Abstract
The aim of this work was to compare the antioxidant and angiotensin converting enzyme (ACE) inhibitory properties of Moringa oleifera seed protein isolate (ISO) and its enzymatic protein hydrolysates. ISO was subjected to enzymatic (alcalase, pepsin and trypsin) hydrolysis to obtain alcalase isolate, pepsin isolate and trypsin isolate hydrolysates (AIH, PIH, TIH). Amino acid composition was similar for the samples except that TIH had lower Sulphur-containing amino acids while PIH was lower in tryptophan. All the samples were tested for antioxidant properties through free radical scavenging abilities such as 2,2 diphenyl-1-picrylhydrazyl (DPPH) and hydroxyl radical scavenging assays as well as ferric reducing antioxidant power (FRAP) and metal ion chelation assays. The maximum percentage inhibition obtained for the samples from the different assays are: DPPH, 36% (PIH); FRAP, 0.04% (PIH); hydroxyl radical scavenging activity, 42.98% (ISO); and inhibition of metal ion chelation, 29.46% (AIH). AIH (79.3%) had the highest ACE-inhibitory activity followed by TIH (75.1%) while PIH (43.0%) had the least. Generally, the hydrolysis process produced hydrolysates with improved antioxidant and ACE-inhibitory properties when compared to the isolate. We conclude that enzymatic hydrolysis with alcalase, pepsin and trypsin may be used to produce M. oleifera seed protein hydrolysates with potential to be used as ingredients for the formulation of functional foods and nutraceuticals.
Keywords: Food science, Food analysis
1. Introduction
Moringa oleifera is widely grown in many tropical and subtropical countries. It is a highly valued plant because almost every part is useful for food or other uses. The seeds, leaves, flower and pods are used in cooking as spices, while the oil is valuable for cosmetic and health-related applications [1]. The high nutritional value and the array of health benefits have prompted a number of research works on moringa [1, 2]. Some of the reported health benefits of the moringa tree include antiulcer, antipyretic, anti-inflammatory, antihypertensive, antiepileptic, antidiabetic, hepatoprotective anti-bacterial, anti-fungal and cholesterol lowering ability [1, 2, 3]. Universally, many plant species have been used for the traditional treatment of different diseases and other health-related issues [4, 5]. In many cases, such knowledge is not documented and is only transferred verbally from one generation to the other. Recently, efforts have been geared toward consumption of foods with disease prevention abilities in addition to their basic nutritional function [4]. An added advantage is the ability to produce peptides from readily available and underutilized plant products with little or no side effects but with protective abilities against deleterious substances. Food proteins are rich in several bioactive peptides (BAPs), which are capable of exerting positive physiological functions beyond their basic roles of providing nutritional benefits [6]. These BAPs are specific protein fragments, which are inactive within the parent polypeptide. Depending on the primary amino acid sequence of the parent protein and the specificity of the hydrolysing enzyme employed, they can provide a varied degree of physiological functions such as antihypertensive, antimicrobial, opioid, immunomodulatory and antioxidant activities [7]. Several studies have investigated the potential antioxidant properties of plant proteins including hydrolysates and their fractions with positive results [8, 9, 10]. The in-vitro antihypertensive property of the protein isolates and hydrolysates was assayed through the ACE inhibition test. The Angiotensin converting enzyme (ACE) is responsible for the conversion of angiotensin I, an inactive decapeptide to angiotensin II, a very potent vasoconstrictor that also enhances sodium (fluid) retention. It is also responsible for inactivating the vasodilator bradykinin [11]. For this dual role in the maintenance of blood pressure and fluid and electrolyte homeostasis, inhibition of ACE has been successfully used for the treatment of hypertension and congestive heart failure. While many health and health-related benefits of M. oleifera have been reported such as antioxidant properties of methanol extracts of the leaves, stem and root barks [12], glucosinolates and phenolics in vegetative and reproductive tissue [13], or in vitro and in vivo antioxidant properties of different fractions of the leaves [14], there is little or no information on antioxidant properties of the seed protein isolate and its enzymatic hydrolysates. Therefore, the aim of this work was to determine the effects of the different proteases on the antioxidant properties of M. oleifera seed protein hydrolysates.
2. Materials and methods
2.1. Materials
M. oleifera seeds were purchased from Oba market, Akure, Ondo State, Nigeria. Alcalase, pepsin and trypsin, 1,1-diphenyl-2-picrylhydrazyl (DPPH), reduced glutathione (GSH), and other antioxidant reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other analytical grade reagents were purchased from Fisher Scientific (Oakville, ON, Canada).
