Abstract
This update is a conversation with our trauma medical director about the initial evaluation and management of the injured patient. After a brief historical perspective, we discuss the immediate treatment of life-threatening injuries at local hospitals within the context of definitive care at a level 1 trauma center. Our journey is colored by recent cases we have treated at our institution with the hope that we save more Missourian lives.
Historical Perspective
The impact of medical history is often lost on the contemporary graduate, but I believe it is extraordinarily helpful to set the stage for our discussion. Have you read Accidental Death and Disability: The Neglected Disease of Modern Society1? Often referred to as the “white paper” of trauma care, the piece was published in the fall of 1966 by the National Academy of Sciences. If you haven’t studied it, you should. The general public was insensitive to the trauma disease process. Prevention efforts and research were sparse. Medical training for responders was suboptimal if present at all. Organized rehabilitation didn’t exist. Patients with long bone fractures died from an alarmingly high rate of venous thromboembolism, yet it was rarely documented in the medical record as a cause of death.2 In short, we didn’t know what we didn’t know.
This visionary paper established standards for educating the lay public and training emergency responders. A foundation for data acquisition and gap analysis occurred at a regional and national level. A mechanism for emergency department and hospital accreditation was established; the precursor for today’s American College of Surgeon’s Committee on Trauma Verification Review Committee. It established “the system” and multiple works demonstrated improved outcomes.3 I didn’t steer off course unintentionally. Knowing your role in a trauma system becomes vitally important to survival, especially in the rural environment where geography and resource-scarce areas comprise the adversary of optimal care. Especially in a place like Missouri.
Initial Evaluation
Let’s get down to the business at hand. Conjure up a typical scenario presenting at your institution where you are the only provider in a rural emergency department (ED). You have two nurses, a clerk, and a radiology/lab technician. There is no surgical capability in-house. The local EMS provider calls in with a young female injured in a car crash: lower extremity fractures, abdominal bruising, and she is “acting like a head injury.” A farmer catches his leg in an auger – they’ve used trauma shears to complete the amputation and he’s in shock. Perhaps a young man involved in an assault sustains gunshot wounds to the proximal thigh and left thoraco-abdomen with significant trouble breathing. You’ve only dealt with these ten times or less in the previous year… so we need to buckle down and get to work.
First, I cannot say enough about standardization of practice. There is a best-practice model to follow: The Advanced Trauma Life Support Course (ATLS™)4 is the most well-known and complete reference to a stepwise, safe approach to evaluating the seriously injured. This common approach means no matter who staffs the ED, physicians, nurses, and support personnel know the expectations. They understand the preparation, actions, and correct sequence for successful resuscitation. Standardization minimizes variation among providers and improves quality of care. It also helps develop a level of comfort to a potentially very stressful situation, keeping you in charge of the team and on the right track. For those of you practicing at a critical access facility, the College’s Rural Trauma Team Development Course (RTTDC™) is an excellent alternative.
When the patient arrives, listen to your pre-hospital providers. They often provide clues that will direct your search for injury. However, resist the temptation to enter what I call a CSI interrogation of the EMT or paramedic… none of the sensational, often inaccurate details change the next steps; the primary and secondary survey. “Annie, I’m Dr. Coughenour. What happened to you; are you ok?” I say it while I feel for a radial pulse and scan the body for life-threatening bleeding. In less than 10 seconds I’ve assessed the patency of the airway, adequacy of breathing and ventilation, circulatory status and presence (or absence) of life-threatening hemorrhage and evaluated the patient’s neurologic capability. A few additional details and we’ve completed the primary survey. Any immediate threats to life should be treated without delay. If there are none, proceed with adjuncts to the primary survey such as application of monitors, obtaining a manual blood pressure reading, and plain radiographs of the chest and pelvis. If ultrasound is available to you, focused assessment with sonography for trauma (FAST) assists in identification of cavitary hemorrhage, pericardial tamponade or pneumothorax. If there are still no injuries that require immediate attention, focus on a head-to-toe physical examination known as the secondary survey.
