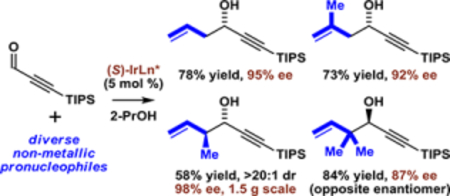
Abstract
Cyclometalated π-allyliridium C,O-benzoates modified by (S)-SEGPHOS or (S)-Cl,OMe-BIPHEP catalyze enantioselective 2propanol-mediated reductive couplings of diverse non-metallic allyl pronucleophiles with the acetylenic aldehyde TIPSC≡CCHO. Absolute stereochemistry of the resulting secondary homoallylic-propargylic alcohols were assigned using Rychnovsky’s competing enantioselective conversion (CEC) method.
Graphical Abstract
Despite decades of study on enantioselective carbonyl allylation, crotylation, and related syntheses of homoallylic alcohols,1 the asymmetric allylation of acetylenic aldehydes (ynals) has received relatively little attention. To date, only two systematic studies of asymmetric ynal allylation appear in the literature,2 along with isolated examples involving reagents based on boron,3 tin4 and zinc.5 These methods invariably require cryogenic conditions and the use of premetalated reagents, which limits their utility. Our laboratory has developed the first alcohol-mediated carbonyl reductive couplings,6 including highly enantioselective carbonyl allylations and propargylations.6d These processes do not require cryogenic conditions and exploit tractable non-metallic allyl pronucleophiles, for example, allyl acetate. In connection with collaborative efforts toward the total synthesis of phosdiecin A,7 a systematic study of enantioselective, iridium-catalyzed ynal reductive couplings mediated by 2-propanol was undertaken. Here, we report that the acetylenic aldehyde TIPSC≡CCHO participates in a diverse range of allylative carbonyl additions to form highly enantiomerically enriched secondary homoallylic-propargylic alcohols.8 Additionally, as an inversion in enantiofacial selectivity was previously observed in response to the steric features of the allyl donor,9 Rychnovsky’s competing enantioselective conversion (CEC) method was used to corroborate absolute stereochemistry, and, in doing so, was expanded to encompass a diverse set of chiral propargyl alcohols.
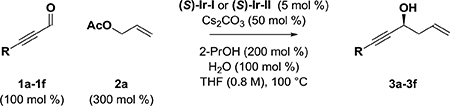
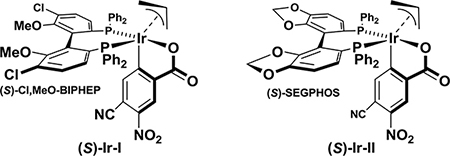
Due to the high kinetic reactivity of alkynes under the conditions of iridium catalysis, it was necessary to evaluate terminally substituted ynals 1a-1d for their ability to participate in the parent enantioselective reductive iridiumcatalyzed carbonyl allylation mediated by 2-propanol.10 For this purpose, the chromatographically purified cyclometallated π-allyliridium C,O-benzoate complexes (S)-Ir-I and (S)-Ir-II, modified by (S)-Cl-MeO-BIPHEP and (S)-SEGPHOS, respectively, were utilized. Acetylenic aldehydes 1a and 1b bearing terminal phenyl and silyloxymethyl moieties, respectively, decomposed upon exposure to the standard reaction conditions, and the desired products of carbonyl allylation were not observed (Table 1, entries 1–4). It was reasoned that a more sterically demanding substituent at the alkyne terminus would mask the alkyne and mitigate decomposition. Indeed, the acetylenic aldehyde 1c bearing a 2-(2-silyloxy)propyl substituent provided the desired product of allylation 3c in modest yield, but with excellent levels of enantiomeric enrichment (Table 1, entries 5 and 6). A series of trialkylsilyl-terminated acetylenic aldehydes 1d-1f were evaluated. While the TMS-substituted acetylenic aldehyde 1d decomposed upon exposure to allylation conditions (Table 1, entries 7 and 8), the TBS-substituted acetylenic aldehyde 1e provided highly enantiomerically enriched allylation product 3e, albeit in poor yield (Table 1, entries 9 and 10). Finally, using the TIPS-substituted acetylenic aldehyde 1f, the targeted product of allylation 3f could be obtained in good yields and excellent enantioselectivities (Table 1, entries 11 and 12). Optimal results were obtained using the catalyst (S)-Ir-I modified by (S)-Cl-MeO-BIPHEP. The optical rotation of 3f matched the literature value,11 and its absolute stereochemical assignment is consistent with the enantiofacial preference generally observed in related aldehyde allylations catalyzed by (S)-IrI and (S)-Ir-II.6d,10
Table 1.