2.2. Production of protein isolate and hydrolysate M. oleifera
Seed protein isolate (ISO) was obtained using a previously described method [9] with some modifications. MSM was dispersed in 0.1 M NaOH (1:20, w/v), mixed for 1 h and centrifuged at room temperature (8000xg for 1 h). The supernatant was adjusted to pH 5 and left in the refrigerator at 4 °C for 8–12 h. The acidified mixture was then centrifuged (8000xg, 4 °C, 1 h), the precipitate freeze dried as the ISO and stored at -20 °C. Moringa protein hydrolysate (MPH) was obtained by hydrolysis of the ISO, which was conducted using each of the following enzyme and reaction conditions as previously reported [15]: alcalase (50 °C, pH 8.0), trypsin (37 °C, pH 8), and pepsin (37 °C, pH 2.0). The ISO was dispersed in deionized water (4% w/v, protein weight basis) in a beaker, stirred continuously, heated to the appropriate temperature and adjusted to the appropriate pH value as stated above for each enzyme. Each enzyme was added to the ISO mixture at 4% (substrate protein weight basis) level. Digestion was performed for 3 h (pH maintained constant by addition of 1 M NaOH or 1 M HCl) after which the enzymes were inactivated by adjusting to pH 5.0 with 1 M HCl or 1 M NaOH, heated at 95 °C in a water bath for 15 min and cooled rapidly in an ice bath. The undigested proteins were sedimented by centrifugation (8000×g for 1 h min at 4 °C) and the supernatant containing target peptides was freeze dried as the respective enzyme MPH.
2.3. Analyses
2.3.1. Determination of amino acid composition
The amino acid profiles were determined using HPLC system, after samples were hydrolyzed with 6 M HCl as previously described [16]. The cysteine and methionine contents were determined after performic acid oxidation [17] while the tryptophan content was determined after alkaline hydrolysis [18].
2.3.2. Determination of DPPH radical-scavenging activity
The scavenging activity of samples against the DPPH radical was determined using a previously described method [9] with slight modifications for a 96-well clear flat-bottom plate. Samples were dissolved in 0.1 M sodium phosphate buffer, pH 7.0 containing 1% (w/v) Triton X-100. DPPH was dissolved in methanol to a final concentration of 100 μM. Peptide samples (100 μL) were mixed with 100 μL of the DPPH solution in the 96-well plate to a final assay concentration of 1 mg/mL and incubated at room temperature in the dark for 30 min. The absorbance values of the control (Ac) and samples (As) were measured at 517 nm. The control consisted of buffer in place of the peptide sample while GSH was used as the positive control. The percent DPPH radical scavenging activity of the samples was determined using the following equation:
where Ac and As are the absorbance of control and sample respectively.
2.3.3. Determination of ferric reducing antioxidant power (FRAP)
The ferric reducing antioxidant power of samples was measured according to a previously described method [19] with some modifications for a microplate reader. Briefly, the FRAP reagent was freshly prepared by mixing 300 mM acetate buffer (sodium acetate buffer, pH 3.6), 10 mM 4,6-tripryridyls-triazine (TPTZ) in 40 mM HCl and 20 mM ferric chloride in a ratio 5:1:1 (v/v) before evaluation. Two hundred μL of FRAP reagent (preheated to 37 °C) was added to 40 μL of sample or GSH in a 96 well microplate. Absorbance at 593 nm was measured relative to a reagent blank. Ferrous sulphate (0.0625–1 mM) was used to prepare a standard curve and the results of the samples were expressed in mM FeSO4.
2.3.4. Determination of chelation of metal ions
The metal chelating activity was measured using a modification of a previous method [20]. Five hundred μL peptide sample solution or GSH (final assay concentration of 1 mg/ml) was combined with 25 μL of 2 mM FeCl2 and 925 μL double distilled water in a reaction tube. Fifty μL ferrozine solution (5 mM) was added and mixed thoroughly. The mixture was then allowed to stand at room temperature for 10 min and a 200 μL was pipetted into a clear bottom 96-well plate. A control was also conducted by replacing the sample with 500 μL of double distilled water. The absorbance values of control (Ac) and sample (As) at 562 nm were measured using a spectrophotometer. Percentage chelating effect (%) was calculated using the following equation:
2.3.5. Determination of hydroxyl radical scavenging assay (HRSA)
The HRSA was modified based on a previously described method [21]. Sample or GSH and 1, 10-phenanthroline (3 mM) were each separately dissolved in 0.1 M phosphate buffer (pH 7.4) while FeSO4 (3 mM) and 0.01% hydrogen peroxide were each separately dissolved in distilled water. An aliquot (50 μL) of sample or GSH (equivalent to a final assay concentration of 1 mg/ml) or buffer (control) was first added to a clear, flat bottom 96-well plate followed by additions of 50 μL of 1, 10-phenanthroline and 50 μL of FeSO4. To initiate the Fenton's reaction in the wells, 50 μL of a hydrogen peroxide solution was added to the mixture, which was then covered. Thereafter, the absorbance of the mixtures was measured at 536 nm every 10 min for a period of 1 h at 37 °C with shaking. The hydroxyl radical scavenging activity was calculated as follows based on changes in absorbance (ΔA):
2.3.6. Determination of angiotensin converting enzyme (ACE) inhibition
The ability of MPH and ISO to inhibit in vitro activity of ACE was measured using the modification of a previously described spectrophotometric method [22] with FAPGG as the substrate. Briefly, 20 μL sample (dissolved in 50 mM Tris-HCl buffer containing 0.3 M NaCl, pH 7.5) was mixed with 10 μL ACE (1 U/mL, final activity of 20 mU). Then 170 μL of 0.5 mM FAPGG (dissolved in same buffer) was then added. The final concentration of samples was 0.5 mg/mL based on protein content. The rate of decrease in absorbance at 345 nm was recorded for 30 min at room temperature. The buffer was used instead of sample solutions for the blank. ACE activity was expressed as the rate of reaction (ΔA/min) and inhibitory activity was calculated as:
Where and represent ACE activity in the presence and absence of the MPH or peptide fractions, respectively.
