Abstract
CACNA2D2 encodes an auxiliary subunit of the voltage-dependent calcium channel. To date, there have only been two reports of individuals with early-infantile epileptic encephalopathy due to CACNA2D2 mutations. In both reports, patients were homozygous for the identified variants. Here, we report a patient with epileptic encephalopathy and cerebellar atrophy who was found to have two novel variants in the CACNA2D2 gene: c.782C>T (p.Pro261Leu) and c.3137T>C (p.Leu1046Pro), by whole-exome sequencing. The variants were shown to be inherited in trans and the unaffected parents were confirmed to be heterozygous carriers. This is the third report of recessive CACNA2D2 variants associated with disease and the first report of compound heterozygous variants. The clinical description of this new case highlights the phenotypic similarities amongst individuals with CACNA2D2-related disease and suggests that CACNA2D2 should be considered as a differential diagnosis in individuals with cerebellar dysfunction and multiple seizure types that begin in the first year of life.
1. Introduction
CACNA2D2 encodes the alpha-2 and delta-2 auxiliary subunits of the voltage-dependent calcium channel. Expression of the alpha-2-delta-2 (α2δ2) subunit enhances calcium currents produced by the alpha-1 (α1) pore-forming subunit of the calcium channel by modulating the assembly, trafficking, and localization of the α1 subunit [1].
To date, there have been two reports of individuals with early-infantile epileptic encephalopathy due to CACNA2D2 variants [2, 3]. Edvardson and colleagues identified the homozygous CACNA2D2 missense variant c.3119A>G (p.Leu1040Pro) in three affected siblings from a consanguineous family [2]. Functional analysis in Xenopus oocytes revealed that the mutant α2δ2 (L1040P) subunit was unable to enhance currents produced by α1 channels, suggesting a loss-of-function mechanism [2]. In addition, a homozygous variant of unknown significance in CELSR3, which is proximal to CACNA2D2 on chromosome 3, was also identified in the three affected siblings. In a second report, Pippucci and colleagues identified a homozygous frameshifting CACNA2D2 variant (c.1295delA, p.Asn432Thrfs∗35) in a proband from another consanguineous family [3]. A muscle biopsy from the proband revealed reduced CACNA2D2 mRNA and protein levels. Notably, this individual also carried a homozygous CELSR3 missense variant.
Here, we describe an individual with epileptic encephalopathy and compound heterozygous missense variants in CACNA2D2. Unlike previous reports, this individual was not homozygous for any CELSR3 variants.
2. Case Presentation
The proband is the male offspring of nonconsanguineous parents born at 39 weeks by cesarean section secondary to fetal distress. Pregnancy was complicated by hypertension and preeclampsia as well as maternal hypothyroidism and migraines. At discharge he was noted to have low muscle tone and unusual eye movements that were jerky with frequent intervals of up gaze. Brain MRIs at one month and five months of age were normal.
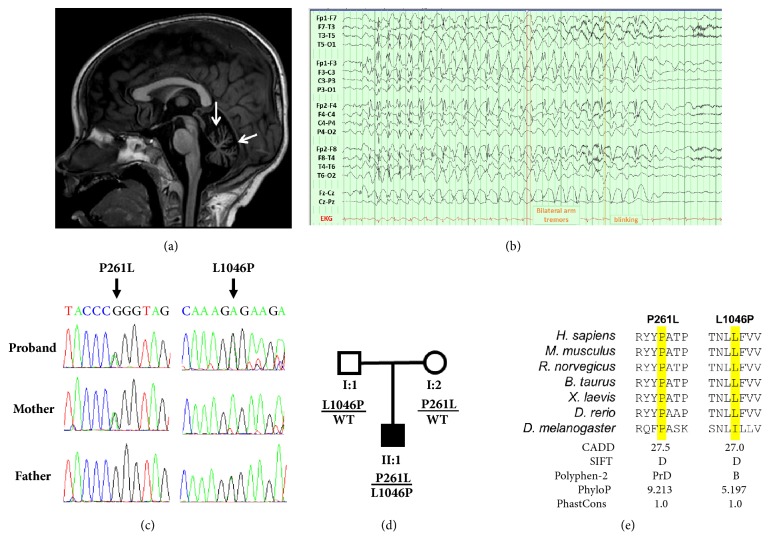
At seven months of age, he had onset of seizures with left arm jerking that spread to the left leg and left side of the face with loss of interaction, followed by whole body jerking that lasted 40 minutes. Video EEG over the next 48 hours recorded several additional seizures with multifocal onsets. He was placed on phenobarbital, levetiracetam, and clonazepam. An EEG at eight months of age showed 3-4 Hz slowing. Seizure frequency increased from two seizures per month to 5-10 per day by 14 months of age, at which point he was started on the ketogenic diet. This resulted in six months of seizure freedom; however, seizures recurred. While brain MRIs were repeatedly normal during the first two years of life, an MRI at 29 months of age showed prominence of cerebellar fissures with a normal brainstem, consistent with cerebellar atrophy (Figure 1(a)).
