Abstract
Background
Right ventricular (RV) systolic dysfunction has been associated with adverse outcomes in tetralogy of Fallot (TOF). However, the role and etiology of diastolic dysfunction remains incompletely defined. We sought to assess the association between traditional echocardiographic measures of diastolic function with catheter-based RV end-diastolic pressure (RVEDP) and identify clinical characteristics independently associated with diastolic dysfunction.
Methods
Single-center, retrospective cohort study of surgically repaired TOF patients undergoing cardiac catheterization with echocardiograms within three months prior to catheterization. Tricuspid inflow and tissue Doppler measurements (E/A, E/e′, and deceleration time) defined diastolic dysfunction, graded as impaired relaxation, pseudonormal or restrictive physiology. Regression analyses tested associations between echocardiographic parameters, RVEDP, and clinical characteristics.
Results
Ninety-four subjects were included. Age at catheterization was 8.9 years (Interquartile range 4.4, 15.9). RVEDP was 9.5±2.5 mm Hg. Sixty-one (65%) subjects had echocardiographic evidence of diastolic dysfunction. RVEDP was not associated with any echocardiographic parameter of diastolic function (grade of dysfunction, E/e′, or E/A). Higher RVEDP was associated with larger right atrial and RV end-diastolic area, independently of weight and degree of pulmonary or tricuspid regurgitation, though was not associated with indexed right atrial or RV end-diastolic area. Greater number of interim procedures was associated with higher RVEDP, E/e′, and presence of diastolic dysfunction by echocardiography.
Conclusions
Diastolic dysfunction, as determined by echocardiography-derived and catheter-based (RVEDP) measures, is prevalent in this population of TOF. These measures are not associated with each other, therefore echocardiographic parameters of diastolic function are not reflective of RVEDP. Development of noninvasive parameters that are associated with filling pressures is required.
Keywords: Tetralogy of Fallot, Doppler echocardiography, Diastolic dysfunction, Congenital heart disease
INTRODUCTION
Despite excellent long-term survival, patients with tetralogy of Fallot (TOF) are at risk for right ventricular (RV) dysfunction. Increased cardiovascular morbidity, including ventricular arrhythmias and sudden death, has been associated with RV systolic dysfunction, but the prevalence and impact of RV diastolic dysfunction remains incompletely defined.1–3
The American Society of Echocardiography (ASE) has established criteria for determining RV diastolic dysfunction in adults, grading it as normal, stage 1 (impaired relaxation: E/A <0.8), stage 2 (pseudonormal: E/A 0.8–2.1 with E/e′ >6), and stage 3 (restrictive filling: E/A >2.1 with Deceleration time <120ms).4 Echocardiographic measures of diastolic dysfunction, particularly E/e′, have been shown to have high sensitivity and specificity for elevated right atrial pressures.5–7 While echocardiographic measures of RV diastolic function have established normal values in children,8–10 these measures have not been validated, and few studies have evaluated these measures in patients with TOF.11,12
Although studies in adults with TOF have demonstrated an association between diastolic dysfunction and important markers of disease severity, such as exercise intolerance and arrhythmias,11,13–17 little is known about diastolic dysfunction in mid-term follow up, and whether clinical factors such as neonatal TOF repair (<1 month of age), type of TOF anatomy or surgical repair could influence diastolic function in the long-term. The goals of this study were to determine the association of echocardiographic measures of diastolic function with catheter-based RV end-diastolic pressure (RVEDP) and to define clinical characteristics associated with diastolic dysfunction and elevated filling pressures. We hypothesized that RV end-diastolic pressure (RVEDP) would directly correlate with stage of diastolic dysfunction by echocardiography. Further, diastolic dysfunction would be prevalent in this population and associated with neonatal repair, type of repair, and greater number of interval procedures.
METHODS
Study Population and Data Collection
We conducted a retrospective cohort study of patients with TOF undergoing cardiac catheterization at our institution between January 2006 and December 2015, with echocardiograms performed within three months prior to cardiac catheterization. Inclusion criteria included complete repair of TOF (defined as ventricular septal defect closure ± relief of RV outflow tract obstruction) and presence of pulse-wave-sampled tricuspid inflow and RV free wall spectral tissue Doppler imaging. Subjects were excluded if there was evidence of heart block, mixed forms of disease (such as TOF with complete atrioventricular canal or significant left-sided anatomic heart disease), or if there were any changes in medical or surgical management between echocardiogram and cardiac catheterization. If multiple catheterizations were available, the earliest catheterization with complete echocardiographic data was used. The study was approved by the Institutional Review Board for the Protection of Human Subjects at the Children’s Hospital of Philadelphia.
Echocardiograms
Archived echocardiographic images were reviewed offline for the presence of both pulse-wave tricuspid inflow Doppler and spectral tissue Doppler imaging at the tricuspid annulus of the RV free wall and absence of heart block. When multiple echocardiograms were present, those closest to catheterization were utilized for review.
