Abstract
In this study aqueous extracts from salal berry (SB) and blackcurrant pomace (BCP) were used to reformulate yogurt and the anti-diabetic properties of the beverage were investigated during 4 weeks of cold storage at 4 °C. Results indicated that α-amylase, α-glucosidase and DPP-IV inhibitory activities increased with storage time for all samples. At the end of storage period α-amylase, α-glucosidase and DPP-IV inhibition were >61%, 62% and 56% respectively for all yogurt types. This increase in bioactivity during cold storage is attributed to the viability of lactic acid bacteria (∼108 cfu/g), which is maintained for 4 weeks. Enzyme inhibition increased similarly for all yogurt types at 4 °C except for α-glucosidase. Yogurt with BCP showed the highest potency to inhibit α-glucosidase (>90%) with an IC50 value of 0.20 mg/ml (week 4). A peptidomic approach based on liquid chromatography coupled with mass spectrometry (LC-MS) was used for the separation and identification of peptides generated in three types of yogurt. A total of 486 peptides mainly from caseins were identified, of which 15 have documented bioactivity, predominantly as antimicrobial agents or ACE-inhibitors.
Keywords: Food analysis, Food safety, Food science, Food technology, Nutrition
1. Introduction
Yogurt is one of the most popular dairy products which is produced from the acid coagulation of milk proteins during fermentation by lactic acid bacteria, typically Lactobacillus bulgaricus and Streptococcus thermophilus (Gharibzahedi and Chronakis, 2018). The popularity of yogurt is primarily owed to its sensory properties, which are appreciated widely by consumers around the world, in addition to its well-established nutritional value (Pereira, 2014). Yogurt may exert beneficial effects on metabolic health by controlling body weight, energy homeostasis and glycemic control and is therefore often considered a functional food with health-promoting and disease-preventing properties (Panahi et al., 2017). The latter are largely attributed to the addition of probiotic bacteria and/or the release of a range of bioactive peptides (Oliveira et al., 2015; Morell et al., 2017). The bioactive peptides identified in yogurt derive predominantly from the proteolytic action of lactic acid bacteria on milk proteins and have a wide range of physiological activities such as antihypertensive, antioxidant, antithrombotic, opioid, antimicrobial, cytomodulatory, immuno-modulatory, and miscellaneous peptides (Mann et al., 2017). These functions relate to human wellness or a reduced risk of certain chronic diseases.
Type 2 diabetes (T2D) is a chronic metabolic disorder that occurs either due to defective insulin production or action and is typically manifested by elevated sugar levels in blood, formerly known as hyperglycemia. Consumption of dairy proteins has been linked with serum glucose regulatory properties in humans, which is attributable to the action of bioactive peptides released during gastrointestinal digestion (Lacroix and Li-Chan, 2013). Milk-protein derived peptides can simulate the secretion of gut-derived hormones and/or inhibit enzymes involved in glycaemia homeostasis such as dipeptidyl peptidase IV (DPP-IV), α-amylase and α-glucosidase (Mann et al., 2017). The structural properties, gastrointestinal fate, absorption, bioavailability and mode of action of milk-protein derived peptides in relation to T2D regulation has been described in detail (Oseguera-Toledo et al., 2014; Patil et al., 2015).
In recent decades, yogurt recipe has diversified in response to consumers' demands for healthier and tastier products, which led to the development of a range of products acquiring different flavors, consistencies and texture (Morell et al., 2015). In particular, the inclusion of fruits in yogurt recipe, either at industrial scale or domestically, is one of the common practices adopted in yogurt-making. Meanwhile, the addition of fruits or fruits extracts has a major impact on the physico-chemical and nutritional properties of yogurt (Oliveira et al., 2015). This effect is fruit-specific and relates to its nutrient and non-nutrient composition. For instance, commonly added fruits to yogurts such as berries, are good sources of phenolic compounds (Matilla et al., 2006). These in turn are known to interact with milk proteins and form protein-polyphenol complexes (Charlton et al., 2002). These type of interactions, which are mediated predominantly by hydrophobic bonding between amino acid side chains and polyphenol aromatic rings and to a lesser extent by hydrogen or covalent bonding, determine the bioaccessibility and thus bioavailability of the ingested nutrients (Jakobek, 2015). Furthermore, molecular interactions between proteins and polyphenols may affect the susceptibility of the former to proteolytic activity by fermenting bacteria or digestive enzymes during passage through the gastrointestinal tract.
Salal (Gaultheria shallon) and blackcurrant (Ribes nigrum) are both common fruits in Scotland, which although novel and underutilized respectively, show potential for food applications due to their phenolic composition (McDougall et al., 2016). In this research, the effect of yogurt reformulation with SB and BCP extract on the inhibition of key enzymes for T2D management was monitored during refrigerated storage. An “omic” approach including peptidomics and proteomics was adopted to identify bioactive peptides from the soluble fraction and monitor changes in whey protein electrophoretic mobility between reformulated and plain yogurt. Thus, the objective of the present study was to correlate T2D enzyme inhibitory activities with bovine milk-derived bioactive peptides released from yogurt reformulated with aqueous berry extracts. Results can be used to identify functional food ingredients for the management of type 2 diabetes.
2. Materials and methods
2.1. Materials
Dried skimmed cow's milk powder (SMP) Marvel® brand, was obtained from Tesco supermarket (Aberdeen, UK). Freeze-dried yogurt starter culture containing Lactobacillus bulgaricus and Streptococcus thermophilus (Goat Nutrition Ltd., Ashford, England) was used to prepare yogurt starter. Dried and powdered SB and BCP were kindly donated by James Hutton Institute (Dundee, Scotland). Pure Whey IsolateTM 97 powder (WPI) was used as emulsifier and was purchased from Bulk Powders (Colchester, UK). A-glucosidase type I from baker's yeast, amylase activity assay, L-Serine and O-Phthaldialdehyde reagent solution was purchased from Sigma-Aldrich (Dorset, UK). Amicon® Ultra-0.5 (3kDa) centrifugal filter units were purchased from Sigma-Aldrich (Dorset, UK). Precast gels and all reagents used for protein electrophoresis were purchased from Bio-Rad Laboratories Ltd (Hertfordshire, UK). All other reagents used were of analytical grade.
2.2. Preparation and storage of aqueous fruit extracts and yogurt beverages
80 ml of purified water was added to 8 g of fruit for preparing each aqueous extract. The extracts were mixed for 1 h on a Stuart SRT6 tube roller (Cole-Palmer, Staffordshire, UK) at room temperature and then centrifuged at 2290 x g for 15 min using an EppendorfTM 5702R (Fisher Scientific, Loughborough, UK). The supernatant was collected and filtered with butter muslin squares (Lakeland, Aberdeen, UK) to remove any residues. The extraction process was repeated 3 times and liquid extracts were combined and stored at -20 °C.
Yogurt was made up to 0.5 kg for each sample using milk powder, filtered water, freshly prepared yogurt starter and aqueous fruit extracts. Yogurt starter was prepared by dissolving the freeze-dried culture (5 g) in 840 g of water and adding 155 g of SMP (0.5 g lactic culture/100 g milk). The recipe for all samples included 16% (w/w) of dried milk powder and 3% (w/w) of yogurt starter. 20% (w/w) of fruit extract in liquid form was added to the reformulated samples and the water content was adjusted accordingly for the control sample. Yogurt mixes (milk powder, water and fruit extracts if applicable) were heated to 80 °C for 10 min and then immediately cooled down to a temperature of approximately 45 °C. This was followed by the addition of the 30g of yogurt starter to the mixes. Samples were then poured in a sterile container and placed in a yogurt fermenter (Lakeland, Aberdeen, UK) set at 44 °C. A portable food and dairy pH meter (Hanna Instruments Ltd, Leighton Buzzard, UK) was used to measure the changes in pH of the samples during fermentation on an hourly basis until a pH of 4.5 was reached. At the end of the fermentation process samples were diluted (1:1) on a weight basis and samples were then processed in a single stage valve homogenizer (APV-1000, SPX Flow Technology, West Sussex, UK). Each sample was passed twice through the homogenizer to ensure complete homogenization. Homogenization pressure was set at 50 bar for both the passages through the homogenizing valve. Two different batches for every yogurt beverage (500 g) were prepared, which were stored at 4 °C for 4 weeks. Sampling was performed on a weekly basis for subsequent analysis. Samples were centrifuged at 13,000 x g for 10 min using an Eppendorf® MiniSpin® Plus (Sigma-Aldrich, MO, USA) and the supernatant (aqueous extract) was used for all subsequent analyses.