Enantioselective iridium-catalyzed allylation of ynals 1a-1f with allyl acetate via 2-propanol-mediated reductive coupling.a
 | ||||
|---|---|---|---|---|
| entry | 1a-1d | catalyst | yield (%) | ee (%) |
| 1 | 1a, R = Ph | (S)-lr-l | — | — |
| 2 | 1a, R = Ph | (S)-lr-ll | — | — |
| 3 | 1b, R = CH2OTBDPS | (S)-lr-l | — | — |
| 4 | 1b, R = CH2OTBDPS | (S)-lr-ll | — | — |
| 5 | 1c, R = CMe2OTIPS | (S)-lr-l | 63 | 95 |
| 6 | 1c, R = CMe2OTIPS | (S)-lr-ll | 53 | 94 |
| 7 | 1d, R = TMS | (S)-lr-l | — | — |
| 8 | 1d, R = TMS | (S)-lr-ll | — | — |
| 9 | 1e, R = TBS | (S)-lr-l | 24 | 94 |
| 10 | 1e, R = TBS | (S)-lr-ll | 22 | 96 |
| 11 | 1f, R = TIPS | (S)-lr-l | 78 | 95 |
| 12 | 1f, R = TIPS | (S)-lr-ll | 61 | 93 |
 | ||||
Yields are of material isolated by silica gel chromatography. Enantioselectivity was determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details.
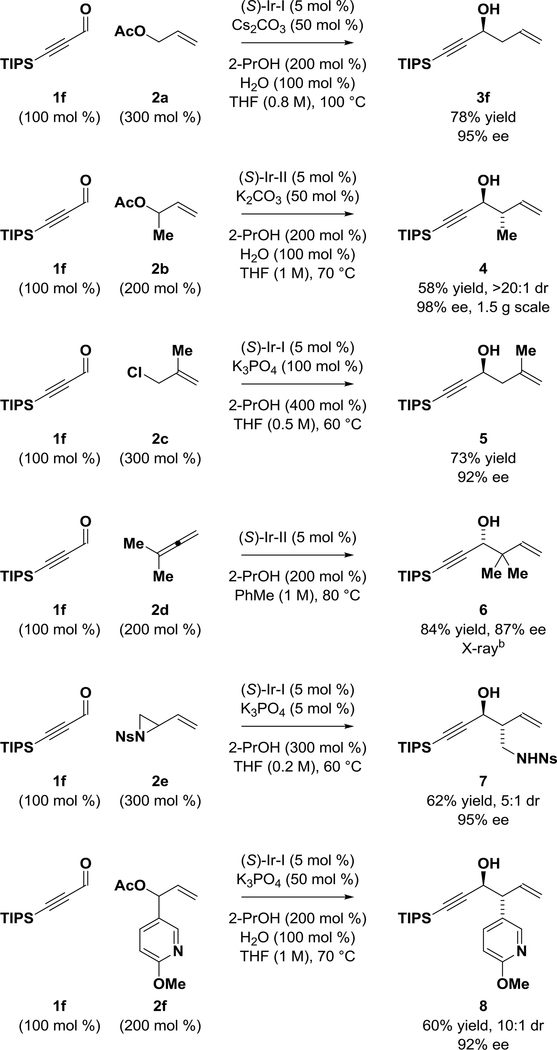
The TIPS-substituted acetylenic aldehyde 1f was reacted with a range of allyl pronucleophiles 2a-2f to assess the diversity of products potentially accessible (Scheme 1). Beyond allylation,10 anti-diastereo- and enantioselective crotylation to form compound 4 occurred in good yield with excellent control of relative and absolute stereochemistry.12 Similarly, methallylation of aldehyde 1f delivered adduct 5 in good yield with high levels of enantiomeric enrichment.13 Using dimethylallene 2d as the pronucleophile, tert-prenylation of aldehyde 1f occurs efficiently and, as anticipated on the basis of prior observations,9 with inversion of enantiofacial selectivity. Aldehyde 1f reacts with vinyl aziridine 2e to form the product of α-(aminomethyl)allylation 7 in an anti-diastereo- and enantioselective manner.14 Finally, using allyl donor 2f, which incorporates a 3-(6-methoxypyridine) substituent, the product of α-(aryl)allylation 8 is formed with good levels of stereoselectivity.15 Attempted reactions from the propargyl alcohol oxidation level were less efficient (20% lower yields), as the inductive effect of the alkyne raises the barrier for alcohol dehydrogenation.16
Scheme 1.