2.4. Statistical analyses
Sample measurements were performed in triplicate (except amino acid composition) and the data analyzed with SPSS version 22 while the means were separated for significant differences (p < 0.05) using Duncan's Multiple Range test. The results are presented as mean.
3. Results and discussion
3.1. Amino acid composition
According to Malomo & Aluko [23], antioxidant properties of peptides are greatly influenced by the amino acid composition of the peptide. Amino acid compositions of the M. oleifera seed protein isolate and hydrolysates are shown in Table 1. Overall, the enzymatic hydrolysis process did not have any measurable effect on the constituent amino acids. Slight changes were observed in the amino acid composition of the protein hydrolysates when compared to the precursor protein (ISO). A similar trend was also observed for hemp seed protein isolate and its protein meal [23]. Generally, the isolate and the hydrolysates showed high contents of glutamic acid + glutamine (Glx), glycine and arginine. The apparently low content of cysteine for trypsin hydrolysate (TIH) sample when compared to others might be due to the specificity of the trypsin for peptide bonds involving mainly lysine and arginine. The combined summary of the compositions reveals the presence of more hydrophobic amino acids (HAA) and the negatively charged amino acids (NCAA) in the samples. Some of these amino acids, especially Tyr, Met, His and Lys have been shown to play specific roles in improving antioxidant properties of peptides [24, 25]. Besides, aromatic amino acids with a large side group such as His (imidazole group) and Trp (indolic group) contribute to the antioxidant potency of peptides because they act as hydrogen donors [26] According to Sarmadi [27], the interaction of peptides with lipids or entry into target organs can be enhanced by the hydrophobic properties, which helps in promoting the antioxidant effects of peptides. Furthermore, hydrophobicity of peptides, which helps to improve their solubility in lipid medium, has been reported to also improve their antioxidant potentials [28, 29]. The ISO and hydrolysates also show high contents of essential amino acids, which is an indication of their high nutritional values.
Table 1.
Amino acid composition of Moringaoleifera seed protein isolate and hydrolysates.
| AA | ISO | AIH | PIH | TIH |
|---|---|---|---|---|
| ASX | 6.27 | 6.87 | 6.86 | 7.44 |
| THR | 3.35 | 3.63 | 3.70 | 4.10 |
| SER | 3.19 | 3.33 | 3.32 | 3.34 |
| GLX | 25.70 | 22.03 | 24.90 | 23.02 |
| PRO | 5.90 | 6.05 | 4.71 | 5.01 |
| GLY | 5.98 | 6.77 | 8.43 | 8.08 |
| ALA | 4.41 | 4.83 | 4.83 | 5.04 |
| CYS | 3.42 | 2.93 | 2.31 | 1.50 |
| VAL | 4.46 | 5.09 | 4.52 | 4.93 |
| MET | 1.93 | 1.85 | 1.58 | 1.37 |
| ILE | 3.00 | 3.27 | 3.05 | 3.10 |
| LEU | 6.33 | 6.70 | 5.99 | 6.22 |
| TYR | 2.27 | 2.44 | 2.22 | 2.59 |
| PHE | 5.35 | 5.62 | 5.60 | 6.56 |
| HIS | 2.95 | 3.08 | 3.07 | 2.98 |
| LYS | 1.29 | 1.46 | 2.51 | 2.49 |
| ARG | 13.19 | 13.19 | 11.95 | 11.35 |
| TRP | 0.99 | 0.86 | 0.45 | 0.91 |
| AAA | 8.61 | 8.92 | 8.26 | 10.05 |
| BCAA | 13.79 | 15.07 | 13.56 | 14.25 |
| HAA | 38.07 | 39.64 | 35.26 | 37.22 |
| PCAA | 17.43 | 17.73 | 17.53 | 16.81 |
| NCAA | 31.97 | 28.90 | 31.76 | 30.46 |
| SCAA | 5.35 | 4.77 | 3.90 | 2.87 |
| EAA | 42.85 | 44.75 | 42.42 | 44.00 |
ISO, protein isolate; AIH, alcalase hydrolysate; PIH, pepsin hydrolysate; TIH, trypsin hydrolysate.
HAA – hydrophobic amino acids – alanine, valine, isoleucine, leucine, tyrosine, phenylalanine, tryptophan, proline, methionine and cysteine.
PCAA – positively charged amino acids – histidine, lysine.
NCAA – negatively charged amino acids – ASX(asparagine + aspartic acid) and GLX (glutamine + glutamic acid).
AAA – aromatic amino acids- phenylalanine, tryptophan and tyrosine.
SCAA – sulphur containing amino acids- cysteine and methionine.
BCAA – branched chain amino acids – leucine and isoleucine.
EAA – essential amino acids – threonine, valine, methionine, isoleucine, leucine, phenylalanine, histidine, lysine, arginine and tryptophan.