At completion, you now can summarize to the team:
what injuries are known or suspected
begin to develop a plan of care, and most importantly
can we take care of this patient or do we need to begin the transfer process? All less than 15 minutes from patient arrival! You’re doing great, stay calm, and keep moving forward.
Allow me to address two misconceptions concerning the initial evaluation: lab tests and CT imaging. First, you don’t need every lab test available. In fact, you only need three. First, arterial blood gas analysis signals the degree of cellular dysfunction and anaerobic metabolism; a venous sample is adequate and many ABG analyses can include a lactate, basic electrolytes, and a hemoglobin (if you just can’t resist). Second, coagulation parameters evaluate for early post-traumatic coagulopathy, a very worrisome and often deadly finding. Third, if you have blood products available, a type and crossmatch will enable the patient to receive type-specific product as soon as possible. Everything else is of questionable benefit. The severely injured human hasn’t had time to equilibrate to significant blood loss so a CBC will be deceiving. Electrolytes and renal function are not helpful. Liver function tests might add to the complexity of the billing, but they contribute nothing to saving the patient’s life. Focus on guiding your resuscitation decision-making.
Patients demonstrating a clear reason to initiate the transfer process to a higher level of care do not require imaging. In fact, I would recommend against it.5 Assuming you have begun treatment for injuries identified in your primary and secondary survey but find yourself needing more information to determine proper disposition for the patient, CT imaging can be considered. Radiation exposure, incidental findings, and appropriate study selection can make this a complicated decision. Let’s focus on the major issue at hand; if you’re going to scan the patient, get the appropriate information necessary for subsequent decision-making. If you aren’t sure what to scan or how to do it, engage your regional trauma center. They will likely share imaging protocols so you can standardize the process. Omitting intravenous contrast and failing to scan certain areas (CT angiogram of the neck for instance) are the most common reasons we have to repeat studies at the trauma center. Interestingly, a recent study from Iowa noted no improvement in time from arrival at the trauma center to operative management for brain injuries when a CT was performed at the referring center.6 Assure YOU need the test. Do not assume it is a requirement of the trauma center.
Initial Management
Now that we’ve covered the principles that will allow for a rapid but accurate patient assessment, let’s discuss what you might have to do. In reality, there are only a few procedures that will handle the majority of cases, but they can be anxiety-provoking and chaotic if you aren’t prepared. Remember that earlier bit about standardization? As I said, when you find a problem during completion of the primary survey, act immediately. Airway management may consist of nasal or oral airway placement with bag-mask ventilation while equipment is prepared, followed by endotracheal intubation. A variety of rescue devices are available for the difficult airway – decide which ones your institution or group agrees to use and train often. Patients can sustain for quite a while in shock, but they’ll only tolerate a few minutes of significant hypoxemia. Write out your approach for drug-assisted intubation and hang it on the wall. Avoid hypotension by volume resuscitating critically ill patients prior to airway intervention.
Quite possibly the most important initial management maneuver, and the most frightening, is the surgical airway. When a cuffed tube cannot be placed in the trachea, and rescue devices are ineffective, proceed immediately to cricothyroidotomy. I strongly advise the Three-Step Method, supported by literature for its economy of motion and success.7 In cases where the anatomy cannot be discerned, make a vertical incision and divide the subcutaneous tissue with your finger, feeling for the anterior wall of the trachea. The anatomy should become apparent, and landmarks will guide you. Pass a bougie through the incised cricothyroid membrane and advance an endotracheal tube until the balloon disappears. Remove the bougie, inflate the cuff and begin ventilation. Most anatomy will accommodate tubes from 5.5mm (smallest that will still slide over a traditional adult bougie) to 7.0mm. Use caution as anxiety will promote mainstem intubation. Lastly, the small anterior jugular veins can bleed and will create quite a spectacle. Plan on doing the procedure by feel not by sight.
