Abstract
Background
HIV-associated Kaposi sarcoma (KS), among the most frequent cancers seen in sub-Saharan Africa, is associated with a high prevalence of lymphedema. Lymphedema causes progressive functional impairment marked by swelling, physical discomfort, disfiguring changes, skin hardening from fibrosis, poor wound healing, and recurrent skin infection. While compression therapy is considered a major component of lymphedema management, this intervention has never been evaluated in HIV-associated KS lymphedema.
Methods/design
The Kenyan Improvised Compression for Kaposi Sarcoma (KICKS) study is a randomized, controlled trial. Due to variable lymphedema stage, we will use block randomization with a 1:1 allocation to assign participants to one of two groups: “Immediate compression” or “Delayed compression.” Those randomized to “Immediate compression” intervention arm will receive weekly two-component compression bandages while receiving chemotherapy, whereas those in the “Delayed compression” control arm will be followed during chemotherapy and then receive compression after chemotherapy is completed. The primary outcome is change in Lower Extremity Lymphedema Index from enrollment at Week 0 to blinded outcome assessment at Week 14 between intervention and control arms. Secondary outcomes are change in leg lymphedema-specific quality of life (LYMQOL) and change in overall health quality of life in cancer (EORTC QLQ C30).
Discussion
This represents the first study in sub-Saharan Africa to assess a lymphedema-directed intervention for KS, and the intervention—locally sourced two-component compression bandages—is affordable and available. Thus, the KICKS study is an important step towards developing an evidence-based path for regionally relevant management of HIV-associated KS lymphedema.
Trial registration
This trial was registered at ClinicalTrials.gov on January 19, 2018: identifier NCT03404297.
Keywords: Kaposi sarcoma, Lymphedema, Compression, Unna boot, Paste bandage, Randomized controlled trial
1. Introduction
1.1. Background
HIV-associated Kaposi sarcoma (KS), among the most frequent cancers seen in sub-Saharan Africa [[1], [2], [3], [4], [5]], is associated with a high prevalence of lymphedema, ranging from 32% to 52% [[6], [7], [8]]. Lymphedema causes progressive functional impairment marked by swelling, physical discomfort, disfiguring changes, skin hardening from fibrosis, poor wound healing, and recurrent skin infection [9]. These changes lead to a significant impact on quality of life [[10], [11], [12]]. Antiretroviral therapy and chemotherapy are used to treat HIV-associated KS, but even after treatment, KS lymphedema may persist [8]. Compression therapy is considered a major component of lymphedema management for all causes of lymphedema [9]. It has been studied specifically for classic KS lymphedema but has not been evaluated in HIV-associated KS lymphedema [13].
Limited availability of wound care and dermatologic supplies are a major barrier to providing compression therapy in sub-Saharan Africa. In western Kenya, locally available elastic stockings are priced at 10-15USD (1000-1500kshs) a pair. Pre-packaged brand name kits are not locally available or affordable for patients, as imported kits costs 7-20USD (700-2000kshs) per package. At the Academic Model Providing Access to Healthcare (AMPATH), an initial donation of compression bandages were used for KS lymphedema in Oncology clinics, resulting in anecdotal patient-reported and provider-reported improvement in swelling and ease of walking. Subsequent collaboration between AMPATH Dermatology and Pharmacy led to use of locally available wound care materials to develop two-component compression bandages with a cost of 2USD (200kshs) to cover supplies required to dress one affected leg for one week. AMPATH is a partnership between Moi Teaching and Referral Hospital and Moi University College of Health Sciences in Kenya and a consortium of North American academic medical centers. AMPATH, a President's Emergency Plan for AIDS Relief-United States Agency for International Development (PEPFAR-USAID)-supported implementing partner, collaborates with the Ministry of Health to serve a catchment area of over 4 million people and has supported HIV care delivery for over 150,000 patients at over 500 sites across western Kenya. Using the infrastructure and healthcare delivery model created through HIV care, AMPATH has been providing care for cancer and chronic diseases.
While the epidemiology of KS lymphedema in western Kenya has not been reported to-date, the age-standardized-incidence rate (ASIR) of KS in East Africa is 334/100,000 person‐years, as compared to the most common cancer in women in the United States (breast cancer with ASIR of 125/100,000 person‐years) and the most common cancer in men (prostate cancer with ASIR of 148/100,000 person‐years) [4]. If the prevalence of lymphedema in HIV-associated KS is similar to what has been reported in other world regions [[6], [7], [8]], at least 30% of the KS population in East Africa are afflicted with lymphedema and stand to benefit from lymphedema-directed interventions.
