Abstract
Advances in the management of burn patients have contributed to significant improvements in morbidity and mortality over the last century. The physiologic insult from this injury pattern, however, still requires extensive surgical intervention, resuscitation and multidisciplinary care. This paper will review the standard of care of these patients in the context of a recent case study from our institution.
Introduction
In spite of a decreasing frequency of burn-related injuries in the 21st century due to improved manufacturing production of commercial goods, thermal injury in the United States is still a major injury pattern. Over 200,000 patients in the United States alone were burned between 2005 and 2016, resulting in over 6000 deaths.1 Mankind has been dealing with thermal injuries for thousands of years, yet “modern” burn care has evolved exponentially over the last 50–60 years. Advances in resuscitation, operative care and grafting techniques, infection prevention and treatment, and mitigation of hypermetabolism have all improved survival and recovery. In spite of these advances, however, questions and controversies regarding best practices are still prevalent, and numerous burn centers and laboratories across the United States continue to research various aspects of burn care, from the resuscitative phase to the reconstructive and recovery phase.
These advances in burn care have improved burn survival from a near 100% mortality seen with a burn size of 30% in the early 1900s,2 to survival estimates over 50% in young, healthy patients with burn sizes up to 95%.3 Nonetheless, the acute phase of resuscitation still generates significant controversy and is not a standardized process. One can query the resuscitation protocols of various burn units throughout the country and find many variations, from the usage of crystalloid-only formulae to adding colloid at various time points in the acute period, to the usage of “rescue therapies” and what they constitute and when to use them. While this review will not go into great detail of the variations, we will describe our initial burn evaluation, subsequent resuscitation, and overall management plan in caring for a seriously thermally-injured patient.
Case Presentation
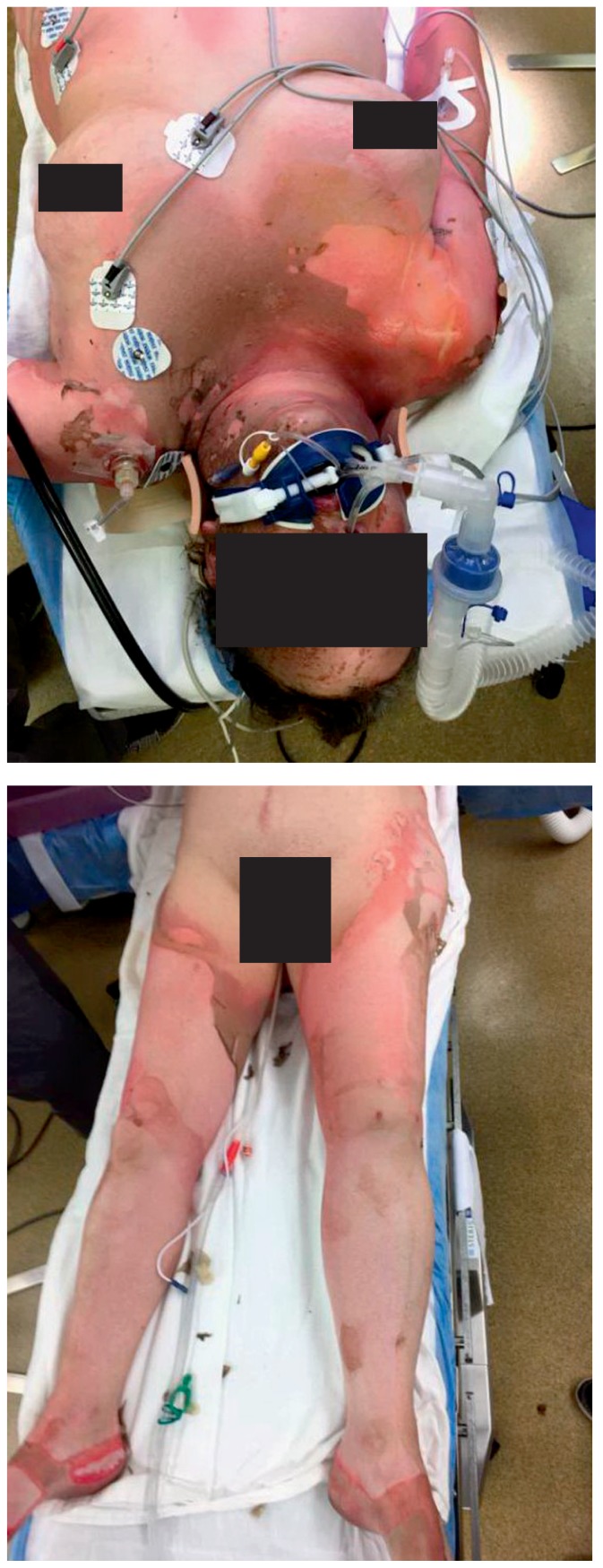
A 58-year-old female patient was activated as a Level 1 trauma alert after being involved in a house explosion with resultant fire. She was awake and alert with no loss of consciousness at the scene but sustained significant thermal injuries per EMS report. She was intubated pre-hospital for “airway protection” out of concern for inhalation injury with facial burns. Initial evaluation revealed an older woman, orally intubated with