Abstract
We review recent findings on medical aspects of marijuana use in order to identify those who are at greatest risk of marijuana-related medical problems. We analyze the impact of medical marijuana laws on health, in particular the disproportionate effects on adolescents and children. Chronic marijuana use predominantly affects certain areas of the brain that overlap the default mode network, linked hubs in the brain that play a supervisory role in critical thought processes such as attention, memory, and social interactions. Disruption of the default mode network areas has been documented in schizophrenia and Alzheimer’s disease, illnesses with symptoms and brain changes that parallel findings in marijuana abusers. These findings counter the claim that marijuana is a harmless drug and are a cause for alarm in persons with cannabis dependence.
Introduction
As the November general election nears with three ill-conceived referenda which would legalize ‘medical’ marijuana, physicians should carefully consider the many medical, social, educational and law enforcement problems that other states, notably Colorado, have encountered after enacting similar legislation. If legalization occurs, physicians should think how they will respond to demands for marijuana prescriptions and the potential liability for adverse marijuana caused side effects, illnesses or accidents.
Consider this statement from the Colorado Medical Association: “Other states facing legislative efforts to legalize marijuana should consider Colorado’s experience as a cautionary tale. Approving medical treatments by ballot initiatives sets a dangerous precedent for public health. This will be one of the great social experiments of the century.” Source: http://www.cms.org/articles/marijuana-legalization.
In this article, we review properties of marijuana, to identify who may be at increased risk of problems if marijuana becomes widely available. We review the general impact of putative medical marijuana laws, including changes in marijuana use and diversion to non-medical and under-age users. Finally, we identify adverse changes in the brain that can occur in chronic marijuana users. Here marijuana or equivalently “cannabis” refers to all parts of the plant in the genus Cannibis, including stems seeds, stalks, and any extracts derived from the plant. We consider all forms of administration including ingestion by vaporization or smoking, ingestion by eating or drinking or by contact with the skin of any cannabis products or chemicals, whether natural or synthesized, collectively referred to as “cannabinoids.” The cannabinoids we discuss include ‘high/mood-altering’ delta-9-tetrahydrocannabinol, commonly referred to as THC, which is used in the recreational use products and the ‘non-high/mood-altering’ cannabidiol, or CBD, which is the form in which medicinal properties reside.
Medical Marijuana Laws and Marijuana Use after Legislation
Thirty-one states and the District of Columbia have passed medical marijuana laws (MML) as of September 2018. Few of these states maintain medical marijuana registries, so the National Academy of Sciences report on therapeutic marijuana relied on patients reporting qualifying illness(es) in Oregon and Colorado to obtain information on the proportion of patients reporting qualifying illnesses of medical marijuana users.1 By far the largest proportion of these users (90–93%) reported severe pain. Other conditions reported by 2% or more of medical marijuana users are muscle spasms and multiple sclerosis (25–29%), nausea (12–13%), PTSD (8%), cancer (4–6%) and seizures (3%).1 (More than one condition may be reported). Only 1% of medical marijuana users listed glaucoma as the medical qualifying condition.
The level of evidence (quality of studies available) for marijuana therapy for these indications is moderate or substantial for only three indications: chronic pain, chemotherapy-induced nausea and vomiting and insomnia (improving short-term sleep outcomes) (Table 1). 1 For these conditions, alternate therapies are either more effective or have fewer adverse effects. For example, marijuana alleviates chemotherapy-induced nausea, yet it can induce the cannabis hyperemesis syndrome. 2–5
Table 1.
Medical indications for marijuana and level of evidence1
| Indication | Level of Evidence |
|---|---|
| Anxiety (individuals with social anxiety disorders) | Limited |
| Chemotherapy-induced nausea, vomiting | Substantial |
| Chronic pain | Substantial |
| Glaucoma | Limited |
| Improving clinician-measured multiple sclerosis spasticity symptoms | Limited |
| Improving clinician-measured multiple sclerosis spasticity symptoms | Limited |
| Improving symptoms of Tourette syndrome | Limited |
| Increasing appetite/decreasing weight loss with HIV/AIDS | Limited |
| Insomnia (improving short-term sleep outcomes) | Moderate |
| PTSD | Limited |
Marijuana Laws, Marijuana Use and Adolescents
A large proportion of youth in the US are exposed to marijuana. For US youth 15 to 16 years old, 35.8% are said to have used marijuana.6,7 One critical question is whether adolescent use of marijuana increases after marijuana laws are passed. Here we consider both medical marijuana laws as well as recreational marijuana laws.