2.3. Total phenol assay of dried SB and BCP
Powdered dried sample (0.8 g) was extracted with 19.2 ml ethanol (50%) for 2 h with shaking at room temperature. After centrifugation at 3220 × g (Eppendorf® 5810R, Fisher Scientific, Loughborough, UK) for 1 h at 4 °C, the supernatant was collected and the extraction was repeated one more time. The supernatants were combined and dried using a rotary evaporator (Rotavapor R-114, Büchi Labortechnik AG, Switzerland) and the remaining solution was freeze-dried (FreeZone, Labconco Corporation, MO, USA). The dried extract was reconstituted in DMSO (100%) at 200 mg/ml, aliquoted and stored at -20 °C until analyzed. YE were used for all analyses unless otherwise stated. Phenol content was measured using a modified Folin–Ciocalteau method (Deighton et al., 2000). In brief, 50 μl of extract were mixed with 250 μl ml of Folin–Ciocalteu reagent and incubated at room temperature for 1 min. Following the addition of 200 μl of 7.5 % (w/v) sodium carbonate to the mixture, total polyphenols were determined after 1 h of incubation in the dark at room temperature. The absorbance of the reaction mixture was determined at 765 nm against blank sample using a SpectraMax 190 microplate reader (Molecular Devices Limited, Berkshire, UK). Quantification was done with respect to the standard curve of Gallic acid and results are expressed as Gallic acid equivalents (GAE).
2.4. Determination of α-Amylase inhibitory activity
The α-amylase inhibition assay was adapted from Apostolidis et al. (2006) with slight modifications. 40 μl yogurt aqueous extracts and 10 μl α-amylase solution (0.5 mg/ml) were incubated at 25 °C for 10 min. This was followed by the addition of 100 μl amylase substrate mix (amylase activity assay kit, Sigma-Aldrich, Dorset, UK). The reaction mixtures were incubated at 25 °C for 10 min and the absorbance were read at 405 nm using a SpectraMax 190 microplate reader (Molecular Devices Limited, Berkshire, UK). The readings were compared to a control which contained 40 μl of buffer solution instead of the extract. The enzyme inhibition was calculated as below:
2.5. Determination of α-Glucosidase inhibitory activity
The α-glucosidase inhibitory activity was determined according to the method described by Arce et al. (2016) with slight modifications. 50 μl yogurt aqueous extracts and 10 μl α-glucosidase solution (1 U/ml) were incubated at 37 °C for 10 min followed by the addition of 50μL of 0.4 mM/L ρ-nitrophenyl-α-D-glucopyranoside (α-NPG) solution in 0.1 mol/L phosphate buffer (pH 7.4). After incubated at 37 °C for 15 min, 50 μl of 0.2 mol/L Na2CO3 were added into the reaction mixture to end the reaction, and its absorbance was measured at 405 nm in a 96 well plate using a using a SpectraMax 190 microplate reader (Molecular Devices Limited, Berkshire, UK). The same procedure was followed for the samples which have been previously fractionated using the Amicon® Ultra-0.5 centrifugal filter devices (3 kDa). The enzyme inhibition was calculated as below:
The IC50 values (concentrations of supernatants to induce a 50% inhibition of the enzyme activity) were determined from the equations generated by fitting the data from the plot of percent α-glucosidase inhibition at week 4 against linear hydrolysate concentrations ranging from 0.15 to 0.3 mg/mL (final assay concentration).
2.6. Determination of DPP-IV inhibitory activity
The Dipeptidyl-peptidase 4 (DPP-IV) inhibitory activity was determined using a DPP-IV inhibitor screening kit (Sigma-Aldrich, Dorset, UK). Yogurt aqueous extracts (25 μl) were pipetted onto a black 96-well microplate containing the DPP-IV enzyme (50 μl). This was followed by the addition of 25 μl Enzymatic Reaction Mix, and the fluorescence (λex = 360/λem = 460) was measured every minute on a plate fluorimeter (SpectraMax, GEMINI XS, Molecular Devices Ltd., Wokingham, UK) in kinetic mode at 37 °C for 15 min. The negative control contained 25 μl DPP-IV Assay Buffer instead of the extract. The enzyme inhibition was calculated as below:
2.7. Degree of hydrolysis
The OPA method was used to determine the degree of hydrolysis (DH) of the yogurt samples stored for different time (Rao et al., 2018). The assay was carried out by adding 400 μl of sample to 3 ml of OPA reagent. After vortexing, the samples were incubated for 2 min at room temperature and the absorbance was measured at 340 nm using a spectrophotometer (SpectraMax 190, Molecular Devices Limited, Berkshire, UK). L-serine (0.9516 meq/L) was used as positive control and distilled water as negative control.
2.8. Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis (PAGE)
SDS-PAGE was carried out according to the method described by Laemmli (1970) using a Mini-Protean® 3 electrophoresis cell unit (Bio-Rad, Hertfordshire, UK). Proteins were analyzed on a 4–20% Mini-Protean® TGX™ precast gel. The migration buffer contained 25 mM Tris, 192 mM glycine and 0.1% SDS (pH 8.3). Supernatants (aqueous extracts) were diluted (x10) with dH2O and were then dispersed in an equal volume of sample buffer (31.5 mM Tris-HCl, pH 6.8, 10% glycerol, 1% SDS, 0.005% Bromophenol Blue). Samples were heat-denatured at 100 °C for 2 min and 10 μl of each sample were loaded on the gel. SDS-PAGE under reducing conditions was carried out with the addition of 2-mercaptoethanol. Electrophoretic migration was performed at 150 V (constant) for 40 min. The gel was stained with Coomassie Brilliant Blue R-250 staining solution for 1 h with gentle agitation and destained for 2 h. The gel was scanned with a GS-800™calibrated densitometer (Bio- Rad, Hertfordshire, UK). Precision Plus Protein™ unstained standards were used from protein band identification as molecular weight markers (MWM).
2.9. Peptide identification
2.9.1. Sample preparation
Sample preparation was according to Kunda et al. (2012) with the addition of a size separation step to select proteins smaller than 3 kDa. Briefly, 0.2 mL yogurt sample was centrifuged at 12000 x g for 30 min in Amicon® Ultra-0.5 centrifugal filter device (3 kDa), and the filtrate (<3 kDa) collected. The resulting filtrate was diluted with 2.5 ml of reduction buffer (6 M urea, 5 mM sodium citrate, 5 mM DTT, pH 8.0) and incubated for 1 h at room temperature, followed by centrifugation at 3200 x g for 30 min. Any resulting fat layer was removed by pipetting and the clear solutions were filtered through 0.22 μm PES (Millex GP filter Unit, Millipore) before solid-phase extraction (SPE) with Bond Elut Plexa (Agilent, UK) polymeric SPE cartridges (60 mg of sorbent). The SPE cartridges were conditioned with 2 mL of methanol followed by 2 mL of water. Sample (2 mL) was applied to the SPE column, rinsed with two 1 ml washes (water), and the retained compounds were eluted with 1 ml of 80:20 (v/v) methanol: water and 0.1% (v/v) of formic acid. Flow rate was maintained at approximately 1 ml/min during SPE using Cerex System 48 pressure processor. Eluate was evaporated to dryness and final residue was reconstituted with 98% water, 2% acetonitrile, 0.1% formic acid before LC-MS experiments.