Tricuspid E and A waves, deceleration time, and spectral tissue Doppler e′ and a′ were assessed by a single observer (MPD) who was blinded to clinical characteristics. Images were obtained on iE33 echo machines (Philips Medical Systems, Andover, MA). To ensure the reproducibility of the tricuspid measurements, quantification of E/A, E/e′, and deceleration time on 10 random samples was repeated on three occasions one week apart. These 10 samples were also repeated by a second observer (LMR) to determine inter-reader variability. Diastolic function was classified as normal, impaired relaxation (E/A<0.8), pseudonormal (E/A 0.8–2.1 with E/e′ ≥6), restrictive (E/A >2.1 with deceleration time <120msec).4
Other echocardiographic parameters included qualitative assessment of RV function, tricuspid regurgitation, and pulmonary regurgitation, RV pressure estimate, and outflow and branch pulmonary artery obstruction. Qualitative assessment of RV function, pulmonary regurgitation, and tricuspid regurgitation was evaluated by a single observer (MPD).5 Fractional area change was measured on apical four-chamber images and calculated as ((end-diastolic area – end-systolic area)/end-diastolic area). Right atrial area was measured at end-systole on apical four-chamber images.
Catheterization data
Catheterization information included age and indication for catheterization, sedation or intubation status, RV systolic pressure, pulmonary vascular resistance, RV outflow tract and branch pulmonary artery gradients, and transpulmonary gradient. RVEDP was measured at end-expiration and considered abnormally elevated if ≥ 10 mm Hg. RVEDP was utilized as the outcome measure due to collinearity with right atrial pressure. All available cardiac waveforms were manually reviewed for data collection.
Clinical data
The following clinical parameters were recorded: TOF anatomy (pulmonary stenosis, pulmonary atresia, absent pulmonary valve), gender, age at time of surgical repair, history of prematurity (< 37 weeks), genetic syndromes, and other medical comorbidities (e.g. gastro-intestinal malformations or neurologic abnormalities), surgical times (bypass, cross-clamp, and circulatory arrest), type of RV outflow tract intervention (transannular patch or RV to pulmonary artery (PA) conduit), and number of interim catheterizations or surgeries between initial surgical repair and evaluated catheterization.
Statistical analysis
Descriptive statistics were presented as frequency counts and percentages for categorical variables and mean ± standard deviation or median (Interquartile range) for continuous variables, as appropriate. Intraclass correlation coefficients for inter- and intra-reader variability were calculated for E/A, E/e′, and deceleration time. The intraclass correlation coefficients for intra-reader variability was 0.98 (95% CI 0.94, 0.99), 0.94 (0.84, 0.98), and 0.96 (0.81, 0.99) for EA, E/e′, and deceleration time, respectively. Intraclass correlation coefficients for inter-rater reliability was 0.93 (0.76, 0.98), 0.93 (0.73, 0.98), and 0.84 (0.21, 0.96) for EA, E/e′, and deceleration time, respectively.
Based on the distribution of the data, one-way ANOVA or the Kruskal-Wallis test was used to assess baseline differences in echocardiographic parameters among stages of diastolic dysfunction. Linear regression was used to determine the association between RVEDP and stage of diastolic dysfunction. Multivariable linear regression was used to adjust for covariates. Interaction effects between individual echocardiographic parameters and age at echocardiogram, fractional area change, and RV end-diastolic area were a priori explored. Agreement between presence of diastolic dysfunction by echocardiogram and elevated RVEDP was explored using Cohen’s kappa coefficient.
Factors associated with diastolic dysfunction were evaluated using univariable and multivariable linear regression for the continuous outcomes RVEDP or E/e′, or logistic regression for the binary outcome diastolic dysfunction by echocardiography. Factors potentially associated with diastolic dysfunction included TOF anatomy, gender, age at surgery, type of repair, length of time between repair and catheterization, and number of interval catheterizations and surgeries. Clinical factors were introduced using stepwise forward linear regression with an entry criteria of p<0.2 for inclusion in the multi-variable model. If there was evidence of collinearity, the variable with the more significant association with the primary outcome variable was included in the final model. The two-sided statistical significance level was set at 0.05. All data analysis was performed using STATA 14.1 (StataCorp, College Station, TX).
RESULTS
Ninety-four patients were included in the study. There was an even distribution of males and females. TOF with pulmonary stenosis was present in 45 (48%), and the majority of subjects received a transannular patch for primary surgical repair (51, 56%). The median age of repair was 107 days (Interquartile range 19–227). Twenty-seven patients (29%) underwent neonatal surgical repair (Table I).
Table I.
