Abstract
Dysregulated long noncoding RNAs (lncRNAs) and microRNAs (miRNAs) mediating chemotherapeutic drug effects and metastasis in pancreatic cancer (PC) are key reasons for the poor prognosis of this disease. lncRNA growth arrest-specific 5 (GAS5) is reported to be a tumor suppressor in multiple cancers. However, the functions of GAS5 and its related miRNAs in PC are poorly understood. This study explored the potential functions and mechanisms of GAS5 in PC gemcitabine resistance and metastasis. The results show that overexpression of GAS5 suppressed the proliferation, migration, gemcitabine resistance, stem cell-like properties, and epithelial-mesenchymal transition (EMT) of PC cells by directly binding to and suppressing miR-221 expression and enhancing suppressor of cytokine signaling 3 (SOCS3) expression. The effects of miR-221 overexpression on proliferation, migration, gemcitabine resistance, stem cell-like properties, and EMT inhibition were reversed by SOCS3 overexpression in PC cells. Additionally, GAS5 promoted gemcitabine-induced tumor growth and metastasis inhibition, as determined by Ki-67 staining and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), bioluminescence imaging, and the detection of cell-like properties and EMT in vivo. Thus, lncRNA GAS5 functioned as a competing endogenous RNA for miR-221, and it suppressed cell growth, metastasis, and gemcitabine resistance in PC by regulating the miR-221/SOCS3 pathway mediating EMT and tumor stem cell self-renewal.
Keywords: lncRNA GAS5, miR-221, SOCS3, pancreatic cancer, gemcitabine resistance
Introduction
One of the most common lethal malignancies is pancreatic cancer (PC), with an overall 5-year survival rate less than 5% due to the advanced stage of disease at initial diagnosis, frequent recurrence, and the lack of effective therapies.1 Surgical resection and chemotherapy are considered to be the main treatment options for PC.2 Currently, gemcitabine-based chemotherapy forms first-line treatment for PC.3 However, chemotherapy resistance seriously impedes PC treatment. Increasing evidence indicates that the epithelial-to-mesenchymal transition (EMT) and accumulation of cancer stem cells (CSCs) are important contributors to PC chemoresistance, recurrence, and metastasis.4, 5, 6 Previous studies found that suppressor of cytokine signaling 3 (SOCS3) is an important tumor suppressor, which is often aberrantly inactivated in various tumors.7, 8 Overexpression of SOCS3 significantly suppressed EMT9 and enhanced chemosensitivity.10 It has also been found that SOCS3 is involved in CSC regulation11 and influences gemcitabine resistance and metastasis, although the underlying molecular mechanisms involved in these processes remain unclear.
Long noncoding RNAs (lncRNAs) of more than 200 nt modify chromatin, and they act as transcriptional or post-transcriptional regulators of gene expression. lncRNAs are involved in many biological processes, such as the cell cycle, cell differentiation, and apoptosis. Dysregulated lncRNAs have oncogenic or tumor-suppressive effects in multiple cancers.12, 13, 14 lncRNA growth arrest-specific 5 (GAS5) is one of the most common noncoding RNAs, and it has been reported to be involved in tumor proliferation, migration, and the EMT in osteosarcoma.15 The regulatory mechanisms of GAS5 in PC are still unknown; therefore, we initiated this study to determine if GAS5 could regulate chemoresistance, recurrence, and metastasis of PC by controlling the EMT and CSCs.
MicroRNAs (miRNAs) are small, non-protein-coding RNAs. Increasing evidence indicates that miRNAs are important in carcinogenesis through post-transcriptional gene silencing. Dysregulation of miRNAs is involved in many cancers, and some miRNAs function as tumor suppressors or oncogenes.16, 17 miR-221 has been identified as an oncogene in many types of cancers, including colorectal cancer,18 breast cancer,19 and osteosarcoma.20 Bioinformatics analysis found that miR-221 was a target of GAS5. GAS5 and miR-221 are suggested to interact with each other to regulate chemoresistance, recurrence, and metastasis of PC. However, the precise mechanisms remain unknown and require further investigation.