Enantioselective iridium-catalyzed reductive couplings of ynal 1f with allyl pronucleophiles 2a-2f to form homoallylic-propargylic alcohols 3f, 4-8.a
aYields are of material isolated by silica gel chromatography. Enantioselectivity was determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details. bXRay data obtained after removal of the TIPS group and conversion to the 3,5-dinitrobenzoate.
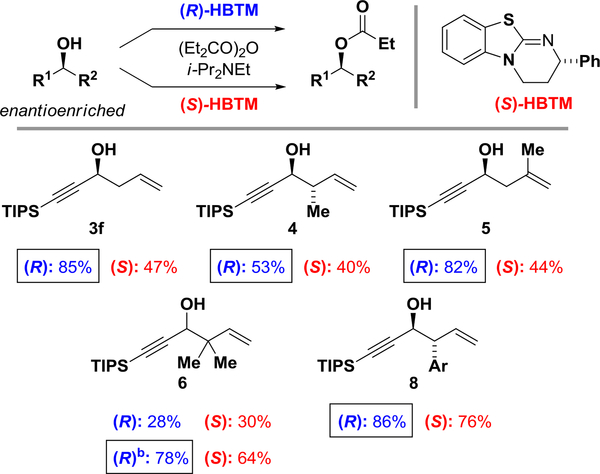
The configuration of the alcohol products were analyzed using the CEC method (Scheme 2).17 Enantioselective acylation of the chiral nonracemic alcohols with (R)- or (S)HBTM, Birman’s catalyst,18 are anticipated to proceed with higher conversions with the stereochemically matched catalyst. In the event, the acylation catalyzed by (R)-HBTM was faster for alcohols 3f, 4, 5, and 8, indicating these alcohols are of the (S)-configuration, that is, the configuration of the alcohols are forward, as shown. Acylation of alcohol 7 was anomalously fast and unselective, and no assignment could be made. Alcohol 6, the tert-prenylation product, was problematic. Acylation of alcohol 6 was essentially unselective at room temperature. At 0 °C, alcohol 6 showed modestly higher conversion with the (R)-HBTM catalyst. The standard mnemonic suggests that the alcohol should be of the (S)-configuration, however, X-ray analysis establishes an (R)-configuration.19 Empirically, while the CEC method is useful for alcohols that display reasonable selectivity at room temperature, lowering the temperature to augment selectivity in normally unselective CEC substrates should be avoided.
Scheme 2.
Analysis of alcohol configurations using the competing enantioselective conversion (CEC) method.a
aParallel acylation reaction were run with (R)- or (S)-HBTM catalyst (10 mol %), DIPEA (300 mol %) and propionic anhydride (300 mol %) at room temperature. The conversions were analyzed by 1H NMR after 1560 min. With the directing group to the left, faster reactions with the (R)HBTM indicate that the alcohol configuration is forward. See Supporting Information for more details. b0 °C, HBTM (26 mol %).
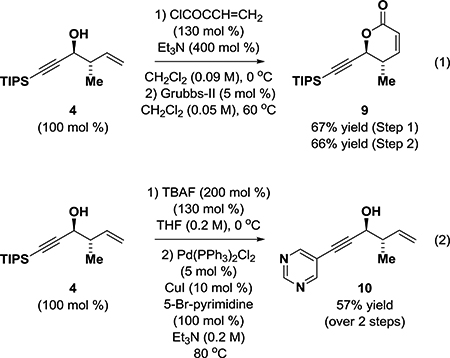
To illustrate how homoallylic-propargylic alcohols 3d, 4-8 might serve as building blocks in chemical synthesis, the product of anti-crotylation, compound 4, was converted to the acrylic ester and subjected to ring-closing metathesis to form the α,β-unsaturated δ-lactone 9(eq 1).6c Alternatively, removal of the TIPS-protecting group of compound 4 using TBAF followed by Sonogashira coupling of the crude terminal alkyne with 5-bromopyrimidine provides the heteroaryl-substituted alkyne 10 in good yield over two steps(eq 2).
