Abstract
Liposarcoma of the uterine corpus is extremely rare. We performed a laparotomy on a 55-year-old woman with the complaints of abdominal distension and genital bleeding who was found to have a uterine tumor, 17 × 16 cm in diameter. The preoperative diagnosis was a lipoma or lipoleiomyoma of the uterine corpus. However, pathological examination revealed proliferation of mature adipocytes and lipoblast-like atypical cells with small, weakly pleomorphic nuclei and foamy or vacuolated cytoplasm present within a fibrous septum. Immunohistochemistry showed that the tumor cells were focally positive for mouse double minute 2 homolog (MDM2). The final pathological diagnosis was a well-differentiated liposarcoma of International Federation of Gynecology and Obstetrics (FIGO) stage IB (pT1bNxM0). On magnetic resonance imaging (MRI), T1 -weighted and fat-saturated images showed high and low intensity in the tumor, respectively, suggesting that this tumor contained a fat component. The septum inside the tumor had a contrast enhancement on T1-weighted, gadolinium-enhanced imaging. The septum was nonuniformly thickened and partially nodular. In hindsight, these findings may have suggested a well-differentiated liposarcoma in the uterine corpus rather than a lipoma or lipoleiomyoma. Clinicians should be aware of the possibility of a liposarcoma of the uterine corpus when a neoplasm contains adipose tissue and a nonuniformly thickened or partially nodular septum on MRI.
Keywords: Liposarcoma, MDM2, MRI, uterine corpus, well-differentiated type
Highlights
-
•
A rare case with a well-differentiated liposarcoma originating from uterine corpus.
-
•
The tumor cells were positive for MDM2 and S-100 protein.
-
•
On MRI, T1WI and T2WI showed high intensity in the tumor with fat- suppression.
-
•
The septum inside the tumor had a low signal on T1WI with contrast enhancement.
-
•
The septum was nonuniformly thickened and partially nodular.
1. Introduction
Lipomatous tumors arising from the uterus are extremely rare, accounting for 0.03% to 0.2%(Chu et al., 2012) of all uterine tumors. They are generally classified as pure lipomas, mixed lipomas (e.g., lipoleiomyoma, angiolipoma, fibrolipoma), and liposarcomas. Liposarcomas are categorized into 4 groups by the World Health Organization classification system (2013): well-differentiated, dedifferentiated, myxoid, and pleomorphic subtypes (Fletcher et al., 2013).
A well-differentiated liposarcoma has a generally favorable prognosis; however, there is a 10% risk of dedifferentiation with local recurrence or metastasis (Fletcher et al., 2013). Therefore, forming an accurate preoperative diagnosis is very important, especially to distinguish this lesion from a benign lipoma. However, making an accurate diagnosis is difficult because liposarcoma, an especially well-differentiated type of neoplasm, is extremely rare in the uterine corpus and there are no reports of the definitive findings on preoperative imaging evaluation.
This is a report of an extremely rare instance of a well-differentiated liposarcoma developing in the uterine corpus. Herein, we describe the clinical, morphologic, and magnetic resonance imaging (MRI) features that typically determine the diagnosis. We also perform a review of the literature.
2. Case report
A 55-year-old Japanese woman, gravida 6 para 3, presented to Dokkyo Medical University Hospital with the complaints of abdominal distension and genital bleeding. She underwent menopause at 53 years of age. She was followed by internal medicine for a history of a myocardial infarction, sleep apnea, diabetes mellitus, and dyslipidemia. Her family history was unremarkable. Her gynecologic history was significant for a uterine myoma, enlarged to the height of the umbilicus, at 45 years of age. At that time, she underwent treatment with a gonadotropin releasing hormone agonist to avoid surgery because of her medical complications; she was observed for 10 years after treatment. She then presented to our hospital with the complaint of abdominal distension.