Figure 1.
Clinical and genetic findings from proband with compound heterozygous CACNA2D2 variants. (a) Sagittal T1 weighted image from MRI demonstrating cerebellar volume loss (arrows). (b) Awake EEG showing spontaneous generalized 3 Hz spike and wave seizure activity with eye fluttering and low amplitude arm flinches. (c) Sanger sequencing traces showing CACNA2D2 variants inherited in a compound heterozygous fashion. Note: The CACNA2D2 gene is encoded by the minus strand of DNA. (d) Pedigree showing the inheritance pattern of the two variants. (e) Species protein alignment showing the evolutionary conservation of the two amino acid positions and prediction scores from in silico algorithms. D, deleterious; PrD, probably damaging; B, benign.
At three years and four months of age, he was experiencing five atonic seizures per day and 2-3 seizures per week with apnea and tonic arm flexion. On exam, he could make brief eye contact and smile with a variety of sounds but produced no words. At three years and 11 months of age, seizures consisted of eye fluttering that lasted 2-5 seconds and occurred 1-20 times per day. Treatment with ethosuximide was ineffective. An EEG demonstrated a photoparoxysmal response at 9, 15, 18, and 27 Hz. Several spontaneous (Figure 1(b)) and light-induced seizures were recorded by EEG, characterized by 2.5-3 Hz frontally predominant generalized spike and wave discharges accompanied by eye fluttering and arm flinches.
Physical examination at five years of age showed no dysmorphic features. Global hypotonia was noted with unsteady control and mild titubation of the head and torso and moderate ataxia on reaching. He can sit with minimal support but cannot stand or walk. He remains on the ketogenic diet, valproic acid, and clonazepam, with up to 150-day intervals of seizure freedom. Currently, seizures occur only in times of illness, consisting of eye fluttering and behavioral arrest for 3-5 seconds.
Previous biochemical testing was nondiagnostic. Gene panel analysis (Epilepsy and Seizure Disorders Panel), and, later, trio-based whole-exome sequencing (WES), was performed by EGL Genetics. No pathogenic variants were identified by gene panel analysis. During reanalysis of the WES, it was noted that the proband carried two rare missense variants in the CACNA2D2 gene (p.Pro261Leu and p.Leu1046Pro), which were inherited on separate alleles (Figures 1(c) and 1(d)).
Gene panel analysis of 110 genes associated with epilepsy and seizure disorders was performed on DNA from the proband as described previously [4]. Whole-exome sequencing was performed on DNA from the proband and his parents using the SureSelect Clinical Research Exome V1 enrichment kit (Agilent Technologies, Santa Clara, CA). Ninety-seven percent of coding regions were sequenced at >20X coverage. Variant inheritance was confirmed by Sanger sequencing after written consent. The CACNA2D2 variants were annotated according to RefSeq accessions NM_006030.2 and NP_006021.2. The following primer pairs were used for PCR amplification and sequence analysis of the two variants: 261_F-GTGGCTGAGGGAGGAGAGAA, 261_R-CCTGGATAGGCCGAGAACAG, 1046_F-GTCGCGTTGTAGTCGAAGCA, and 1046_R-CTCGGTAAACGCCTCCTACA. This study was approved by the Institutional Review Board of Emory University.
3. Discussion
The two CACNA2D2 variants identified during WES reanalysis are classified as variants of uncertain significance according to the ACMG-AMP classification criteria [5]; however, there are several lines of evidence to suggest these variants contribute to disease. First, the patient has compound heterozygous variants in a gene associated with autosomal recessive epilepsy and shares many of the clinical features observed for the previously reported patients with homozygous CACNA2D2 mutations (Figures 1(c) and 1(d), Table 1). Second, both variants are extremely rare in the general population; the p.L1046P variant is absent from the gnomAD database, while p.P261L is only observed once out of 245,184 alleles. Third, both variants are located in functional domains of the α2δ2 protein and the affected amino acid residues are evolutionarily conserved (Figure 1(e)). Fourth, both variants were predicted to be deleterious by in silico algorithms, including CADD and SIFT (Figure 1(e)). Finally, one of the proband's variants, p.L1046P, is in close proximity to the previously reported disease variant p.L1040P, which was demonstrated to reduce the function of the α2δ2 protein [2]. Additionally, no other potentially causative variants were identified from WES.
Table 1.