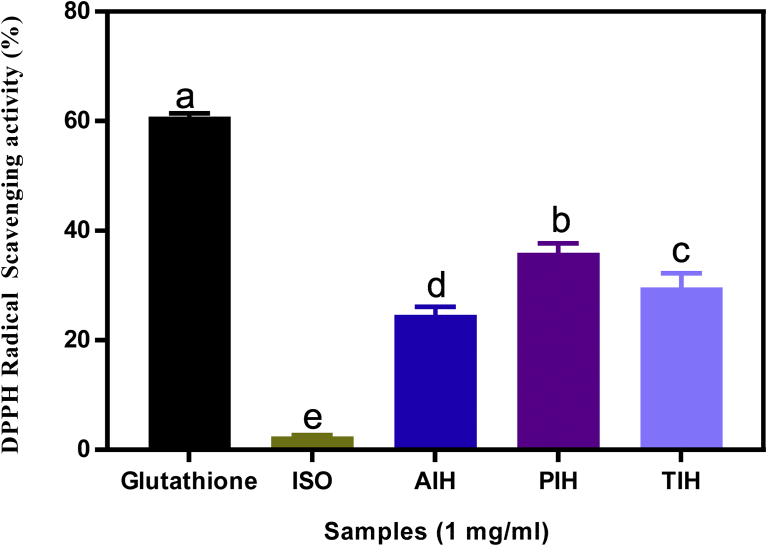
3.2. DPPH radical scavenging assay
DPPH radical scavenging assay is commonly used to measure the radical scavenging ability of a sample. It is a stable free radical with maximum absorbance at 517 nm in methanol/ethanol. It has a proton-accepting tendency; therefore, when there is a compound that has a free proton to donate, such as antioxidants, the DPPH free radical is scavenged leading to reduction in the measured absorbance [29]. The antioxidant property of such a compound is thus expressed as its ability to scavenge DPPH radicals. Fig. 1 shows that the radical scavenging ability of the isolate and its hydrolysates are significantly different (P < 0.05) with the isolate having the least scavenging activity of all the samples at 2.44% followed by alcalase hydrolysate (AIH), TIH and pepsin hydrolysate (PIH) at 24.62, 29.62 and 35.99% respectively. This shows that the different enzymes produced peptides with improved inhibitory potentials against the DPPH radicals when compared to the precursor protein isolate. The scavenging ability of the peptide was not a function of the presence of more hydrophobic amino acid as earlier reported [30]. A summary of the amino acid composition of the samples was not very different; therefore, we cannot conclude that the hydrophobic amino acid had an effect on the observed DPPH scavenging activity. The improved radical scavenging potentials of the hydrolysates might be due to the smaller size of the peptides resulting from the hydrolysis and the isolation or concentration of more electron-donating substances in the hydrolysates. The AIH, PIH and TIH samples used in this study, showed better inhibitory potential than kidney bean [31] and marble vine seed [10] hydrolysates, which lacked DPPH radical scavenging activity. The values obtained in this study are however lower than the 48–58 % reported for barley hydrolysate at 0.5 mg/mL [32].
Fig. 1.
Percentage (mean ± standard deviation, n = 3) of DPPH radical scavenging ability of Moringa oleifera seed protein isolate (ISO) and hydrolysates (AIH, alcalase hydrolysate; PIH, pepsin hydrolysate; TIH, trypsin hydrolysate). Bars with different letters have means values that are significantly different (p < 0.05).
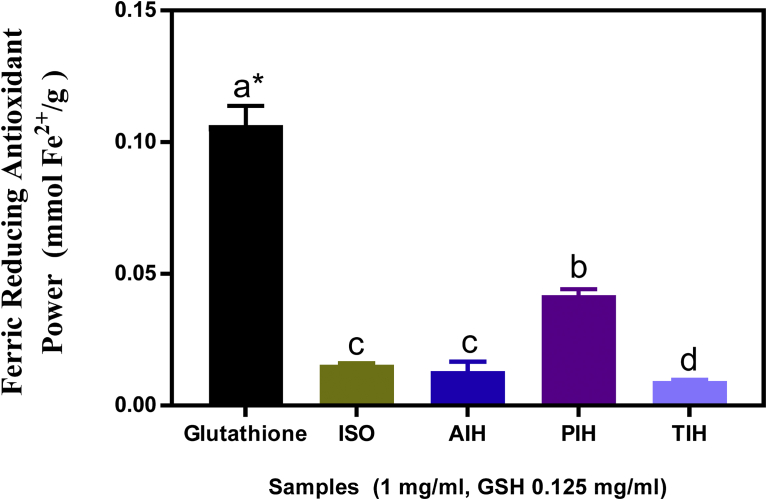
3.3. Ferric reducing antioxidant power (FRAP)
FRAP values for the isolate and protein hydrolysates are shown in Fig. 2. FRAP evaluates the electron donating potential of an antioxidant compound such as peptides whereby the Fe3+/ferricyanide complex is reduced to the ferrous form [33]. The antioxidant potential of a compound is directly related to its reducing ability [34]. Previous study had also confirmed the direct relationship between antioxidant properties and the ferric reducing abilities of bioactive compounds [30]. The ferric reducing power involves an antioxidant probe accepting an electron from the antioxidant analyte (e.g. peptides) and converts into the reduced probe which is colored [35]. Apart from the PIH, there were no significant differences (p < 0.05) among the samples. The control sample (GSH) even at lower concentration (0.125 mg/mL) showed better reducing ability (∼0.11 mmoL Fe2+/g) than the hydrolysates at 1 mg/ml. PIH had the highest FRAP at 0.04 mmol Fe 2+/g while the isolate, AIH and TIH had 0.015, 0.013 and 0.01 mmoL Fe2+/g respectively. The results show that only pepsin was able to release a high amount of hydrogen or electron donating amino acid responsible for Fe3+ reduction. All the values are however, lower than those obtained for flaxseed protein that was hydrolyzed with different enzymes [36]. FRAP has also been reported for other protein samples such as African yam bean [8] and rapeseed protein hydrolysates [9]. However, direct comparisons cannot be made with the results obtained in this study because previous works did not quantify the amount of Fe3+ reduced. Different factors such as the enzymes used for hydrolysis, molecular weight of the peptides and amino acid composition can be responsible for the ability of protein hydrolysates to reduce ferric iron [9].
