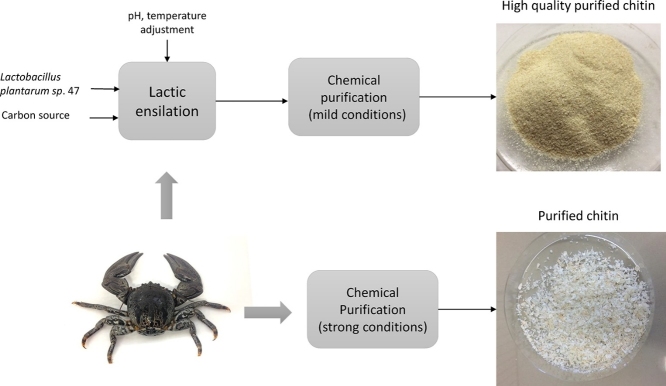
Graphical abstract
Abbreviations: DM, demineralization; DP, deproteinization; DD, deacetylation degree; TTA, total titratable acids
Keywords: Silage, Lactic acid, Demineralization, Deproteinization, Molecular weight, Degree of deacetylation
Highlights
-
•
Establishment of an optimized method to extract high quality chitin from A. punctatus crab by lactic acid fermentation.
-
•
The method generate Lactobacillus plantarum sp. 87 high growth rate, high lactic acid production and prevent spoilage.
-
•
Lactic acid fermentation developed method improves yield and quality of Chitin obtained compared to a chemical method.
Abstract
Chitin extraction from Allopetrolisthes punctatus, a crab species proliferating in Chile and Peru seashores, was carried out applying preliminary lactic ensilation. For this purpose, Lactobacillus plantarum sp. 47 isolated from Coho salmon was inoculated in crab biomass. Previously, fermentation parameters (carbon source, inoculum concentration and incubation temperature) to obtain peak lactic acid production and bacterial growth were studied. The optimal fermentation conditions were 10% inoculum, 15% sucrose and 85% crab biomass, producing 17 mg lactic acid/ g silage. Extracted and purified chitin, after 60 h fermentation, showed 99.6 and 95.3% demineralization and deproteinization, respectively, using low concentrated acids and bases. As a means of comparison, chitin was also extracted by chemical hydrolysis using high concentrated acids and bases, giving a lower yield and lower quality product.
1. Introduction
The crab Allopetrolisthes punctatus is a crustacean that lives in the coast area between Ancud (Perú) and the North of Arauco (Chile). Its population is particularly abundant at San Vicente (Chile), feeding on commercially important species and, therefore, causing a negative impact in small size fisheries. Since this crab has a very small size for commercial application, an alternative use is as a chitin source, extracted from the exoskeleton.
Chitin is a natural, insoluble cationic polysaccharide formed by a repetition of N-acetyl-d-glucosamine dimers. It is the second most abundant biopolymer in nature, only after cellulose [1,2]. Commercial interest in chitin has increased due to its outstanding properties as a biocompatible material, as well as its adsorption and chelating ability. Consequently, it is a key element in functional and innovative material production [3].
Chitin is commercially extracted from the exoskeleton of crustaceans, shrimp and prawns included, although there are published reports on chitin isolation from crabs, such as Callinectes sapidus and Chionoecetes opilio [4], as well as Podophthalmus vigil [5].
The most common industrial method to extract chitin from marine sources is by the addition of concentrated acid solutions in order to dissolve minerals (mainly calcium carbonate), and subsequent alkaline protein hydrolysis with concentrated basic solutions [6]. Protein and minerals are almost completely removed with this process [7]. However, the acid-alkaline process also results in chitin deacetylation and production of low molecular oligomers, reducing chitin quality. Besides, it reduce protein functional quality and increase pigment destruction [8]. In addition, the acid-alkaline process is highly environmentally unfriendly [9].