Keyes et al. and Sarvet et al. found no overall increase in adolescent use of marijuana after the passage of MMLs.8,9 Chu commented on the Sarvet study, “MMLs could encourage existing casual users to become regular or heavy users. As the majority of social costs are incurred by a small number of heavy users, the effects of MMLs on the intensive margin [heavy users of marijuana] must be evaluated carefully, as this is a significant policy implication.10 Chu further cited five studies that all showed an increase in adult use of marijuana after passage of MMLs. Chu asked, “Why do MMLs affect adolescents differently from adults? For example, could MMLs reduce the black-market supply and so limit adolescents’ access to marijuana? Or could making a substance legal or semi-legal reduce teenagers’ preference for it?”
In another study, Chu analyzed marijuana arrests among adult males following the passage of MMLs and found an increase in marijuana arrests among adult males by about 15–20%.11 Admissions to rehabilitation facilities for marijuana treatments similarly increased after the passage of MMLs. There is some concern that the perception of harmfulness of marijuana, possibly a key factor in lowering rates of adolescent marijuana use, may fall after MML passage. A fall in perceived harmfulness of marijuana in the general population has been noted8 and confirmed by Cerdá et al. in Washington but not Colorado.12
Hasin et al. analyzed US national survey data collected in three periods from 1991 to 2013 from 118,497 participants. They determined that the risk for cannabis use and cannabis use disorders increased at a significantly greater rate in states that passed MMLs than in states that did not.13
After recreational marijuana use was legalized in the state of Washington, both eighth and tenth graders in that state perceived marijuana use as more harmless.12 Marijuana use increased in both grades following the passage of recreational marijuana laws. In Oregon, following similar legislation, rates of undergraduate marijuana use increased from pre- to post-2015, at a rate that was similar to the increase that was also observed at other universities in states where marijuana was still illegal.14 Increases in rates of undergraduate marijuana use were significantly greater in Oregon than in comparison institutions, but only among students reporting recent heavy alcohol use, an indication of co-morbidities in students with substance abuse.
In contrast, Colorado did not exhibit any differential change in perceived harmfulness or past-month adolescent marijuana use following legalization. Yet Jones et al. found the frequency of marijuana use in Colorado college students is much higher than the national average, especially the percentage of daily or almost daily users.15 The same study found significant differences between the marijuana non-users and the once a week or more marijuana users in grade point average.15 Cerdá suggests investment in evidence-based adolescent substance use prevention programs in any additional states that may legalize recreational marijuana use.12
Marijuana Laws, Children, Emergency Rooms, and Schools
Calls to Poison Control Centers have reported an increase in marijuana exposure among younger children.1 Accidental ingestion of marijuana edibles has resulted in respiratory failure and coma.16,17 An increase in overall pediatric exposures has followed MML enactment. From 2000 to 2013, the annual rate of Poison Control Center calls for marijuana exposure for children younger than six was 2.82 times higher in states that had MMLs prior to 2000, than in states where marijuana remained illegal as of 2013.18 Sergeant P. Bret Hinkle of the Marijuana Unit of the Denver Police Department cited instances of parents that grow marijuana and make it available to children.19
Emergency rooms are seeing a relatively new syndrome, the marijuana hyperemesis syndrome.2,5 Persistent vomiting is observed in cannabis users, at a rate that has increased significantly in states where marijuana has been legalized.2 The number of emergency room visits for cyclic vomiting, including cannabis-induced vomiting, doubled since legalization in Colorado.3 Although hot showers are often recommended for cannabis hyperemesis, these are of variable effectiveness, as marijuana cessation appears to be the best remedy.4 Phelps County Regional Medical Center ER physician Dr. Francina Hoffman has seen her first cases in recent years. She said, “They [marijuana users] come in because they can’t stop vomiting. We prescribe Phenergan or Zofran, but they are often noncompliant. On the third ER visit, they get admitted.”5
Overall, the yearly rate of emergency department visits related to marijuana increased 35 percent after the legalization of recreational marijuana in Colorado.20 The number of hospitalizations related to marijuana also increased steadily during this period, nearly doubling from 2011 to 2014.20