Investigation using MRI revealed a 14.5 × 14-cm mass, unchanged compared with previous imaging. The radiologic diagnosis was leiomyoma or lipoleiomyoma. The MRI was repeated after 6 months because of her complaints of gradually increasing abdominal distension and postmenopausal genital bleeding. Repeat MRI revealed an obviously enlarged, solid tumor, 17 × 16 cm in diameter, occupying the uterine corpus. T1 and T2-weighted images showed high intensity in the tumor, and T1-weighted, fat-saturated images showed low intensity, suggesting this mass contained a fat component. A septum inside the tumor had a low signal on T1-weighted imaging and a high signal on T1-weighted imaging with fat saturation. The septum was enhanced on T1-weighted, fat-saturated, and gadolinium-enhanced imaging. The septum was nonuniform in thickness (Fig. 1). Diffusion-weighted imaging did not show any areas of abnormally high intensity, and an apparent diffusion coefficient map showed a high signal in some parts. Cervical and endometrial cytology were negative for malignancy. Based on these findings, the tumor was diagnosed as a uterine lipoma or lipoleiomyoma.
Fig. 1.
Pelvic MRI findings. MRI revealed a solid tumor, 17 × 16 cm in diameter, occupying the uterine corpus. T1 and T2-weighted images showed high intensity in the tumor, and T1-weighted, fat-saturated images showed low intensity, suggesting this mass contained a fat component. A septum inside the tumor had a low signal on T1-weighted imaging and a high signal on T1-weighted imaging with fat saturation. The septum was enhanced on T1-weighted, fat-saturated, and gadolinium-enhanced imaging. The septum was nonuniform in thickness. (a; T2-weighted image, b; T1-weighted image, c; T1-weighted imaging with fat saturation, d; T1-weighted, fat-saturated, and gadolinium-enhanced imaging).
After obtaining informed consent, we performed a hysterectomy and bilateral salpingo-oophorectomy to relieve her complaints and to obtain a precise pathological diagnosis. Intraoperative inspection revealed the uterus to be round and enlarged to the size of over 15 cm in diameter, with a smooth surface; the ovaries and fallopian tubes appeared normal, and there were no other abnormalities noted. The tumor was encapsulated and well separated from the myometrium of the uterine corpus, occupying almost the entire corpus. The cut surface of the tumor was yellowish, with an elastic, soft consistency. There was a greyish part and a fibrous septum. There was no apparent hemorrhage or necrosis (Fig. 2a).
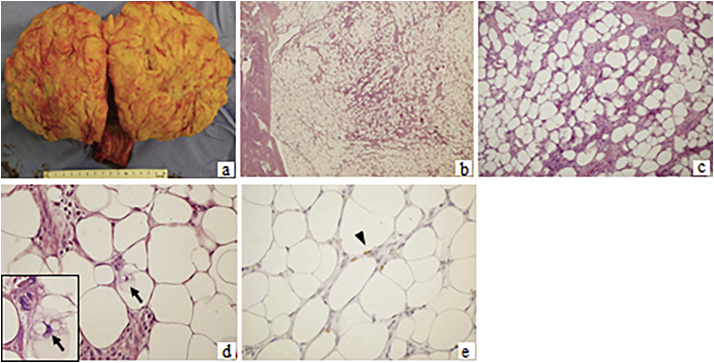
Fig. 2.
Macroscopic findings and pathological examination. The tumor was encapsulated and well separated from the myometrium of the uterine corpus, occupying almost the entire corpus. The cut surface of the tumor was yellowish, with an elastic, soft consistency. There was a greyish part and a fibrous septum. There was no apparent hemorrhage or necrosis (a). Pathological examination revealed massive proliferation of mature adipocytes and spindle-shaped cells with atypia in the fibrous septum. Lipoblast-like atypical cells with relatively small, hyperchromatic, weakly pleomorphic nuclei and foamy or vacuolated cytoplasm were observed (arrow). There were no areas of hemorrhage, degeneration, or necrosis. Rare mitotic figures were seen in the tumor cells. Immunohistochemistry revealed that the tumor cells were weakly positive for MDM2 (arrowhead). (b; H&E, original magnification x4, c; H&E x10, d; H&E x40, e; MDM2 immunostaining x40).