An alternative to the chemical process, based on lactic fermentation, has been reported by several authors [10]. Protein hydrolysis can be carried out by proteolytic enzymes produced by the inoculated lactic acid bacteria, or present in the waste and activated by the low pH of the medium [11]. This process has the advantage of allowing the recovery of enzymes, proteins and pigments for further application in the food industry [12].
The objective of the present work was to extract chitin by lactic fermentation from A. punctatus. The inoculum was Lactobacillus plantarum sp. 47 (LPS47), isolated from Coho salmon. L. plantarum sp. 47 is a Gram positive, catalase negative, obligate heteromefermentative bacteria, that showed to produce high lactic acid concentrations in De Man, Rogosa and Sharpe agar medium culture [13]. Consequently, LPS47 can be an adequate alternative to elaborate a silage with Allopetrolisthes punctatus raw material, for its conservation and chitin extraction. Also, LPS47 is a probiotic, therefore the process allows obtaining chitin and to recover soluble protein and a probiotic from the fermentation broth, with possible application as fish feed.
2. Materials and methods
2.1. Inoculum preparation
L. plantarum sp.47 was isolated from Coho Salmon (DSM 23602, deposited I DSMZ-Deutsche Sammlung von Mikro-organismen und Zellkulturen GmbH, Germany). The isolated strain, stored at −20 °C, was activated using a commercial de Man, Rogosa and Sharpe agar (MRS) medium [14] and incubated for 24 h at 32 °C. Then, it was cultured under the same conditions in two successive MRS transfers at 32 °C for 24 h. The fermentation broth was centrifuged and the pellet was resuspended in saline solution until obtain 108 CFU mL−1, measured by spectrophotometry at 540 nm.
2.2. Silage elaboration
Crabs were collected at San Vicente (VIII Region, Chile), frozen at −20 °C and transported to our laboratory at Universidad de Concepción. This biomass was frozen until used. The crabs were thawed and ground in a meat grinder; final particle size was approximately 5 mm. Molasses, glucose or sucrose was added as carbon source at 5, 10, 15 or 20% w/w. Biomass pH was adjusted with acetic acid to pH 6 ± 0,1, inoculated with L. plantarum sp.47 (0, 5, 10 or 20% v/w), and incubated at 32 °C.
2.3. Analysis of samples
The bacterial population was determined by total viable count. Kinetics modelling was carried out through the Gompertz equation. pH was measured using a potentiometer (Hanna HI9126, Rhode Island, USA). Lactic acid concentration during the fermentation stationary phase was determined by total titratable acids (TTA), as reported by Pacheco et al. [15].
2.4. Chitin extraction and purification
Chitin was obtained at 0, 24, 48 and 60 h of fermentation. Silage was diluted with distilled water and subsequently filtered, retaining the solid fraction. The obtained solids were bleached using methanol- chloroform-water solution (1:2:4). Crude chitin was purified following the method reported by Cira et al. [16].
2.5. Chemical extraction chitin
The chemical hydrolysis method, reported by Sierra et al. [17] was also studied. Previously, crab biomass was ground and washed with distilled water to remove the protein fraction. The solid fraction was treated with 3,5 % NaOH solution at 95 °C. It was then filtered and neutralized with distilled water. Demineralization was carried out using 2 N HCl solution. Finally, purification was performed with NaOH 2% at 100 °C for 1 h. Samples were filtered, washed and dried at 70 °C.
2.6. Chitin analysis
Moisture was determined by gravimetry. Fat percentage was measured by distillation with diethyl ether using a Soxhtec HT1043 equipment. Ash was determined by calcination in muffle at 550 °C. Total protein nitrogen was determined by Kjedhal method [18]. Chitin percentage was obtained using the method reported by Black and Schwartz [19] method.
The purification yield was obtained from Eq. (1):
| (1) |
where: X0 and XR are protein or ash content percentages in raw and fermented samples, respectively. S0 and SR are raw and fermented sample weight (g), respectively. Total extraction yield (%YT) was calculated according to Eq. (2):
| (2) |
Where SR is weight of recovered chitin, and S0 is initial raw material weight.