Baseline patient Characteristics
| Gender, Male | 49 (52%) |
|
| |
| Ethnicity | |
| Non-Hispanic | 84 (89%) |
| Hispanic | 7 (7%) |
| Not answered | 3 (4%) |
|
| |
| Race | |
| African American | 10 (11%) |
| Caucasian | 69 (73%) |
| Asian | 3 (3%) |
| Other | 12 (13%) |
|
| |
| Anatomy | |
| Pulmonary Stenosis | 45 (48%) |
| Pulmonary Atresia | 23 (25%) |
| Pulmonary Atresia/Collaterals | 20 (21%) |
| Absent Pulmonary Valve | 6 (6%) |
|
| |
| Genetic syndrome | 30 (32%) |
|
| |
| Age at surgery, days | 107 (19, 227) |
| Neonate (n=27) | 9 (4, 16) |
| Non-neonate (n=67) | 151 (98, 321) |
|
| |
| Surgical repair | |
| Transannular Patch | 51 (56%) |
| RV-PA conduit | 25 (27%) |
| Ventricular Septal Defect only | 1 (1%) |
| RV-PA conduit + unifocalization | 15 (16%) |
|
| |
| Number of interval procedures | 2 (1, 4) |
|
| |
| Number of interval surgeries | |
| None | 44 (47%) |
| 1 | 34 (36%) |
| 2 | 13 (14%) |
| 3 | 3 (3%) |
|
| |
| Number of interval interventional catheterizations | |
| None | 32 (34%) |
| 1 | 23 (24%) |
| 2 | 15 (16%) |
| >2 | 24 (26%) |
|
| |
| Indication for catheterization | |
| Hemodynamic catheterization | 12 (13%) |
| Pulmonary artery intervention | 36 (38%) |
| Main pulmonary artery stent/balloon | 15 (16%) |
| Pulmonary valve replacement | 14 (15%) |
| Atrial septal defect closure | 5 (5%) |
| Drug study | 4 (4%) |
| Multiple indications | 8 (9%) |
Data are presented as frequency (percentage) or median (Interquartile range).
RV, Right ventricle
The median age at time of catheterization was 8.5 years (Interquartile range 4.1, 15.1) with echocardiograms performed at a median of 30 days (Interquartile range 6, 54) prior to catheterization. There was overall high-normal RVEDP (9.6 ± 2.6 mm Hg). Forty-eight subjects (51%) had an elevated RVEDP. Pulmonary vascular resistance was normal overall (1.9 (Interquartile range 1.3, 2.5) indexed Wood units).
Median age at echocardiogram was 8.4 years (Interquartile range 4.0, 14.9). There was overall mildly diminished RV systolic function and low-normal fractional area change. The majority had an echocardiographic diagnosis of diastolic dysfunction (65%). Among stages of diastolic dysfunction, heart rate was significantly lower in subjects with restrictive physiology, and there was suggestion that right atrial area was higher in the restrictive group. There was no significant difference in age, qualitative RV function, fractional area change, tricuspid regurgitation, or pulmonary regurgitation by grade of diastolic dysfunction (Table II).
Table II.
Echocardiographic data by grade of diastolic dysfunction
| Echocardiogram parameter | Overall N=94 |
Normal N=24 |
Impaired relaxation N=20 |
Pseudo-normal N=38 |
Restrictive N=3 |
Uncategorized N=9 |
p* |
|---|---|---|---|---|---|---|---|
| Age | 8.4 (4, 15) | 11.9 (5, 25) | 4.3 (2, 15) | 9.3 (5, 13) | 17.5 (14, 20) | 8.4 (7, 9) | 0.08 |