Pathological examination revealed massive proliferation of mature adipocytes and spindle-shaped cells with atypia in the fibrous septum (Fig. 2b-e). Lipoblast-like atypical cells with relatively small, hyperchromatic, weakly pleomorphic nuclei and foamy or vacuolated cytoplasm were observed in the fibrous septum (Fig. 2b-e). There were no areas of hemorrhage, degeneration, or necrosis. Rare mitotic figures were seen in the tumor cells, with a mitotic count of <1 per 10 high-power fields. The tumor itself was composed of nonepithelial cells with no apparent epithelial components. Immunohistochemistry revealed that the tumor cells were focally and weakly positive for mouse double minute 2 homolog (MDM2) and S-100 protein, and negative for phospho-histone H3 (PHH3). The labeling index of Ki-67 was 1%. The peritoneal washing cytology was negative for malignancy. The final pathologic diagnosis was a well-differentiated liposarcoma of International Federation of Gynecology and Obstetrics (FIGO) stage IB (pT1bNxM0).
After surgery, the patient underwent close follow-up without adjuvant therapy. She is currently alive 19 months after surgery, with no evidence of recurrence.
3. Discussion
The incidence of lipomatous tumors in the uterus is low, and liposarcoma in particular is extremely rare. Many reported patients with liposarcoma have a neoplasm originating from the uterine cervix rather than the corpus. To the best of our knowledge, there are only 9 patients, including ours, reported in the English-language literature with a liposarcoma occurring in the uterine corpus(Bapat & Brustein, 1989; Sosnik et al., 2006; Hong et al., 2008; McDonald et al., 2011; Schoolmeester et al., 2016; Fadare & Khabele, 2011) (Table 1). Patients with lipoleiomyosarcoma or mesenchymal malignant tumors having only a small part of liposarcomatous differentiation are excluded from this review.
Table 1.
Liposarcoma of the uterine corpus: review of literature.
| Authors | Age (years) | Symptoms | Tumor size (cm) | Preoperative diagnosis | Operation | Histological subtype | Histo-genesis | FIGO stage | Postoperative treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Bapat (Bapat & Brustein, 1989) | 55 | Genital bleeding | 12x7x6 | N/A | ATH + BSO +PLN + PAN |
Mixed (myxoid+pleomorphic)+leiomyosarcoma | N/A | IB | CT | 12 M Rec |
| Sosnik (Sosnik et al., 2006) | 71 | Genital bleeding | 10.8×12.9 | N/A | ATH + BSO | Pleomorphic | N/A | IB | RT | 8Y NED |
| Hong (Hong et al., 2008) | 48 | Dysmenorrhea, abdominal mass | 21×18 | N/A | ATH + BSO | Myxoid | Leiomy-oma | N/A | – | 2 M NED |
| McDon-ald (McDonald et al., 2011) | 49 | Abdominal mass | 10.5 | N/A | ATH + BSO | Pleomorphic | Lipoleio-myoma | N/A | – | 1Y NED |
| 58 | Genital bleeding | 18 | N/A | ATH + BSO | Mixed (myxoid+pleomorphic) | Lipoleio-myoma | N/A | – | 2Y NED |
|
| 70 | Abdominal mass | 10 | N/A | ATH + BSO | Myxoid | Lipoleio-myoma | N/A | – | 20Y NED |
|
| School-meeste (Schoolmeester et al., 2016) | 70 | Dysuria | 9x8x7.5 | N/A | MRH + BSO +PLN |
Pleomorphic | Lipoleio-myoma | N/A | Lung meta: CT (GEM +DTX) |
3 M DOD |
| Fadare (Fadare & Khabele, 2011) | 62 | Abdominal pain | 7 × 6.3×4.5 | Uterine fibroid | ATH + BSO | Pleomorphic+leiomyosarcoma | Leiomy-oma | N/A | CT (after Rec) |
2 M Rec |
| Present case | 58 | Abdominal distension, genital bleeding | 16 × 17 | Lipoleiomyo-ma or lipoma | ATH + BSO | Well-differentiated | Unclear | IB | – | 1Y NED |
N/A: not applicable, MRH: modified radical hysterectomy, ATH: abdominal total hysterectomy, BSO: bilateral salpingo-oophorectomy, PLN: pelvic lymphadenectomy, PAN: paraaortic lymphadenectomy, OMT: omentectomy, meta: metastasis, RT: radiotherapy, GEM: gemcitabine, DTX: docetaxel, CT: chemotherapy, NED: no evidence of disease, DOD: dead of disease, Rec: recurrence.