2.7. Chitin physicochemical characterization
FTIR analysis was carried out using IR spectroscopy in a Thermo-Nicolet NEXUS equipment (USA, DTGS Detector, 64 scan, resolution 2). Samples were treated with potassium bromide (KBr) and absorbance was measured from 450 to 4000 cm−1 wavelength. The degree of acetylation was calculated from the correlation reported by Sannan et al. [20] (Eq. (3):
| (3) |
The molecular weight was calculated from the sample intrinsic viscosity. Chitin (0,08% w/v) was mixed with dimethylacetamide (DMAc) and 5% LiCl, stirred at 40 °C, and placed in Cannon Fenske capillary tubes (size 150) in a water bath at 30 °C. a and K constants for Mark-Houwink Sakurada equation, were 0.71 dlg−1 and 8,93 × 10-4 respectively [21].
2.8. Experimental design and statistical analysis
The sources of variation were: 1) Carbon source (glucose, molasses and sucrose), at 10%; the inoculum was fixed at 10% (v/w); 2) Sucrose-raw material ratio at 5, 10, 15, 20% (w/w); the inoculum was fixed at 10% (v/w); 3) Inoculum (0–20%), constant sucrose-raw material ratio. 3) Temperature (25 °C–32 °C); surcrose concentration and inoculum were fixed at 15% (w/w) and 10% (v/w), respectively. The response variables were: pH, total titrable acidity and bacterial growth. All experiments were carried out in triplicate. Crude chitin extracted was studied at different fermentation times (0, 24, 48 and 60 h) for %DP and %DM determination.
Data were subjected to ANOVA, 95% confidence interval, using a SPSS 22 package, and Tukey test [22] for multiple comparisons between three or more tests, 95% confidence interval.
3. Results and discussion
3.1. Effect of carbon source
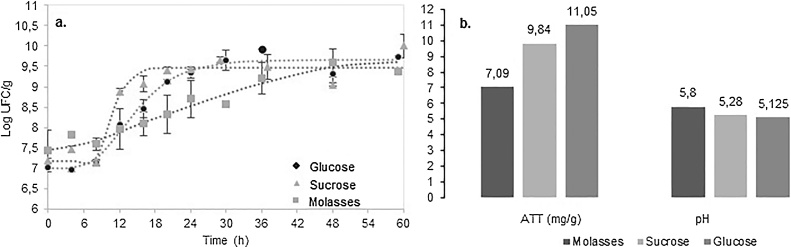
Significantly different growth rates were observed in treatments added with sucrose as compared to molasses (Fig. 1a) (p < 0.05). Although the lag phase in media with glucose and sucrose started at the same time (approximately 9 h), microbial growth rate in the glucose-containing medium is lower. This was probably due to the ability of the selected strain to hydrolyze glucosyl-fructose β-union, metabolizing both monomers more efficiently than glucose. However silages with glucose and sucrose had similar pH and TTA (p > 0.05) unlike biomass-containing molasses that result in lower lactic acid concentration (Fig. 1b).
Fig. 1.
a. Kinetics of L. plantarum LPS47 in silages with different carbon sources (● glucose,  sucrose,
sucrose,  molasses) b. Peak lactic acid concentration (mg/g) and minimum pH in silages with different carbon sources.
molasses) b. Peak lactic acid concentration (mg/g) and minimum pH in silages with different carbon sources.
L. plantarum LPS47 basic requirement for acid production is abundant sugar availability; slow acid production allows spoilage microorganisms to growth. Sucrose and glucose fulfill the strain’s sugar need. Conversely, poor acidification was observed in biomass mixed with molasses. Although molasses contain high sucrose concentration [23], its thickness and high viscosity did not allow the media to become fully incorporated into the silage, the carbon source was not easy reached by the bacteria resulting in slow carbon metabolism. Due to the lower cost of sucrose as compared to glucose, further studies were carried out using sucrose as carbon source.
