDD transplants.
For heart recipients, unadjusted 5-year patient survival rates were 79.2%, 77.5%, and 74.4% for ODD, TDD, and MDD transplants, respectively. Standardized 5-year survival was similar for ODD heart recipients compared with TDD and MDD heart recipients (sRDs, 0.8% [CI, −3.4% to 4.3%] and 3.9% [CI, −0.3% to 7.5%], respectively).
For lung recipients, unadjusted 5-year patient survival rates were 56.5%, 55.7%, and 54.4% for ODD, TDD, and MDD transplants, respectively. Standardized 5-year survival was slightly higher for ODD transplants than TDD and MDD transplants (sRDs, 3.9% [CI, −5.3% to 8.5%] and 5.2% [CI, −4.5% to 9.8%], respectively).
Standardized survival curves are shown in the Appendix Figure (available at Annals.org).
Death-Censored Graft Survival With ODD Transplants
Unadjusted death-censored 5-year graft survival was slightly higher for ODD kidney, liver, and heart grafts than for TDD and MDD grafts (Table 2). Standardized 5-year graft survival was similar for ODD versus TDD kidneys (sRD, 0.2% [CI, −1.6% to 1.9%]) and was higher for ODD versus MDD kidneys (sRD, 4.9% [CI, 2.9% to 6.5%]). Standardized 5-year graft survival was lower for ODD versus TDD livers (sRD, −3.2% [CI, −6.2% to −0.4%]) and was similar for ODD and MDD livers (sRD, −1.0% [CI, −4.2% to 1.7%]). Standardized graft survival was slightly higher for ODD versus TDD hearts (sRD, 1.9% [CI, 0.3% to 3.2%]) and MDD hearts (sRD, 2.6% [CI, 1.1% to 4.0%]). For lungs, standardized graft survival was similar across donor types.
Discard of Organs From ODDs
We identified 1665 kidneys, 501 livers, 117 hearts, and 23 lungs that were recovered from ODDs but subsequently discarded. Discard of ODD organs was higher than that of TDD organs (kidneys, 14.1% vs. 8.8%; livers, 8.8% vs. 6.8%; hearts, 1.0% vs. 0.6%; lungs, 8.1% vs. 5.9%) but was, with the exception of lungs, lower than that of MDD organs (kidneys, 14.1% vs. 26.1%; livers, 8.8% vs. 12.5%; hearts, 1.0% vs. 1.5%; lungs, 8.1% vs. 6.1%). After standardization, discard remained higher for ODD kidneys than TDD kidneys (20.0% vs. 14.9%; sRD, 5.2% [CI, 3.7% to 6.7%]) (Table 3). With further standardization for HCV and IRD status, ODD and TDD kidney discard rates did not differ (16.5% vs. 16.1%) (Table 3). After additional standardization, a lower percentage of ODD kidneys than MDD kidneys were discarded (sRD, −2.1% [CI, −3.9% to −0.2%]). A higher percentage of ODD livers than TDD livers were discarded (sRD, 1.8% [CI, 0.4% to 3.2%]); this difference remained after standardization for HCV and IRD status (sRD, 1.6% [CI, 0.1% to 3.1%]). Standardized discard rates were similar between ODD and TDD hearts and lungs and among ODD and MDD livers, hearts, and lungs.
Table 3.
Standardized Absolute Risk for Discard and Standardized Risk Differences for ODD Organs Compared With TDD and MDD Organs*
| Organ | Standardized Risk for Discard, % | Standardized Risk Difference (95% CI), % | ||||||
|---|---|---|---|---|---|---|---|---|
| ODD | TDD | MDD | ODD vs. TDD | P Value | ODD vs. MDD | P Value | ||
| Standardized by donor factors† | ||||||||
| Kidney | 20.0 | 14.9 | 18.6 | 5.2 (3.7 to 6.7) | <0.001 | 1.5 (−0.5 to 3.4) | 0.137 | |
| Liver | 10.1 | 8.3 | 10.3 | 1.8 (0.4 to 3.2) | 0.013 | −0.2 (−1.9 to 1.6) | 0.83 | |
| Heart | 0.9 | 0.7 | 1.4 | 0.3 (−0.2 to 0.7) | 0.21 | −0.4 (−0.9 to 0.04) | 0.073 | |
| Lung | 7.3 | 5.5 | 6.5 | 1.8 (−1.1 to 4.8) | 0.22 | 0.8 (−1.5 to 3.0) | 0.50 | |
| Further standardized by HCV and IRD status | ||||||||
| Kidney | 16.5 | 16.1 | 18.5 | 0.4 (−1.3 to 2.0) | 0.67 | −2.1 (−3.9 to −0.2) | 0.031 | |
| Liver | 10.0 | 8.4 | 10.2 | 1.6 (0.1 to 3.1) | 0.035 | −0.2 (−2.1 to 1.6) | 0.82 | |
| Heart‡ | 0.8 | 0.7 | 1.3 | 0.1 (−0.3 to 0.6) | 0.58 | −0.5 (−1.1 to 0.2) | 0.137 | |
| Lung‡ | 6.8 | 5.5 | 6.6 | 1.4 (−2.0 to 4.7) | 0.42 | 0.2 (−2.2 to 2.6) | 0.86 | |
HCV = hepatitis C virus; IRD = increased-infectious risk donor; MDD = medical-death donor; ODD = overdose- death donor; TDD = trauma-death donor.