To evaluate the efficacy of a low-cost locally sourced two-component compression bandage system for HIV-associated KS lymphedema, we will conduct a randomized controlled trial of immediate vs. delayed compression therapy. We will compare the change in our primary outcome measure before and after compression therapy between the immediate vs. delayed compression arms. We present the hypothesis, objectives, and methodology being used to assess this intervention.
2. Material and methods
2.1. Study hypothesis
The effect of compression therapy on HIV-associated KS lymphedema will vary depending on baseline lymphedema severity, as determined by Campisi Clinical Staging. We hypothesize that compression therapy will lead to the following effect on lymphedema:
-
•
Stage 1B & Stage 2: decrease in lymphedema
-
•
Stage 3: no progression of lymphedema
-
•
Stage 4: no progression of lymphedema
2.2. Study objectives
2.2.1. Primary objective
To determine, in participants with HIV-associated KS lymphedema, if compression therapy with two-component compression bandages as compared to no compression therapy leads to an improvement in:
-
•
Lower Extremity Lymphedema Index
2.2.2. Secondary objective
To evaluate, in participants with HIV-associated KS lymphedema, if compression therapy with two-component compression bandages as compared to no compression therapy leads to an improvement in:
-
•
Leg lymphedema-specific quality of life
-
•
Overall health quality of life in cancer
2.3. Trial design
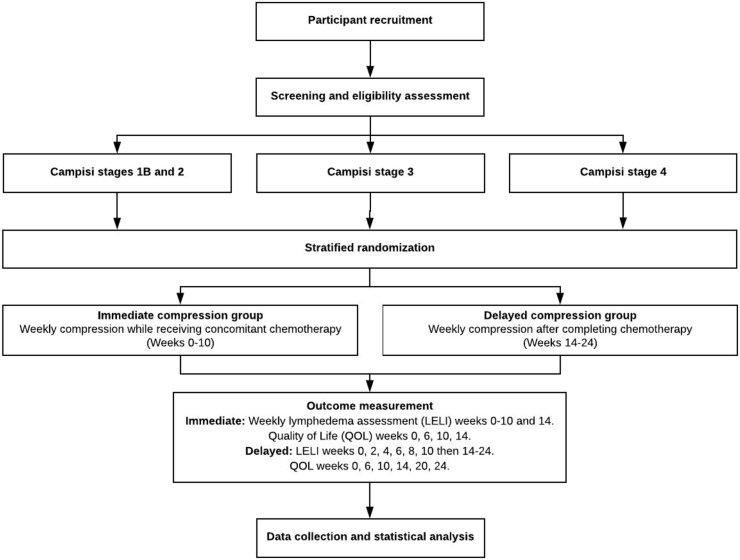
The KICKS study is a randomized, controlled trial with two parallel groups: 1) Intervention: Immediate compression group will receive compression therapy during chemotherapy and 2) Control: Delayed compression group will be followed during chemotherapy and then receive compression after chemotherapy is completed. The primary endpoint is change in Lower Extremity Lymphedema Index from enrollment at Week 0 to blinded outcome assessment at Week 14 between intervention and control arms. The secondary endpoints are change in leg lymphedema-specific quality of life (LYMQOL) and change in overall health quality of life in cancer (EORTC QLQ C30) from enrollment at Week 0 to blinded outcome assessment at Week 14 between intervention and control arms. Randomization will be performed as block randomization with a 1:1 allocation. Fig. 1.
Fig. 1.
Overall study design schematic.
Trial registration: This trial was registered at ClinicalTrials.gov on January 19, 2018: identifier NCT03404297.
2.4. Setting
Participants will be recruited from the AMPATH Oncology clinic in Chulaimbo, which is located at the Chulaimbo sub-district health center.
2.5. Eligibility
2.5.1. Inclusion criteria
-
•
Older than 18 years of age
-
•
Diagnosis of biopsy-proven Kaposi sarcoma with associated leg lymphedema
-
•
Leg lymphedema consistent with Campisi Clinical Stage 1B, 2, 3, 4 (Table 1). At these stages, lymphedema is clinically evident and not yet permanently fibrotic, sclerotic, or indurated with verrucous change.
-
•
HIV positive
-
•
On anti-retroviral therapy
-
•
Willingness to participate for the entire study duration, ranging from at least 14 weeks and up to 24 weeks, depending on randomization.
-
•
Provision of written Informed Consent
Table 1.