Fig. 2.
Ferric reducing antioxidant power of glutathione (GSH), Moringa oleifera seed protein isolate (ISO) and hydrolysates (AIH, alcalase hydrolysate; PIH, pepsin hydrolysate; TIH, trypsin hydrolysate). Bars (mean ± standard deviation, n = 3) with different letters have means values that are significantly different (p < 0.05).
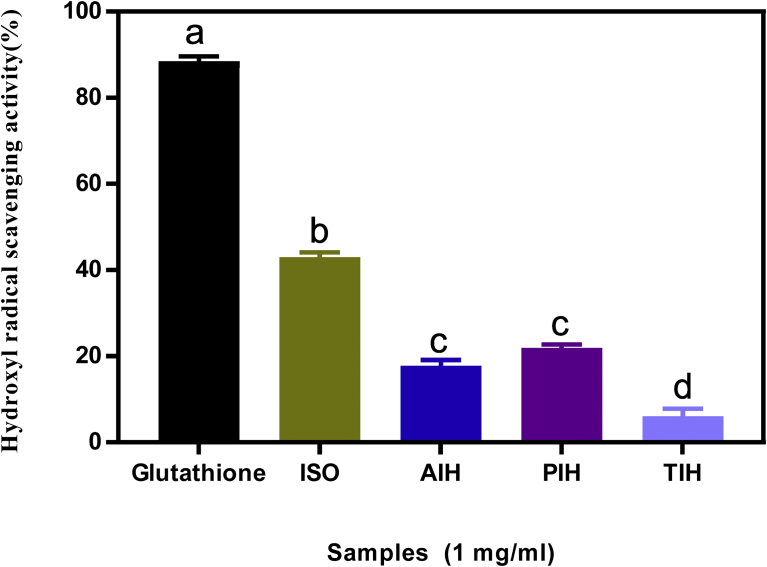
3.4. Hydroxyl radical scavenging ability
The hydroxyl radical scavenging ability of the isolate and hydrolysates is shown in Fig. 3. Prolonged and severe oxidative stress in humans can result in the initiation or promotion of several chronic disease conditions. The hydroxyl radical reacts with aromatic amino acids, where it can add on across the double bond leading to the generation of the hydroxycyclohexadienyl radical [37]. The hydroxyl radical (·OH) is generated from the conversion of superoxide and hydrogen peroxide as well as metal-catalyzed processes. This can lead to the oxidation of virtually all organic cell constituents including the DNA, lipids and proteins [37]. The seed protein isolate showed better hydroxyl radical scavenging ability (43%) than each of the hydrolysates (AIH- 17%, PIH- 21% and TIH 6%). The higher HRSA of the isolate might be due to synergistic effect of the proteins and other component present in the un-hydrolyzed sample. The results obtained in this work are similar to those reported for the hemp protein hydrolysate (15.7–23.7%) [21] as well as other samples reported in literature such as kidney bean protein hydrolysates and its membrane fractions [31] and African yam bean and its membrane fractions [8]. The hydroxyl radical scavenging ability of a sample is a result of the combined effect of the reducing power, donation of hydrogen atoms and the scavenging of active oxygen [38]. The results suggest that M. oleifera seed isolate may provide moderate level of hydroxyl radical scavenging ability thereby helping to offer a defensive shield against the hydroxyl radical, which has been implicated as one of the major causative agents in aging and in many other chronic diseases within the body [39].
Fig. 3.
Percentage (mean ± standard deviation, n = 3) of hydroxyl radical scavenging ability of Moringa oleifera seed protein isolate (ISO) and hydrolysates (AIH, alcalase hydrolysate; PIH, pepsin hydrolysate; TIH, trypsin hydrolysate). Bars with different letters have means values that are significantly different (p < 0.05).