| Heart Rate | 86 ± 20 | 82 ± 20 | 96 ± 18 | 84 ± 15 | 59 ± 10 | 92 ± 36 | 0.01 |
| RVEDP (mm Hg) | 9.6 ± 2.6 | 9.8 ±2.2 | 9.1 ± 3.2 | 9.5 ± 2.5 | 11.7 ± 4.7 | 9.7 ± 1.2 | 0.5 |
| Tricuspid E/A | 1.3 ± 0.6 | 1.4 ± 0.3 | 0.7 ± 0.08 | 1.4 ± 0.4 | 2.2 ± 0.2 | 1.3 ± 1.3 | <0.001 |
| Tricuspid E/e′ | 6.2 (4.6, 8.8) | 4.5 (3.9, 5) | 5.2 (4.3, 6.2) | 8.6 (7, 10.5) | 6.1 (5.9, 7.6) | 6.7 (0, 9.7) | <0.001 |
| Tricuspid deceleration time | 150 ± 63 | 153 ± 53 | 172 ± 70 | 154 ± 53 | 117 ± 2 | 88 ± 87 | 0.01 |
| RV dysfunction | |||||||
| Normal | 10 (11%) | 14(58%) | 10 (50%) | 18 (47%) | 1 (33%) | 6(67%) | 0.9 |
| Mild | 69 (73%) | 7 (29%) | 6 (30%) | 13 (34%) | 2 (67%) | 2 (22%) | |
| Moderate | 3 (3%) | 2 (9%) | 3 (15%) | 7 (19%) | 0 | 1 (11%) | |
| Severe | 12 (13%) | 1 (4%) | 1 (5%) | 0 | 0 | 0 | |
| Right atrial area (cm2) | 10.8 (8, 16) | 15.7 (8, 17) | 9.0 (6, 14) | 9.6 (8, 16) | 19 (17, 21) | 15 (10, 15) | 0.07 |
| RV End-diastolic area (cm2) | 19 (14, 27) | 22 (15, 30) | 16 (9, 21) | 17 (15, 26) | 28 (27, 30) | 19 (15, 23) | 0.1 |
| Fractional area change | 0.29 ± 0.09 | 0.29 ± 0.8 | 0.31 ± 0.1 | 0.28 ± 0.07 | 0.25 ± 0.07 | 0.3 ± 0.09 | 0.2 |
| Tricuspid regurgitation | |||||||
| None/Trivial | 28 (27%) | 7 (29%) | 8 (40%) | 10 (26%) | 2 (67%) | 2 (23%) | 0.6 |
| Mild | 54 (52%) | 15 (63%) | 9 (45%) | 19 (50%) | 1 (33%) | 3 (33%) | |
| Moderate | 18 (18%) | 2 (8%) | 3 (15%) | 8 (21%) | 0 | 3 (33%) | |
| Severe | 3 (3%) | 0 | 0 | 1 (3%) | 0 | 1 (11%) | |
| Pulmonary Regurgitation | |||||||
| None/Trivial | 9 (10%) | 2 (8%) | 2 (10%) | 4 (11%) | 0 | 1 (11%) | 0.6 |
| Mild | 13 (14%) | 3 (13%) | 9 (45%) | 6 (16%) | 1 (33.3%) | 3 (33%) | |
| Moderate | 34 (36%) | 8 (33%) | 9 (45%) | 13 (34%) | 1 (33.3%) | 3 (33%) | |
| Severe | 38 (40%) | 11 (46%) | 0 | 15 (39%) | 1 (33.3%) | 2 (23%) | |
Data are presented as frequency (percentage), mean ± standard deviation, or median (Interquartile range).
RV, Right Ventricle; RVEDP, RV end-diastolic pressure
p-values determined using ANOVA or Kruskal-Wallis test, as appropriate
Association between RVEDP and echocardiographic parameters
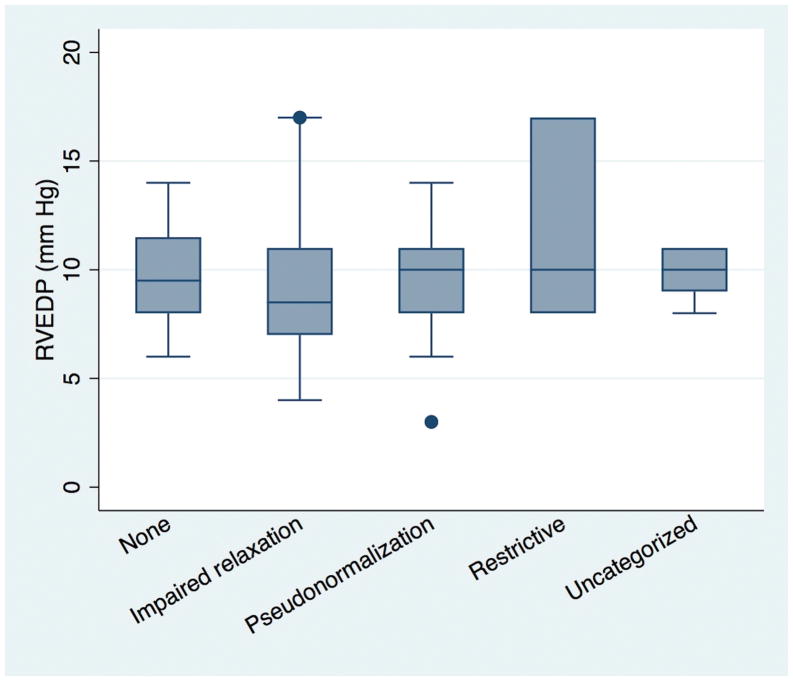
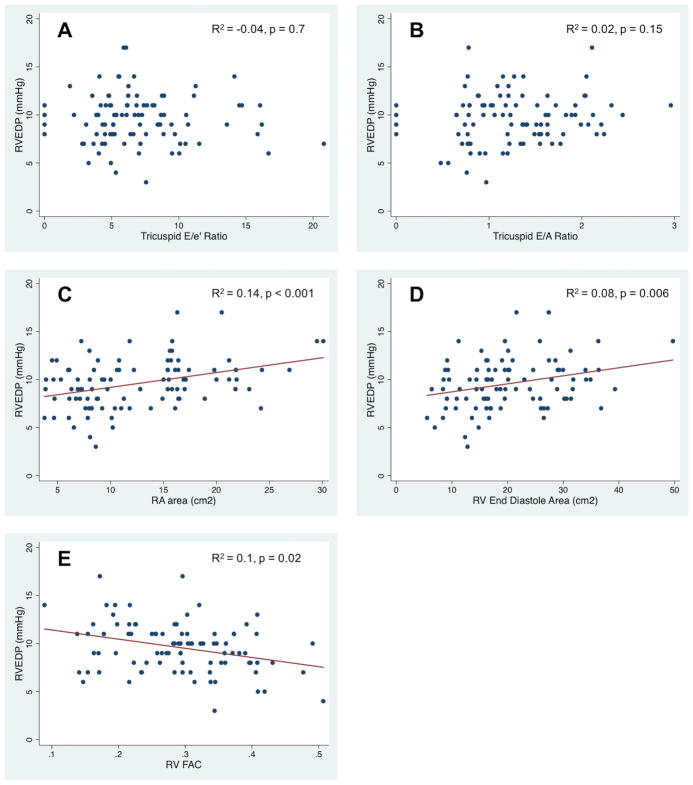
There was no significant difference in RVEDP based on grade of diastolic dysfunction (p=0.5, Figure 1) on unadjusted or adjusted analyses (age and body surface area at time of catheterization, heart rate, and RV end-diastolic area or fractional area change). There was no association between RVEDP and either E/e′ or E/A on unadjusted or adjusted analysis (Figure 2A and B). Interactions with age, fractional area change, and RV end-diastolic area were also not significant. There was poor agreement between an elevated RVEDP and the presence of diastolic dysfunction by echocardiogram (Kappa = 0.04, Table III) or an elevated E/e′ (Kappa = 0.04).