Lower extremity lymphedema index & campisi staging classification system.
| Campisi's Lymphedema Clinical Staging System | |||
|---|---|---|---|
| Stage 1A | No edema with presence of lymphatic dysfunction | Campisi Stage | Average LEL Index (SD) [14] |
| Stage 1B | Mild edema, reversible with inclined position and night rest | Stage 1 | 210 (11) |
| Stage 2 | Persistent edema that regresses only partially with inclined position and night rest | Stage 2 | 242 (8) |
| Stage 3 | Persistent edema that continually becomes more severe | Stage 3 | 293 (29) |
| Stage 4 | Fibrotic lymphedema (with initial lymphostatic warts) and column-shaped limb | Stage 4 | 336 (14) |
| Stage 5 | Elephantiasis with severe limb deformation, firm, bound-down skin, and widespread lymphostatic warts | ||
2.5.2. Exclusion criteria
-
•
HIV negative
-
•
Leg lymphedema consistent with Campisi Clinical Stage 1A or 5 (Table 1). At these stages, lymphatic dysfunction is not yet clinically evident (Stage 1A) or lymphedema has become permanently fibrotic, sclerotic, or indurated with verrucous change (Stage 5).
-
•
History of another cancer diagnosis
-
•
Concomitant peripheral arterial disease as documented via history or peripheral vascular physical examination
-
•
Concomitant diagnosis of decompensated heart failure, acute phase dermatitis (at the time of the study), rheumatoid arthritis, acute phase deep vein thrombosis
-
•
Diagnosis of medical conditions that may also lead to lower extremity lymphedema, including: congestive heart failure, filiariasis, previous vein stripping or peripheral vascular surgery
-
•
Current use of medications known to cause edema, (i.e. calcium channel blockers, systemic corticosteroids)
-
•
Pregnant females
2.5.3. Withdrawal criteria
-
•
Wishes to withdraw consent to participate in trial
-
•
Unable to tolerate compression therapy due to pain or discomfort
-
•
Development of skin reaction to compression bandages
2.6. Interventions
2.6.1. Study intervention
The study intervention is 10 weeks of weekly clinic-based application of two-component compression bandages. The inner layer is comprised of zinc oxide-impregnated gauze, a modification of the Unna boot using locally available materials. The outer layer is elastic crepe. These compression bandages are assembled by AMPATH Pharmacy in Eldoret and delivered to AMPATH pharmacies throughout western Kenya [15]. For this study, the compression bandages will be applied by a trained research study nurse; the same individual will be applying the bandages at all visits for all participants.
2.6.2. Modifications
Pain/Discomfort: Individuals tend to have varying levels of tolerance for compression therapy. For pain, a clinical assessment will be made to ensure that the compression wrap has not been applied too tightly and to confirm that clinical signs of arterial disease are absent (which would be a contraindication to compression as indicated in 2.3.2. Exclusion Criteria). If pain persists, a trial of paracetamol (acetaminophen) will be given. If the participant continues to be unable to tolerate the compression therapy, then the compression therapy will be stopped and the participant withdrawn from the study.
Skin Reactions: Zinc oxide is inert. Skin reactions have not been reported with its frequent use in a variety of clinical settings. The study team will monitor for any skin reactions. If this is suspected, the intervention will be stopped for the participant. This will be reported as an adverse event.
2.6.3. Adherence
Face-to-face adherence reminder sessions will take place at enrollment, which occurs on the same day as the initial study visit, and each study visit thereafter. This session will include:
-
•
Importance of coming to each study visit
-
•
Importance of keeping compression wraps dry and intact on leg until the next study visit
-
•
Importance of calling study team personnel if experiencing problems
Study personnel will also call participants weekly to remind them of their upcoming study visit.
2.6.4. Concomitant care
Leg Ulcer/Wound: If an ulcer is present, then routine wound care will also be administered. Routine wound care is dictated by clinical practice standards: If poor granulation tissue is present, mechanical debridement is performed, as tolerated by patient.
Infection: If there are clinical signs of bacterial infection (pus, foul smell, pain), topical and/or systemic antibiotics will be administered as indicated. If there are clinical signs of fungal infection (scale, pain/itch, interdigital maceration), topical antifungals will be administered as indicated.
Washing: Washing lymphedematous skin with soap and clean water is a standard part of lymphedema care. In other lymphedematous conditions, washing is often performed by the patient at home or with a group of other patients who also have lymphedema. In this study, washing will be conducted by the research study nurse at each study visit for both intervention and control arms before outcome measures are performed and new compression bandages are placed. Washing will only be performed at clinic, by the study nurse, at study visits to minimize the following threats to internal validity: variability between participants in their washing technique or frequency, intervention group cannot conduct washing at home as leg will have compression bandage.