The superintendent of Rolla schools, Dr. Aaron Zalis, said that medical marijuana laws add work for school administrators.21 “There is more to unwind,” said Dr. Zalis. “Alcohol, opiates and marijuana, right now, all are illegal for juveniles … The schools are required to check out all the drugs that students have … The distinction of medical marijuana makes it a lot more complicated … There will be more documentation and compliance with new regulations. Additionally, any legislation that makes marijuana available could lead to other substance abuse. I’ve always viewed marijuana as a gateway. Based on the counselors that we work with, over a 34-year period … [marijuana] leads to other things.21
Groups at Increased Risk of Adverse Effects of Marijuana
Epidemiologic evidence shows strong correlation between heavy marijuana use during adolescence and schizophrenia.22 Other evidence shows increased risk for schizophrenia with early use of marijuana in males. Onset of marijuana use in early adolescence (before age 16) has been associated with more severe psychosis.7,23–26 Recent findings using a polygenic risk score for schizophrenia and cerebral cortical thickness obtained from MRI imaging showed progressive thinning of the cerebral cortex in males with a high genetic risk for schizophrenia and early marijuana use.7 The researchers found no increased risk for females. Two factors may be responsible for increased vulnerability of males to schizophrenia with marijuana use: earlier onset of schizophrenia in males and sex differences in dynamics of brain development, with greater changes in male white and gray matter volumes in male adolescents compared to females.27,28 The anatomic locations in the brain for both schizophrenia and brain alterations with chronic use of marijuana are similar, involving especially the hippocampus and pre-frontal cortex.29
Users of High-Potency Weed: “Skunk”
There has been a general increase in the potency of available marijuana, with available marijuana quadrupling in strength from the 1980s to 2012.29,30 Sometimes called “skunk,” a name apparently generated by users’ comments about odor, the high-potency weed has higher levels of active cannabinoids. Marijuana potency is usually judged according to concentrations of THC, the primary psychoactive constituent in marijuana. Marijuana also contains other cannabinoids such as CBD, which has been thought to lack most of the psychoactive effects of THC and even to moderate the effect of THC.31 Uncertainty about the ameliorative effect of CBD on THC was raised by recent animal studies showing an increased THC level mediated by cannabidiol and cannabidiol inhibits the metabolism of THC.32,33 There is no limit on the potency of marijuana in the US, as there is for example in some nations, e.g. Uruguay.31,34 Fifteen states do have limits on THC content for a different purpose—to allow access to what are considered “non-psychoactive” products such as cannabidiol oil.1
Volkow et al. noted in a review in the New England Journal of Medicine that higher marijuana potency raises concerns that the “consequences of marijuana use may be worse now than in the past,” and “may account for the significant increases in emergency department visits by persons reporting marijuana use and the increases in fatal motor-vehicle accidents.”29,35,36 The Volkow group also said, “This increase in THC potency over time also raises questions about the current relevance of the findings in older studies on the effects of marijuana use, especially studies that assessed long-term outcomes.”
Freeman and Winstock showed that high-potency skunk marijuana resulted in greater memory impairment and likelihood of paranoia.31 Eighty-two percent of skunk users had memory impairment as opposed to 4% of users of “other types of grass.” Skunk was also more likely to induce a feeling of paranoia than other types (78% vs. 7%), yet it remained by far the most preferred type of marijuana, giving the “best high” and was the most available type of marijuana.31
Adverse Effects of Marijuana on the Lungs: Chronic Bronchitis Symptoms
The organ with best evidence for adverse effects from chronic marijuana use, besides the brain, is the lower respiratory system. The National Academy of Sciences report1 found association between smoking marijuana on a regular basis and an increase in chronic cough and phlegm production as well as more frequent chronic bronchitis episodes.1 Quitting marijuana smoking reduces these adverse effects. Tetrault et al. found a lower FEV1 in long-term marijuana exposure.37 Hancox et al. found higher forced vital capacity (FVC), although other studies varied in their conclusions on objective measures such as FEV1 and FVC.1,38
Brain Changes in Regular Users of Marijuana
Lorenzetti et al. reviewed anatomic brain changes in chronic marijuana users.39 Chronic marijuana use was defined as exposure to marijuana on a daily or almost daily basis. Most users in the study started smoking marijuana between the ages of 15 to 17 and continued for a duration between two and 23 years. Lifetime episodes of marijuana use, typically referring to the number of joints smoked, ranged from 402 to 5,625. The brain abnormalities found were associated with higher levels of marijuana use, taking into consideration dosage, age of onset, and duration.