Mechanism of death reported as drug intoxication in ODDs; blunt injury, drowning, gunshot, stab wound, asphyxiation, seizure, electric shock, or sudden infant death syndrome in TDDs; and intracranial hemorrhage, stroke, myocardial infarction, natural causes, or other in MDDs. Kidneys and livers from ODDs were more likely to be discarded than those from TDDs. After HCV and IRD status were accounted for, discard risk was similar, with the exception of ODD versus TDD livers and ODD versus MDD kidneys.
Deceased donors were standardized by age, sex, race, body mass index, history of hypertension, history of diabetes, donation after circulatory death, serum creatinine level >133 μmol/L (>1.5 mg/dL), and calendar year of recovery using multinomial logistic regression. Standardization weights were then applied to logistic regression models with organ discard as the dependent variable, and separate models were run for each organ.
Further standardized by IRD status only because <1% of recovered hearts and lungs were HCV-positive.
Sensitivity Analyses
Inferences related to posttransplant outcomes remained unchanged after additional stratification across transplant programs in an IPW Cox regression model. Recipients of ODD kidneys and livers had a lower risk for death than MDD kidney and liver recipients; recipients of ODD and TDD kidneys and livers had similar risk for death and graft loss (see the Appendix). Recipients of ODD kidneys had a 17% lower risk for death (standardized hazard ratio, 0.83 [CI, 0.72 to 0.96]) than MDD kidney recipients. The observed hazard ratio in favor of ODD transplants could be explained away by an unmeasured confounder that was associated with both the exposure (ODD kidneys) and the outcome (mortality) by a risk ratio of 1.53 above and beyond the measured confounders, but weaker confounding could not do so. Similar levels of potential unmeasured confounding in terms of risk ratios ranged from 1.09 to 1.94 for cases in which receipt of ODD organs seemed to be protective. Even greater confounding would be needed to explain our observed results if mortality rates were higher for recipients of ODD organs (additional details are provided in the Appendix).
Discussion
In this national study of 138 565 deceased donors and 337 934 solid organ transplant recipients, we found a 24-fold increase in ODD transplants, from 149 in 2000 to 3533 in 2016. Unadjusted rates of 5-year patient and graft survival for recipients of ODD organs were equivalent to or marginally higher than those for recipients of TDD organs (who are generally considered optimal donors) and MDD organs, indicating that outcomes were acceptable given the overall population of ODDs. After standardization, receipt of an ODD organ (independent of donor quality) resulted in similar 5-year patient survival; standardized survival differences ranged from 3.1% lower for ODD kidney recipients to 5.2% higher for ODD lung recipients. Although the upper bounds of the 95% CIs exceeded 0.0% in all cases, the CIs are narrow. Therefore, our findings suggest that for most of the organs studied, the strength of the evidence slightly favors ODD organs over non-ODD organs or at least supports noninferiority of ODD organs.
We found that ODDs account for a growing proportion of the national pool of deceased donors (13.4% in 2017) and reflect the demographic characteristics of those most affected by the opioid overdose epidemic, as reported by the CDC: young white adults concentrated in the Northeast and in the Midwestern belt from Virginia to Iowa (1, 3). These findings and the observation that ODDs were more likely to have IRD behaviors and HCV infection are also consistent with recent editorials about the opioid epidemic and organ donation (10, 11, 26).
Despite favorable donor characteristics, kidneys and livers from ODDs were discarded at a higher rate than those from TDDs (sRDs, 5.2% and 1.8%, respectively). This is likely attributable to designation of ODDs as IRDs and higher prevalence of HCV infection among ODDs. Increased infectious risk is a behavioral designation meant to identify donors at risk for recent HIV, HBV, or HCV acquisition (27, 28). However, with viral nucleic acid and antibody testing, the true risk for a window-period infection for IRD organ recipients is extremely low (<1 in 1000 for HCV and <1 in 10 000 for HIV) (29, 30). Furthermore, candidates who accept IRD kidneys have better survival than those who wait for another organ (12). Despite this survival benefit, IRD kidneys continue to be discarded (12); this may be driven by administrative burdens of specialized consent (31), medical-legal concerns, or stigma associated with the IRD designation (32, 33). Our findings of elevated discard associated with the IRD designation are consistent with these prior studies and suggest that these organs might be unnecessarily discarded in the context of an organ shortage.