3.2. Effect of carbon source concentration
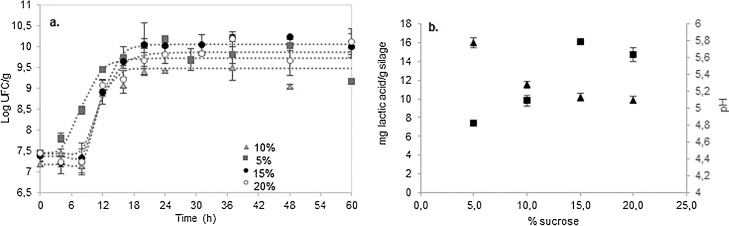
L. plantarum LPS47 followed similar growth rate in all treatments (Fig. 2a). Nonetheless, silages with 15–85% (sucrose-raw material) had the highest TTA and lowest pH was observed (Fig. 2b). Higher TTA and pH values were observed at 30 h of incubation. Furthermore this values remained constant during the whole fermentation period.
Fig. 2.
a. Kinetic curves of L. plantarum LPS47 in silages with different sucrose concentrations ( 10%,
10%,  5%, ○ 20%, ● 15%) b. Maximum lactic acid concentration (■) and and minimum pH (▲) in silages with different sucrose percentages.
5%, ○ 20%, ● 15%) b. Maximum lactic acid concentration (■) and and minimum pH (▲) in silages with different sucrose percentages.
3.3. Inoculum effect
As negative control, no inoculum was added to silage with 15–85% (sucrose raw material), generating 312 mg of lactic acid/g of silage and pH 665 at 24 h of incubation. Also, the silage presented spoilage signs, therefor it was discarded.
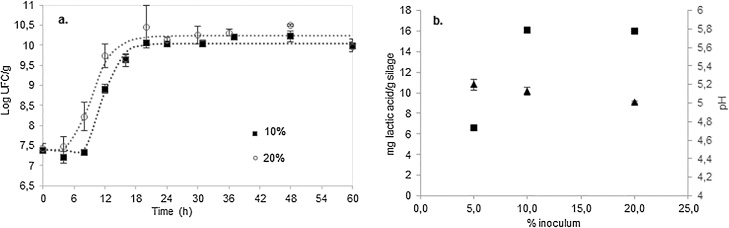
At 5% inoculum, L. plantarum LPS47 did not inhibit the growth of the native microbiota in crab. Even though, growth rate, pH and TTA were not significantly different at 10 and 20% inoculum (p > 0.05) (Fig. 3a and b), the lag phase lasted 8,9 and 5,8 h for 10 and 20% inoculum, respectively. Similar results were reported by Stuardo [24], who demonstrated that, even doubling the inoculum concentration of strain LPS47, the maximum growth rate remained constant. We concluded that 10% inoculum was the minimum required to obtain the maximum growth rate and acid production, as well as to inhibit pathogens and to prevent spoilage.
Fig. 3.
a. Kinetic curves of L. plantarum LPS47in silages with different inoculum percentages (■ 10%, ○ 20%). b. Maximum lactic acid concentration (■) and minimum pH (▲) achieved in silages with different inoculum percentages.
3.4. Temperature effect
Temperature effect was studied under the conditions previously determined for providing the highest growth rate and peak lactic acid production. L. plantarum LPS47 optimum growth temperature is 32 °C, although growth at 25 °C has been also reported [14]. Lower fermentation temperature means a reduction in the process cost. However, according to Foo et al. [24] the optimum growth temperature for Lactobacillus sp. is between 30 and 40 °C. When lactic fermentation was carried out at 25 °C, slower growth and reduced lactic acid production (3,72 mg/ g) was observed as compared to 32 °C. Therefore, silages underwent spoilage at a lower fermentation temperature.