Once the airway is secure, breathing is next on our priority list. Needle decompression and tube thoracostomy are used to decompress pneumothoraces and hemothoraces following injury. While conventional teaching places a long, large-bore needle in the 2nd interspace along the mid-clavicular line, newer cadaveric studies promote a 5th interspace approach along the anterior axillary line.8 Either is acceptable, but assure you’ve entered the pleural space to correct the problem. Because the catheter is prone to malposition following withdrawal of the needle, I advocate for a rapidly placed chest tube. Unfortunately, this may be a lost art in many emergency departments. That’s right… I said it. Many physicians staffing EDs in our state can’t place a chest tube. I’ve seen the attempts and I’ve listened to nurses who tearfully describe the weak effort put forth by physicians they work with. Be proactive and refresh your skills. Or take yourself off the ED schedule.
Vascular access is usually straight forward. Peripheral intravenous access with a 14 to 18 gauge catheter is still preferred. Using an ultrasound probe for the difficult stick or moving straight to an intraosseous device for patients in life-threatening shock is best. If you are comfortable with central venous access the subclavian position works well but carries a risk of inadvertent arterial puncture and pneumothorax. If you select central access, utilize an appropriate line – temporary dialysis catheters or pulmonary artery catheter introducers are short and have a large lumen, thus they can instill a large volume of fluid in a hurry. The traditional “triple-lumen” catheter has no place in the initial resuscitation following trauma.
I feel obligated to briefly discuss resuscitation. The literature has been clear since 1994 as the beginning of the movement away from crystalloid infusion towards earlier blood product transfusion.9 Patients bleed whole blood. Packed cells are helpful, but the plasma component is equally vital. Have a system to mobilize what you have in a timely manner. If you don’t have access to blood components, use crystalloid to maintain a radial pulse and mentation. Avoid the temptation to ‘normalize’ vital signs… and move the patient rapidly to a place capable of stopping the bleeding. Albumin and other colloids aren’t worth the increased cost to stock, so we avoid them entirely. Since uncontrolled hemorrhage is implicated in as many as 20% of preventable deaths following trauma, we should discuss bleeding. Direct pressure, compression dressings with hemostatic gauze, and tourniquet application may be necessary. Reduce and stabilize open long-bone fractures. Yes, reduce them all! Returning the compartment to its normal anatomic alignment slows bleeding. “Splinting in position found” has a place in the medical archives, not present-day trauma care (joint dislocations being the one notable exception). Don’t forget often overlooked sources of hemorrhage such as the scalp, especially in the anticoagulated medically-frail elderly. I like to say I’ve never seen a death related to hemorrhage shock from a scalp laceration…but I can’t.
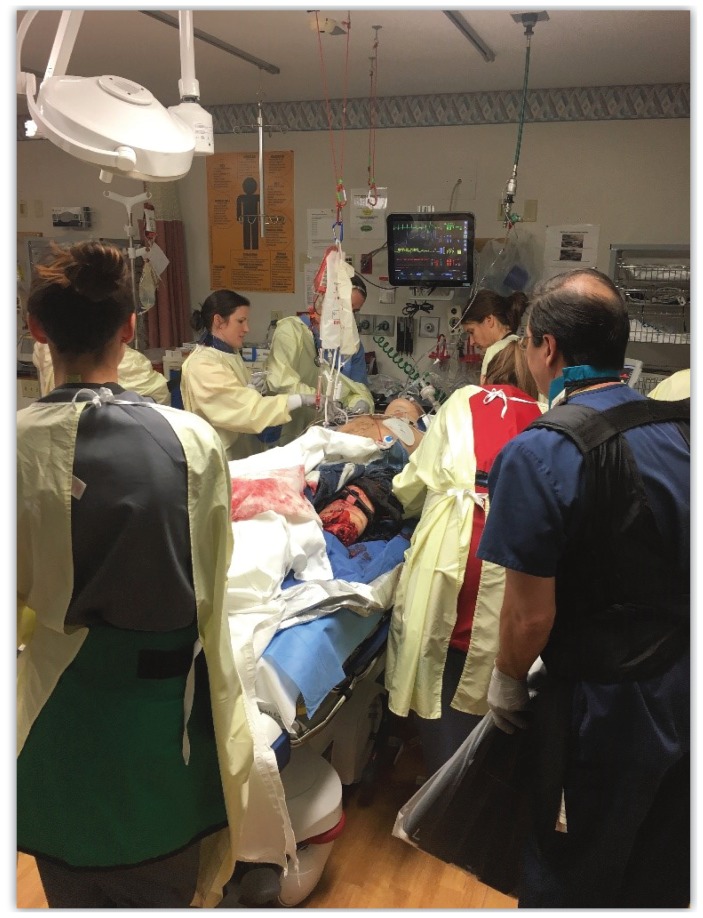