Figure 1.
Box-whisker plot of median, interquartile range, and range of RVEDP for each grade of diastolic dysfunction.
Figure 2.
Scatter plot and regression coefficients of RVEDP vs. A) Tricuspid E/e′ B) Tricuspid E/A C) Right atrial area D) RV end-diastolic area E) Fractional area change.
Table III.
Two-by-two table of diastolic dysfunction by echocardiography and catheterization. RVEDP, Right ventricular end-diastolic pressure.
| Diastolic Dysfunction (Echocardiography) | ||||
|---|---|---|---|---|
|
| ||||
| Yes | No | Total | ||
|
| ||||
| RVEDP (mm Hg) | <10 | 12 | 30 | 42 |
| >=10 | 12 | 31 | 43 | |
|
| ||||
| Total | 24 | 61 | 85 | |
|
| ||||
There was a significant association between RVEDP and right atrial area, RV end-diastolic area, and fractional area change (Figure 2C–E). There was a modest but significant association between right atrial area and RVEDP after adjusting for weight and degree of tricuspid regurgitation (R2 = 0.13, p=0.001). There was a similarly modest association between RV end-diastolic area and RVEDP after adjusting for weight and degree of pulmonary regurgitation (R2=0.08, p=0.05). However, there was no significant association between indexed right atrial or end-diastolic areas and RVEDP, even when adjusting for degree of pulmonary regurgitation.
Clinical parameters associated with diastolic dysfunction
On univariable analysis, older age at catheterization, longer time between catheterization and initial surgical repair, and greater number of follow-up procedures and catheterizations were directly associated with higher RVEDP, although these factors were not significant on multivariable analysis. There was a trend towards significance for the independent association of number of follow-up procedures with RVEDP (coefficient 0.2 mm Hg per procedure (95% CI −0.02, 0.44), p=0.07).
Greater number of follow-up procedures and follow-up surgeries were significantly associated with a higher E/e′, of which number of follow up surgeries was also significant on adjusted analysis, with a 1.7 (95% CI 0.7, 2.7) point increase in the E/e′ ratio for each additional surgery (p=0.001) (Table IV).
Table IV.
Association of Clinical Parameters with E/e′.
| Factor | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p | Beta | 95% CI | p | |
| Gender | −0.9 | −2.5, 0.7 | 0.3 | |||
| Prematurity | 0.1 | −0.13, 0.34 | 0.4 | |||
| Age at catheterization (years) | 0.007 | −0.07, 0.09 | 0.9 | |||
| Time from repair to catheterization | 0.013 | −0.08, 0.10 | 0.8 | |||
| Anatomy | ||||||
| Pulmonary Stenosis | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| Pulmonary Atresia | −1.8 | −3.7, 0.16 | 0.08 | −1.5 | −3.5, 0.36 | 0.11 |
| Pulmonary Atresia/Collaterals | −0.8 | −2.8, 1.2 | 0.4 | −2.0 | −4.1, 0.11 | 0.05 |
| Absent Pulmonary Valve | 2.7 | −0.5, 6.0 | 0.1 | 2.2 | −0.94, 5.4 | 0.17 |
| Age at repair (days) | −0.001 | −0.002, 0.001 | 0.5 | |||
| Neonatal repair | 0.86 | −1.8, 3.5 | 0.5 | |||
| Number of follow-up procedures | 0.4 | 0.12, 0.71 | 0.007 | |||
| Number of follow-up catheterizations | 0.4 | −0.002, 0.7 | 0.051 | |||
| Number of follow-up surgeries | 1.6 | 0.64, 2.5 | 0.001 | 1.7 | 0.72, 2.7 | 0.001 |
| Type of repair | ||||||
| Transannular | (ref) | (ref) | (ref) | |||
| RV to pulmonary artery conduit | 0.4 | −1.5, 2.3 | 0.7 | |||
| Ventricular septal defect only | 0.7 | −7.3, 8.6 | 0.9 | |||
| RV-PA conduit+Unifocalization | 0.1 | −2.2, 2.4 | 0.9 | |||
RV, Right ventricle
Beta represents change in E/e′ per each unit change in variable of interest.