Finally, it can be suggested that optimum parameters for Allopetrolisthes punctatus silage using L. plantarum sp.47 are 85% w/w raw material, 15% w/w sucrose, 10% inoculum, incubation at 32 °C for at least 30 h. At this conditions, LPS47 can growth and produce it maximum lactic acid concentration, inhibiting spoilage and demineralizing crab exoskeleton. This result may be due to the capacity of the strain LPS47 to metabolize sucrose quickly, achieving in 30 h of fermentation, highest pH and TTA values. The strain hydrolyze glucosyl-fructose β-union and generate lactate from α-glucopyrasose and β-fructofurase, which releases to the silage as lactic acid. Besides, at this time pH and TTA values remains constant, probably due to the demineralization process, which releases salts by reaction of lactic acid and calcium carbonate, to produce calcium lactacte. The strain continues metabolizing sucrose to form lactic acid, but increasing of free salts concentration generates a buffer effect, keeping constant pH and TTA values.
3.5. Extraction time effect
The solid fraction containing chitin was obtained after ensilation, bleached and purified according to the method reported by Cira et al. [16]. Table 1 shows a decrease in protein and ash percentages as the fermentation time increased. However, after 48 h fermentation protein content in the solid fraction did not significantly changed. Similarly, %DP remain constant after 48 h, but %DM reached a maximum at 60 h while chitin content increased with fermentation time. It was concluded that the 60 h was the optimal fermentation time.
Table 1.
Chitin composition during ensilation process.
| 0 (control) | 0 | 24 | 48 | 60 | |
|---|---|---|---|---|---|
| Moisture (%) | 2,5 | 2,0 | 2,9 | 2,85 | 2,11 |
| Fat (%) | 1,4 | 0,7 | 3,35 | 1,13 | 4,05 |
| Ashes (%) | 66,4 | 57,3 | 42,1 | 34,0 | 2,02 |
| Proteins (%) | 13,6 | 10,6 | 13,3 | 2,08 | 4,26 |
| Chitin (%) | 7,9 | 23,9 | 34,8 | 66,6 | 93,4 |
| %DM | 0 | 64,3 | 83,6 | 80,5 | 99,6 |
| %DP | 0 | 67,7 | 74,7 | 95,5 | 95,3 |
Rao et al. [26] reported that chitin extracted from silages with extended fermentation time had more mineral removal but the protein content remained constant after 24 h.
3.6. Effect of hydrolysis type on chitin yield
The chitin yield was also affected by the hydrolysis method, lactic acid fermentation or chemical hydrolysis. Moisture, ash and protein percentage in chitin obtained by lactic fermentation and chemical treatment were not significantly different, although chemical hydrolysis reduced fat content (Table 2). Demineralization yield also showed similar percentages: 99.9 and 99.6% in lactic fermentation and chemical hydrolysis, respectively. Conversely, %DP in chitin extracted by the chemical method was lower (91.7%) than by lactic fermentation (95.3%), probably due to the presence of protein remaining in the crab waste after washing the raw material. No et al. [27] and Armenta et al. [28] concluded that proteins are covalently bound to chitin reducing the effectiveness of deproteinization.
Table 2.
Composition and yield of chitins obtained by lactic fermentation and chemical hydrolysis methods.
| Lactic fermentation | Chemical hydrolysis | |
|---|---|---|
| Moisture (%) | 2,11 | 3,54 |
| Fat (%) | 4,05 | 0,16 |
| Ash (%) | 2,02 | 0,33 |
| Protein (%) | 4,26 | 7,36 |
| Chitin (%) | 93,35 | 86,71 |
| %DM | 99,55 | 99,87 |
| %DP | 95,33 | 91,73 |
| Total yield (%) | 6,93 | 7,47 |
Preliminary lactic fermentation of crab waste allowed the chemical hydrolysis using milder conditions, as well as removal of protein and minerals. Pacheco et al. [15] reported yields from 63 to 98.8% (%DP) and 61 to 99.7% (%DM). Inoculating two strains, Lactobacillus paracasei and Serratia marcescens in co-fermentation of crab waste, Jung et al. [10] obtained 94.3 and 68.9% (%DM and DP% respectively). From these data, it was concluded that the process studied in this work gave high extraction yields.