What about surgical capability? General or subspecialty surgeons should be willing to help plan your standardized approach to trauma. Use them as a resource. If you are a surgeon, please support your emergency care colleagues in their efforts to better prepare the hospital’s response to major injury. Most importantly, discuss mobilization of the operating room and the necessary components to operate on a critically ill patient. Is it something the institution is willing and able to do? A hemorrhage control operation prior to transfer can be life-saving, demonstrating that complex surgical problems often demand simple surgical solutions. A gunshot wound to the trunk may require suture ligation of a single bleeding vessel. Packing and a temporary closure can then be followed by transfer to the trauma center for a second-look surgery and definitive closure. Splenectomy and surgical control of extremity hemorrhage are other examples of straight-forward operations that can have significant positive impact. Surgery at a non-designated hospital or lower-level trauma center (III or IV) is a vital component of a larger trauma system, and sadly it hardly ever occurs (Figure 1).
Figure 1.
Trauma resuscitation.
Decision to Transfer
Military trauma care has evolved around the concept of echelons of care, where far-forward surgical teams provide immediate care with limited resources. Patients are then moved to higher levels of care for definitive treatment and rehabilitation. A mature civilian trauma system should mirror this concept, where patients that need immediate intervention are taken to the local ED with subsequent transfer to a regional level 1 trauma center. The idea, coined ‘minimally acceptable care’, was described in a 2002 edition of Missouri Medicine by one of my mentors, Thomas Helling.10 A word of caution; moving patients through the “echelons” of care takes time. I rarely see a patient stop at the local ED and depart for the trauma center in under 90 minutes. Evaluate your local hospital and EMS resources and strive for an initial ED stay of less than 60 minutes. Plan which patients may be best served bypassing the nearest hospital to more rapidly arrive at a center capable of operative hemorrhage control. Always keep quality care in mind; rapid transfer of an untreated pneumothorax – still bad.
I visualize these patient transfer decisions in three basic categories. First, pre-hospital providers can provide enough information for the most severely injured to move to a higher level of care; you have no surgical or critical care capability and the patient has a GCS of 4, transfer without a CT scan. Second, some patients with minor injuries can and should be managed locally. Rib fractures requiring 24 hours of pain management or a ground-level fall that requires a medical evaluation are good examples. These patients will be happy to receive quality care close to home. Third, and often most difficult, are the patients that appear stable until they’re not. Unrecognized bleeding or significant solid organ injuries may take time to identify. Follow the mantra of RTTDC™, and work to complete the primary and secondary survey and make a transfer decision within 15 minutes of patient arrival11, understanding some patients may require additional diagnostics before demonstrating a need for transfer.
Allow me to make some statements on a few odds and ends. Consider what the EMTALA statutes say, but most importantly what they don’t say. You are required to treat a patient based on your facility’s available resources. It is perfectly acceptable to transfer a hypotensive patient with on-going bleeding if you have no surgeon. Be willing to have difficult discussions with families regarding end of life care. When a 93-year-old patient falls and suffers massive, non-survivable intracranial hemorrhage, a neurosurgeon doesn’t necessarily need to be the one to relay that news. Especially when the family would decline surgical intervention if offered. In some cases, telephone or telehealth consultation may suffice. Lastly, demand quality from the providers moving your patients. Just as all hospitals are not created equal, all ambulances and helicopters aren’t either. Work closely with your EMS folks to establish expectations and participate in their ongoing education.