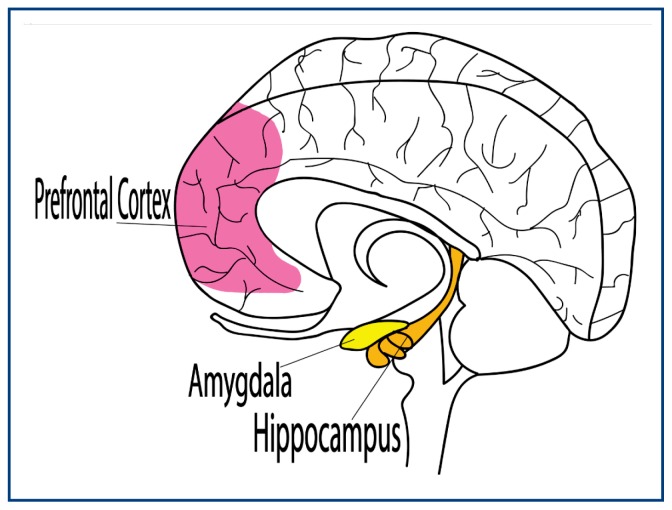
The review found substantial overlap between the location of the changes in the brains of marijuana users and the location of concentrated THC receptors.39,40 The most consistent alterations, in comparison to control subjects, were found in the hippocampus and pre-frontal cortex. Yucel and Ashtari found a 12–13% volume reduction in the hippocampus.41,42 Additional groups found other changes in the hippocampus such as shape and gray matter content.43–46 Changes in amygdala were also well documented.39 Figure 1 shows the three areas of the brain for which morphological and functional changes are best documented.
Figure 1.
Three areas of the brain for which morphological and functional changes are best documented.
The brain regions most affected in regular users of marijuana, the hippocampus and regions of the cerebral cortex (parietal cortex, inferior frontal gyrus), are “integral components of the brain reward, memory, and executive-attention systems.” This may explain the deficits that marijuana users show in these areas.39,47–51 Rueda et al. cited work by Posner and Raichle showing that the regulatory effects of these executive areas “apply just as well to brain areas involved in processing the semantics of words, storing information in memory, and generating emotions such as fear and sadness.52,53
Risks for Psychoses
Alongside changes in major brain structures, marijuana has been repeatedly linked to an increased risk for psychosis. The review committee for the National Academy of Sciences found multiple high-quality studies that confirmed association between marijuana use and development of schizophrenia and other psychoses, including manic-depressive disorder, hypomania, depression, and suicide, as well as well as a marijuana and psychosis dose-response relationship.1 In a recent review, Marconi et al. found substantial evidence for the ability of marijuana “to induce long-lasting psychotic disorders.”54 Increased marijuana use was associated with a “higher risk of schizophrenia and other psychosis-related outcomes compared to nonusers.” Marijuana use has been “widely reported to induce acute psychotic experiences,55–57 to affect the severity of psychotic symptoms,58–59 and previous meta-analyses have reported a two-fold increase in the risk to develop a psychotic disorder in [marijuana] users compared to nonusers.”54 Following this pattern, in another study where “the history of [marijuana] use and a spot urine test for cannabinoids were obtained” for 137 persons diagnosed with schizophrenia in treatment, the “subjects who were using cannabis during the six-month observation period presented with a significantly higher degree of delusional and hallucinatory activity than those who did not. Moreover, the group using cannabis made a higher average number of visits to the hospital during the same period.”61 Not only does marijuana work to induce and increase severity of psychotic episodes,63 but marijuana use is also linked to “increased risk of relapse,64 earlier relapse,65 longer inpatient stay,66 and an increased risk of violence and criminal activity67 as well as being associated with higher rates of transition to psychosis.68”69 Substance abuse (including marijuana, amphetamines, and cocaine) leads to increased mortality from overdoses.70 Rosen et al. found that substance abuse-linked illness, including overdoses, accounted for 27.6% of deaths among 169,051 male Vietnam-era veteran psychiatric patients, vs. 8.8% among psychiatric patients in the same group of veterans without substance abuse.70 In summarizing the link between chronic marijuana use and psychoses, the National Academy review stated: “The higher the use, the greater the risk..”1
Persons with schizophrenia and substance abuse, including marijuana, amphetamines and cocaine, are at risk of increased risk of relapse of schizophrenia symptoms71 as well as increased mortality from overdoses.70 Rosen et al. found that substance abuse-linked illness, including overdoses, accounted for 27.6% of deaths among 169,051 male Vietnam-era veteran psychiatric patients, vs. 8.8% among psychiatric patients in the same group of veterans without substance abuse.70