The second factor that is probably driving ODD organ discard is the prevalence of HCV infection, which increased from 7.8% in 2000 to 30.0% in 2017. Discard of HCV-positive kidneys and livers might be warranted in certain circumstances; some studies of HCV-positive recipients have shown worse posttransplant outcomes with HCV-positive versus HCV-negative kidneys and livers (13, 14). However, studies have shown that patients who accept HCV-positive organs have reduced wait times (14) and outcomes similar to those with HCV-negative organs (34). Despite this, HCV-positive organs continue to be discarded at higher rates than HCV-negative organs of similar quality (14, 34–37). Because direct- acting antivirals are now widely available to cure HCV infection, including in transplant recipients (38–40), organs from infected donors should be considered more broadly. In 2 recent single-center pilot trials (41, 42), kidneys from HCV-infected donors were successfully used for transplant in uninfected recipients in combination with direct-acting antivirals. This innovative practice, coupled with the increase in HCV- positive ODDs with few other comorbidities, might allow for safe expansion of the pool of deceased donors.
Several limitations of this study merit consideration. In our identification of ODDs and analyses of state overdose death rates, we were unable to distinguish between deaths due to opioid versus nonopioid overdose. Furthermore, states and jurisdictions vary in their reporting of specific drugs implicated in overdose deaths to the CDC. However, according to 2015 data from 28 states with high-quality reporting, 63% of overdose deaths involved opioids (2). Moreover, use of organs for transplant after death due to other types of chemical poisoning is rare (43, 44). We could not determine the specific behavior for which ODDs were labeled as IRDs, and behaviors may differ between TDDs and MDDs who are labeled as IRDs. Although reasons for IRD labeling may vary, the risks associated with each behavioral criterion are low and are not expected to affect posttransplant survival (29, 30). Finally, as with any registry-based study, unmeasured factors might have affected our inferences. Our reported E-values, which quantify the strength of potential unmeasured confounding needed to explain away the results that suggest ODD organs might confer lower risk to recipients, support the robustness of our findings.
In conclusion, organ donation after overdose death has increased dramatically in parallel with the opioid epidemic in the United States, and we found that recipients of ODD organs had noninferior patient and graft survival. Although this is not an ideal or sustainable solution to the organ shortage, use of ODD organs should be optimized. Potential risks attributable to IRD and HCV status should be carefully weighed against the benefit these organs can provide to transplant candidates.
Acknowledgment:
The authors thank John Ward, MD; Deborah Holtzman, PhD; Scott Holmberg, MD, MPH; Jon E. Zibbell, PhD; and Michele K. Bohm, MPH, from the Division of Viral Hepatitis at the CDC for their advice and data support for the manuscript.
Grant Support: Dr. Durand is supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (grant K23CA177321–01A1). Drs. Kucirka, Massie, Cameron, and Segev are supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grants F30DK095545 [Dr. Kucirka], K23DK101677 [Dr. Massie], R01DK111966 [Dr. Cameron], and K24DK101828 and R01AI120938 [Dr. Segev]). Dr. Sulkowski is supported by the National Institute of Allergy and Infectious Diseases (grant K24DA034621).
Primary Funding Source: National Institute of Diabetes and Digestive and Kidney Diseases and National Institute of Allergy and Infectious Diseases.
“This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
Appendix: Sensitivity Analyses
Addressing Potential Confounding by Variation Across Transplant Programs Through Stratification To identify potential confounding introduced by variation in practices and outcomes across transplant programs, we used IPWs to estimate the standardized hazard of patient death and graft loss using Cox regression models with and without stratification by transplant program (Appendix Table 3). Stratification allowed the baseline hazard of mortality to vary across transplant programs; thus, coefficients can be interpreted as being adjusted for variation in types of organ donors across programs. All models adjusted SEs for within-center clustering.
Potential Unmeasured Confounding Needed to Explain Away Observed Results: E-value Estimation E-values are a form of sensitivity analysis to estimate the strength of unmeasured confounding needed to explain away an observed association between an exposure (such as donor type) and an outcome (such as mortality). For purposes of exposition, our examples for mortality are on the commonly used hazard ratio scale. For example, the hazard ratio of patient mortality for recipients of ODD kidneys compared with MDD kidneys was 0.83 (CI, 0.72 to 0.96), with an E-value of 1.53 (lower 95% confidence bound, 1.20) (Appendix Table 4). The primary result is interpreted as a 17% lower hazard of mortality for ODD versus MDD kidneys. The E-value suggests that an unmeasured confounder with a level of association between the confounder and ODD exposure and between the confounder and mortality (after adjustment or standardization for all observed confounders) as measured by risk ratios of 1.53 would be needed to explain away the observed hazard ratio of 0.83 between ODD kidney transplant and mortality. The degree of confounding needed to move the observed upper confidence bound (0.96) to include the null would be lower (E-value = 1.2).
The E-value can also be used to estimate the degree of confounding needed to move an association that might be near the null, as in the present example, to any other clinically important level, even if that level is in the opposite direction from the observed level. For judgments of noninferiority of outcomes, the other clinically important level might be a higher mortality rate for ODD kidneys than other donor kidneys, with the goal of reducing the discard rate of ODD kidneys. For example, suppose that a recipient would tolerate a 5% relative increase in the hazard of mortality from an ODD kidney that might otherwise be discarded. The question then becomes the degree of unmeasured confounding that would explain away an observed hazard ratio of 0.83 versus a true hazard ratio of 1.05. In this case, the estimated E-value becomes 1.84, which represents a larger degree of unmeasured confounding necessary to explain away this nonnull alternative outcome. The corresponding E-value needed to shift the upper bound of the hazard ratio (0.96) to include the hazard ratio of 1.05 is 1.41. Thus, when noninferiority is the clinical question of importance and the goal is to be able to rule out worse outcomes from otherwise discarded donated organs, the comparison of observed associations of organ type and outcome might be with hazard ratio alternatives greater than 1.0. Our reported data, together with the online E-value calculator at www.hsph.harvard.edu/tyler-vanderweele/tools-and-tutorials, can be used to evaluate the robustness of our findings to any nonnull alternative that might reflect the tradeoffs inherent in the investigation of noninferior outcomes.