Therefore, the aim of this study was to identify the regulatory mechanism of GAS5/miR-221 in chemoresistance, recurrence, and metastasis of PC. Specifically, we investigated if the regulatory effect involved the EMT and CSCs. Our data may provide new directions for the future development of potential molecular therapies aimed at improving therapeutic outcomes for PC patients.
Results
Identifying the Expression of GAS5, miR-221, and SOCS3 in Human PC
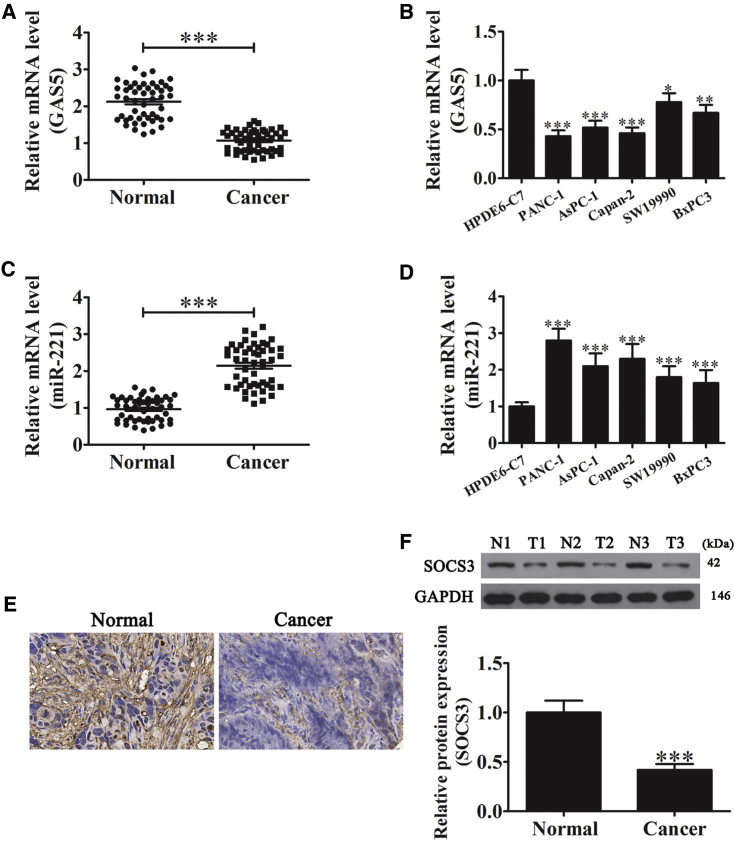
Chemotherapy resistance, recurrence, and metastasis are important reasons for the poor prognosis of PC. Increasing evidence shows that noncoding RNAs (including lncRNAs and miRNAs) are crucial for tumorigenesis and tumor progression.21, 22 Previous studies have shown that lncRNA GAS5 expression was downregulated in gastric cancer; furthermore, upregulation of GAS5 expression is known to suppress cell proliferation.23 In this study, we found that expression of GAS5 was downregulated in PC tissue compared with adjacent normal pancreatic tissues (Figure 1A). In addition, GAS5 expression levels in the PC cell lines PANC-1, AsPC-1, Capan-2, SW19990, and BxPC3 were significantly downregulated compared with normal pancreatic epithelial cells (HPDE6-C7; Figure 1B). The results suggested that downregulation of GAS5 was involved in the poor prognosis of PC.
Figure 1.
Expression of GAS5, miR-221, and SOCS3 in Human PC
(A) RT-PCR of GAS5 expression in pancreatic tissues. Data are means ± SD (n = 3). ***p < 0.001 versus normal group. (B) RT-PCR of GAS5 in human normal pancreatic epithelial cells (HPDE6-C7) and five pancreatic cancer cell lines (PANC-1, AsPC-1, Capan-2, SW19990, and BxPC3). Data are means ± SD (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001 versus HPDE6-C7 group. (C) RT-PCR of miR-221 in pancreatic tissues. Data are means ± SD (n = 3). ***p < 0.001 versus normal group. (D) RT-PCR of miR-221 in human normal pancreatic epithelial cells (HPDE6-C7) and PC cell lines (PANC-1, AsPC-1, Capan-2, SW19990, and BxPC3). Data are means ± SD (n = 3). ***p < 0.001 versus HPDE6-C7 group. (E) IHC staining for SOCS3 in human PC (right) and paired adjacent noncancerous tissues (left). Original magnification, 400×. (F) Western blots of SOCS3 in pancreatic tissues. Data are means ± SD (n = 3). ***p < 0.001 versus normal group. T, tumor; N, normal.