bilateral breath sounds, mildly tachycardic in the 100s, moderately hypertensive in the 160s/90s, with readily apparent full-thickness burns to the face, neck, anterior torso, bilateral arms, and bilateral legs (Photos 1 and 2). Secondary survey and imaging revealed no further injuries. At this juncture, it’s important to remember and remind the non-burn or trauma center practitioner that a thermally-injured patient is still a “trauma” patient. While a large, third degree burn certainly elicits a significant morbid response in many observers, spending significant time managing the burn wounds while neglecting potential internal hemorrhage will invariably lead to a delay in treatment and worse outcomes.
Photos 1 and 2.
Patient Initial Burns
The patient was immediately taken to our specialized Burn Operating Room once other injuries were ruled out and both non- and -excisional debridement of her burn wounds occurred, with resultant wound dressing application. Her upper body burns were debrided and dressed with antimicrobial dressings. She then resuscitated for the next 48 hours, ultimately receiving approximately 3.3 mL/kg/%TBSA in the first 24 hours post-injury based on a TBSA of 63%, primarily full-thickness (third degree). Resuscitation continued over the first 48 hours, and the patient underwent serial excisional debridement and wound preparation procedures over the next few weeks. Given the size of her burns, we opted to utilize cultured epidermal autografts for assistance with skin/wound coverage. It is important to note that during the entirety of our patient’s two-month hospitalization she received attentive multidisciplinary care including efforts from nutrition services, therapy services, social work, as well as the nursing and physician teams. After continued local wound and graft care the patient was discharged on HD61 to a rehabilitation facility, where she stayed for approximately three weeks until discharge home, where she now lives independently and is continuing to improve.
Discussion
Evaluating overall burn size can, likewise, be difficult to the untrained. While we expect that many in the medical field have heard of “the rule of 9s”, establishing the rule in practice is trickier. Many studies have evaluated overall accuracy of pre-burn center size estimates from both EMS and referring hospitals, many of which are incorrect. What is perhaps more troubling is that the inaccuracies run in both direction, i.e. overestimating burn size is as frequent as underestimating. The American Burn Association (ABA) has a list of Burn Center Referral Criteria as well as a helpful guide to the “Rule of 9s for public usage on their main website, reprinted in this summary (Figure 1).
Figure 1.