2.7. Outcomes
Outcome measures were carefully selected after a review of literature for reported measures of leg lymphedema disease activity, leg lymphedema quality of life, and cancer-related quality of life.
2.7.1. Primary outcome measure
-
•Comparison of change in Lower Extremity Lymphedema Index (LELI) score Week 0 to Week 14 between intervention and control arm
-
•The LELI is calculated by taking the sum of the squares of the circumference in 5 areas of a lower extremity and dividing it by the BMI. The LEL indices are significantly correlated with clinical stages and can be used as a severity scale. The average LEL index (SD) in Campisi's clinical stage 1 is 210 (11), stage 2 is 242 (8), stage 3 is 293 (29), stage 4 is 336 (14). [14], as shown in Table 1.
-
•
2.7.2. Secondary outcome measures
-
•Comparison of change in LYMQOL (leg lymphedema-specific quality of life) from Week 0 to Week 14 between intervention and control arm
-
•The Lymphedema Quality-of-Life measure (LYMQOL) has separate tools for assessing arm lymphedema and leg lymphedema [16].
-
•
-
•Comparison of change in EORTC QLQ C30 (overall health quality of life in cancer patients) from Week 0 to Week 14 between intervention and control arm
-
•The EORTC QLQ-C30 is a questionnaire developed by the European Organization for Research and Treatment of Cancer to assess the quality of life of cancer patients: http://groups.eortc.be/qol/eortc-qlq-c30
-
•
2.8. Participant timeline
Consecutive patients with Kaposi sarcoma lymphedema who meet eligibility criteria (see 2.5) and provide consent will be enrolled at Week 0. Baseline assessment at Week 0 will occur prior to randomization. Baseline assessment will include demographics, baseline Kaposi sarcoma disease characteristics, baseline health characteristics including HIV disease characteristics, lymphedema assessment (which includes the primary outcome measure LELI), and quality of life assessment. These assessments will be conducted by study personnel using an interview format. For both arms, de-identified photographs of the affected leg(s) will be taken at Week 0, Week 10, and Week 14.
Those randomized to the immediate compression arm will receive 10 weeks of weekly compression therapy while receiving chemotherapy (6 cycles every 2 weeks of bleomycin-vincristine or gemcitabine, pending local clinic availability). The lymphedema assessment will be administered every week. At Week 6, Week 10, and Week 14, the secondary outcome quality of life measurement tools (LYMQOL, EORTC CLC-Q30) will be administered. Week 14 represents the 1st follow-up oncology visit after completion of the course of chemotherapy. After Week 14, participants in the immediate arm will have the remainder of their care directed by clinic. Participants are eligible for an additional 10 weeks of weekly compression therapy. At Week 24, a follow-up phone call will be made to determine the status of the participant's lymphedema.
Those randomized to the control arm will not receive compression therapy until after completion of chemotherapy (6 cycles every 2 weeks of bleomycin-vincristine or gemcitabine, pending local clinic availability). The lymphedema assessment will be administered every 2 weeks at the time of routine oncology care (Week 2, 4, 6, 8, 10). At Week 6, Week 10, and Week 14, the secondary outcome quality of life measurement tools (LYMQOL, EORTC CLC-Q30) will be administered. Week 14 represents the 1st follow-up oncology visit after completion of the course of chemotherapy. After Week 14, participants in the delayed arm will cross over to 10 weeks of weekly compression therapy. Once this is completed, participants are eligible for an additional 10 weeks of weekly compression therapy. At Week 24, a follow-up phone call will be made to determine the status of the participant's lymphedema.
This is summarized schematically in Table 2.
Table 2.
KICKS study schedule.