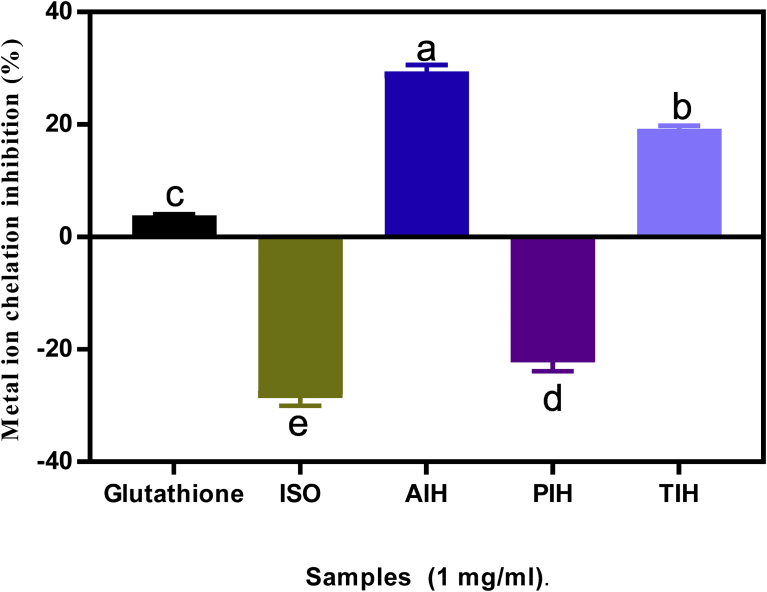
3.5. Metal chelating activity
Iron has been reported to be involved in many pathogenic processes in a number of degenerative diseases [31]. The highly reactive hydroxyl radical can be generated from the redox-active Fe (II) when it comes in contact with hydrogen peroxide through the Fenton reaction. Production of high level of hydroxyl radicals may lead to the onset of various oxidant-induced metabolic disorders. This may explain the critical link between the level of Fe(II) and oxidative stress in humans [31]. The metal chelating activity of the protein isolate and hydrolysates is shown in Fig. 4. While AIH and TIH showed metal chelation activities (29.5 and 19.3% respectively), the isolate and PIH did not have measurable effects. The observed difference among the hydrolysates might be due to the specificity of alcalase and trypsin enzymes in liberating particular amino acids with metal ion chelation ability. The results suggest that AIH and TIH may be able to provide moderate metal ion chelation. The presence of some side group such as amino and carboxyl of the acidic (Glycine and Asx) and basic (Lys, His and Arg) in amino acids have been reported to be involved in chelating metal ions [40].
Fig. 4.
Percentage (mean ± standard deviation, n = 3) of metal ion chelation of Moringa oleifera seed protein isolate (ISO) and hydrolysates (AIH, alcalase hydrolysate; PIH, pepsin hydrolysate; TIH, trypsin hydrolysate). Bars with different letters have means values that are significantly different (p < 0.05).
3.6. In vitro antihypertensive properties
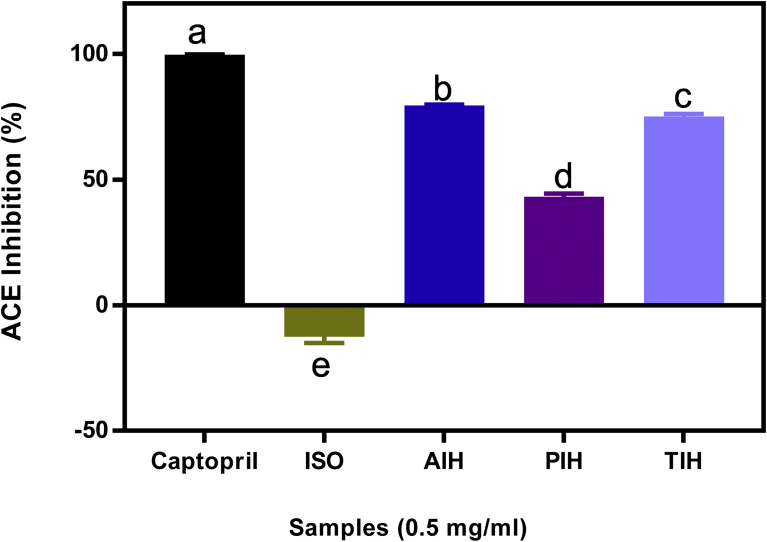
ACE-inhibitory potentials of the protein isolates and hydrolysates are shown in Fig. 5. The protein isolate showed no inhibitory potential against ACE activity up to the maximum 0.5 mg protein/mL concentration used in this study. The results confirm that enzymatic hydrolysis is responsible for the release of biologically active peptides with potent ACE-inhibitory properties [7]. Many reports have adduced the ACE-inhibitory potentials of proteins or peptides to the presence of hydrophobic amino acids and the size of the peptides (the smaller the stronger the potency) [10]. The nature as well as the specific location of amino acids in peptide chain has also been suggested to possibly be an essential factor in the antihypertensive potentials of peptides [31]. Since the ISO was not hydrolyzed, it is not surprising that it lacked ACE-inhibitory ability because the enzyme hydrolysis process is very crucial in releasing peptides with bioactive properties. In a previous study [41], there was also lack of ACE-inhibitory activity for unhydrolysed globulin fraction of amaranth grain. AIH showed higher potency (79.30%) when compared to the 43.01 and 75.05% for PIH and TIH, respectively. The improved activity of the hydrolysates might be due to the liberation of peptides with hydrophobic amino acids, which have been reported to be responsible for enhanced ACE-inhibitory properties of peptides. This is supported by the amino acid composition (Table 1) where AIH with the best ACE-inhibitory ability also had the highest (39.64%) total hydrophobic amino acid contents. Though Table 1 revealed ISO possessed comparable hydrophobic amino acid content with AIH, the fact that the ISO was not hydrolysed might be responsible for the lack of potency observed against ACE. The ACE-inhibitory properties observed in this study, especially the AIH are comparable to the 80% reported for alcalase-hydrolysed kidney bean seed protein [31].