2.9.2. Proteomics-Q Exactive LC-MS
Peptide samples (purified by SPE) in 1 mL 80% methanol were dried in a SpeedVac SC110A (Savant Instruments Pvt. Ltd., Hyderabad, India) and dissolved in 100 μL 0.1% trifluoroacetic acid (TFA) for purification on ZipTip C18 stage tips (Merck Millipore, MA, USA) according to the manufacturer's instructions. Peptides were eluted in 5 μL 70% acetonitrile, 0.1% TFA in a 96-well format microtitre plate, dried in a SpeedVac, and dissolved in 10 μL loading solvent for LC-MS analysis. The LC-MS system comprised a UltiMate 3000 RSLCnano liquid chromatography system (Thermo Scientific Dionex, MA, USA) configured for pre-concentration onto a nano column fitted to an EASY-Spray ion source (Thermo Scientific) and connected to a Q Exactive Plus quadrupole – Orbitrap hybrid mass spectrometer (Thermo Scientific). Sample (2 μL) was loaded onto a pre-column (C18 PepMap 100 300 μm i.d. x 5 mm (Thermo Scientific)) in loading solvent (2% acetonitrile, 0.1% formic acid in water) at 10 μL/min. After 5 min flow through the pre-column was switched to nanoflow and the sample was reversed-flushed to the nano column (PepMap RSLC C18 75 μm i.d. x 25 cm (Thermo Scientific)) at 300 nL/min using a gradient of acetonitrile in 0.1% formic acid in water. Solvent A was 0.1% formic acid in water and solvent B was 80% acetonitrile, 0.1% formic acid in water. The gradient was 3–10% solvent B from 5-10 min, 10–40% solvent B from 10-40 min, 40–80% solvent B from 40-45 min, 80% solvent B from 45-53 min, and finally held at 3% solvent B from 54-69 min. The Q Exactive Plus was operated from 5-65 min in full MS/data-dependent MS2 mode using a ‘Top 10’ method. Full MS scans were performed from 375-1750 m/z with a resolution of 70,000, an automatic gain control (AGC) target of 3e6, and a maximum injection time (IT) of 50 ms. The 10 most abundant precursor ions with a charge state of +2 to +5 were selected from each MS scan for sequential trapping and fragmentation by higher-energy collisional dissociation (normalized collision energy 26%). MS2 scans were performed at a resolution of 17,500, an AGC target of 5e4, and a maximum IT of 100 ms. Previously selected ions were dynamically excluded for 40 s, and peptide ions were preferred. Q Exactive raw data files were processed with Proteome Discoverer v1.4 (Thermo Scientific) using a workflow that included a Mascot v2.5 (Matrix Science) and calculation of protein area values based on the extracted ion chromatograms of the three most abundant peptides. Searches were carried out against the Bos taurus sequences (UniProtKB proteome UP000009136) using the following parameters: Enzyme = none; Maximum missed cleavages = 0; Instrument = FT-ICR; Precursor mass tolerance = 10 ppm; Fragment mass tolerance = 20 mmu; Dynamic modification = Oxidation (M); Static modification = none.
2.10. Microbiological analysis of yogurt
The most important indices of yogurt microflora were measured during the storage period at days 1, 14, 21 and 28 at 4 °C. The total lactic acid bacteria (LAB) were counted (after appropriate dilutions in Oxoid buffered peptone water (CM0509) in 41782 MRS agar (Sigma-Aldrich, Dorset, UK) after incubation at 37 °C for 4 days, while Lactobacillus bulgaricus was inoculated in Lactobacillus selective agar (83920 Rogosa Agar, Sigma-Aldrich, Dorset, UK) and incubated at 37 °C for 4 days, and Streptococcus thermophillus was cultivated in M-17 agar (Sigma-Aldrich, Dorset, UK) and incubated at 37 °C for 2 days. The microbial populations were analyzed in duplicate and the average values are depicted here.
2.11. Statistical analysis
Results are expressed as mean ± standard deviation (SD) of at least three replicates. Statistical analysis of the data was performed using the statistical software SPSS Statistics 22 (IBM). Data were analyzed by analysis of variance (ANOVA) and significant differences (p < 0.05) were detected by the Scheffé’s post hoc test.
3. Results and discussion
3.1. Determination of α-amylase and DPP-IV inhibitory activities
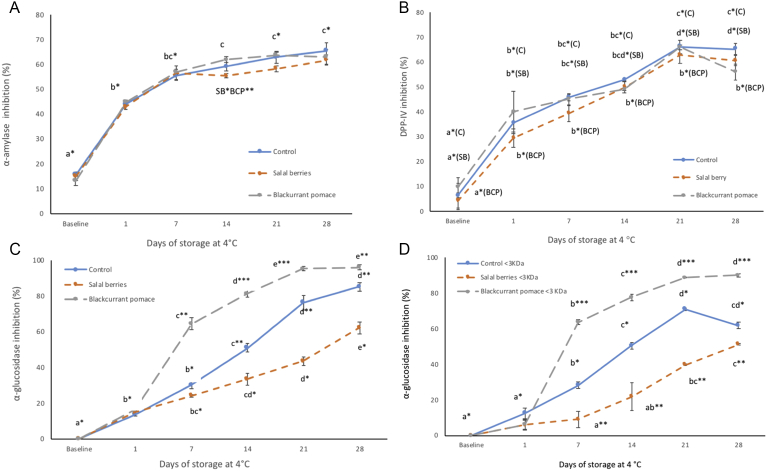
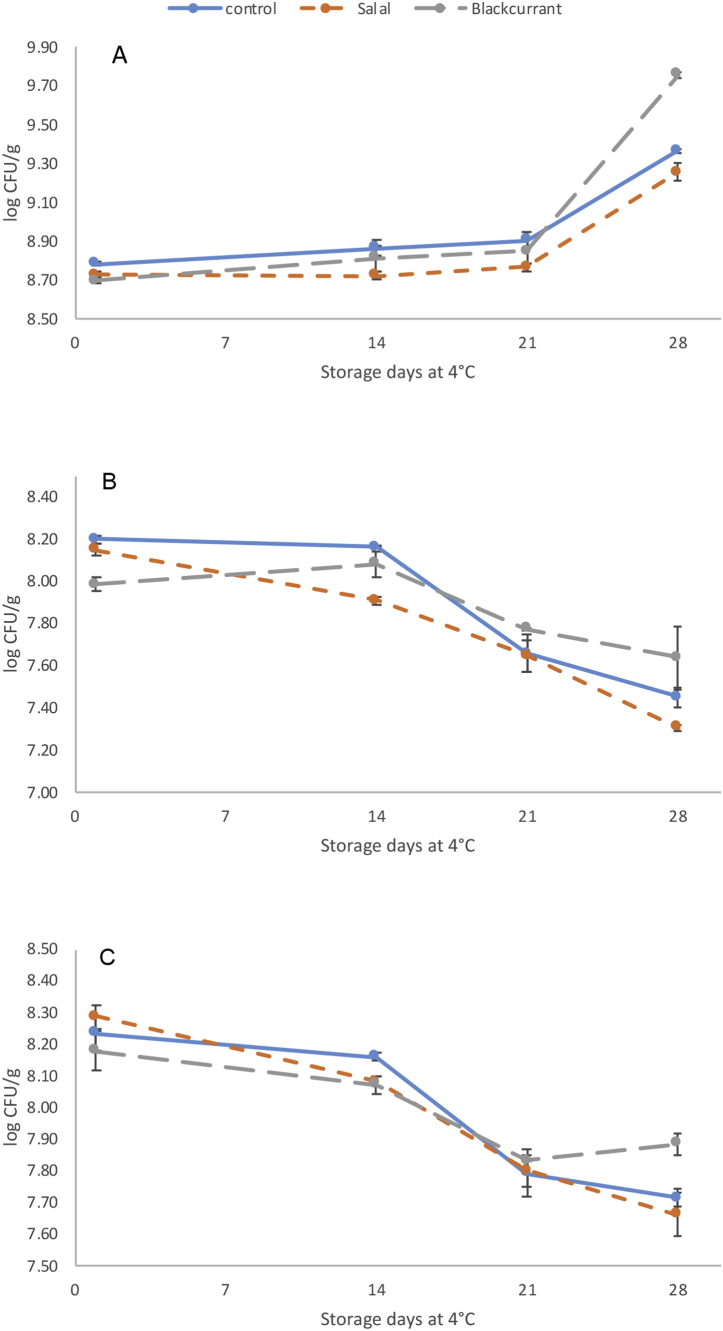
Alpha-amylase inhibition is considered an important mechanism involved in the management of T2 diabetes, which delays carbohydrate digestion by preventing the breakdown of starch (Saeidnia et al., 2016). Plant proteins and particularly cereals, legumes and wheat, as well as soybean and cumin have been reported to be the main sources of peptides with α-amylase inhibitory effects (Daliri et al., 2017; Oseguera-Toledo et al., 2014). Furthermore, a variety of fruits are known to display α-amylase inhibitory activity, which is attributed to their polyphenolic composition such as flavonoids and tannins (McDougall et al., 2005). In this study, the α-amylase inhibition increased significantly for all types of yogurt after the fermentation step compared to the non-fermented samples (baseline) and the inhibitory effect was further increased during storage at 4 °C (Fig. 1A). At day 7, the α-amylase inhibitory activities of all three samples were over 60%, and remained at this level throughout the total storage period of 28 days. To the best of our knowledge, the present study is the first to report α-amylase inhibitory activity from fermented milk proteins during cold storage. This effect may be attributed to the release of peptides with moderate α-amylase inhibitory activity during cold storage as a result of the continuous proteolytic activity of the lactic acid bacteria. As indicated by Fig. 2, the viability of lactic acid bacteria was similar for all three types of yogurts and in accordance to the requirements for viable counts in fermented dairy products (>7 log CFU/ml) (Oliveira et al., 2015). Viable counts remained stable during 14 days of refrigerated storage, followed by a decline for L. bulgaricus strain for the remaining of the storage period and an increase for S. thermophillus during the last week of storage. Our results are in agreement with previously published data which suggests that viable counts are higher for S. thermophillus compared with L. bulgaricus, possibly due to the documented oxygen sensitivity of the latter bacterial strain (Beshkova et al., 2002). Nevertheless, the total number of lactic acid bacteria decreased during storage and this effect was more evident for storage periods longer than 14 days (Fig. 2C).