To conclude, despite decades of study on asymmetric carbonyl allylation, only two systematic studies on ynal electrophiles appear in the literature, which require cryogenic conditions and premetalated reagents.2 Here, using the concept of alcohol-mediated carbonyl addition, we report general catalytic enantioselective methods for ynal allylations that are non-cryogenic and utilize nonmetallic pronucleophiles. Additionally, the CEC method for determination of absolute stereochemistry was applied to homoallylic-propargylic alcohols 3f, 4-6, 8 and, notwithstanding compound 6, which bears a quaternary carbon directly adjacent to the carbinol stereocenter, was proven effective for this class of secondary alcohols. Future studies will be focused on the development of related C-C bond forming transfer hydrogenations, including transfer hydrogenative imine additions.
Supplementary Material
Acknowledgments.
Acknowledgment is made to the Robert A. Welch Foundation (M.J.K. F-0038), the NIH-NIGMS (M.J.K. RO1-GM069445), the UT Austin Center for Green Chemistry and Catalysis (M.J.K.) and the National Science Foundation (S.D.R. CHE 1361998 & 1764380) for partial support of this research. Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) is acknowledged for postdoctoral fellowship support (G.B. 2017/00734–5) and research internship scholarship support (F.D.F. 2017/18487–4). The China Scholarship Council is acknowledged for research internship scholarship support (G.L. CSC.201707060020).
Footnotes
The authors declare no competing financial interest.
Supporting Information Available. Spectral data for all new compounds (1H NMR, 13C NMR, IR, HRMS). Single crystal X-ray diffraction data. Conversion and selectivity data for the CEC reactions in Scheme 2. This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- (1).For selected reviews on asymmetric carbonyl allylation, crotylation and related processes, see: Ramachandran PV Aldrichim. Acta 2002, 35, 23.Denmark SE; Fu J Chem. Rev 2003, 103, 2763.Yu C-M; Youn J; Jung H-K Bull. Korean Chem. Soc 2006, 27, 463.Marek I; u G Chem. Commun 2007, 1683.Hall DG Synlett 2007, 1644.Hargaden GC; Guiry PJ Adv. Synth. Catal 2007, 349, 2407.Lachance H; Hall DG Org. React 2008, 73, 1.Han SB; Kim IS; Krische MJ Chem. Commun 2009, 7278.Yus M; González-Gómez JC; Foubelo F Chem. Rev 2011, 111, 7774.Moran J; Krische MJ Enantioselective Carbonyl Allylation and Crotylation from the Alcohol Oxidation Level via C-C Bond Forming Transfer Hydrogenation Asymmetric Synthesis– The Essentials II; Christmann M,Bräse S Eds. Wiley-VCH: Weinheim, 2012, p 187.Yus M; GonzalezGomez JC; Foubelo F Chem. Rev. 2013, 113, 5595.Huo H-X; Duvall JR; Huang M-Y; Hong R Org. Chem. Front. 2014, 1, 303.Kumar P; Tripathi D; Sharma BM; Dwivedi N Org. Biomol. Chem. 2017, 15, 733.
- (2).For systematic studies of asymmetric ynal allylation, see: BouzBouz S; Pradaux F; Cossy J; Ferroud C; Falguières A Tetrahedron Lett.2000, 41, 8877.Urmibhusan B; Sullivan E; Hall DG Tetrahedron 2014, 70,678.
- (3).For isolated examples of the asymmetric ynal allylboration, see: Roush RR; Park JC J. Org. Chem. 1990, 55, 1143.Smith AB III; Ott GR J. Am. Chem. Soc. 1996, 118, 13095.Reddy YK; Falck JR Org. Lett., 2002, 4, 969.Gravel M;Lachance H; Lu X; Hall DG Synthesis 2004, 1290.Rauniyar V; Hall DG Angew. Chem. Int. Ed. 2006, 45, 2426. Robles O; McDonald FE Org. Lett. 2008, 10, 1811. Xing C-H; Liao Y-X; Zhang Y; Sabarova D; Bassous M; Hu Q-S Eur. J. Org. Chem. 2012, 1115. Sabitha G; Rao AS; Sandeep A; Yadav JS Eur. J. Org. Chem. 2014, 455.
- (4).For isolated examples of the asymmetric ynal allylstannation, see: Marshall JA; Tang Y Synlett 1992, 653.Park S-K; Kim S-I; Cho I-H Bull. Korean Chem. Soc. 1995, 16, 12.Marshall JA; Palovich MR J. Org. Chem. 1998, 63, 4381.Yu C-M; Jeon M; Lee J-Y; Jeon J Eur. J. Org. Chem. 2001, 1143. Denmark SE; Wynn TJ Am. Chem. Soc. 2001,123, 6199.Kurosu M; Lorca M Tetrahedron Lett.2002, 43, 1765.De Fátima A; Pilli RA Tetrahedron Lett. 2003, 44, 8721.Kurosu M; Lorca M Synlett 2005, 1109.Veerasamy N; Ghosh A; Li J; Watanabe K; Serrill JD; Ishmael JE; McPhail KL; Carter RG J. Am. Chem. Soc. 2016, 138, 770.