| Study Period |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrollment |
Post-Allocation |
Close-out |
|||||||||||||||||||||||
| Allocation | |||||||||||||||||||||||||
| Timepoint (weeks) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14∗ | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| Screening | |||||||||||||||||||||||||
| Campisi lymphedema clinical stage assessment | X | ||||||||||||||||||||||||
| Inclusion/exclusion criteria | X | ||||||||||||||||||||||||
| Enrollment | |||||||||||||||||||||||||
| Informed consent | X | ||||||||||||||||||||||||
| Demographic profile | X | ||||||||||||||||||||||||
| Kaposi Sarcoma disease characteristics | X | ||||||||||||||||||||||||
| Health characteristics† | X | ||||||||||||||||||||||||
| Randomization (stratified)‡ | X | ||||||||||||||||||||||||
| Intervention | |||||||||||||||||||||||||
| Compression therapy | I | I | I | I | I | I | I | I | I | I | I | C | C | C | C | C | C | C | C | C | C | C | |||
| Routine clinical care | |||||||||||||||||||||||||
| Chemotherapy | X | X | X | X | X | X | |||||||||||||||||||
| Assessments | |||||||||||||||||||||||||
| LELI | X | I | X | I | X | I | X | I | X | I | X | X | C | C | C | C | C | C | C | C | C | C | |||
| LYMQOL | X | X | X | X | C | C | |||||||||||||||||||
| EORTC QLQ-C30 | X | X | X | X | C | C | |||||||||||||||||||
| De-identified photographs | X | X | X | ||||||||||||||||||||||
| 3 month follow-up: visit or phone call | X | ||||||||||||||||||||||||
∗Endpoint for primary and secondary outcome assessments.
† Including HIV disease characteristics.
‡ Randomization occurs after all baseline assessments complete.
C-Control group (delayed compression).
EORTC QLQ-C30-European organization for research and treatment of cancer quality of life.
I-Intervention group (immediate compression).
LELI-Lower extremity lymphedema index.
LYMQOL-Lymphedema quality-of-life measure.
X-All Participants.
2.9. Sample size
With standard 0.05 Type I error rate and a sample size of 30 participants with 15 participants in each arm, assuming a standard deviation in the LELI change score of 10, we will be able to detect a change in LELI of 10.6 points with 80% power. Table 3 describes how ease of enrollment will influence our ability to detect an effect size at varying levels of participant retention during the study. Please note that based on Yamamoto et al., the standard deviation of the LELI across patients similar to ours is approximately 20. No references on the standard deviation of the change score are available, but assuming a within-subject correlation coefficient of 0.875, the standard deviation of the change score will be 10.
Table 3.
Detectable effect size (with 80% power and alpha = 0.05) for different levels of enrollment and follow-up.
| Enrolled Participants | Participant Follow-Up |
|||
|---|---|---|---|---|
| 100% | 90% | 80% | 70% | |
| 30 | 10.6 | 11.5 | 12.0 | 13.3 |
| 40 | 9.1 | 9.6 | 10.2 | 11 |
| 50 | 8.1 | 8.6 | 9.1 | 9.9 |
| 60 | 7.4 | 7.8 | 8.3 | 8.9 |
| 70 | 6.8 | 7.2 | 7.6 | 8.3 |
| 80 | 6.3 | 6.7 | 7.1 | 7.6 |
| 90 | 6.0 | 6.3 | 6.7 | 7.2 |
| 100 | 5.7 | 6.0 | 6.3 | 6.8 |
2.10. Recruitment
Potential study participants will be recruited from the AMPATH oncology clinic in Chulaimbo. Oncology clinicians at these sites will be made aware of this study, and contact info for study personnel will be made available.
2.11. Randomization
2.11.1. Sequence generation
Participants will be randomly assigned to either intervention (immediate compression) or control (delayed compression) group with a 1:1 allocation as per a computer generated randomization schedule. A randomized block design will be utilized with stratification by lymphedema stage. There will be three stratifications based on Campisi's lymphedema clinical staging (Table 1): Stage 1B & Stage 2; Stage 3; Stage 4. Stage 1B and Stage 2 will be combined into one stratum because we anticipate fewer participants with Stage 1B or Stage 2, as compared to Stage 3 or Stage 4, based on clinical experience. Block sizes will not be disclosed in order to ensure concealment.
2.11.2. Allocation concealment mechanism
Taking into consideration unreliable phone and data connectivity, participants will be randomized using sequentially numbered, opaque, sealed envelopes (color coded by stratified lymphedema stage). Randomization will occur only after enrollment and completion of baseline assessments, thus ensuring allocation concealment.
2.11.3. Implementation
There will be complete separation of individuals involved in the sequence generation and allocation concealment mechanism from those involved in implementation of study group assignments. A study team member having no interaction with individuals implementing the study group assignments will generate the allocation list and store it securely. Those enrolling and assigning participants will not have access to this list.
2.12. Blinding
The Week 14 outcome assessments will be blinded. A study member who has not participated in enrollment, randomization, or administration of study assessments will perform the Week 14 outcome assessments. As outlined in Section 2.6.4. Concomitant Care, washing of the lymphedematous legs will occur prior to performing of outcome assessments at all study visits. Thus, any residual zinc oxide paste on the limbs of participants in the intervention arm will be removed prior to performing the Week 14 outcome assessments.