Fig. 1.
Alpha-amylase (A), DPP-IV (B) and alpha-glucosidase (C,D) inhibitory activities of yogurt beverages. Each point is the mean of three replicates (n = 3)±SE. Different letters depict significant differences (P < 0.05) between means for days of storage (per yogurt type). Asterisks depict significant differences (P < 0.05) between means for yogurt types (per days of storage).
Fig. 2.
Viable counts of S. thermophilus (A) L. bulgaricus (B) and total lactic acid bacteria (LAB) (C) in plain and fruit yogurt beverages during 4 weeks of storage at 4 °C. Each point is the mean of two replicates (n = 2)±SD.
DPP-IV inhibitors enhance the ability to control blood glucose by increasing the active levels of incretin hormones in the body (Barnett, 2006). Peptides with documented DPP-IV inhibitory activity can be generated from various food sources including salmon skin, rice bran, tuna cooking juice, skin gelatin and milk (Patil et al., 2015). In particular, 172 entries for DPP-IV inhibitory peptides (of which 64 unique sequences) are deposited in the milk bioactive peptide database (Nielsen et al., 2017). These peptides range from 2 to 14 amino acids in length and half of the unique sequences have a proline at the second position from the N-terminal, indicating a possible structural pattern for DPP-IV inhibitory peptides (Nongonierma and FitzGerald, 2014). Bovine casein proteins and in particular β-casein has a great potential to serve as precursor for DPP-IV inhibitors due to the high number of proline residues in the amino acid sequence (Lacroix and Li-Chan, 2012). In the present study, the DPP-IV inhibitory activities of all types of yogurt are similar to the α-amylase inhibitory activities (Fig. 1B). The DPP-IV inhibitory activity significantly increased after the fermentation stage and continued to increase at steady rate during the storage period of 28 days. The maximum inhibitory effect (>60%) was observed after 21 days of storage for all three types of yogurt and may be explained by the release of DPP-IV inhibitory peptides as a result of the hydrolytic action of lactic acid bacteria. In conclusion, yogurt beverages containing SB and BCP had similar α-amylase and DPP-IV inhibitory activities with plain yogurt at all sampling points, which suggests that the addition of fruit extracts had no significant effect (P < 0.05) on the viability of the fermenting bacteria or the release of peptides with α-amylase and DPP-IV inhibitory activities.
3.2. Determination of α-glucosidase inhibitory activity
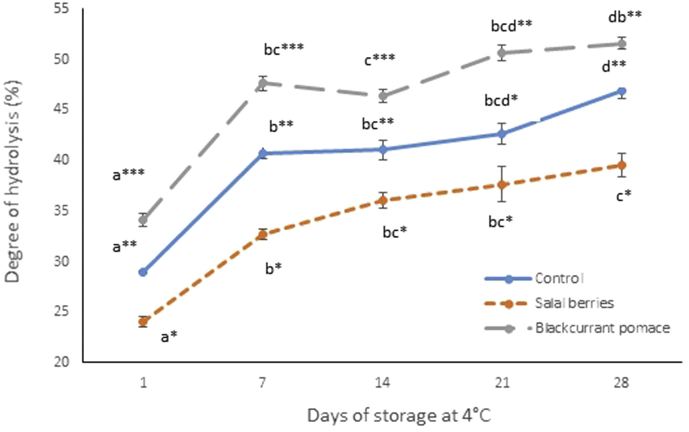
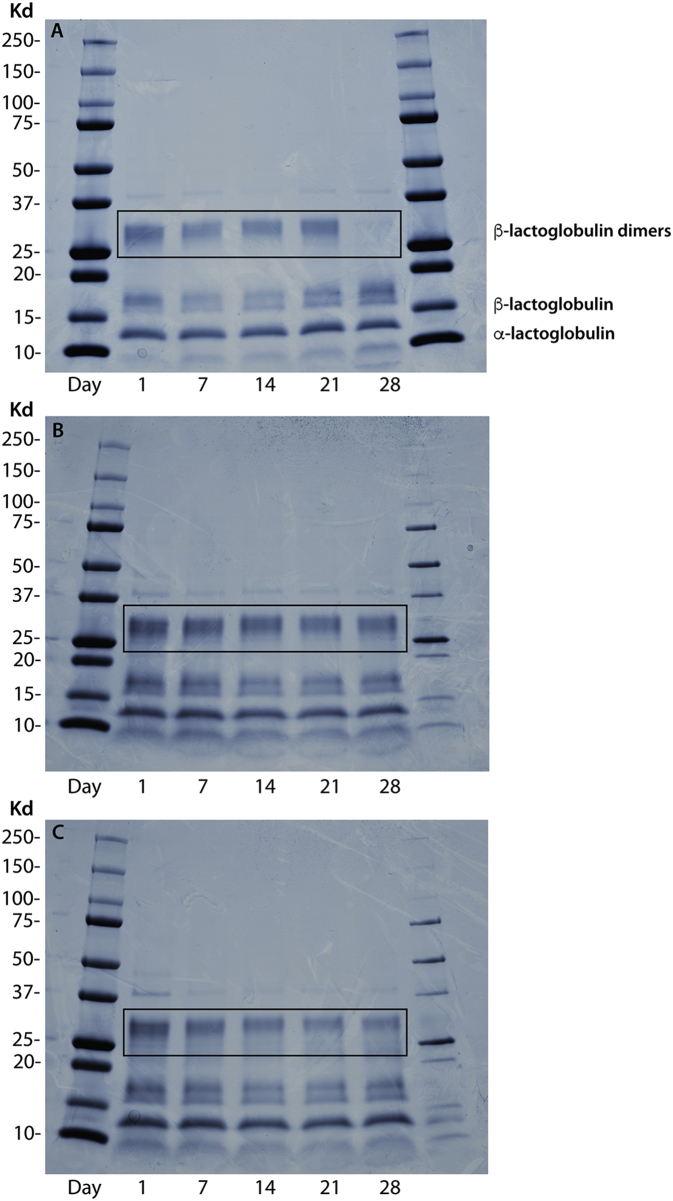
Alpha-glucosidase is another key enzyme involved in diabetes control, which is located in the brush border of the enterocytes of the jejunum in the small intestine and catalyzes the breakdown of carbohydrates by cleaving glycosidic bonds (Yao et al., 2010). Hence, α-glucosidase inhibitors can retard the release and absorption of monosaccharides from complex carbohydrates, resulting in reduced postprandial plasma glucose levels. A-glucosidase inhibitors such as acarbose, are currently used for the treatment of diabetic individuals with postprandial hyperglycemia (Patil et al., 2015). As presented in Fig. 1C, all three types of yogurt exhibited α-glucosidase inhibitory activities, and the inhibitory effect increases with increasing storage time. During storage time, the α-glucosidase inhibitory activity of yogurt beverages containing SB was significantly lower (P < 0.05) compared with the yogurt formulated with BCP (day 7 onwards) and the plain yogurt (day 14 onwards). At the end of the storage period, the α-glucosidase inhibitory activities of the yogurt beverages followed the order BC > C >> SB. The calculated IC50 values of the yogurt beverages (BC = 0.20 mg/ml, C = 0.20 mg/ml and SB = 0.28 mg/ml) were in agreement with this order and were lower (higher potency) compared to values reported for whey protein hydrolysates (Lacroix and Li-Chan, 2013). The exact mechanism by which peptides inhibit α-glucosidase activity is unknown, but it has been suggested that non-saccharide compounds may exert their inhibitory activity by binding to the enzyme's active site via hydrophobic interactions (Bharatham et al., 2008). To further investigate the effect of the fruit extracts on the observed α-glucosidase inhibitory activity in yogurt, 10 kDa and 3 kDa centrifugal filters were used to fractionate peptides based on their molecular mass. The retentates over 3 kDa showed no α-glucosidase inhibitory activity for all yogurt types (data not shown), whereas the 3 kDa filtrates showed similar α-glucosidase inhibitory activities to the non-filtered samples and followed the same trends with respect to storage time and fruit specificity (Fig. 1D). This