3.7. Total extraction yields
Based on crab weight, we obtained 7.5% yield by chemical hydrolysis and 6.9% yield by lactic fermentation. However, these yields were lower as compared to the ones reported in the literature: 20, 30 and 45% [10,15,29]. It is important to take into account that total chitin percentage in Allopetrolisthes punctatus is 14.2%, therefore 50% is lost during the purification processes.
3.8. Comparison of physicochemical characteristics between lactic fermentation methods and chemical hydrolysis
The degree of deacetylation (DD) of chitin extracted by lactic fermentation and chemical method was determined in order to compare the biopolymers quality. In the deacetylated chitin form, chitosan, the acetyl group attached to carbon 2 in the monomer is eliminated, leaving a free amino group. Since not all acetyl groups are removed, DD is an average of the acetyl groups removed. Chitins have DD less than 40%, whereas commercial chitosans have more than 60%, resulting in considerably different properties such as solubility in diluted acids, biodegradability, biocompatibility, adhesion, film forming, and antimicrobial and antioxidant properties, among others.
Chitins obtained by the biological method had 5.95% DD, however the biopolymer extracted by chemical hydrolysis had 5.97% DD. DD values generally are between 2 and 8% [8,30]. Particularly, chitins obtained from crabs have approximate 5%, which is in agreement with our results. These figures also indicate that obtained chitin was not significantly altered, even after a chemical treatment; reducing DD. Subsequent deacetylation can be carried out to obtain chitosan for specific applications.
The molecular weight of chitins obtained from each extraction method was also calculated. Chitin obtained by chemical hydrolysis had 3,34 E + 5 gmol−1 molar mass; whereas chitin extracted by lactic fermentation had 9,67E + 5 gmol−1. The mass difference could be caused by the treatment with a strong acid and base, used in the chemical method, resulting in chitin depolymerization [31]. Pacheco et al. [8] reported 1,6E + 6 gmol−1 in chitin extracted by lactic fermentation, whereas Pillai et al. [32] indicated that the molecular weight of natural chitin ranges from 1,03 to 2,5E + 6 gmol−1. Furthermore, commercial chitin extracted by chemical methods has 6,4E + 5 gmol-1 molecular weight [33]. As mentioned before, chitin extracted by the chemical method had lower molecular weight, reducing the polymer quality and its subsequent application. Therefore, the chitin obtained by the biological method preserves its native physicochemical properties unlike the product obtained by the chemical method.
4. Conclusions
L. plantarum strain 47 (LPS47) is able to ferment crab solid waste generating lactic acid as a fermentation product. In addition, the most efficient silage with respect to kinetic parameters, metabolite production and the use of minimum input was a silage with 10% inoculum, 15% sucrose and 85% of crustacean waste, generating 17 mg of lactic acid / g silage. To achieve the maximum extraction yield and to keep chitin physicochemical properties, it is recommended to ferment the biomass for 60 h, with a subsequent solid fraction purification with 0.4 M NaOH and 0.5 M HCl. The chitin extracted by the chemical method had lower extraction yield and reduced molecular weight than chitin obtained by the biological method. Therefore, application of lactic fermentation is a viable, economic and ecological way to extract high quality chitin, also allowing to recovery proteins and probiotics that can be used as ingredients for aquaculture diets.
Declaration of interest
All authors disclose there is not any financial and personal relationships with other people or organizations that could inappropriately influence their work, as employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Acknowledgement
This study was supported by FONDEF (Grant D13R20011).