Fig. 5.
Percentage (mean ± standard deviation, n = 3) angiotensin converting enzyme inhibition ability of captopril, Moringa oleifera seed protein isolate (ISO) and hydrolysates (AIH, alcalase hydrolysate; PIH, pepsin hydrolysate; TIH, trypsin hydrolysate). Bars with different letters have means values that are significantly different (p < 0.05).
4. Conclusion
Three different enzymes – alcalase, pepsin and trypsin were used for the hydrolysis of M. oleifera seed protein isolate. The hydrolysates had improved antioxidant and ACE-inhibitory properties when compared to the isolate (except for the hydroxyl radical activity where the isolate had better activity). Peptide hydrophobicity may have contributed to increased ACE-inhibitory properties of the alcalase protein hydrolysate. We can conclude that enzymatic protein hydrolysis released peptides that contributed to the greater antioxidant and ACE-inhibitory properties of hydrolysate when compared to the unhydrolyzed protein isolate. Further studies that involve peptide purification and in vivo studies are needed to confirm the observed in vitro antioxidant properties and provide indications of potential health benefits.
Declarations
Author contribution statement
Taiwo A Aderinola: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tayo N. Fagbemi, Victor N. Enujiugha: Conceived and designed the experiments; Analyzed and interpreted the data.
Adeola M. Alashi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Rotimi E. Aluko: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
Taiwo A. Aderinola was supported by the Tertiary Education Trust Fund (TETFund) of Nigeria. Rotimi E. Aluko was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Rebecca H.S.U., Sharon M., Arbainsyah A., Lucienne D. International Course on Economic Botany, National Herbarium Leiden; Netherlands: 2006. Moringa oleifera: Medicinal and Socio-economic Uses; pp. 2–6. [Google Scholar]
- 2.Sileshi T., Makonnen E., Debella A., Tesfaye B. Antihyperglycemic and subchronic toxicity study of Moringa stenopetala leaves in mice. J. Coast. Life Med. 2014;2:214–221. [Google Scholar]
- 3.Mehta L.K., Balaraman R., Amin A.H., Baffa P.A., Gulati O. Effects of fruits of M. oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003;86:191–195. doi: 10.1016/s0378-8741(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 4.Dike I.P., Obembe O.O., Adebiyi F.E. Ethnobotanical survey for potential anti-malarial plants in south-western Nigeria. J. Ethnopharmacol. 2012;144:618–626. doi: 10.1016/j.jep.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Oosthuizen D., Goosen N., Stander M., Ibrahim A., Pedavoah M.-M., Usman G. Solvent extraction of polyphenolics from the indigenous african fruit ximenia caffra and characterization by LC-HRMS. Antioxidants. 2018;7:103. doi: 10.3390/antiox7080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray B.A., FitzGerald R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: biochemistry, bioactivity and production. Curr. Pharmaceut. Des. 2007;13:773–791. doi: 10.2174/138161207780363068. [DOI] [PubMed] [Google Scholar]
- 7.Korhonen H., Pihlanto A. 2003. Food-derived Bioactive Peptides – Opportunities for Designing Future Foods; pp. 1297–1308. [DOI] [PubMed] [Google Scholar]
- 8.Ajibola C.F., Fashakin J.B., Fagbemi T.N., Aluko R.E. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int. J. Mol. Sci. 2011;12:6685–6702. doi: 10.3390/ijms12106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He R., Girgih A.T., Malomo S.A., Ju X., Aluko R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods. 2013;5:219–227. [Google Scholar]
- 10.Akinyede A.I., Girgih A.T., Osundahunsi O.F., Fagbemi T.N., Aluko R.E. Effect of membrane processing on amino acid composition and antioxidant properties of marble vine seed ( Dioclea reflexa ) protein hydrolysate. J. Food Process. Preserv. 2016;00:1–9. [Google Scholar]
- 11.Nakahara T., Sano T., Yamaguchi H., Sugimoto K.R.I., Chikata H., Kinoshita E. Antihypertensive effect of peptide-enriched soy sauce-like seasoning and identification of its angiotensin I-converting enzyme inhibitory substances. J. Agric. Food Chem. 2010;58:821–827. doi: 10.1021/jf903261h. [DOI] [PubMed] [Google Scholar]
- 12.Atawodi S.E., Atawodi J.C., Idakwo G.A., Pfundstein B., Haubner R., Wurtele G. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. J. Med. Food. 2010;13:710–716. doi: 10.1089/jmf.2009.0057. [DOI] [PubMed] [Google Scholar]
- 13.Amaglo N.K., Bennett R.N., Lo Curto R.B., Rosa E.A.S., Lo Turco V., Giuffrida A. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010;122:1047–1054. [Google Scholar]