ABA Burn Referral Criteria
Historically, “burn surgery” consisted of reconstruction and scar release if the patient survived. Lieutenant Colonel C.P. Artz, in 1955, discussed “exposing” the burn wounds to air until eschar forms, “[i]n full-thickness burns there is dehydration of the pearly white or charred dead skin, and it is converted into a protective eschar....This eschar serves as a temporary physiological cover until liquefaction occurs beneath it in 14 to 21 days.”4 It wasn’t until the 1970s that a Yugoslavian surgeon, Zora Janzekovic, described her experience tangentially excising deep partial- and full-thickness burn wounds in over 1,600 patients that “burn surgery” truly developed into a surgical subspecialty.5 Most modern burn units excise deep-partial and full-thickness burn wounds “early,” typically 24–72 hours post-injury. In our experience, “early” means at or near admission, as burned tissue is a nidus of the inflammatory cascade that potentially leads to the “burn shock” phenomenon.6
Fluid resuscitation of the thermally injured patient is, in many ways, the most important early aspect of burn care, and likely contributes most to overall improvement in burn survival. The need for fluid resuscitation was first recognized in modern times in the 1920s. A physiologist at Yale Hospital, Dr. Frank Underhill, while caring for 20 burn-injured patients, discovered upon evaluation of burn-blister fluid a composition quite similar to plasma. He correctly theorized that “burn shock,” or the hemodynamic instability that occurs after a major burn injury, was a hypovolemic state and that an intravascular volume-based treatment was necessary.7 In 1942 the “Cocoanut Grove” nightclub, a popular, Pacific-Island themed club in Boston, Ma., caught fire. Drs. O. Cope and F. D. Moore cared for a majority of the patients between Boston City and Massachusetts General Hospitals, and in so doing helped codify the relationship between patient size and overall burn size as they related to fluid resuscitation. This resuscitation work was further advanced by C. Baxter and T. Shires at Parkland Hospital in Dallas, Texas, in the 1960s and 70s, ultimately leading to the Parkland™ formula, or 4mL/kg/%TBSA of Lactated Ringers solution, which is the most common burn resuscitation formula used in the United States.8
Large body-surface area burns are typically very difficult to close in an expedited manner, primarily due to lack of donor site autograft to use. To combat this issue numerous tissue substitutes have been developed over the last several decades. C. P. Artz, described earlier, relates using “postmortem homografts,” “removed from the body of a deceased person under aseptic conditions soon after death…”.4 In modern times, “postmortem homografts” are now standardly called allografts and are typically utilized in a cryopreserved rather than fresh fashion. These are nearly always temporary dressings meant for wound coverage and desiccation prevention. Concerns of disease transmission, skin supply, and expense make the use of allografts somewhat problematic. Bioengineered “skin substitutes” have been utilized in large body surface area burns for decades. The first, and perhaps most widely used is a bilaminar product called Integra.™ Developed in the 1980s, it combines bovine collagen and shark cartilage-derived chondroitin-6-sulfate, and allows for vascularization and formation of a neodermis when placed on full-thickness burns. The outer layer is a Silastic silicone-based material that acts like an epidermis and allows for protection of the fragile underlying collagenous material. Once the collagen is engrafted in a few weeks, it allows for placement of an ultra-thin split-thickness skin graft. Similar materials have been developed for use in burn care, including another bovine collagen product called Primatrix™ as well as a Hyaluronic acid derivative called Hyalomatrix.™ In very large burns with extremely limited donor sites, cultured “skin” has been utilized with great success at wound closure. Called Epicel,™ confluent sheets are grown into epidermis within two to three weeks from a sample of patients’ full-thickness skin sample by a company in Cambridge, Massachusetts. These sheets can then be implanted on a prepared wound and are FDA-approved for compassionate use in large burns. The end-result is a closed but imperfect wound as the thin epidermal layer offers little resistance to blistering or shearing. The expense associated with its usage is also considerable. An offshoot of “cultured” skin is a product commonly called “spray-on skin” in social media and by the public. ReCell™ is a real-time non-cultured skin graft alternative that is presently being evaluated by the FDA. By obtaining a small full-thickness skin sample, a clinician prepares a suspension containing basal keratinocytes using a proprietary kit in real-time that may be “sprayed” on an excised and prepared wound bed in an approximate 80:1 expansion, which is truly donor-site sparing.9 Thus, on HD2 a 6 × 2 cm full-thickness skin sample from our patient was sent for growth of epidermal sheets which were implanted without complication approximately one month after injury.