supports further the hypothesis that the α-glucosidase inhibitory activity shown by the yogurt samples is primarily owned to the release of bioactive peptides. The degree of protein hydrolysis following the fermentation process and during storage of the yogurt beverages was determined to assess differences between the samples (Fig. 3). The results implied that milk protein was gradually hydrolyzed during storage and the degree of hydrolysis varied depending on the fruit extract. The pattern was consistent with the results obtained from the α-glucosidase inhibitory activity assay and suggests that the addition of BCP promoted the release of peptides with α-glucosidase inhibitory activity. On the contrary, yogurt beverages with SB were less susceptible to enzymatic hydrolysis, which may have hindered the release of bioactive peptides. This effect may be attributed to the formation of complexes between milk proteins and polyphenols by hydrophobic interactions or hydrogen bonds (Oliveira et al., 2015). Caseins and whey proteins are known to interact with plant phenolics, which may induce protein unfolding and the formation of insoluble complexes (Hasni et al., 2011; Zhang et al., 2014). Furthermore the complex formation is affected by the phenol concentration, which in turn determines the extent of the aggregation phenomena (von Staszewksi et al., 2011). Our results indicate that SB contains significantly higher concentration of phenolic compounds compared to blackcurrant pomace (SB: 12.9 M and BCP: 7.4M Gallic acid equivalents), which may explain the reduced susceptibility to proteolytic cleavage for the yogurt beverages with salal fruit. Further evidence from proteomic analysis of the yogurt samples during storage, suggests that phenols may play a significant role in protein structure (Fig. 4). β-lactoglobulin exists as a mixture of monomers and dimers in the samples and the latter are stabilized by noncovalent interactions (reducing conditions). At the end of the storage period, the band corresponding to the dimer cannot be detected for plain yogurt and the intensity of the band corresponding to the monomer increases (Fig. 4A). The same but less noticeable effect can be observed for the sample containing BCP (Fig. 4C), whereas the intensity of the dimer band for the yogurt with SB remains high throughout the storage period (Fig. 4B). Our results suggest that β-lactoglobulin dimer is more stable in the yogurt sample with SB, possibly due to increased interactions with phenolic compounds, which may contribute to protein crosslinking.
Fig. 3.
Extent of hydrolysis of yogurt beverages during storage at 4 °C. Each point is the mean of three replicates (n = 3)±SE. Different letters depict significant differences (P < 0.05) between means of each yogurt type for days of storage (per yogurt type). Asterisks depict significant differences (P < 0.05) between means for yogurt types (per days of storage).
Fig. 4.
Electrophoretic migration pattern analysis of whey proteins and molecular weight markers for plain (A), SB (B) and BCP (C) yogurt by SDS-PAGE (reducing conditions) during refrigerated storage.
3.3. Identification of (bioactive) peptides in yogurt drinks using Q Exactive LC-MS
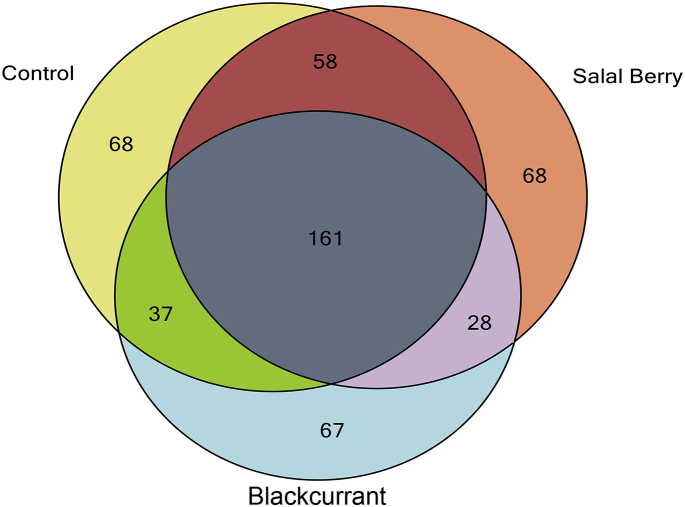
Peptidomics is becoming an increasingly valuable tool for the characterization of food samples including dairy products. It is considered a sub-field of proteomics with a primary focus on the composition, interactions, and properties of peptides present in a food matrix (Gagnaire et al., 2009). The recent developments of peptidomic technology for the identification of bioactive peptides from dairy proteins have been documented in detail (Giacometti and Buretić-Tomljanović, 2017; Sánchez-Rivera et al., 2014). Peptidomic approaches have been employed for identifying peptides in yogurt generated from fermented milk proteins with lactic acid bacteria (Jin et al., 2016; Kunda et al., 2012). By using different combinations of parameters and milk matrices, various bioactive peptide fragments can be obtained (Bernardi et al., 2015). In this study, aqueous extracts from berry fruits were used as additional ingredients for yogurt manufacture. Samples from each yogurt type from the end of the storage period (4 weeks) were selected for peptidomic analysis. Results revealed a total 487 peptides of which 161 were common in all types of yogurts (Fig. 5). The vast majority of the peptides identified belong to caseins and based on their abundance follow the order: beta-casein, alpha-S1-casein, kappa-casein and alpha-S2-casein. Peptides from whey proteins were not detected, possibly because at this pH (4.5), whey proteins are insoluble (isoelectric precipitation). The casein peptides identified (407) were screened for bioactivity using the Milk Bioactive Peptide Database (MBPDB, http://mbpdb.nws.oregonstate.edu/) (Nielsen et al., 2017). The bioactive peptides generated from the different types of yogurt are presented in Table 1. According to the literature, these peptides have ACE-inhibitory or antimicrobial activity but no peptides with documented anti-diabetic activity were detected. The peptides identified exclusively in yogurt formulated with BCP (potent α-glucosidase inhibitors) are summarized in Table 2. Only two peptides (MPFPKYPVEP and YQEPVLGPVR) from the list (57 in total) have been identified as bioactive previously (ACE-inhibitors). Further research using in silico screening for the peptides identified in BCP yogurt is required to assess their potential as α-glucosidase inhibitors.