Brain Regions Affected by Marijuana, Schizophrenia, and Earliest Areas of Alzheimer’s Disease Overlap and Symptoms are Similar
Sheline, Raichle et al. studied a group of functionally normal persons with PET scans using Pittsburgh Compound B (PIB) imaging.72 The PIB-positive PET scan patients were considered to have an early marker predictive of later-onset Alzheimer’s disease.73 Early changes were found in and around the hippocampus, anterior and dorsal cingulate area of the prefrontal cortex and precuneus; most of these areas are associated with abnormalities from chronic marijuana use.39,74
Further studies of preclinical Alzheimer’s disease used functional MRI for imaging with similar findings.75 Studies of schizophrenia have shown abnormalities in related brain regions.76–78 The clinical similarity between schizophrenia and dementia was observed in early descriptions of schizophrenia, which was termed “dementia praecox,” used to describe “a deteriorating process involving intellectual functions and behavior but no involvement of consciousness.”79 Clinical descriptions of schizophrenia, early dementia, and chronic marijuana use overlap.79
Importance of the Default Mode Network
The discovery of the default mode network by Raichle and other researchers at Washington University was widely cited and confirmed by other laboratories.80,81 These linked hubs within the brain have been described as “orchestra conductors,” directing other specialized parts of the brain that can be compared to orchestra sections. Raichle said, “The [default mode network] is thought to behave something like an orchestra conductor, issuing timing signals, much as a conductor waves a baton, to coordinate activity among different brain regions.”82
The functioning of the default mode network, the orchestra director in the brain, was further described by Andrews-Hanna.83 She surveyed new findings in psychology bolstered by imaging studies that showed the function of these midline core areas. The default mode network allows humans to “spontaneously and deliberately” engage in “introspective and adaptive mental activities,” thinking about the past and planning the future, placing high value on “personally significant information,” especially about ‘friends, family members, and romantic partners.” 83
The areas of the brain83 affected by chronic marijuana use are very closely associated with the default mode network defined originally by Raichle and colleagues. The results of chronic marijuana use are believed to negatively influence the ability to focus attention, to retain information, and to succeed in social roles.84
Conclusions
In this review, we discussed the general impact of medical marijuana laws, changes in marijuana use after medical marijuana laws are passed, and groups at increased risk when restrictions on the availability of marijuana are loosened. Changes that occur in the brain with chronic use of marijuana are of primary concern, especially for males. Earlier use of marijuana, stronger marijuana, and those with schizophrenia or polygenic risk for schizophrenia are at increased risk for adverse changes in the brain. Similar areas in the brain are affected in schizophrenia, Alzheimer’s disease, and chronic marijuana use. Research findings raise critical concerns for chronic marijuana users. Marijuana should not be considered a medicine or pure drug.
Footnotes
William V. Stoecker, MD, MS, MSMA member since 1984, is with S&A Technologies and The Dermatology Center, Rolla, Missouri, and the Department of Dermatology, University of Missouri Health Sciences Center, Columbia, Missouri. Emily E. Rapp is a student in Chemical Engineering, Missouri University of Science and Technology, Rolla, Missouri. Joseph M. Malters, MD, is with The Dermatology Center, Rolla, Missouri.
Contact: wvs@mst.edu
Disclosure
None reported.