Using diastolic dysfunction by echocardiography as a binary outcome (present or absent), older age at catheterization and longer time between surgical repair and catheterization were protective for diastolic dysfunction, while greater number of follow up procedures and catheterizations were associated with increased odds of diastolic dysfunction on univariable analysis. Of these, age at catheterization (OR 0.92 (95% CI 0.86, 0.99) per year increase in age, p=0.02) and greater number of follow-up procedures (OR 1.6 (95% CI 1.1, 2.3) per follow-up procedure, p=0.02) were independent predictors of diastolic dysfunction.
DISCUSSION
Echocardiographic measures of diastolic dysfunction and risk factors for its development in repaired TOF are poorly understood. In this study, we investigated the association between diastolic dysfunction by echocardiography and cardiac catheterization, and factors associated with diastolic dysfunction in this population. We found no association between echocardiography-derived diastolic parameters and RVEDP, but a significant association between right atrial and RV end-diastolic area with RVEDP, and an association between number of interim procedures with diastolic dysfunction either by echocardiography or catheterization.
Association between RVEDP and echocardiographic parameters
Diastolic dysfunction was prevalent in this cohort utilizing either method, with over three quarters of the study population having either an elevated RVEDP or diastolic dysfunction by echocardiography. However, there was no association between RVEDP and either presence of diastolic dysfunction by echocardiography or individual echocardiographic parameters. Prior studies in adults without congenital heart disease have shown modest to excellent correlation between right atrial pressures and tricuspid valve E/A and E/e′ in varied populations,5–7,18 as well as associations between echocardiographic parameters and improved clinical status in response to therapy in patients with pulmonary hypertension and subclinical hypothyroidism.19,20 These findings would suggest that tricuspid inflow and tissue Doppler parameters are valid measures of RV filling pressures.
However, our findings are similar to prior studies in patients with TOF, which have shown no association between E/e′ and RV filling pressures in both pediatric and adult populations.12, 21 One explanation for this finding could be the use of adult guidelines, which may not be reflective of the age-related variation in echocardiographic parameters. Additionally, this lack of association could be attributed to a pulmonary regurgitation-induced volume-loaded state following TOF repair resulting in elevated RVEDP, a known load-dependent parameter. This may also explain the high-normal RVEDP that is present even in those with normal diastolic function parameters by echocardiography, as well as the association of RVEDP with RV end-diastolic area and right atrial area. Prior conductance catheter studies demonstrating significant differences in diastolic stiffness without differences in RVEDP between restrictive and non-restrictive TOF patients as measured by end-diastolic forward flow suggest that RVEDP is more reflective of load, rather than lusitropy.22
Differences in the intrinsic hemodynamic properties being measured may explain the lack of association between parameters. E-wave velocities do reflect load (based on the contribution of right atrial and RV pressure differences during early diastole), but additionally reflect rate of RV relaxation. E′ velocities reflect RV relaxation, restoring forces, and myocardial stiffness. Alternatively, RVEDP may solely be a function of load. Echocardiographic parameters may therefore more accurately reflect abnormalities in myocardial relaxation. Though incompletely elucidated, underlying fibrosis may play a role in this finding, and is certainly supported by the association between fibrosis by histopathology and e′ in post-operative TOF patients.23–26 These modalities each may therefore reflect different facets of diastolic function, which would explain their lack of agreement with each other, yet their independent association with clinical markers of disease severity, such as greater number of procedures.
Association with clinical parameters
Greater number of interim procedures was associated with a higher RVEDP, diastolic dysfunction by echocardiography, and a higher E/e′. It has been previously demonstrated that re-interventions in TOF are associated with myocardial fibrosis and lower peak VO2 by exercise stress test, findings that likely reflect disease severity and potential underlying myocardial dysfunction.26–28 Our findings would therefore suggest that greater disease burden, as measured by number of interim procedures, is associated with diastolic dysfunction. Further, diastolic dysfunction by echocardiography in patients with TOF has previously been demonstrated to be prevalent and associated with adverse outcomes, including arrhythmias, worse aerobic capacity, and more unscheduled hospitalizations.11, 13–17 We additionally demonstrated that younger age at time of catheterization was independently associated with the presence of diastolic dysfunction on echocardiography. While we would have expected older age at catheterization to be associated with diastolic dysfunction, a possible explanation for this our findings is that older subjects had less significant disease overall and therefore were protected from developing dysfunction.