- (5).Whereas highly enantioselective allylzincation of acetylenic ketones has been reported (ref a), corresponding ynal allylzincation display significantly lower levels of asymmetric induction (ref b): Nakamura M; Hirai A; Sogi M; Nakamura E J. Am. Chem. Soc. 1998, 120, 5846.Fürstner A; Schlecker A Chem. Eur. J. 2008, 14, 9181.
- (6).For recent reviews of alcohol-mediated carbonyl addition, see: Ketcham JM; Shin I; Montgomery TP; Krische MJ Angew. Chem. Int. Ed. 2014, 53, 9142.Shin I; Krische MJ Top. Curr. Chem. 2016, 372, 85.Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ Science 2016, 354, 300. Kim SW; Zhang W; Krische MJ Acc. Chem. Res. 2017, 50, 2371.
- (7).Thomasi SS; Ladeira C; Ferreira D; Sprenger R.d. F.; Badino AC; Ferreira AG; Venâncio T Helv. Chim. Acta 2016, 99, 281. [Google Scholar]
- (8).For recent reviews on the chemistry of propargyl alcohols, see: Miyake Y; Uemura S; Nishibayashi Y ChemCatChem 2009, 1, 342.Cadierno V; Crochet P; Garcia-Garrido SE; Gimeno J Dalton Trans.2010, 39, 4015.Bauer EB Synthesis 2012, 44, 1131.Wang Q; Pu L Synlett 2013, 24, 1340.Zhu Y; Sun L; Lu P; Wang Y ACS Catal. 2014, 4, 1911.Ayers BJ; Chan PW H. Synlett 2015, 26, 1305.Zhang L; Fang G; Kumar RK; Bi X Synthesis 2015, 47, 2317.
- (9).(a) Han SB; Kim IS; Han H; Krische MJ J. Am. Chem. Soc. 2009, 131, 6916. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Han SB; Kim IS; Han H; Krische MJ J. Am. Chem. Soc. 2010, 132, 12517. [Google Scholar]
- (10).(a) Kim IS; Ngai M-Y; Krische MJ J. Am. Chem. Soc. 2008, 130, 6340. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim IS; Ngai M-Y; Krische MJ J. Am. Chem. Soc. 2008, 130, 14891. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hassan A; Lu Y; Krische MJ Org. Lett. 2009, 11, 3112. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schmitt DC; Dechert-Schmitt A-MR; Krische MJ Org. Lett. 2012, 14, 6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Robles O; McDonald Org. Lett. 2009, 11, 5498 Literature value: [α]D = −27.1 (25 °C, c = 1.2, CHCl3, >99% ee). Observed value: [α]D = −26.1 (25 °C, c = 1.0, CHCl3, 95% ee). [DOI] [PubMed] [Google Scholar]
- (12).(a) Kim IS; Han SB; Krische MJ J. Am. Chem. Soc. 2009, 131, 2514. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gao X; Townsend IA; Krische MJ J. Org. Chem. 2011, 76, 2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hassan A; Townsend IA; Krische MJ Chem. Comm. 2011, 10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wang G; Franke J; Ngo CQ; Krische MJ J. Am. Chem. Soc. 2015, 137, 7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cabrera JM; Tauber J; Zhang W; Xiang M; Krische MJ Submitted for Publication, ja-2018–05725q.
- (16).Sam B; Luong T; Krische MJ Angew.Chem.Int.Ed. 2015, 54, 5465 and reference cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).(a) Wagner AJ; David JG; Rychnovsky SD Org. Lett. 2011, 13, 4470. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wagner AJ; Miller SM; King RP; Rychnovsky SD J. Org. Chem. 2016, 81, 6253. [DOI] [PubMed] [Google Scholar]; (c) Burns AS; Ross CC; Rychnovsky SD J. Org. Chem. 2018, 83, 2504. [DOI] [PubMed] [Google Scholar]
- (18).Birman VB; Li X Org. Lett. 2008, 10, 1115. [DOI] [PubMed] [Google Scholar]
- (19).Alcohols with adjacent quaternary centers have shown unexpected selectivity in the past. See reference 17c.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.