Appendix Figure. Standardized survival curves stratified by donor mechanism of death (overdose, trauma, or medical).
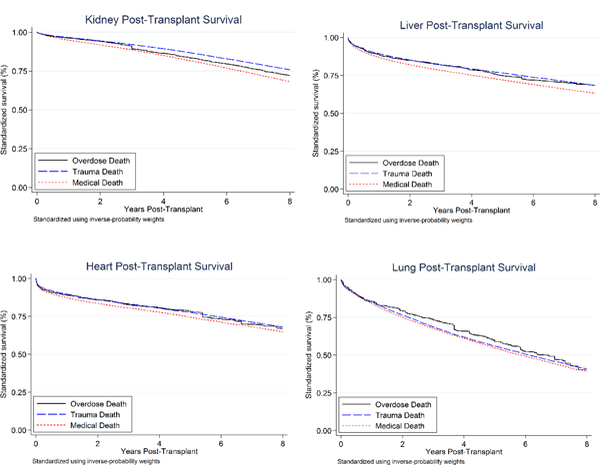
Curves standardized using inverse-probability weights derived from previously specified multinomial logistic regression models..
Appendix Table 1.
Variables Included in Each Posttransplant Outcomes Standardization Model and Respective Missingness*
| Standardization Model | Missingness, % | |
|---|---|---|
| Kidney transplant | ||
| Recipient factor | ||
| Age at transplant | 0.0 | |
| Biological sex | 0.0 | |
| Race/ethnicity | 0.0 | |
| Time receiving dialysis | 0.0 | |
| Indication for transplant | 0.8 | |
| Peripheral vascular disease | 0.4 | |
| Blood type | 0.0 | |
| Calculated panel reactive antibody | 14.9 | |
| Insurance type | 0.0 | |
| HLA A mismatch | 0.01 | |
| HLA DR mismatch | 0.03 | |
| BMI | 6.4 | |
| Left, right, en bloc | 0.0 | |
| Hepatitis C antibody status | 1.0 | |
| Calendar year of transplant | 0.0 | |
| Previous malignancy | 0.3 | |
| Donor factor | ||
| Age at organ recovery | 0.0 | |
| Biological sex | 0.0 | |
| Race/ethnicity | 0.0 | |
| Donation after circulatory death | 0.01 | |
| Cold ischemic time | 5.5 | |
| Blood type | 0.0 | |
| BMI | 0.5 | |
| Creatinine | 0.1 | |
| Shared | 0.0 | |
| Hepatitis C antibody status | 0.2 | |
| Hepatitis B core antibody | 0.3 | |
| Prerecovery diuretics | 2.6 | |
| Prerecovery medications: T4 | 2.4 | |
| Infection in lung | 0.0 | |
| History of cancer | 0.8 | |
| History of cigarette smoking | 1.2 | |
| History of hypertension | 0.7 | |
| History of diabetes | 0.0 | |
| Liver transplant | ||
| Recipient factor | ||
| Age at transplant | 0.0 | |
| Biological sex | 0.0 | |
| Race/ethnicity | 0.0 | |
| Malignant neoplasm | 0.01 | |
| Hepatocellular carcinoma | 0.01 | |
| Hepatitis C antibody status | 9.1 | |
| Portal vein thrombosis | 3.0 | |
| Previous transplant | 0.0 | |
| Previous abdominal surgery | 3.6 | |
| Previous malignancy | 3.6 | |
| Last albumin | 7.2 | |
| Last ascites | 7.2 | |
| Split vs. whole liver | 0.0 | |
| BMI | 4.6 | |
| Insurance | 0.0 | |
| INR | 7.1 | |
| Serum creatinine | 0.2 | |
| Dialysis within previous week | 7.5 | |
| Life support | 0.0 | |
| Diabetes | 1.9 | |
| Blood type | 0.0 | |
| Donor factor | ||
| Age at organ recovery | 0.0 | |
| Biological sex | 0.0 | |
| Race/ethnicity | 0.0 | |
| Donation after circulatory death | 0.01 | |
| BMI | 0.0 | |
| Hepatitis C antibody status | 0.2 | |
| History of diabetes | 0.5 | |
| History of hypertension | 0.3 | |
| Blood type | 0.0 | |
| Cold ischemic time | 5.7 | |
| Shared | 0.0 | |
| Heart transplant | ||
| Recipient factor | ||
| Age at transplant | 0.0 | |
| Biological sex | 0.0 | |
| Race/ethnicity | 0.0 | |
| BMI | 2.0 | |
| Education | 15.1 | |
| Primary indication for transplant | 0.0 | |
| PCW pressure | 12.5 | |
| Total bilirubin | 3.0 | |
| Type ventricular assistance device | 12.0 | |
| Creatinine | 1.2 | |
| Medical condition | 0.0 | |
| CMV status | 6.5 | |
| Previous malignancy | 1.4 | |
| Calendar year of transplant | 0.0 | |
| Donor factor | ||
| Age at recovery | 0.0 | |
| Biological sex | 0.0 | |
| Race/ethnicity | 0.0 | |
| BMI | 0.3 | |
| CMV status | 0.4 | |
| Antihypertensive | 0.3 | |
| History of smoking | 0.9 | |
| History of cocaine use | 1.9 | |