Fig. 5.
Venn diagram of the number of identified peptides in yogurt beverages.
Table 1.
Bioactive peptides identified in yogurt drinks using polymeric SPE cartridges.
| Protein ID | Peptide | Protein description | Species | Intervals | Function | Reference | Control | SB | BCP |
|---|---|---|---|---|---|---|---|---|---|
| P02666 | KYPVEPFTESQSLTL | β-casein | Bos taurus | 128–142 | ACE-inhibitory | Apostolidis et al. (2006) | ✓ | ✓ | |
| P33048 | TEDELQDKIHPF | β-casein | Capra hircus | 56–67 | Antimicrobial | Arce et al. (2016) | ✓ | ✓ | ✓ |
|
P33048 P02666 |
YQEPVLGPVRGPFPI | β-casein | Capra hircus Bos taurus |
206–220 193–207 |
Antimicrobial | Arce et al. (2016), Barnett, (2006) | ✓ | ✓ | |
| P02666 | YQEPVLGPVRGPFPIIV | β-casein | Bos taurus | 193–209 | Immunomodulatory, Antithrombin, Antimicrobial, ACE-inhibitory |
Apostolidis et al. (2006), Barnett, (2006), Bernardi et al. (2015), Beshkova et al. (2002) | ✓ | ✓ | ✓ |
| P02666 | VLPVPQKAVPYPQR | β-casein | Bos taurus | 185–198 | Antimicrobial | Bharatham et al. (2008) | ✓ | ✓ | ✓ |
| P02666 | LLYQEPVLGPVRGPFPIIV | β-casein | Bos taurus | 206–224 | ACE-inhibitory | Apostolidis et al. (2006) | ✓ | ✓ | ✓ |
| P02666 | SQSKVLPVPQ | β-casein | Bos taurus | 181–190 | ACE-inhibitory | Charlton et al. (2002) | ✓ | ✓ | |
| P02666 | LHLPLPL | β-casein | Bos taurus | 148–154 | ACE-inhibitory | Daliri et al. (2017) | ✓ | ✓ | ✓ |
| P02666 | NLHLPLPLL | β-casein | Bos taurus | 147–155 | ACE-inhibitory | Deighton et al. (2000) | ✓ | ✓ | ✓ |
| P33048 | PVVVPPFLQPE | β-casein | Capra hircus | 96–106 | Antimicrobial | Arce et al. (2016) | ✓ | ||
| P02666 | MPFPKYPVEP | β-casein | Bos taurus | 124–133 | ACE-inhibitory | Charlton et al., (2002) | ✓ | ||
| P02666 | YQEPVLGPVR | β-casein | Bos taurus | 208–217 | ACE-inhibitory | Gagnaire et al. (2009) | ✓ | ||
| P02662 | VLNENLLR | α-S1-casein | Bos taurus | 30–37 | Antimicrobial | Gharibzahedi and Chronakis, (2018), Giacometti and Buretić-Tomljanović, (2017), Hasni et al. (2011) | ✓ | ✓ | |
| P02663 | ALNEINQFYQK | α-S2-casein | Bos taurus | 96–106 | ACE-inhibitory | Jakobek, (2015) | ✓ | ||
| P02663 | TKVIPYVRYL | α-S2-casein | Bos taurus | 213–222 | Antimicrobial | Jin et al. (2016) | ✓ | ✓ | ✓ |
Table 2.
Peptides identified exclusively in Blackcurrant pomace yogurt drinks using polymeric SPE cartridges.
| n | Amino acid sequence | Protein precursor | Observed mass (Da) | Ion m/z | Charge | Retention time (min) | IonScore |
|---|---|---|---|---|---|---|---|
| 1 | APKHKEMPFPKYPVEPF | β-casein | 2042.05 | 1021.53 | 2 | 20.72 | 60 |
| 2 | HKEMPFPKYPVEPFTES | β-casein | 2062.99 | 1032.00 | 2 | 23.18 | 58 |
| 3 | MAPKHKEMPFPKYPVEPFT | β-casein | 2274.14 | 1137.58 | 2 | 20.99 | 55 |
| 4 | MHQPHQPLPPTVMFPPQSVL | β-casein | 2281.16 | 1141.08 | 2 | 27.56 | 55 |
| 5 | ENLHLPLPLLQSW | β-casein | 1559.86 | 780.43 | 2 | 38.70 | 55 |
| 6 | MPFPKYPVEPFTESQSLTL | β-casein | 2211.11 | 1106.06 | 2 | 33.76 | 54 |
| 7 | MAPKHKEMPFPKYPVEPFTESQ | β-casein | 2618.27 | 1309.64 | 2 | 20.97 | 54 |
| 8 | EMPFPKYPVEPFTESQSLTLTD | β-casein | 2572.22 | 1286.61 | 2 | 32.17 | 54 |
| 9 | EAMAPKHKEMPFPKYPVEPF | β-casein | 2373.18 | 1187.09 | 2 | 21.96 | 53 |
| 10 | EMPFPKYPVEPFTESQSLTL | β-casein | 2356.14 | 1178.57 | 2 | 32.79 | 53 |
| 11 | LTDVENLHLPLPLLQS | β-casein | 1802.00 | 901.52 | 2 | 36.62 | 52 |
| 12 | LYQEPVLGPVRGPFPII | β-casein | 1895.08 | 948.04 | 2 | 34.93 | 52 |
| 13 | MPFPKYPVEPFTESQSLT | β-casein | 2098.01 | 1049.51 | 2 | 30.73 | 51 |
| 14 | PVVVPPFLQPEVMG | β-casein | 1508.82 | 754.91 | 2 | 39.33 | 51 |
| 15 | VLPVPQKAVPYPQRDMPIQA | β-casein | 2263.22 | 1132.11 | 2 | 21.65 | 50 |
| 16 | SKVLPVPQKAVPYPQRD | β-casein | 1922.09 | 641.37 | 3 | 17.15 | 49 |
| 17 | KEMPFPKYPVEPFTE | β-casein | 1838.90 | 919.96 | 2 | 25.53 | 48 |
| 18 | SLSQSKVLPVPQKAVPYPQR | β-casein | 2222.26 | 741.43 | 3 | 18.89 | 47 |
| 19 | MPFPKYPVEPF | β-casein | 1351.67 | 676.34 | 2 | 29.78 | 47 |
| 20 | MPFPKYPVE | β-casein | 1107.55 | 554.28 | 2 | 23.54 | 46 |
| 21 | HPFAQTQSL | β-casein | 1028.51 | 514.76 | 2 | 16.63 | 46 |
| 22 | NLHLPLPLLQSW | β-casein | 1430.81 | 715.91 | 2 | 38.57 | 45 |
| 23 | SLSQSKVLPVPQKAVPYPQRD | β-casein | 2337.29 | 585.08 | 2 | 19.17 | 45 |