2.13. Data collection methods
2.13.1. Additional data to be collected
In addition to data related to the outcome measures discussed in Section 2.7 Outcomes, the following information will be collected:
-
•Overall health
-
•Underlying chronic diseases: HIV/AIDS, diabetes mellitus, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, asthma, congestive heart failure, chronic kidney disease, osteoarthritis, tobacco use
-
•
-
•KS and HIV disease characteristics
-
•Date of biopsy confirming KS
-
•KS AIDS Clinical Trials Group Staging
-
•HIV diagnosis date
-
•Viral load/CD4 count at time of diagnosis
-
•Current antiretroviral treatment regimen
-
•CD4/viral load at diagnosis and most recent
-
•Prior and current chemotherapy regimen
-
•
2.13.2. Training of personnel
Taking into consideration unreliable data connectivity, data collection will occur on paper forms. Study personnel interacting with study participants will be required to have a clinical background. The research study nurse will receive focused training on assessing and managing leg lymphedema, wounds, and associated infections, as well as washing lymphedematous legs and applying compression bandages. This study nurse and study personnel performing the blinded outcome assessment at Week 14 will also be trained in taking the standard leg measurements for the primary outcome measure, completing study data collection forms for secondary outcome measures, and interviewing participants appropriately to obtain information.
2.13.3. Retention
An anticipated barrier to retention will be costs associated with travel to and from clinic for study visits. For both arms, each participant's transport costs will be subsidized for 12 visits with 750 KShs (7.50 USD) per visit. For the participants in the immediate arm, the following visits will be covered: Week 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 14. For the participants in the delayed arm, the following visits will be covered: Week 0, 2, 4, 6, 8, 10, 14, 15, 16, 17, 18, 19. Transport subsidies will be administered at the end of each study visit. Although the number of study visits is different between both arms, the number of subsidized visits is the same between both arms. This is consistent with transport subsidization schemas used by other research studies in this setting and has been approved by the local institutional review board.
For participants who miss a study visit, they will be contacted by study personnel through phone contact and asked to come into clinic the next day or following day. Three attempts by phone will be made to the participant and to the additional contacts that the participant lists at time of study enrollment. If the participant fails to come to clinic after these phone attempts are made, then the participant will be considered loss to follow-up during study data analysis.
2.14. Data management
Participants will be assigned a unique study ID number, which will be their identifier throughout the study. Only the principal investigator and co-investigators will have access to the master list. Hard copies of all patient consent forms will be kept safely and all electronic data will be kept on a password protected computer database. Paper-based study assessment forms, containing the unique study ID number, will be scanned into an electronic file. Optical recognition software (Captricity®) will be utilized to extract data from scanned electronic forms into an analyzable data format.
2.15. Statistical methods
For the primary and secondary outcome measures, a t-test will be performed to compare the change in outcome measure from baseline to final Week 14 measurement between the immediate and delayed arms. We will also use generalized estimating equations (GEE) to take advantage of the measurements between the baseline and final Week 14 measurement.
2.16. Ethics and dissemination
Regulatory approvals have been obtained from Moi University/Moi Teaching and Referral Hospital Institutional Research and Ethics Committee in Eldoret, Kenya and Indiana University Institutional Review Board in Indianapolis, USA; exemption from the University of California, San Francisco Institutional Review Board in San Francisco, USA. For study participants, compression therapy will be provided without fees. Topical or systemic antibiotics that may be needed for ulcers will be provided without fees during duration of study. Following completion of the study, participants will be eligible for an additional 10 weeks of subsidized compression therapy. The study results will be shared with patients with KS lymphedema, clinicians from AMPATH Oncology clinics, and the general medical community, local and abroad.
3. Discussion
There is limited evidence for the effectiveness of compression therapy for KS lymphedema. To date, one non-randomized controlled study found that 60% of patients with classic KS treated with compression stockings experienced a limb volume reduction, compared to 0% of untreated patients [13]. There are no studies evaluating compression bandages in KS lymphedema. Thus, we designed the Kenyan Improvised Compression for Kaposi Sarcoma (KICKS) study to evaluate the efficacy of using locally sourced two-component compression bandages in managing HIV-associated KS lymphedema in Western Kenya. Despite the burden of KS lymphedema in sub-Saharan Africa, this represents the first study in this world region to assess a lymphedema-directed intervention for KS. Furthermore, the intervention—locally sourced two-component compression bandages—has a price point that has been affordable thus far for patients in clinical care, suggesting this may be a sustainable intervention in similar settings.