| Clinical infection | 3.1 | |
| Lung transplant | ||
| Recipient factor | ||
| Age at transplant | 0.0 | |
| Biological sex | 0.0 | |
| Race/ethnicity | 0.0 | |
| Education | 9.4 | |
| BMI | 1.2 | |
| Primary indication for transplant | 0.0 | |
| Long-term steroid use | 3.4 | |
| Previous transplant | 0.0 | |
| ECMO | 0.0 | |
| Total bilirubin | 2.2 | |
| Creatinine | 0.5 | |
| Medical condition | 0.0 | |
| FVC | 3.5 | |
| FEV1 | 3.7 | |
| Donor-recipient weight ratio | 0.6 | |
| Donor-recipient height ratio | 1.1 | |
| Calendar year of transplant | 0.0 | |
| Donor factor | ||
| Age at organ recovery | 0.0 | |
| Biological sex | 0.0 | |
| Race/ethnicity | 0.0 | |
| BMI | 0.2 | |
| CMV status | 0.3 | |
| Donation after circulatory death | 0.01 | |
| Prerecovery diuretics | 1.7 | |
| Infection of the blood | 0.0 | |
| Total bilirubin | 0.3 | |
| Antihypertensive | 0.2 | |
| History of cocaine use | 1.6 | |
| History of diabetes | 0.3 | |
| Creatinine | 0.0 | |
BMI = body mass index; CMV = cytomegalovirus; ECMO = extracorporeal membrane oxygenation; INR = international normalized ratio; PCW = pulmonary capillary wedge.
For variables missing <1%, complete-case adjustment was used. For missingness >1%, a missing indicator was included in the multinomial regression model (initial step of propensity score-weighted standardization) to achieve covariate balance across transplant groups for the levels of missing covariate values. More details on this approach for missing values using propensity scores can be found in reference 21, Appendix B.
Appendix Table 2.
Public Health Services Behavioral Criteria for Donors at Increased Risk for Recent HIV, HBV, or HCV Infection
| 1. People who have had sex with a person known or suspected to have HIV, HBV, or HCV in the preceding 12 months. |
| 2. Men who have had sex with men in the preceding 12 months. |
| 3. Women who have had sex with a man with a history of MSM behavior in the preceding 12 months. |
| 4. People who have had sex in exchange for money or drugs in the preceding 12 months. |
| 5. People who have had sex with a person who had sex in exchange for money or drugs in the preceding 12 months. |
| 6. People who have had sex with a person who has injected drugs by intravenous, intramuscular, or subcutaneous route for nonmedical reasons in the preceding 12 months. |
| 7. A child who is ≤18 years of age and born to a mother known to have or be at increased risk for HIV, HBV, or HCV infection. |
| 8. A child who has been breastfed within the preceding 12 months whose mother is known to have or be at increased risk for HIV infection. |
| 9. People who have injected drugs by intravenous, intramuscular, or subcutaneous route for nonmedical reasons in the preceding 12 months. |
| 10. People who have been in lockup, jail, prison, or a juvenile correctional facility for more than 72 hours in the preceding 12 months. |
| 11. People who have been newly diagnosed with or have been treated for syphilis, gonorrhea, chlamydia, or genital ulcers in the preceding 12 months. |
| 12. Donors who meet the following criteria should be identified as being at increased risk for recent HCV infection only: |
People who have been on hemodialysis in the preceding 12 months.
HBV = hepatitis B virus; HCV = hepatitis C virus; MSM = men who have sex with men.
Appendix Table 3.