References
- 1.Aytekin O., Elibol M. Cocultivation of Lactococcus lactis and Teredinobacter turnirae for biological chitin extraction from prawn waste. Bioprocess Biosyst. Eng. 2009;33:393–399. doi: 10.1007/s00449-009-0337-6. [DOI] [PubMed] [Google Scholar]
- 2.Tzoumaki M.V., Moschakis T., Scholten E., Biliaderis C.G. In vitro lipid digestion of chitin nanocrystal stabilized o/w emulsions. Food Funct. 2013;4:121–129. doi: 10.1039/c2fo30129f. [DOI] [PubMed] [Google Scholar]
- 3.Barber P.S., Shamshina J.L., Rogers R.D. A “green” industrial revolution: using chitin towards transformative technologies. Pure Appl. Chem. 2013;85:1693–1701. [Google Scholar]
- 4.Hayes M., Carney B., Slater J., Brück W. Mining marine shellfish wastes for bioactive molecules: chitin and chitosan – part a: extraction methods. Biotechnol. J. 2008;3:871–877. doi: 10.1002/biot.200700197. [DOI] [PubMed] [Google Scholar]
- 5.Das S., Ganesh A. Extraction of chitin from trash crabs (Podophthalmus vigil) by an eccentric method. Curr. Res. J. Biol. Sci. 2010;2:72–75. [Google Scholar]
- 6.Tharanathan R.N., Kittur F.S. Chitin—the undisputed biomolecule of great potential. Crit. Rev. Food. Sci. Nutr. 2003;43:61–87. doi: 10.1080/10408690390826455. [DOI] [PubMed] [Google Scholar]
- 7.Percot A., Viton C., Domard A. Optimization of chitin extraction from shrimp shells. Biomacromolecules. 2003;4:12–18. doi: 10.1021/bm025602k. [DOI] [PubMed] [Google Scholar]
- 8.Pacheco N., Garnica-González M., Gimeno M., Bárzana E., Trombotto S., David L. Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules. 2011;12:3285–3290. doi: 10.1021/bm200750t. [DOI] [PubMed] [Google Scholar]
- 9.Armenta R.E., Guerrero-Legarreta I. Amino acid profile and enhancement of the enzymatic hydrolysis of fermented shrimp carotenoproteins. Food Chem. 2008;112:310–315. [Google Scholar]
- 10.Jung W., Jo G., Kuk J., Kim K., Park R. Extraction of chitin from red crab shell waste by cofermentation with Lactobacillus paracaseisubsp. tolerans KCTC-3074 and Serratia marcescens FS-3. Appl. Microbiol. Biotechnol. 2006;71:234–237. doi: 10.1007/s00253-005-0126-3. [DOI] [PubMed] [Google Scholar]
- 11.Mukundan M., Antony P., Nair M. A review on autolysis in fish. Fish Res. 1986;4:259–269. [Google Scholar]
- 12.Xu Y., Gallert C., Winter J. Chitin purification from shrimp wastes by microbial deproteination and decalcification. Appl. Microbiol. Biotechnol. 2008;79:687–697. doi: 10.1007/s00253-008-1471-9. [DOI] [PubMed] [Google Scholar]
- 13.Toledo N., Ferrer J., Bórquez R. Drying and storage stability of a probiotic strain incorporated into a fish feed formulation. Drying Technol. 2010;28:508–516. [Google Scholar]
- 14.de Man J.D., Rogosa M., Sharpe M.E. A medium for the cultivation of Lactobacilli. J Appl. Bact. 1960;23:130–135. [Google Scholar]
- 15.Pacheco N., Garnica-González M., Ramírez-Hernández J.Y., Flores-Albino B., Gimeno M., Bárzana E. Effect of temperature on chitin and astaxanthin recoveries from shrimp waste using lactic acid bacteria. Bioresour. Technol. 2009;100:2849–2854. doi: 10.1016/j.biortech.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Cira L., Huerta S., Hall G.M., Shirai K. Pilot scale lactic acid fermentation of shrimp wastes for chitin recovery. Process. Biochem. 2002;37:1359–1366. [Google Scholar]