We had initially designed a longitudinal cohort study with pre-intervention, intra-intervention, and post-intervention data points. After consideration of the heterogeneous population and potential confounders, with respect to degree of HIV disease control, antiretroviral therapy regimen, lymphedema stage, and chemotherapy regimen, we felt that it would be difficult to draw conclusions from a study without a control group and thus opted for a randomized controlled trial design. Given unpredictable attendance at oncology clinic, we created Table 3 as a model for determining effect size based on varying levels of enrollment and loss-to-follow-up. Additionally, anecdotal experience suggests that those with earlier stage disease are more likely to have reversible changes, compared to those with later stage disease. The exact boundaries between earlier stage and later stage are not clear. Although the anticipated sample size is not large, the potential variability in response to compression therapy that could be dependent on baseline lymphedema stage prompted us to utilize stratified randomization by lymphedema stage.
One of the fundamental challenges with designing this study protocol was balancing scientific rigor with real world applicability. As the first study to evaluate compression therapy in HIV-associated KS lymphedema, we wanted to be able to attribute any detected changes to the compression therapy intervention. However, we also wanted the study findings to be relevant to real-world settings in sub-Saharan Africa, where KS patients have different stages of lymphedema, varying levels of HIV disease control, and variable chemotherapy access. We also believed the study should reflect best practices in lymphedema management, since local healthcare providers would likely try to incorporate the study interventions into KS lymphedema care. Taking into consideration these study goals and our available resources, we ultimately designed a randomized controlled trial with elements of a pragmatic clinical trial. These pragmatic elements include: 1) integrating the intervention within the chemotherapy administration schedule and 2) including wound care, evaluation for infection, and leg washing as part of concomitant care. A heterogeneous population remains one of the major limitations of our study. While we hope that stratified randomization will account for this, our study data may ultimately need to be interpreted through the lens of a pragmatic clinical trial.
Despite its limitations, the KICKS study is an important step towards developing an evidence-based path for regionally relevant management of KS lymphedema. If the data support the use of compression therapy in KS lymphedema, next steps will include evaluation of self-based and community-based care delivery models, as have occurred for podoconiosis and lymphatic filariasis, other lymphedematous conditions prevalent in resource-limited settings [[17], [18], [19]].
Funding
This study is funded with support from the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. This project was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1TR001872. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The funders had no role in writing this report or the decision to submit this article for publication. This research was also supported by the President's Emergency Plan for AIDS Relief through the US Agency for International Development under the terms of Cooperative Agreement No. AID-623-A-12-0001. The contents of this article are the sole responsibility of the authors and do not necessarily reflect the views of USAID or the US government.
Acknowledgements
The authors wish to thank Dr. Lisa Hoo, staff physician at Laguna Honda Hospital, San Francisco, California, for her generous donation of compression bandages to AMPATH that inspired this work.
References
- 1.Dryden-Peterson S., Medhin H., Kebabonye-Pusoentsi M., Seage G.R., 3rd, Suneja G., Kayembe M.K., Mmalane M., Rebbeck T., Rider J.R., Essex M. Cancer Incidence following Expansion of HIV Treatment in Botswana. PloS One. 2015;10(8) doi: 10.1371/journal.pone.0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mutyaba I., Phipps W., Krantz E.M., Goldman J.D., Nambooze S., Orem J., Wabinga H.R., Casper C. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo county, Uganda, 1999-2008. J. Acquir. Immune Defic. Syndr. 2015;69(4):481–486. doi: 10.1097/QAI.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Msyamboza K.P., Dzamalala C., Mdokwe C., Kamiza S., Lemerani M., Dzowela T., Kathyola D. Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC Res. Notes. 