Summary
The patient with serious injury is a high-acuity, low-occurrence event. Material and human resources may be limited. Important adjuncts to optimal care such as experience, education, blood components, or surgical expertise are often unavailable. Standardize your institution’s approach and train often. Perform a rapid primary and secondary survey, treating life-threatening injuries as they are identified. Airway management, tube thoracostomy, and various maneuvers to control hemorrhage may be necessary. Practice the concept of ‘minimally acceptable care;’ treating the patient within the hospital’s capability and promptly transferring the more severely injured to a higher level of care. Engage your regional trauma centers and use them as a resource.
Footnotes
Jeffrey Coughenour, MD FACS, is Associate Professor of Surgery, Trauma Medical Director, Division of Acute Care Surgery, Department of Surgery, University of Missouri School of Medicine, Columbia, Missouri.
Contact: coughenourj@health.missouri.edu
Disclosure
None reported.
References
- 1.Accidental Death and Disability: The Neglected Disease of Modern Society. National Academy of Sciences and National Research Council Committee on Trauma, National Research Council Committee on Shock. Washington, DC: National Academies Press; 1966. [PubMed] [Google Scholar]
- 2.An analysis of 950 fatal injuries. Fitts WT, et al. Surgery. 1964;56:663–668. [PubMed] [Google Scholar]
- 3.A systematic review and meta-analysis comparing outcome of severely injured patients treated in trauma centers following the establishment of trauma systems. Celso B, Tepas J, Langland-Orban B, Pracht E, Papa L, Lottenberg L, Flint L. J Trauma. 2006 Feb;60(2):371–8. doi: 10.1097/01.ta.0000197916.99629.eb. discussion 378. [DOI] [PubMed] [Google Scholar]
- 4.American College of Surgeons. Advanced Trauma Life Support. https://www.facs.org/quality-programs/trauma/atls.
- 5.Imaging may delay transfer of rural trauma victims: a survey of referring physicians. Lee CY, Bernard AC, Fryman L, Coughenour J, Costich J, Boulanger B, Chang P, Kearney PA. J Trauma. 2008 Dec;65(6):1359–63. doi: 10.1097/TA.0b013e31818c10fc. [DOI] [PubMed] [Google Scholar]
- 6.Head CT before transfer does not decrease time to craniotomy for TBI patients. Sidwell RA, et al. Am Surg. 2018 Feb 1;84(2):201–207. [PubMed] [Google Scholar]
- 7.Emergent surgical airway: comparison of the three-step method and conventional crico-thyroidotomy utilizing high-fidelity simulation. Quick JA, MacIntyre AD, Barnes SL. J Emerg Med. 2014 Feb;46(2):304–7. doi: 10.1016/j.jemermed.2013.08.065. [DOI] [PubMed] [Google Scholar]
- 8.Cadaveric comparison of the optimal site for needle decompression of tension pneumo-thorax by prehospital care providers. Inaba K, Karamanos E, Skiada D, Grabo D, Ham-mer P, Martin M, Sullivan M, Eckstein M, Demetriades D. J Trauma Acute Care Surg. 2015 Dec;79(6):1044–8. doi: 10.1097/TA.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 9.Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. Bickell WH, Wall MJ, Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mat-tox KL. N Engl J Med. 1994 Oct 27;331(17):1105–9. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 10.Minimal acceptable care as a vital component to Missouri’s trauma system. Helling TS. Mo Med. 2002 Jul;99(7):275–8. [PubMed] [Google Scholar]
- 11.American College of Surgeons, Rural Trauma Team Development Course. 4th Edition. [Accessed 6 April 2018]. https://www.facs.org/quality-programs/trauma/education/rttdc.