- 14.Verma A.R., Vijayakumar M., Mathela C.S., Rao C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009;47:2196–2201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.He R., Alashi A., Malomo S.A., Girgih A.T., Chao D., Ju X. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013;141:153–159. doi: 10.1016/j.foodchem.2013.02.087. [DOI] [PubMed] [Google Scholar]
- 16.Bidlingmeyer B.A., Cohen S.A., Tarvin T.L. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. B Biomed. Sci. Appl. 1984;336:93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- 17.Gehrke C., Wall L., Absheer J., Kaiser F., Zumwalt R. Sample preparation for chromatography of amino acids: acid hydrolysis of proteins. J. Assoc. Off. Anal. Chem. 1985;68:811–821. [Google Scholar]
- 18.Landry J., Delhaye S. Simplified procedure for the determination of tryptophan of foods and feedstuffs from barytic hydrolysis. J. Agric. Food Chem. 1992;40:776–779. [Google Scholar]
- 19.Benzie I., Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z., Huang J., Xu X., Jin Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008;111:370–376. doi: 10.1016/j.foodchem.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 21.Girgih A.T., Udenigwe C.C., Aluko R.E. In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. JAOCS J. Am. Oil Chem. Soc. 2011;88:381–389. [Google Scholar]
- 22.Girgih A.T., Udenigwe C.C., Li H., Adebiyi A.P., Aluko R.E. Kinetics of enzyme inhibition and antihypertensive effects of hemp seed (Cannabis sativa L.) protein hydrolysates. JAOCS J. Am. Oil Chem. Soc. 2011;88:1767–1774. [Google Scholar]
- 23.Malomo S.A., He R., Aluko R.E. Structural and functional properties of hemp seed protein products. J. Food Sci. 2014;79:1512–1521. doi: 10.1111/1750-3841.12537. [DOI] [PubMed] [Google Scholar]
- 24.Samaranayaka A.G.P., Li-chan E.C.Y. Food-derived peptidic antioxidants: a review of their production , assessment , and potential applications. J. Funct. Foods. 2011;3:229–254. [Google Scholar]
- 25.Udenigwe C.C., Aluko R.E. Food protein-derived bioactive peptides: production, processing, and potential health benefits. J. Food Sci. 2012;77 doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- 26.Nam K.A., You S.G., Kim S.M. Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates. J. Food Sci. 2008;73:C249–C255. doi: 10.1111/j.1750-3841.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- 27.Sarmadi B.H., Ismail A. Peptides Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Rajapakse N., Mendis E., Byun H., Kim S. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005;16:562–569. doi: 10.1016/j.jnutbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992;40:945–948. [Google Scholar]
- 30.Wang D., jun Wang L., xue Zhu F., ye Zhu J., Chen X.D., Zou L. In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese salt-fermented soybean food) Food Chem. 2008;107:1421–1428. [Google Scholar]
- 31.Mundi S., Aluko R.E. Inhibitory properties of kidney bean protein hydrolysate and its membrane fractions against renin , angiotensin converting enzyme, and free radicals. Austin J. Nutr. Food Sci. 2014;2:1008–1019. [Google Scholar]
- 32.Bamdad F., Wu J., Chen L. Effects of enzymatic hydrolysis on molecular structure and antioxidant activity of barley hordein. J. Cereal. Sci. 2011;54:20–28. [Google Scholar]
- 33.Yıldırım A., Mavi A., Kara A.A. Determination of antioxidant and antimicrobial activities of rumex crispus L. Extracts. 2001:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 34.Oktay M., Gülçin İ., Küfrevioğlu Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci. Technol. 2003;36:263–271. [Google Scholar]
- 35.Berker K.I., Güçlü K., Tor İ., Apak R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta. 2007;72:1157–1165. doi: 10.1016/j.talanta.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Karamać M., Kosińska-Cagnazzo A., Kulczyk A. Use of different proteases to obtain flaxseed protein hydrolysates with antioxidant activity. Int. J. Mol. Sci. 2016;17:1027. doi: 10.3390/ijms17071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du J., Gebicki J.M. Proteins are major initial cell targets of hydroxyl free radicals. Int. J. Biochem. Cell Biol. 2004;36:2334–2343. doi: 10.1016/j.biocel.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Yen G., Hsieh P. 1995. Antioxidative Activity and Scavenging Effects on Active Oxygen of Xylose-lysine Maillard Reaction Products; pp. 415–420. [Google Scholar]
- 39.Zhu K., Zhou H., Qian H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006;41:1296–1302. [Google Scholar]
- 40.Saiga A., Tanabe S., Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003;51:3661–3667. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- 41.Tovar-Pérez E.G., Guerrero-Legarreta I., Farrés-González A., Soriano-Santos J. Angiotensin I-converting enzyme-inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain. Food Chem. 2009;116:437–444. [Google Scholar]