The reason for the difference in incidence of liposarcoma of the uterine cervix versus the uterine corpus remains unclear. The corpus does not usually contain any adipose tissue, while adipose tissue is reportedly identified within the cervical stroma in about 15% of patients(Doldan et al., 2009). This anatomical and histological distinction may be one reason for the difference in the incidence of liposarcoma development between the cervix and the corpus.
There are various theories on how liposarcoma may develop from the uterine corpus: origination from pluripotential stem cells (Scurry & Hack, 1990), malignant transformation of adipocytes in the myometrium from fat metaplasia of uterine mesenchymal tissue, migration of embryonic adipocytes or perivascular fat tissue, and transfer of adipocytes into the myometrium by manipulation (Sosnik et al., 2006; Fadare & Khabele, 2011). Malignant transformation from leiomyomata and lipoleiomyomata has also been reported (McDonald et al., 2011; Fadare & Khabele, 2011). The pathogenesis of progression from a leiomyoma to a liposarcoma like as the present case is unclear; it is likely a malignant transformation of adipose metaplasia upon secondary degeneration of the leiomyoma.
Although most liposarcomas of soft tissue are well-differentiated, the subtypes of liposarcomas seen in the uterine corpus are of the myxoid or pleomorphic type (or a combination)(McDonald et al., 2011). Our patient, however, had a well-differentiated subtype (Table 1). In our review of 8 reported patients with myxoid or pleomorphic liposarcomas, 3 relapsed or died, even with postoperative adjuvant chemotherapy. The biologic characteristics and optimal treatment strategy for these tumors, including possible chemotherapy regimens, has not been investigated because of the rarity of the lesions, and their prognosis remains unfavorable.
In contrast, well-differentiated types of soft-tissue tumors are lower-grade malignancies with a generally better prognosis, but even these have a risk for dedifferentiation with subsequent local recurrence and metastasis (Enzinger & Weiss, 2001). Therefore, distinguishing these neoplasms from a lipoma, especially in the preoperative time period, is particularly important. Well-differentiated liposarcoma is characterized by amplification of the 12q13–15 region, and overexpression of the MDM2 and cyclin-dependent kinase 4 genes is useful in making the diagnosis (McDonald et al., 2011). In our patient, immunostaining for MDM2 was slightly positive, supporting the histopathological diagnosis.
On MRI, pure lipomas generally do not have any nonadipose components, appearing as a homogeneous tumor with a large amount of fat and a decreased signal on fat- saturated imaging. In contrast, lipoleiomyoma has an inhomogeneous signal with fat and nonfat soft-tissue components; only part of the tumor demonstrates a decreased signal on fat-saturated imaging (Chu et al., 2012). To the best of our knowledge, this is the first report describing the detailed MRI findings in a liposarcoma developing from the uterine corpus.
Lipomas and liposarcomas usually occur in the extremities or the retroperitoneum. Reportedly, simple lipomas can be diagnosed with 100% specificity when the following 4 features are present on MRI: few or no thin septae, linear septae, no areas of enhancement, and no areas of high signal on T2-weighted imaging (Gaskin & Helmes, 2004). In contrast, well-differentiated liposarcomas are diagnosed with 100% sensitivity and 83% specificity when the following 3 features are present on MRI: a thickened or nodular septum (generally >2 mm), areas of enhancement, and areas of high signal on T2-weighted imaging (Gaskin & Helmes, 2004). In hindsight, the MRI findings in our patient were consistent with these criteria. Therefore, these MRI criteria should be considered when forming the differential diagnosis in a patient with a uterine tumor containing adipose tissue. This is the same as for soft tissue nongynecologic neoplasms. In addition to these MRI findings, various symptoms, such as abdominal discomfort or genital bleeding, or postmenopausal enlargement of a uterine tumor may be useful signs of liposarcoma of the uterine corpus.
In conclusion, we describe herein a patient diagnosed with a rare liposarcoma originating from the uterine corpus. The rarity of liposarcoma of the uterine corpus poses a challenge for diagnosis and treatment. Currently, case reports and case series are the most viable option for characterizing this tumor and for developing optimal treatment strategies.