Standardized Hazard Ratios of Patient Mortality and Death-Censored Graft Loss With and Without Accounting for Variation Across Transplant Programs
| Variable | Hazard Ratio (95% CI) |
|||
|---|---|---|---|---|
| ODD vs. TDD | ODD vs. MDD | |||
| Without Stratification by Center |
With Stratification by Center |
Without Stratification by Center |
With Stratification by Center |
|
| Patient mortality | ||||
| Kidney | 1.10 (0.94-1.29) | 1.08 (0.96-1.23) | 0.83 (0.71-0.96) | 0.83 (0.72-0.96) |
| Liver | 1.08 (0.94-1.23) | 1.01 (0.90-1.12) | 0.89 (0.78-1.01) | 0.85 (0.77-0.94) |
| Heart | 1.07 (0.89-1.28) | 1.01 (0.85-1.19) | 0.94 (0.79-1.13) | 0.90 (0.76-1.06) |
| Lung | 0.90 (0.76-1.05) | 0.93 (0.81-1.06) | 0.86 (0.74-1.01) | 0.88 (0.77-1.01) |
| Death-censored graft loss | ||||
| Kidney | 0.98 (0.86-1.12) | 0.95 (0.84-1.06) | 0.70 (0.62-0.80) | 0.68 (0.61-0.77) |
| Liver | 1.34 (1.02-1.77) | 1.25 (0.97-1.61) | 1.00 (0.76-1.31) | 0.95 (0.74-1.22) |
| Heart | 1.03 (0.67-1.57) | 0.93 (0.69-1.24) | 0.94 (0.62-1.43) | 0.79 (0.60-1.04) |
| Lung | 0.89 (0.68-1.17) | 0.90 (0.72-1.13) | 0.82 (0.62-1.07) | 0.82 (0.65-1.03) |
MDD = medical-death donor; ODD = overdose-death donor; TDD = trauma-death donor.
Appendix Table 4.
Hazard Ratios and E-Values for Patient Mortality and Graft Loss*
| Variable | ODD vs. TDD | ODD vs. MDD | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
E-Value (Lower Bound) |
Hazard Ratio (95% CI) |
E-Value (Lower Bound) |
|
| Patient mortality† | ||||
| Kidney | 1.08 (0.96-1.23) | 1.30 (1.00) | 0.83 (0.72-0.96) | 1.53 (1.20) |
| Liver | 1.01 (0.90-1.12) | 1.09 (1.00) | 0.85 (0.77-0.94) | 1.48 (1.26) |
| Heart | 1.01 (0.85-1.19) | 1.09 (1.00) | 0.90 (0.76-1.06) | 1.36 (1.00) |
| Lung | 0.93 (0.81-1.06) | 1.28 (1.00) | 0.88 (0.77-1.01) | 1.39 (1.00) |
| Death-censored graft failure | ||||
| Kidney | 0.95 (0.84-1.06) | 1.23 (1.00) | 0.68 (0.61-0.77) | 1.94 (1.66) |
| Liver | 1.25 (0.97-1.61) | 1.61 (1.00) | 0.95 (0.74-1.22) | 1.23 (1.00) |
| Heart | 0.93 (0.69-1.24) | 1.28 (1.00) | 0.79 (0.60-1.04) | 1.63 (1.00) |
| Lung | 0.90 (0.72-1.13) | 1.39 (1.00) | 0.82 (0.65-1.03) | 1.58 (1.00) |
MDD = medical-death donor; ODD = overdose-death donor; TDD = trauma-death donor.
Hazard ratios were derived from Cox regression models that accounted for clustering within transplant center and allowed the baseline hazard of each outcome to vary across centers through stratification.
Standardized according to the recipient, donor, and transplant factors in Appendix Table 1.
Footnotes
Reproducible Research Statement: Study protocol: Not applicable. Statistical code: Available from Ms. Bowring (e-mail, mbowrin1@jhmi.edu). Data set: Publicly available for purchase atwww.srtr.org.
Requests for Single Reprints: Christine M. Durand, MD, Department of Medicine, Johns Hopkins University School of Medicine, 1830 East Monument Street, Room 450D, Baltimore, MD 21087; e-mail, cdurand2@jhmi.edu.
Disclaimer: The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and should in no way be seen as an official policy of or interpretation by the SRTR, the United Network for Organ Sharing/OPTN, or the U.S. government.
Disclosures: Dr. Desai reports grants, personal fees, and nonfinancial support from Merck outside the submitted work. Dr. Sulkowski reports grants from AbbVie, Gilead, Merck, Janssen, and the National Institutes of Health and personal fees from AbbVie, Gilead, Merck, Janssen, and Trek outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M17–2451.
Contributor Information
Christine M. Durand, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Mary G. Bowring, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Alvin G. Thomas, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Lauren M. Kucirka, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Allan B. Massie, Johns Hopkins University School of Medicine and Johns Hopkins School of Public Health, Baltimore, Maryland.
Andrew Cameron, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Niraj M. Desai, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Mark Sulkowski, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Dorry L. Segev, Johns Hopkins University School of Medicine and Johns Hopkins School of Public Health, Baltimore, Maryland, and Scientific Registry of Transplant Recipients, Minneapolis, Minnesota.
References
- 1.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–82. [PMID: ] doi : 10.15585/mmwr.mm6450a3 [DOI] [PubMed] [Google Scholar]
- 2.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–52. [PMID: ] doi: 10.15585/mmwr.mm655051e1 [DOI] [PubMed] [Google Scholar]
- 3.Rudd RA, Paulozzi LJ, Bauer MJ, Burleson RW, Carlson RE, Dao D, et al. ; Centers for Disease Control and Prevention (CDC). Increases in heroin overdose deaths—28 states, 2010 to 2012. MMWR Morb Mortal Wkly Rep. 2014;63:849–54. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. Organ Procurement and Transplantation Network. 2017. Accessed at https://optn.transplant.hrsa.gov on 1 June 2017.