| 24 | MPFPKYPVEP | β-casein | 1204.61 | 602.81 | 2 | 24.73 | 45 |
| 25 | YQEPVLGPVR | β-casein | 1157.63 | 579.32 | 2 | 20.13 | 44 |
| 26 | TPVVVPPFLQPEVM | β-casein | 1568.84 | 784.92 | 2 | 37.21 | 43 |
| 27 | FPKYPVEPFTES | β-casein | 1440.70 | 720.86 | 2 | 25.98 | 43 |
| 28 | VLPVPQKAVPYPQRDM | β-casein | 1837.10 | 613.34 | 3 | 20.96 | 43 |
| 29 | PGPIPNSLPQ | β-casein | 1019.55 | 510.28 | 2 | 24.41 | 43 |
| 30 | TDVENLHLPLPLLQS | β-casein | 1688.92 | 844.96 | 2 | 35.64 | 43 |
| 31 | NLHLPLPLLQSWM | β-casein | 1577.86 | 789.43 | 2 | 37.66 | 42 |
| 32 | ENLHLPLPL | β-casein | 1045.60 | 523.31 | 2 | 53.60 | 40 |
| 33 | LGPVRGPFPIIV | β-casein | 1264.78 | 632.89 | 2 | 31.87 | 35 |
| 34 | SDIPNPIGSE | α-s1-casein | 1028.49 | 514.75 | 2 | 24.38 | 59 |
| 35 | FFVAPFPEVFGKEKV | α-s1-casein | 1740.93 | 580.98 | 3 | 30.71 | 50 |
| 36 | SFSDIPNPIGSENSEK | α-s1-casein | 1720.80 | 860.90 | 2 | 23.86 | 46 |
| 37 | YTDAPSFSDIPNPIGSEN | α-s1-casein | 1923.86 | 962.43 | 2 | 32.52 | 46 |
| 38 | PFPEVFGKEKV | α-s1-casein | 1276.69 | 638.85 | 2 | 23.80 | 45 |
| 39 | APFPEVFGKEK | α-s1-casein | 1248.66 | 624.84 | 2 | 26.12 | 44 |
| 40 | FPEVFGKEKVN | α-s1-casein | 1293.68 | 431.90 | 3 | 19.31 | 43 |
| 41 | YGLNYYQQKPVAL | κ-casein | 1556.81 | 778.91 | 2 | 24.61 | 56 |
| 42 | QYVLSRYPSYGLNYYQQKPV | κ-casein | 2466.24 | 822.75 | 3 | 24.40 | 54 |
| 43 | PSYGLNYYQQKPVAL | κ-casein | 1740.90 | 870.95 | 2 | 25.31 | 51 |
| 44 | YYQQKPVALINNQFLPYPYYAKPA | κ-casein | 2889.49 | 963.83 | 3 | 29.07 | 51 |
| 45 | VLSRYPSYGLNYYQQKPV | κ-casein | 2175.12 | 725.71 | 3 | 22.12 | 51 |
| 46 | SRYPSYGLNYYQQKP | κ-casein | 1863.90 | 621.97 | 3 | 19.26 | 51 |
| 47 | SRYPSYGLNYYQQKPVALINN | κ-casein | 2488.27 | 830.09 | 3 | 24.38 | 48 |
| 48 | NQFLPYPYYA | κ-casein | 1275.60 | 638.30 | 2 | 32.83 | 47 |
| 49 | INNQFLPYPYYAKPAAVRSPAQ | κ-casein | 2508.30 | 836.77 | 3 | 24.63 | 46 |
| 50 | SRYPSYGLN | κ-casein | 1056.51 | 528.76 | 2 | 17.32 | 42 |
| 51 | LPYPYYAKPA | κ-casein | 1182.61 | 591.81 | 2 | 20.78 | 42 |
| 52 | ALNEINQFYQKFPQ | α-s2-casein | 1739.88 | 870.44 | 2 | 27.53 | 52 |
| 53 | EINQFYQKFPQ | α-s2-casein | 1441.71 | 721.36 | 2 | 23.57 | 52 |
| 54 | LPQYLKTVYQ | α-s2-casein | 1252.69 | 626.85 | 2 | 23.14 | 47 |
| 55 | NAVPITPTLNREQ | α-s2-casein | 1452.78 | 726.89 | 2 | 19.79 | 42 |
| 56 | NAVPITPTLNREQL | α-s2-casein | 1565.87 | 783.44 | 2 | 23.63 | 41 |
| 57 | KVIPYVRYL | α-s2-casein | 1150.70 | 575.85 | 2 | 21.95 | 41 |
4. Conclusions
The present study adopted a bioactive peptide approach to investigate the potential of adding aqueous berry extracts to the yogurt matrix for the development of a beverage with anti-diabetic properties. Results demonstrated that enzyme inhibition (α-amylase, α-glucosidase and DPP-IV) increases with storage time at 4 °C, regardless the yogurt type. These findings suggest that yogurt may have the potential to improve blood glucose regulation by means of its ability to slow both the inactivation of the incretin hormones and the intestinal digestion of carbohydrates. The beverage formulated with BCP showed the highest potential for inhibiting the activity of α-glucosidase, which is possibly due to the peptides released from caseins during the fermentation process. Differences in α-glucosidase inhibition between yogurts with BPC and SB extracts are attributed to the formation of complexes between milk proteins and phenolic compounds for the latter, which may induce changes in protein conformation and can affect the susceptibility to enzymatic hydrolysis by lactic acid bacteria. Further research is needed to assess the bioactivity of the peptides generated in yogurt with BCP and particularly their potential as α-glucosidase inhibitors. In addition, once the bioactivity of the reformulated yogurts has been established, the effects on sensory attributes will need to be evaluated. This will require an instrumental approach to determine microstructural and rheological properties as well as consumer-based studies to obtain essential information on sensory perception in the initial stages of product reformulation.
Declarations
Author contribution statement
He Ni: Performed the experiments; Wrote the paper.
Helen E. Hayes, David Stead: Performed the experiments.