Acknowledgments
Acknowledgement
We are immensely grateful to our esteemed colleagues from department of pathology and radiology who provided histopathological and radiological findings for this article.
Conflict of interest
No conflicts of interest to declare.
Author contribution
1. Kaori Kiuchi; drafting of the manuscript and first author.
2. Kiyoshi Hasegawa; corresponding author, supervision.
3. Shoko Ochiai; acquisition of data, especially photo.
4. Nobuaki Kosaka; acquisition of data, especially clinical data and course.
5. Hajime Kuroda; pathological and immunohistochemical diagnosis, and interpretation.
6. Yasushi Kaji; radiological diagnosis, and interpretation.
7. Ichio Fukasawa; supervision throughout this manuscript.
References
- Bapat K., Brustein S. Uterine sarcoma with liposarcomatous differentiation: report of case and review of literature. Int. J. Gynecol. Obstet. 1989;28:71–75. doi: 10.1016/0020-7292(89)90547-x. [DOI] [PubMed] [Google Scholar]
- Chu C.Y., Tang Y.K., Chan T.S., Wan Y.H., Fung K.H. Diagnostic challenge of lipomatous uterine tumors in three patients. World J Radiol. 2012;4:58–62. doi: 10.4329/wjr.v4.i2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doldan A., Otis C., Pantanowitz L. Adipose Tissue: A Normal Constituent of the Uterine Cervical Stroma. Int. J. Gynecol. Pathol. 2009;28:396–400. doi: 10.1097/PGP.0b013e318192cd20. [DOI] [PubMed] [Google Scholar]
- Enzinger F.M., Weiss S.W. 4th edn. Mosby; St Louis: 2001. Soft tissue tumors; pp. 670–687. [Google Scholar]
- Fadare O., Khabele D. Pleomorphic Liposarcoma of the Uterine Corpus with Focal Smooth Muscle Differentiation. In J Gynecol Pathol. 2011;30:282–287. doi: 10.1097/PGP.0b013e31820086a4. [DOI] [PubMed] [Google Scholar]
- Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F. IARC Press; Lyon: 2013. WHO classification of tumours of soft tissue and bone. [Google Scholar]
- Gaskin C.M., Helmes C.A. Lipomas, lipoma variants, and well-differentiated liposarcomas (atypical lipomas): results of MRI evaluations of 126 consecutive fatty masses. Am. J. Roentgenol. 2004;182:733–739. doi: 10.2214/ajr.182.3.1820733. [DOI] [PubMed] [Google Scholar]
- Hong R., Lim S.C., Jung H. A myxoid liposarcoma arising in a leiomyoma of the uterus:a case report. Arch. Gynecol. Obstet. 2008;277:445–448. doi: 10.1007/s00404-007-0486-2. [DOI] [PubMed] [Google Scholar]
- McDonald A.G., Cin P.D., Ganguly A., Campbell S., Imai Y., Rosenberg A.E. Liposarcoma Arising in Uterine Lipoleiomyoma: A Report of 3 Cases and Review of the Literature. Am. J. Surg. Pathol. 2011;35:221–227. doi: 10.1097/PAS.0b013e31820414f7. [DOI] [PubMed] [Google Scholar]
- Schoolmeester J.K., Stamamakos M.D., Moyer A.M., Park K.J., Fairbairn M., Fader A.N. Pleomorphic Liposarcoma Arising in a Lipoleiomyosarcoma of the Uterus: Report of Case With Genetic Profiling by a Next Generation Sequencing Panel. Int. J. Gynecol. Pathol. 2016;35:321–326. doi: 10.1097/PGP.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scurry J., Hack M. Leiomyosarcoma arising in a lipoleiomyoma. Gynecol. Oncol. 1990;39:381–383. doi: 10.1016/0090-8258(90)90271-l. [DOI] [PubMed] [Google Scholar]
- Sosnik H., Jelen M., Sosnik K., Pomorska M. Liposarcoma of the uterine corpus coexisting with preinvasive cervical cancer- a case report. Pol. J. Pathol. 2006;57:171–173. [PubMed] [Google Scholar]