References
- 1.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): National Academies Press (US); 2017. Jan 12, [Accessed September 8, 2018]. An Evidence Review and Research Agenda. Book Available Online at https://www.ncbi.nlm.nih.gov/books/NBK423845/ [PubMed] [Google Scholar]
- 2.Kim HS, Monte AA. Colorado cannabis legalization and its effect on emergency care. Ann Emerg Med. 2016;68(1):71–5. doi: 10.1016/j.annemergmed.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HS, Anderson JD, Saghafi O, et al. Cyclic vomiting presentations following marijuana liberalization in Colorado. Acad Emerg Med. 2015;22(6):694–9. doi: 10.1111/acem.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorensen CJ, DeSanto K, Borgelt L, Phillips KT, Monte AA. Cannabinoid Hyperemesis Syndrome: Diagnosis, Pathophysiology, and Treatment-a Systematic Review. J Med Toxicol. 2017 Mar;13(1):71–87. doi: 10.1007/s13181-016-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Interview with Dr. Francina Hoffman, Phelps County Regional Medical Center Emergency Physician. Sep 16, 2018.
- 6.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. Monitoring the Future: National Results on Drug Use: 1975–2013. [Google Scholar]
- 7.French L, Gray C, Leonard G, Perron M, Pike GB, Richer L, et al. Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. JAMA Psychiatry. 2015 Oct;72(10):1002–11. doi: 10.1001/jamapsychiatry.2015.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keyes KM, Wall M, Cerdá M, Schulenberg J, O’Malley PM, Galea S, Feng T, Hasin DS. How does state marijuana policy affect US youth? Medical marijuana laws, marijuana use and perceived harmfulness: 1991–2014. Addiction. 2016 Dec;111(12):2187–2195. doi: 10.1111/add.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarvet AL, Wall MM, Fink DS, Greene E, Le A, Boustead AE, Pacula RL, et al. Medical marijuana laws and adolescent marijuana use in the United States: a systematic review and meta-analysis. Addiction. 2018 Jun;113(6):1003–1016. doi: 10.1111/add.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu YL. Commentary on Sarvet et al. 2018. What do we still need to know about the impacts of medical marijuana laws in the United States? Addiction. 2018 Jun;113(6):1017–1018. doi: 10.1111/add.14167. [DOI] [PubMed] [Google Scholar]
- 11.Chu YW. The effects of medical marijuana laws on illegal marijuana use. J Health Econ. 2014 Dec;38:43–61. doi: 10.1016/j.jhealeco.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Cerdá M, Wall M, Feng T, et al. Association of State Recreational Marijuana Laws With Adolescent Marijuana Use. JAMA pediatrics. 2017;171(2):142–149. doi: 10.1001/jamapediatrics.2016.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasin D, Sarvet AL, Cerda M, Keyes KM, Stohl MS, Galea S, et al. U.S. adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991–1992 to 2012–2013. JAMA Psychiatry. 2017;74:579–588. doi: 10.1001/jamapsychiatry.2017.0724. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr DCR, Bae H, Phibbs S, Kern AC. Changes in undergraduates’ marijuana, heavy alcohol and cigarette use following legalization of recreational marijuana use in Oregon. Addiction. 2017;112(11):1992–2001. doi: 10.1111/add.13906. [DOI] [PubMed] [Google Scholar]
- 15.Jones J, Nicole Jones K, Peil J. The impact of the legalization of recreational marijuana on college students. Addict Behav. 2018 Feb;77:255–259. doi: 10.1016/j.addbeh.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Amirav I, Luder A, Viner Y, Finkel M. Decriminalization of cannabis--potential risks for children? Acta Paediatr. 2011 Apr;100(4):618–9. doi: 10.1111/j.1651-2227.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- 17.Appelboam A, Oades PJ. Coma due to cannabis toxicity in an infant. Eur J Emerg Med. 2006 Jun;13(3):177–9. doi: 10.1097/01.mej.0000194405.38206.f2. [DOI] [PubMed] [Google Scholar]
- 18.Onders B, Casavant MJ, Spiller HA, Chounthirath T, Smith GA. Marijuana Exposure Among Children Younger Than Six Years in the United States. Clin Pediatr (Phila) 2016 May;55(5):428–36. doi: 10.1177/0009922815589912. [DOI] [PubMed] [Google Scholar]
- 19.Interview with P. Bret Hinkle, Marijuana Unit. Denver Police Department; Sep 13, 2018. [Google Scholar]
- 20. [Accessed September 16, 2018]. https://www.rmhidta.org/html/FINAL%202017%20Legalization%20of%20Marijuana%0in%20Colorado%20The%20Impact.pdf.