- 5.Danovitch GM, Cohen DJ, Weir MR, Stock PG, Bennett WM, Christensen LL, et al. Current status of kidney and pancreas transplantation in the United States, 1994–2003. Am J Transplant. 2005;5:904–15. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 6.Gill JS, Rose C, Pereira BJ, Tonelli M. The importance of transitions between dialysis and transplantation in the care of end-stage renal disease patients. Kidney Int. 2007;71:442–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 7.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–6. [PMID: ] doi: 10.1097/TP.0b013e3181ac620b [DOI] [PubMed] [Google Scholar]
- 8.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3 Suppl 4:114–25. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 9.Friedewald JJ. Utilization and outcomes of marginal kidneys—using Kidney Donor Risk Index to move beyond the current labels [Editorial]. Am J Transplant. 2012;12:1971–2. [PMID: ] doi: 10.1111/j.1600-6143.2012.04149.x [DOI] [PubMed] [Google Scholar]
- 10.Goldberg DS, Blumberg E, McCauley M, Abt P, Levine M. Improving organ utilization to help overcome the tragedies of the opioid epidemic. Am J Transplant. 2016;16:2836–41. [PMID: ] doi: 10.1111/ajt.13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goss M, Reese J, Kueht M, Vierling JM, Mindikoglu AL, Sussman NL, et al. A surge in cadaveric liver donors and a national narcotic epidemic: is there an association? [Letter]. Liver Transpl. 2017;23:698–700. [PMID: ] doi: 10.1002/lt.24761 [DOI] [PubMed] [Google Scholar]
- 12.Bowring MG, Holscher CM, Zhou S, Massie AB, Garonzik-Wang J, Kucirka LM, et al. Turn down for what? Patient outcomes associated with declining increased infectious risk kidneys. Am J Transplant. 2018;18:617–24. [PMID: ] doi: 10.1111/ajt.14577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen JB, Eddinger KC, Shelton B, Locke JE, Forde KA, Sawinski D. Effect of kidney donor hepatitis C virus serostatus on renal transplant recipient and allograft outcomes. Clin Kidney J. 2017;10:564–72. [PMID: ] doi: 10.1093/ckj/sfx048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant. 2010;10:1238–46. [PMID: ] doi: 10.1111/j.1600-6143.2010.03091.x [DOI] [PubMed] [Google Scholar]
- 15.Montenovo MI, Dick AA, Hansen RN. Donor hepatitis C sero-status does not impact survival in liver transplantation. Ann Transplant. 2015;20:44–50. [PMID: ] doi: 10.12659/A0T.892530 [DOI] [PubMed] [Google Scholar]
- 16.Ballarin R, Cucchetti A, Spaggiari M, Montalti R, Di Benedetto F, Nadalin S, et al. Long-term follow- up and outcome of liver transplantation from anti-hepatitis C virus-positive donors: a European multicentric case-control study. Transplantation. 2011;91:1265–72. [PMID: ] doi: 10.1097/TP.0b013e318219eb8f [DOI] [PubMed] [Google Scholar]
- 17.Weinkauf JG, Puttagunta L, Nador R, Jackson K, LaBranche K, Kapasi A, et al. Long-term outcome of lung transplantation in previous intravenous drug users with talc lung granulomatosis. Transplant Proc. 2013;45:2375–7. [PMID: ] doi: 10.1016/j.transproceed.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. About Underlying Cause of Death, 1999–2016. 2017. Accessed at http://wonder.cdc.gov/ucd-icd10.html on 10 March 2017.
- 19.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79. [PMID: ] doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scientific Registry of Transplant Recipients. SRTR Risk Adjustment Model Documentation: Posttransplant Outcomes. 2018. Accessed at www.srtr.org/reports-tools/risk-adjustment-models- posttransplant-outcomes on 22 January 2018.