Vassilios Raikos: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work is part of the Strategic Research Programme 2016–2021 and is funded by the Scottish Government's Rural and Environment Science and Analytical Services Division (RESAS).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Apostolidis E., Kwon Y.I.I., Shetty K. Potential of cranberry-based herbal synergies for diabetes and hypertension management. Asia Pac. J. Clin. Nutr. 2006;15:433–441. [PubMed] [Google Scholar]
- Arce F.J.V., Concepcion J.E.D., Mayol K.M.C., See G.L.L. In Vitro α-amylase and α-glucosidase inhibition activity of Tabing Abutilon indicum (Linn 1836) root extracts. Int. J. Toxicol. Pharmacol. Res. 2016;8(5):391–396. [Google Scholar]
- Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int. J. Clin. Pract. 2006;60(11):1454–1470. doi: 10.1111/j.1742-1241.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- Bernardi N., Benetti G., Haouet N.M., Sergi M., Grotta L., Marchetti S., Martino G. A rapid high-performance liquid chromatography-tandem mass spectrometry assay for unambiguous detection of different milk species employed in cheese manufacturing. J. Dairy Sci. 2015;98:8405–8413. doi: 10.3168/jds.2015-9769. [DOI] [PubMed] [Google Scholar]
- Beshkova D., Simova E., Frengova G., Simov Z.I., Spasov Z. Effect of oxygen on batch yoghurt cultures. World J. Microbiol. Biotechnol. 2002;18(4):365–369. [Google Scholar]
- Bharatham K., Bharatham N., Park K.H., Lee K.W. Binding mode analyses and pharmacophore model development for sulfonamide chalcone derivatives, a new class of α-glucosidase inhibitors. J. Mol. Graph. Model. 2008;26:1202–1212. doi: 10.1016/j.jmgm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Charlton A.J., Baxter N.J., Lokman, Khan M., Moir A.J.G., Haslam E., Davies A.P., Williamson M.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002;50:1593–1601. doi: 10.1021/jf010897z. [DOI] [PubMed] [Google Scholar]
- Daliri E.B.-M., Oh D.H., Lee B.H. Bioactive peptides. Foods. 2017;6:32. doi: 10.3390/foods6050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton N., Brennan R., Finn C., Davies H.V. Antioxidant properties of domesticated and wild Rubus species. J. Sci. Food Agric. 2000;80:1307–1313. [Google Scholar]
- Gagnaire V., Jardin J., Jan G., Lortal S. Invited review: proteomics of milk and bacteria used in fermented dairy products: from qualitative to quantitative advances. J. Dairy Sci. 2009;92:811–825. doi: 10.3168/jds.2008-1476. [DOI] [PubMed] [Google Scholar]
- Gharibzahedi S.M.T., Chronakis I.S. Crosslinking of milk proteins by microbial transglutaminase: utilization in functional yogurt products. Food Chem. 2018;245:620–632. doi: 10.1016/j.foodchem.2017.10.138. [DOI] [PubMed] [Google Scholar]
- Giacometti J., Buretić-Tomljanović A. Peptidomics as a tool for characterizing bioactive milk peptides. Food Chem. 2017;230:91–98. doi: 10.1016/j.foodchem.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Hasni I., Bourassa P., Hamdani S., Samson G., Carpentier R., Tajmir-Riahi H.-.A. Interaction of milk a- and b-caseins with tea polyphenols. Food Chem. 2011;126:630–639. [Google Scholar]
- Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–567. doi: 10.1016/j.foodchem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Jin Y., Yu Y., Qi Y., Wang F., Yan J., Zou H. Peptide profiling and the bioactivity character of yogurt in the simulated gastrointestinal digestion. J. Proteomics. 2016;141:24–46. doi: 10.1016/j.jprot.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Kunda P.B., Benavente F., Catalá-Clariana S., Giménez E., Barbosa J., Sanz-Nebot V. Identification of bioactive peptides in a functional yogurt by micro liquid chromatography time-of-flight mass spectrometry assisted by retention time prediction. J. Chromatogr. A. 2012;1229:121–128. doi: 10.1016/j.chroma.2011.12.093. [DOI] [PubMed] [Google Scholar]
- Lacroix I.M.E., Li-Chan E.C.Y. Evaluation of the potential of dietary proteins as precursors of dipeptidyl peptidase (DPP)-IV inhibitors by an in silico approach. J. Funct. Foods. 2012;4:403–422. [Google Scholar]
- Lacroix I.S.M.E., Li-Chan C.Y. Inhibition of dipeptidyl peptidase (DPP)-IV and α-glucosidase activities by pepsin-treated whey proteins. J. Agric. Food Chem. 2013;61:7500–7506. doi: 10.1021/jf401000s. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mann B., Athira S., Sharma R., Bajaj R. Bioactive peptides in yogurt. In: Shah N.P., editor. Yogurt in Health and Disease Prevention. Elsevier; Amsterdam: 2017. pp. 411–426. [Google Scholar]
- Matilla P., Hellström J., Törrönen R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006;54(19):193–199. doi: 10.1021/jf0615247. [DOI] [PubMed] [Google Scholar]
- McDougall G.J., Austin C., Van Schayk E., Martin P. Salal (Gaultheria shallon) and aronia (Aronia melanocarpa) fruits from Orkney: phenolic content, composition and effect of wine-making. Food Chem. 2016;205:39–247. doi: 10.1016/j.foodchem.2016.03.025. [DOI] [PubMed] [Google Scholar]
- McDougall G.J., Shpiro F., Dobson P., Smith P., Blake A., Stewart D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005;53:2760–2766. doi: 10.1021/jf0489926. [DOI] [PubMed] [Google Scholar]
- Morell P., Fiszman S., Llorca E., Hernando I. Designing added-protein yogurts: relationship between in vitro digestion behavior and structure. Food Hydrocolloids. 2017;72:27–34. [Google Scholar]
- Morell P., Piqueras-Fiszman B., Hernando I., Fiszman S. How is an ideal satiating yogurt described? A case study with added-protein yogurts. Food Res. Int. 2015;78:141–147. doi: 10.1016/j.foodres.2015.10.024. [DOI] [PubMed] [Google Scholar]
- Nielsen S.D., Beverly R.L., Qu Y., Dallas D.C. Milk bioactive peptide database: a comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017;232:673–682. doi: 10.1016/j.foodchem.2017.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongonierma A.B., FitzGerald R.J. Susceptibility of milk protein-derived peptides to dipeptidyl peptidase IV (DPP-IV) hydrolysis. Food Chem. 2014;145:845–852. doi: 10.1016/j.foodchem.2013.08.097. [DOI] [PubMed] [Google Scholar]
- Oliveira A., Alexandre E.M.C., Coelho M., Lopes C., Almeida D.P.F., Pintado M. Incorporation of strawberries preparation in yoghurt: impact on phytochemicals and milk proteins. Food Chem. 2015;171:370–378. doi: 10.1016/j.foodchem.2014.08.107. [DOI] [PubMed] [Google Scholar]
- Oseguera-Toledo M.E., González de Mejía E., Reynoso-Camacho R., Cardador-Martínez A., Amaya-Llano S.L. Proteins and bioactive peptides. Mechanisms of action on diabetes management. Nutrafoods. 2014;13:147–157. [Google Scholar]
- Panahi S., Fernandez M.A., Marette A., Tremblay A. Yogurt, diet quality and lifestyle factors. Eur. J. Clin. Nutr. 2017;71:573–579. doi: 10.1038/ejcn.2016.214. [DOI] [PubMed] [Google Scholar]
- Patil P., Mandal S., Tomar S.K., Anand S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015;54:863–880. doi: 10.1007/s00394-015-0974-2. [DOI] [PubMed] [Google Scholar]
- Pereira P.C. Milk nutritional composition and its role in human health. Nutrition. 2014;30:619–627. doi: 10.1016/j.nut.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Rao P.S., Mayur A., Harisha N.B., Bajaj R., Mann B. Comparison of OPA and pH stat methods for measurement of degree of hydrolysis of alcalase and flavourzyme digested casein. Indian J. Dairy Sci. 2018;71(1):107–109. [Google Scholar]
- Saeidnia S., Ara L., Hajimehdipoor H., Roger W., Read R.W., Arshadi S., Nikan M. Chemical constituents of Swertia longifolia Boiss with α-amylase inhibitory activity. Res. Pharmaceut. Sci. 2016;11(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rivera L., Martínez-Maqueda D., Cruz-Huerta E., Miralles B., Recio I. Peptidomics for discovery, bioavailability and monitoring of dairy bioactive peptides. Food Res. Int. 2014;63:170–181. [Google Scholar]
- von Staszewski M., Jagus R.J., Pilosof M.R. Influence of green tea polyphenols on the colloidal stability and gelation of WPC. Food Hydrocolloids. 2011;25:1077–1084. [Google Scholar]
- Yao Y., Sang W., Zhou M., Ren G. Antioxidant and alpha-glucosidase inhibitory activity of coloured grains in China. J. Agric. Food Chem. 2010;58:770–774. doi: 10.1021/jf903234c. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yu D., Sun J., Guo H., Ding Q., Liu R., Ren F. Interaction of milk whey protein with common phenolic acids. J. Mol. Struct. 2014;1058:228–233. [Google Scholar]