Clinical features of individuals carrying recessive CACNA2D2 variants.
| This Report | Edvardson et al. 2013 | Pippucci et al. 2013 | |
|---|---|---|---|
| Genomic position | chr3:50418428 | chr3:50402595 | chr3:50416390 |
| chr3:50402577 | |||
| cDNA changea | c.782C>T | c.3119A>G | c.1295delA |
| c.3137T>C | |||
| Protein changea | p.Pro261Leu | p.Leu1040Pro | p.Asn432Thrfs∗35 |
| p.Leu1046Pro | |||
| Sex | Male | 2 Males, 1 Female | Male |
| Seizure Onset | 7 months | 20-60 days | 5 months |
| Epileptic Encephalopathy | + | + | + |
| Developmental delay | + | + | + |
| Cerebellar atrophy | + | + | + |
| Refractory seizures | + | + | + |
| Seizure types | Absence, atonic, tonic, tonic-clonic | Atonic, clonic, tonic | Absence, clonic, tonic-clonic |
| EEG | 2.5-3 Hz frontally predominant generalized spike and wave discharges | Slow background rhythm with multifocal spikes and slow waves | Multifocal spikes over the right centrotemporal and left parietooccipital regions, slowed background activity |
| Other features | Status epilepticus, hypotonia, tremor and ataxia, atypical eye movements | Axial hypotonia, choreiform movements, no eye contact | Status epilepticus, axial hypotonia, dyskinetic movements, tremor, no eye contact, facial dysmorphisms, small head, uncoordinated eye movements |
a CACNA2D2 variants annotated according to Refseq NM_006030.2 and NP_006021.2. EEG, electroencephalogram. + indicates presence of feature.
When the clinical presentation of the proband was compared to the two previous reports, we found striking similarities amongst the affected individuals, including seizure onset in the first year of life and hypotonia (Table 1) [2, 3]. Additionally, all of the reported patients experience multiple treatment-resistant seizure types without focality and involuntary movements. Of note is the consistent observation of cerebellar atrophy (Figure 1(a)), which is also reported in several mouse models of Cacna2d2 dysfunction [6, 7]. Moreover, it is interesting to note the occurrence of prolonged seizures and photosensitivity for both our patient and the patient described by Pippucci and colleagues [3].
Unlike the two previous reports, the proband in the current study only had one heterozygous missense variant in the CELSR3 gene (c.5273A>G, p.Gln1758Arg). This variant is common in the general population and has been observed in the homozygous state more than 4,000 times in the gnomAD database, suggesting it is unlikely to contribute to pediatric disease. Furthermore, the observed phenotypic similarities amongst the affected individuals indicate that altered CACNA2D2 function is the cause of disease.
Because CACNA2D2-derived disease is rare, the optimal treatment regime for these individuals is currently unclear; however, the patient reported here did appear to experience improvement while on the ketogenic diet. The mechanism by which CACNA2D2 dysfunction has been proposed to cause disease involves reduced α2δ2 expression and/or function, leading to reduced α1 cell surface expression and function [2], which is consistent with the partial clinical overlap observed between CACNA2D2- and CACNA1A-related disorders. As whole-exome and genome sequencing technologies are applied to individuals with different diseases, it will be interesting to observe if CACNA2D2 variants are identified in individuals with other disease presentations, such as ataxia or migraine, as has been seen for CACNA1A.
In conclusion, we present the third report of a patient with epileptic encephalopathy and cerebellar atrophy due to recessive variants in CACNA2D2. Importantly, we present the first case with compound heterozygous CACNA2D2 variants and no additional candidate disease variants (e.g., CELSR3), further supporting the relationship between pathogenic CACNA2D2 variants and epileptic encephalopathy with cerebellar atrophy.
Acknowledgments
The authors would like to thank the family for their participation in this study. This work was supported by the Training Grant Appointment 5T32GM008490 to Kameryn M. Butler.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Hoppa M. B., Lana B., Margas W., Dolphin A. C., Ryan T. A. α2δ expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486(7401):122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edvardson S., Oz S., Abulhijaa F. A., et al. Early infantile epileptic encephalopathy associated with a high voltage gated calcium channelopathy. Journal of Medical Genetics. 2013;50(2):118–123. doi: 10.1136/jmedgenet-2012-101223. [DOI] [PubMed] [Google Scholar]
- 3.Pippucci T., Parmeggiani A., Palombo F., et al. A novel null homozygous mutation confirms CACNA2D2 as a gene mutated in epileptic encephalopathy. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0082154.e82154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler K. M., da Silva C., Alexander J. J., Hegde M., Escayg A. Diagnostic Yield From 339 Epilepsy Patients Screened on a Clinical Gene Panel. Pediatric Neurology. 2017;77:61–66. doi: 10.1016/j.pediatrneurol.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine. 2015;17(5):405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanov S. V., Ward J. M., Tessarollo L., et al. Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. The American Journal of Pathology. 2004;165(3):1007–1018. doi: 10.1016/S0002-9440(10)63362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier H. The neuropathology of ducky, a neurological mutation of the mouse - A pathological and preliminary histochemical study. Acta Neuropathologica. 1968;11(1):15–28. doi: 10.1007/BF00692792. [DOI] [PubMed] [Google Scholar]