- 17.Sierra D., Orozco C., Rodríguez M., Villa W. Optimización de un protocolo de extracción de quitina y quitosano desde caparazones de crustáceos. Scientia et Technica. 2013;18:260–266. [Google Scholar]
- 18.A.O.A.C. Official methods of analysis . 2000. Association of Official Analytical Chemists. Washington DC. [Google Scholar]
- 19.Black M.M., Schwartz H.M. The estimation of chitin and chitin nitrogen in crawfish waste and derived products. Analyst. 1950;75:185–189. [Google Scholar]
- 20.Sannan T., Kurita K., Ogura K., Iwakura Y. Studies on chitin: 7. IR spectroscopic determination of degree of deacetylation. Polymer. 1978;19:458–459. [Google Scholar]
- 21.Rathke T.D., Hudson S.M. Review of chitin and chitosan as fiber and film formers. J. Macromol. Sci. Polym. Rev. 1994;34:375–437. [Google Scholar]
- 22.Tukey J.W. Comparing individual means in the analysis of variance. Biometrics. 1949;5:99–114. [PubMed] [Google Scholar]
- 23.Swan H., Karalazos A. Las melazas y sus derivados. Rev. Tecn. Geplacea. 1990;19:78–82. [Google Scholar]
- 24.Stuardo C. Universidad de Concepción; Chile: 2009. Fermentación en sustratos sólidos por bacterias lácticas aisladas de salmón, Tesis para optar al título de Bioingeniero. [Google Scholar]
- 26.Rao M., Munoz J., Stevens W. Critical factors in chitin production by fermentation of shrimp biowastes. Appl. Microbiol. Biotechnol. 2000;54:808–813. doi: 10.1007/s002530000449. [DOI] [PubMed] [Google Scholar]
- 27.No H.K., Meyers S.P., Lee K.S. Isolation and characterization of chitin from crawfish shell waste. J. Agric. Food Chem. 1989;37:575–579. [Google Scholar]
- 28.Armenta R., Guerrero-Legarreta I., Huerta S. Astaxanthin extraction from shrimp waste by lactic fermentation and enzymatic hydrolysis of the carotenoprotein complex. J. Food Sci. 2002;67:1002–1006. [Google Scholar]
- 29.Flores-Albino B., Arias L., Gómez J., Castillo A., Gimeno M., Shirai K. Chitin and L (+)-lactic acid production from crab (Callinectes bellicosus) wastes by fermentation of Lactobacillus sp. B2 using sugar cane molasses as carbon source. Bioprocess Biosyst. Eng. 2012;35:1193–1200. doi: 10.1007/s00449-012-0706-4. [DOI] [PubMed] [Google Scholar]
- 30.Pacheco N. Universidad Autónoma Metropolitana; México: 2010. Extracción biotecnológica de quitina para la producción de quitosanos: caracterización y aplicación, Tesis para obtener el grado de Doctor en Biotecnología. [Google Scholar]
- 31.Kjartansson G., Zivanovic S., Kristbergsson K., Weiss J. Sonication-assisted extraction of chitin from North Atlantic shrimps (Pandalus borealis) J. Agric. Food Chem. 2006;54:5894–5902. doi: 10.1021/jf060646w. [DOI] [PubMed] [Google Scholar]
- 32.Pillai C.K.S., Paul W., Sharma C.P. Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009;34:641–678. [Google Scholar]
- 33.Islas R. Universidad Autónoma Metropolitana; México: 2010. Tratamiento microbiano de residuos de camarón para la obtención de quitina y astaxantina, Tesis para obtener el grado de maestro en Biotecnología. [Google Scholar]