2012;5:149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semeere A., Wenger M., Busakhala N., Buziba N., Bwana M., Muyindike W., Amerson E., Maurer T., McCalmont T., LeBoit P. A prospective ascertainment of cancer incidence in sub-Saharan Africa: the case of Kaposi sarcoma. Canc. Med. 2016;5(5):914–928. doi: 10.1002/cam4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohner E., Valeri F., Maskew M., Prozesky H., Rabie H., Garone D., Dickinson D., Chimbetete C., Lumano-Mulenga P., Sikazwe I. Incidence rate of Kaposi sarcoma in HIV-infected patients on antiretroviral therapy in Southern Africa: a prospective multicohort study. J. Acquir. Immune Defic. Syndr. 2014;67(5):547–554. doi: 10.1097/QAI.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellet C., Kerob D., Dupuy A., Carmagnat M.V., Mourah S., Podgorniak M.P., Toledano C., Morel P., Verola O., Dosquet C. Kaposi's sarcoma-associated herpesvirus viremia is associated with the progression of classic and endemic Kaposi's sarcoma. J. Invest. Dermatol. 2006;126(3):621–627. doi: 10.1038/sj.jid.5700083. [DOI] [PubMed] [Google Scholar]
- 7.Tulpule A., Groopman J., Saville M.W., Harrington W., Jr., Friedman-Kien A., Espina B.M., Garces C., Mantelle L., Mettinger K., Scadden D.T. Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma. Cancer. 2002;95(1):147–154. doi: 10.1002/cncr.10634. [DOI] [PubMed] [Google Scholar]
- 8.Tulpule A., Scadden D.T., Espina B.M., Cabriales S., Howard W., Shea K., Gill P.S. Results of a randomized study of IM862 nasal solution in the treatment of AIDS-related Kaposi's sarcoma. J. Clin. Oncol. 2000;18(4):716–723. doi: 10.1200/JCO.2000.18.4.716. [DOI] [PubMed] [Google Scholar]
- 9.Grada A.A., Phillips T.J. Lymphedema: diagnostic workup and management. J. Am. Acad. Dermatol. 2017;77(6):995–1006. doi: 10.1016/j.jaad.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Vaqas B., Ryan T.J. Lymphoedema: pathophysiology and management in resource-poor settings - relevance for lymphatic filariasis control programmes. Filaria J. 2003;2(1):4. doi: 10.1186/1475-2883-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franks P.J., Moffatt C.J., Doherty D.C., Williams A.F., Jeffs E., Mortimer P.S. Assessment of health-related quality of life in patients with lymphedema of the lower limb. Wound Repair Regen. 2006;14(2):110–118. doi: 10.1111/j.1743-6109.2006.00099.x. [DOI] [PubMed] [Google Scholar]
- 12.Stolldorf D.P., Dietrich M.S., Ridner S.H. A Comparison of the quality of life in patients with primary and secondary lower limb lymphedema: a mixed-methods study. West. J. Nurs. Res. 2016;38(10):1313–1334. doi: 10.1177/0193945916647961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brambilla L., Tourlaki A., Ferrucci S., Brambati M., Boneschi V. Treatment of classic Kaposi's sarcoma-associated lymphedema with elastic stockings. J. Dermatol. 2006;33(7):451–456. doi: 10.1111/j.1346-8138.2006.00108.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T., Matsuda N., Todokoro T., Yoshimatsu H., Narushima M., Mihara M., Uchida G., Koshima I. Lower extremity lymphedema index: a simple method for severity evaluation of lower extremity lymphedema. Ann. Plast. Surg. 2011;67(6):637–640. doi: 10.1097/SAP.0b013e318208fd75. [DOI] [PubMed] [Google Scholar]
- 15.Chang A.Y., Tonui E.C., Momanyi D., Mills A.R., Wasike P., Karwa R., Maurer T.A., Pastakia S.D. Development of low-cost locally sourced two-component compression bandages in western Kenya. Dermatol. Ther. (Heidelb) 2018 Sep;8(3):475–481. doi: 10.1007/s13555-018-0248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeley VC S., Locke J., Veigas D., Riches K., Hilliam R. A quality of life measure for limb lymphoedema (LYMQOL) J. Lymphoedema. 2010;5(1):26–37. [Google Scholar]
- 17.Negussie H., Kassahun M.M., Fegan G., Njuguna P., Enquselassie F., McKay A., Newport M., Lang T., Davey G. Podoconiosis treatment in northern Ethiopia (GoLBet): study protocol for a randomised controlled trial. Trials. 2015;16:307. doi: 10.1186/s13063-015-0818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narahari S.R., Bose K.S., Aggithaya M.G., Swamy G.K., Ryan T.J., Unnikrishnan B., Washington R.G., Rao B.P., Rajagopala S., Manjula K. Community level morbidity control of lymphoedema using self care and integrative treatment in two lymphatic filariasis endemic districts of South India: a non randomized interventional study. Trans. R. Soc. Trop. Med. Hyg. 2013;107(9):566–577. doi: 10.1093/trstmh/trt054. [DOI] [PubMed] [Google Scholar]
- 19.Douglass J., Graves P., Gordon S. Self-Care for Management of Secondary Lymphedema: a Systematic Review. PLoS Neglected Trop. Dis. 2016;10(6) doi: 10.1371/journal.pntd.0004740. [DOI] [PMC free article] [PubMed] [Google Scholar]