- 21.Interview with Dr. Aaron Zalis. Superintendent Rolla Public Schools; Sep 14, 2018. [Google Scholar]
- 22.Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med. 2012;42(6):1321–1328. doi: 10.1017/S0033291711002078. [DOI] [PubMed] [Google Scholar]
- 23.Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 24.Fontes MA, Bolla KI, Cunha PJ, et al. Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry. 2011;198(6):442–447. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- 25.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19(1):1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- 27.Paus T, Nawaz-Khan I, Leonard G, et al. Sexual dimorphism in the adolescent brain: role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm Behav. 2010;57(1):63–75. doi: 10.1016/j.yhbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014 Jun 5;370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ElSohly MA. Potency Monitoring Program quarterly report no 123— reporting period: 09/16/2013–12/15/2013. Oxford: University of Mississippi, National Center for Natural Products Research; 2014. [Google Scholar]
- 31.Freeman TP, Winstock AR. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychol Med. 2015 Nov;45(15):3181–9. doi: 10.1017/S0033291715001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, et al. Cannabidiol potentiatesΔ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology. 2011;218(2):443–457. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- 33.Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27(6):799–810. doi: 10.1097/01.ftd.0000177223.19294.5c. [DOI] [PubMed] [Google Scholar]
- 34.Coombes R. Cannabis regulation: high time for change? BMJ. 2014 May 21;348:g3382. doi: 10.1136/bmj.g3382. [DOI] [PubMed] [Google Scholar]
- 35.Drug Abuse Warning Network. 2011 national estimates of drug-related emergency department visits. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. [Accessed September 8, 2018]. http://www.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. [Google Scholar]
- 36.Brady JE, Li G. Trends in alcohol and other drugs detected in fatally injured drivers in the United States, 1999–2010. Am J Epidemiol. 2014;179:692–9. doi: 10.1093/aje/kwt327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167(3):221–8. doi: 10.1001/archinte.167.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hancox RJ, Poulton R, Ely M, Welch D, Taylor DR, McLachlan CR, et al. Effects of cannabis on lung function: a population-based cohort study. Eur Respir J. 2010 Jan;35(1):42–7. doi: 10.1183/09031936.00065009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenzetti V, Solowij N, Yücel M. The role of cannabinoids in neuroanatomic alterations in cannabis users. Biol Psychiatry. 2016;79:e17–e31. doi: 10.1016/j.biopsych.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997 Mar;77(2):299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 41.Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, et al. Regional brain abnormalities associated with heavy long-term cannabis use. Arch Gen Psychiatry. 2008;65:1–8. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 42.Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, et al. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matochik JA, Eldreth DA, Cadet J, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Demirakca T, Sartorius A, Ende G, Meyer N, Welzel H, Skopp G, et al. Diminished gray matter in the hippocampus of cannabis users: Possible protective effects of cannabidiol. Drug Alcohol Depend. 2011;114:242–245. doi: 10.1016/j.drugalcdep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Battistella G, Fornari E, Annoni J-M, Chtioui H, Dao K, Fabritius J, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014;39:2041–2048. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solowij N, Walterfang M, Lubman DI, Whittle S, Lorenzetti V, Styner M, et al. Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophr Res. 2013;143:179–184. doi: 10.1016/j.schres.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 47.Rueda MR, Posner MI, Rothbart MK. The development of executive attention: Contributions to the emergence of self-regulation. Dev Neuropsychol. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- 48.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 49.Ramus SJ, Davis JB, Donahue RJ, Discenza CB, Waite AA. Interactions between the orbitofrontal cortex and the hippocampal memory system during the storage of long-term memory. Ann N Y Acad Sci. 2007 Dec;1121:216–31. doi: 10.1196/annals.1401.038. [DOI] [PubMed] [Google Scholar]
- 50.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008 Dec;100(6):3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takagi M, Yücel M, Cotton SM, Baliz Y, Tucker A, Elkins K, Lubman DI. Verbal memory, learning, and executive functioning among adolescent inhalant and cannabis users. J Stud Alcohol Drugs. 2011 Jan;72(1):96–105. doi: 10.15288/jsad.2011.72.96. [DOI] [PubMed] [Google Scholar]