Burn care is a true “team effort,” and, in fact, was likely the first “team-centered” surgical subspecialty developed after more than 500 people were killed and 3,000 injured in the “Texas City Disaster” in 1947, still considered to be America’s “worst industrial accident.”10 The injured were cared for by Dr. T. Blocker and the University of Texas Hospital in Galveston, and during their convalescence were cared for by a team comprising nurses, physicians, therapists, nutritionists, and social workers to maximize outcomes. The team recognized the need for early nutrition, early mobilization, and aggressive wound care. These concepts are still followed today.
Conclusion
Care of the patient with a large body-surface area burn is complex, lengthy, and fraught with potential complications. These complications can be anticipated and minimized in burn centers accustomed to the complexities of major burn care; ultimately yielding improved survival and functional outcomes. The patient in this article, in spite of an anticipated initial mortality approaching 70% on admission, survived to discharge with only a few treatable complications, and is currently home, driving, and otherwise living independently. Her burn scars are being managed with outpatient scar exercises such as moisturization, massage, and compression. This positive outcome is the norm in burn centers, and an excellent example of why patients with major burn injuries should be cared for in these specialized centers. Any patient with a significant burn, even if not delineated on the ABA referral list, warrants discussion with a burn-trained surgical team.
Footnotes
Jeffrey S. Litt, DO, FACS, is Assistant Professor of Surgery, Burn Director, Division of Acute Care Surgery, Department of Surgery, University of Missouri School of Medicine, Columbia, Missouri.
Contact: littjs@health.missouri.edu
Disclosure
J. Litt received Acelity/KCI Speaker Honoraria.
References
- 1.Mosier MJ, Bernal N, et al. National Burn Repository. Report Dataset Version 12.0. 2016. pp. 8–12. retrieved from Ameriburn.site-ym.com.
- 2.Cancio LC, Pruitt BA., Jr . Historical Perspective and the Development of Modern Burn Care. In: Phillips, BJ, editor. Pediatric Burns. 1. Amherst, NY: Cambria Press; 2012. [Google Scholar]
- 3.Branski LK, Herndon DN, Barrow RE. A Brief History of Acute Burn Care Management. In: Herndon DN, editor. Total Burn Care. 4th Ed. 1. Elsevier; [Google Scholar]
- 4.Artz CP, Soroff HS. Modern Concepts in the Treatment of Burns. JAMA. 1955;159(5):411–417. doi: 10.1001/jama.1955.02960220001001. [DOI] [PubMed] [Google Scholar]
- 5.Janzekovic Z. A New Concept in the Early Excision and Immediate Grafting of Burns. The Journal of Trauma. 1970;10(12):1103–1108. [PubMed] [Google Scholar]
- 6.Lee JO, Dibildox M, et al. Operative Wound Management. In: Herndon DN, editor. Total Burn Care. 4th Ed. Elsevier; pp. 157–158. [Google Scholar]
- 7.Underhill FP. The Significance of Anhydremia in Extensive Superficial Burns. JAMA. 1930;95(12):852–57. [Google Scholar]
- 8.Warden GD. Fluid Resuscitation and Early Management. In: Herndon, DN, editor. Total Burn Care. 4th Ed. pp. 117–118. [Google Scholar]
- 9.Wood FM, Giles N, Stevenson A, et al. Characterization of the Cell Suspension Harvested from the Dermal Epidermal Junction Using a ReCell Kit. Burns. 2012;38:44–51. doi: 10.1016/j.burns.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Branski LK, Herndon DN, Barrow RE. A Brief History of Acute Burn Care Management. In: Herndon DN, editor. Total Burn Care. 4th Ed. 1. Elsevier; [Google Scholar]