- 21.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–24. doi: 10.1080/01621459.1984.10478078 [DOI] [Google Scholar]
- 22.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 23.Therneau TM, Crowson CS, Atkinson EJ. Adjusted survival curves. January 2015. Accessed at https://cran.r-project.org/web/packages/survival/vignettes/adjcurve.pdf on 22 January 2018. [Google Scholar]
- 24.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. [PMID: ] doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 25.Mathur MB, Ding P, VanderWeele TJ. EValue: sensitivity analyses for unmeasured confounding in observational studies and meta-analyses. Updated 21 December 2017. Accessed at https://CRAN.R-project.org/package=EValue on 25 January 2017. [Google Scholar]
- 26.Weiner SG, Malek SK, Price CN. The opioid crisis and its consequences. Transplantation. 2017;101:678–81. [PMID: ] doi: 10.1097/TP.0000000000001671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seem DL, Lee I, Umscheid CA, Kuehnert MJ; United States Public Health Service. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep. 2013;128:247–343. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Guidelines for preventing transmission of human immunodeficiency virus through transplantation of human tissue and organs. MMWR Recomm Rep. 1994;43:1–17. [PMID: ] [PubMed] [Google Scholar]
- 29.Kucirka LM, Sarathy H, Govindan P, Wolf JH, Ellison TA, Hart LJ, et al. Risk of window period hepatitis-C infection in high infectious risk donors: systematic review and meta-analysis. Am J Transplant. 2011;11:1188–200. [PMID: ] doi: 10.1111/j.1600-6143.2011.03460.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucirka LM, Sarathy H, Govindan P, Wolf JH, Ellison TA, Hart LJ, et al. Risk of window period HIV infection in high infectious risk donors: systematic review and meta-analysis. Am J Transplant. 2011;11:1176–87. [PMID: ] doi: 10.1111/j.1600-6143.2010.03329.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon EJ, Mullee J, Beauvais N, Warren E, Theodoropoulos N, McNatt G, et al. ; Increased Risk Organ and Informed Consent (IROIC) Workgroup. Education and informed consent about increased risk donor kidneys: a national survey of non-physician transplant providers. Transpl Infect Dis. 2014;16:251–60. [PMID: ] doi: 10.1111/tid.12199 [DOI] [PubMed] [Google Scholar]
- 32.Ros RL, Kucirka LM, Govindan P, Sarathy H, Montgomery RA, Segev DL. Patient attitudes toward CDC high infectious risk donor kidney transplantation: inferences from focus groups. Clin Transplant. 2012;26:247–53. [PMID: ] doi: 10.1111/j.1399-0012.2011.01469.x [DOI] [PubMed] [Google Scholar]
- 33.Reese PP, Tehrani T, Lim MA, Asch DA, Blumberg EA, Simon MK, et al. Determinants of the decision to accept a kidney from a donor at increased risk for blood-borne viral infection. Clin J Am Soc Nephrol. 2010;5:917–23. [PMID: ] doi: 10.2215/CJN.08251109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowring MG, Kucirka LM, Massie AB, Luo X, Cameron A, Sulkowski M, et al. Changes in utilization and discard of hepatitis C-infected donor livers in the recent era. Am J Transplant. 2017;17:519–27. [PMID: ] doi: 10.1111/ajt.13976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massie AB, Desai NM, Montgomery RA, Singer AL, Segev DL. Improving distribution efficiency of hard-to-place deceased donor kidneys: predicting probability of discard or delay. Am J Transplant. 2010;10:1613–20. [PMID: ] doi: 10.1111/j.1600-6143.2010.03163.x [DOI] [PubMed] [Google Scholar]
- 36.Marrero WJ, Naik AS, Friedewald JJ, Xu Y, Hutton DW, Lavieri MS, et al. Predictors of deceased donor kidney discard in the United States. Transplantation. 2017;101:1690–7. [PMID: ] doi: 10.1097/TP.0000000000001238 [DOI] [PubMed] [Google Scholar]
- 37.Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting hepatitis C-positive kidneys. N Engl J Med. 2015;373:303–5. [PMID: ] doi: 10.1056/NEJMp1505074 [DOI] [PubMed] [Google Scholar]
- 38.Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS Jr, Hassan MA, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: results from the HCV-TARGET study. Hepatology. 2017;66:1090–101. [PMID: ] doi: 10.1002/hep.29258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown RS Jr, O’Leary JG, Reddy KR, Kuo A, Morelli GJ, Burton JR Jr, et al. ; Hepatitis C Therapeutic Registry Research Network Study Group. Interferon-free therapy for genotype 1 hepatitis C in liver transplant recipients: real-world experience from the Hepatitis C Therapeutic Registry and Research Network. Liver Transpl. 2016;22:24–33. [PMID: ] doi: 10.1002/lt.24366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumortier J, Leroy V, Duvoux C, de Ledinghen V, Francoz C, Houssel-Debry P, et al. Sofosbuvir- based treatment of hepatitis C with severe fibrosis (METAVIR F3/F4) after liver transplantation. Liver Transpl. 2016;22:1367–78. [PMID: ] doi: 10.1002/lt.24505 [DOI] [PubMed] [Google Scholar]
- 41.Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR, et al. Trial of transplantation of HCV-infected kidneys into uninfected recipients [Letter]. N Engl J Med. 2017;376:2394–5. [PMID: ] doi: 10.1056/NEJMc1705221 [DOI] [PubMed] [Google Scholar]
- 42.Durand C, Brown D, Wesson R, Bhair N, Naqvi F, Ostrander D, et al. EXPANDER-1: Exploring Renal Transplants Using Hepatitis-C Infected Donors for HCV-Negative Recipients [Abstract]. Am J Transplant. 2017;17(Suppl 3). [Google Scholar]
- 43.Sutter ME, Daily MF, Owen KP, Daubert GP, Albertson TE. Multiple organ transplantation after suicide by acetaminophen and gunshot wound. West J Emerg Med. 2010;11:506–9. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- 44.Gok MA, Gupta A, Olschewski P, Bhatti A, Shenton BK, Robertson H, et al. Renal transplants from non-heart beating paracetamol overdose donors. Clin Transplant. 2004;18:541–6. [PMID: ] [DOI] [PubMed] [Google Scholar]