- 52.Posner MI, Raichle ME. Images of mind. New York: Scientific American Books; 1994. [Google Scholar]
- 53.Posner MI, Raichle ME. Overview: The neuroimaging of human brain function. Proceedings of the National Academy of Sciences; USA. pp. 763–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophrenia Bulletin. 2016;42(5):1262–1269. doi: 10.1093/schbul/sbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 56.Henquet C0, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 57.Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- 58.Tien AY, Anthony JC. Epidemiological analysis of alcohol and drug use as risk factors for psychotic experiences. J Nerv Ment Dis. 1990;178:473–480. [PubMed] [Google Scholar]
- 59.Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- 60.Hall W, Degenhardt L. Adverse health effects of medical cannabis use. Lancet. 2009;374:8. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 61.Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis affects the severity of schizophrenic symptoms: results of a clinical survey. Psychol Med. 1986;16:515–520. doi: 10.1017/s0033291700010278. [DOI] [PubMed] [Google Scholar]
- 62.Foti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167:987–993. doi: 10.1176/appi.ajp.2010.09020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. Bmj. 2005;330(7481):11. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Linszen DH, Dingemans PM, Nugter MA, Van der Does AJ, Scholte WF, Lenior MA. Patient attributes and expressed emotion as risk factors for psychotic relapse. Schizophr Bull. 1997;23(1):119–30. doi: 10.1093/schbul/23.1.119. [DOI] [PubMed] [Google Scholar]
- 65.Hides L, Dawe S, Kavanagh DJ, Young RM. Psychotic symptom and cannabis relapse in recent-onset psychosis. Prospective study. Br J Psychiatry. 2006;189:137–43. doi: 10.1192/bjp.bp.105.014308. [DOI] [PubMed] [Google Scholar]
- 66.Isaac M, Isaac M, Holloway F. Is cannabis an anti-antipsychotic? The experience in psychiatric intensive care. Hum Psychopharmacol. 2005;20(3):207–10. doi: 10.1002/hup.674. [DOI] [PubMed] [Google Scholar]
- 67.Miles H, Johnson S, Amponsah-Afuwape S, Finch E, Leese M, Thornicroft G. Characteristics of subgroups of individuals with psychotic illness and a comorbid substance use disorder. Psychiatr Serv. 2003;54(4):554–61. doi: 10.1176/appi.ps.54.4.554. [DOI] [PubMed] [Google Scholar]
- 68.Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res 30. 2007;151(1–2):151–4. doi: 10.1016/j.psychres.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O’Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66(4):442–51. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- 70.Rosen CS, Kuhn E, Greenbaum MA, Drescher KD. Substance abuse-related mortality among middle-aged male VA psychiatric patients. Psychiatr Serv. 2008;59:290–296. doi: 10.1176/ps.2008.59.3.290. [DOI] [PubMed] [Google Scholar]
- 71.Swofford CD, Kasckow JW, Scheller-Gilkey G, Inderbitzin LB. Substance use: a powerful predictor of relapse in schizophrenia. Schizophr Res. 1996;20:145–151. doi: 10.1016/0920-9964(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 72.Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010 Mar 15;67(6):584–7. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 74.Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology (Berl) 2012 Aug;222(4):675–84. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol Psychiatry. 2013 Sep 1;74(5):340–7. doi: 10.1016/j.biopsych.2012.11.028. [Sheline13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hugdahl K, Raichle ME, Mitra A, Specht K. On the existence of a generalized non-specific task-dependent network. Front Hum Neurosci. 2015 Aug 6;9:430. doi: 10.3389/fnhum.2015.00430. [Hugdahl15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calhoun VD, Adalı T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [Calhoun12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007 Jul;33(4):1004–12. doi: 10.1093/schbul/sbm052. [Bluhm07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kraepelin E. Psychiatrie. 5th ed. Leipzig: JA Barth; 1896. [Kraepelin96] [Google Scholar]
- 80.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):676–82. doi: 10.1073/pnas.98.2.676. [Raichle01] Proc Natl Acad Sci U S A. 2001 Jan 16, 98 (2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fox MD1, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005 Jul 5;102(27):9673–8. doi: 10.1073/pnas.0504136102. [Fox05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.[Raichle18]Marcus E.Raichle, personal communicaton August31, 2018
- 83.Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012 Jun;18(3):251–70. doi: 10.1177/1073858411403316. [Andrews-Hanna12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and chronic dffects of cannabinoids on human cognition-A systematic review. Biol Psychiatry. 2016 Apr 1;79(7):557–67. doi: 10.1016/j.biopsych.2015.12.002. [DOI] [PubMed] [Google Scholar]