Limitations
We acknowledge the limitations in this study. First, this study included patients referred for cardiac catheterization, and thus represents a distinct subset of TOF with potentially more significant disease. Therefore, these results cannot be generalized to the population of TOF at large. Further, echocardiograms and catheterizations were not performed simultaneously, and most catheterizations required sedation. As a result, the RVEDP obtained at time of catheterization may not be reflective of the RVEDP at echocardiography due to hemodynamic changes that may have occurred due to use of anesthesia, and therefore misclassification may have occurred. As this is a retrospective analysis, there may be some differences in acquisition of echocardiographic parameters with respect to angle of interrogation. However, all studies were reviewed for adequacy of images prior to inclusion. Due to a limited sample size, there were few subjects with restrictive disease, limiting our power for the most severe group of diastolic dysfunction.
CONCLUSIONS
Diastolic dysfunction, as determined by echocardiography-derived measures and catheter-based measures (RVEDP), is prevalent in this population of TOF. These measures are not associated with each other, therefore echocardiographic parameters of diastolic function are not reflective of RVEDP. Development of noninvasive parameters that are associated with filling pressures is required.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health [grant numbers T32 HL007915 (MPD), K01HL125521 (LMR)] and a Pulmonary Hypertension Association supplement to the K01 (LMR).
Abbreviations
- TOF
Tetralogy of Fallot
- RV
Right Ventricle
- RVEDP
RV End-diastolic Pressure
Footnotes
PATIENT CONSENT
No identifiable personal patient information is present in this publication. Consent was therefore not obtained.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of Ethical Advisory Board and the National Institute of Health, and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee (Institutional Review Board, Children’s Hospital of Philadelphia, IRB # 16--12635). Informed consent was waived for this study as it was retrospective and did not present risk to subjects. There was no animal experimentation in this study.
AUTHOR CONTRIBUTIONS
All authors approved of the content of this manuscript. Michael P DiLorenzo designed the study, performed data collection and analysis, and drafted the manuscript. Wei-Ting Hwang contributed to study design, provided statistical analysis and critically revised the article. Elizabeth Goldmuntz contributed to study design and critically revised the article. Bonnie Ky contributed to study concept and design, data analysis, and critical revision of the article. Laura Mercer-Rosa contributed to study concept and design, data analysis, data collection, critical revision of the article, and funding for the article.
References
- 1.Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 2.Geva T, Sandweiss BM, Gauvreau K, et al. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068–1074. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 3.Scherptong RWC, Mollema SA, Blom NA, et al. Right ventricular peak systolic longitudinal strain is a sensitive marker for right ventricular deterioration in adult patients with tetralogy of Fallot. Int J Cardiovasc Imaging. 2009;25:669–676. doi: 10.1007/s10554-009-9477-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Nageh MF, Kopelen HA, Zoghbi WA, et al. Estimation of mean right atrial pressure using tissue Doppler imaging. Am J Cardiol. 1999;84:1448–1451. doi: 10.1016/s0002-9149(99)00595-0. [DOI] [PubMed] [Google Scholar]
- 6.Sade LE, Gulmez O, Eroglu S, et al. Noninvasive Estimation of Right Ventricular Filling Pressure by Ratio of Early Tricuspid Inflow to Annular Diastolic Velocity in Patients with and Without Recent Cardiac Surgery. J Am Soc Echocardiogr. 2007;20:982–988. doi: 10.1016/j.echo.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Sundereswaran L, Nagueh SF, Vardan S, et al. Estimation of left and right ventricular filling pressures after heart transplantation by tissue Doppler imaging. Am J Cardiol. 1998;82:352–357. doi: 10.1016/s0002-9149(98)00346-4. [DOI] [PubMed] [Google Scholar]
- 8.Lopez L, Colan SD, Frommelt PC, et al. Recommendations for Quantification Methods During the Performance of a Pediatric Echocardiogram: A Report From the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Eidem BW, McMahon CJ, Cohen RR, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17:212–221. doi: 10.1016/j.echo.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Roberson DA, Cui W, Chen Z, et al. Annular and Septal Doppler Tissue Imaging in Children: Normal z-Score Tables and Effects of Age, Heart Rate, and Body Surface Area. J Am Soc Echocardiogr. 2007;20:1276–1284. doi: 10.1016/j.echo.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Aboulhosn JA, Lluri G, Gurvitz MZ, et al. Left and Right Ventricular Diastolic Function in Adults With Surgically Repaired Tetralogy of Fallot: A Multi-institutional Study. Can J Cardiol. 2013;29:866–872. doi: 10.1016/j.cjca.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Hayabuchi Y, Sakata M, Ohnishi T, et al. Ratio of Early Diastolic Tricuspid Inflow to Tricuspid Lateral Annulus Velocity Reflects Pulmonary Regurgitation Severity but Not Right Ventricular Diastolic Function in Children With Repaired Tetralogy of Fallot. Pediatr Cardiol. 2013;34:1112–1117. doi: 10.1007/s00246-012-0612-1. [DOI] [PubMed] [Google Scholar]
- 13.Brili S, Alexopoulos N, Latsios G, et al. Tissue Doppler imaging and brain natriuretic peptide levels in adults with repaired tetralogy of Fallot. J Am Soc Echocardiogr. 2005;18:1149–1154. doi: 10.1016/j.echo.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 14.D’Andrea A, Caso P, Sarubbi B, et al. Right ventricular myocardial dysfunction in adult patients late after repair of tetralogy of fallot. Int J Cardiol. 2004;94:213–220. doi: 10.1016/j.ijcard.2003.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Friedberg MK, Fernandes FP, Roche SL, et al. Impaired right and left ventricular diastolic myocardial mechanics and filling in asymptomatic children and adolescents after repair of tetralogy of Fallot. Eur Heart J Cardiovasc Imaging. 2012;13:905–913. doi: 10.1093/ehjci/jes067. [DOI] [PubMed] [Google Scholar]
- 16.Puranik R, Greaves K, Hawker RE, et al. Abnormal Right Ventricular Tissue Velocities After Repair of Congenital Heart Disease? Implications for Late Outcomes. Heart Lung Circ. 2007;16:295–299. doi: 10.1016/j.hlc.2007.02.084. [DOI] [PubMed] [Google Scholar]
- 17.Vogel M, Schmidt MR, Kristiansen SB, et al. Validation of Myocardial Acceleration During Isovolumic Contraction as a Novel Noninvasive Index of Right Ventricular Contractility: Comparison With Ventricular Pressure-Volume Relations in an Animal Model. Circulation. 2002;105:1693–1699. doi: 10.1161/01.cir.0000013773.67850.ba. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Kopelen HA, Zoghbi WA. Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation. 1996;93:1160–1169. doi: 10.1161/01.cir.93.6.1160. [DOI] [PubMed] [Google Scholar]
- 19.Gan CT-J, Lankhaar J-W, Westerhof N, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;132:1906–1912. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 20.Turhan S, Tulunay C, Ozduman Cin M, et al. Effects of Thyroxine Therapy on Right Ventricular Systolic and Diastolic Function in Patients with Subclinical Hypothyroidism: A Study by Pulsed Wave Tissue Doppler Imaging. J Clin Endocrinol Metab. 2006;91:3490–3493. doi: 10.1210/jc.2006-0810. [DOI] [PubMed] [Google Scholar]
- 21.Do DH, Therrien J, Marelli A, et al. Right Atrial Size Relates to Right Ventricular End-Diastolic Pressure in an Adult Population with Congenital Heart Disease: Right Atrial Size and Right Ventricular Diastolic Filling. Echocardiography. 2011;28:109–116. doi: 10.1111/j.1540-8175.2010.01277.x. [DOI] [PubMed] [Google Scholar]
- 22.Apitz C, Latus H, Binder W, et al. Impact of restrictive physiology on intrinsic diastolic right ventricular function and lusitropy in children and adolescents after repair of tetralogy of Fallot. Heart. 2010;96:1837–1841. doi: 10.1136/hrt.2010.203190. [DOI] [PubMed] [Google Scholar]
- 23.Babu-Narayan SV, Kilner PJ, Li W, et al. Ventricular Fibrosis Suggested by Cardiovascular Magnetic Resonance in Adults With Repaired Tetralogy of Fallot and Its Relationship to Adverse Markers of Clinical Outcome. Circulation. 2006;113:405–413. doi: 10.1161/CIRCULATIONAHA.105.548727. [DOI] [PubMed] [Google Scholar]
- 24.Chen C-A, Dusenbery SM, Valente AM, et al. Myocardial ECV Fraction Assessed by CMR Is Associated With Type of Hemodynamic Load and Arrhythmia in Repaired Tetralogy of Fallot. JACC Cardiovasc Imaging. 2016;9:1–10. doi: 10.1016/j.jcmg.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Farah MCK, Castro CRP, Moreira V, de M, et al. The Impact of Preexisting Myocardial Remodeling on Ventricular Function Early after Tetralogy of Fallot Repair. J Am Soc Echocardiogr. 2010;23:912–918. doi: 10.1016/j.echo.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Broberg CS, Huang J, Hogberg I, et al. Diffuse LV Myocardial Fibrosis and its Clinical Associations in Adults With Repaired Tetralogy of Fallot. JACC Cardiovasc Imaging. 2016;9:86–87. doi: 10.1016/j.jcmg.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Ohuchi H, Hayama Y, Negishi J, et al. Heart failure with preserved right ventricular ejection fraction in postoperative adults with congenital heart disease: A subtype of severe right ventricular pathophysiology. Int J Cardiol. 2016;212:223–231. doi: 10.1016/j.ijcard.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Dobson RJ, Mordi I, Danton MH, et al. Late gadolinium enhancement and adverse outcomes in a contemporary cohort of adult survivors of tetralogy of Fallot. Congenit Heart Dis. 2017;12:58–66. doi: 10.1111/chd.12403. [DOI] [PubMed] [Google Scholar]