Bioinformatics analysis (http://starbase.sysu.edu.cn/) found that miR-221 was the target of GAS5. Increasing evidence has shown that miR-221 promotes carcinogenesis, including cervical cancer,24 osteosarcoma,20 and lung cancer.25 We found that miR-221 was upregulated in both PC tissues and cell lines (Figures 1C and 1D). In addition to noncoding RNA, we detected the expression of SOCS3, which was decreased in PC tissues (Figures 1E and 1G). Our study found that downregulation of GAS5 promoted the miR-221/SOCS3-mediated progression of PC.
Overexpression of GAS5 Inhibits Xenograft Tumor Growth, Metastasis, and Gemcitabine Resistance
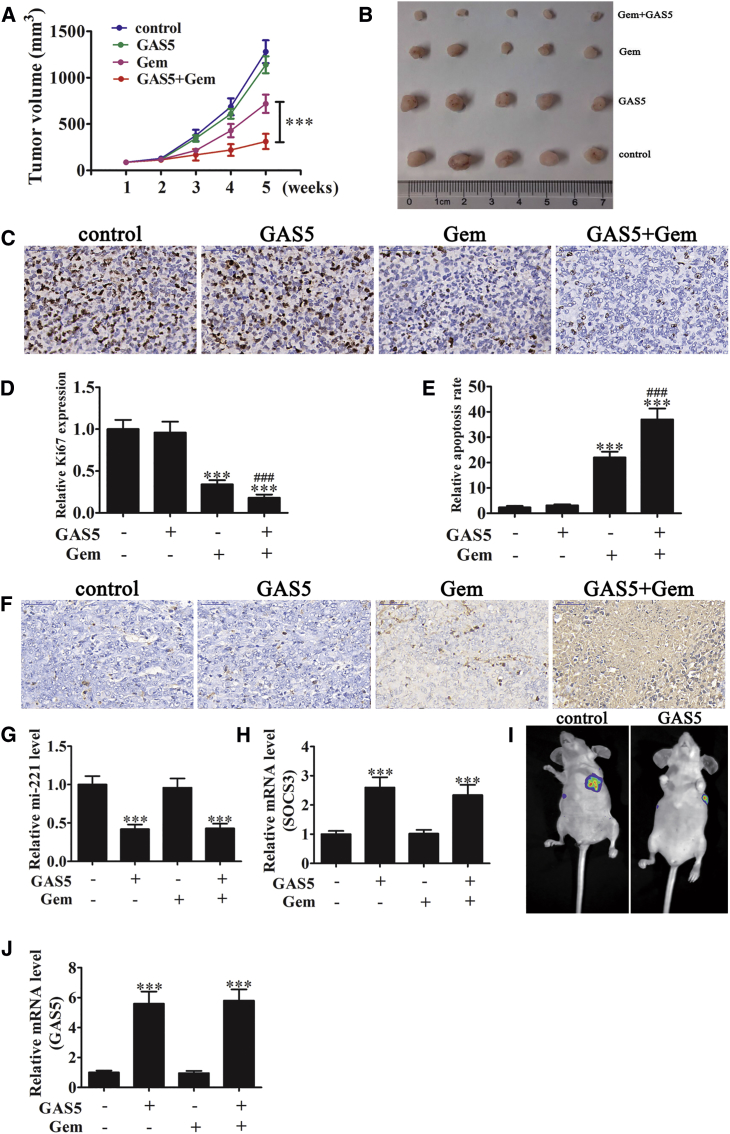
To identify if GAS5 overexpression reversed the promotion of tumor growth, metastasis, and gemcitabine resistance, a GAS5 overexpression vector was constructed and transfected into PANC-1 cells (Figure 3A), followed by injection of 5 × 106 viable cells into the right flanks of nude mice. Beginning 7 days after inoculation, mice received weekly intraperitoneal injections of either gemcitabine (15 mg/kg) or saline (100 μL, negative control [NC]). Tumor sizes were measured every 7 days.
Figure 3.
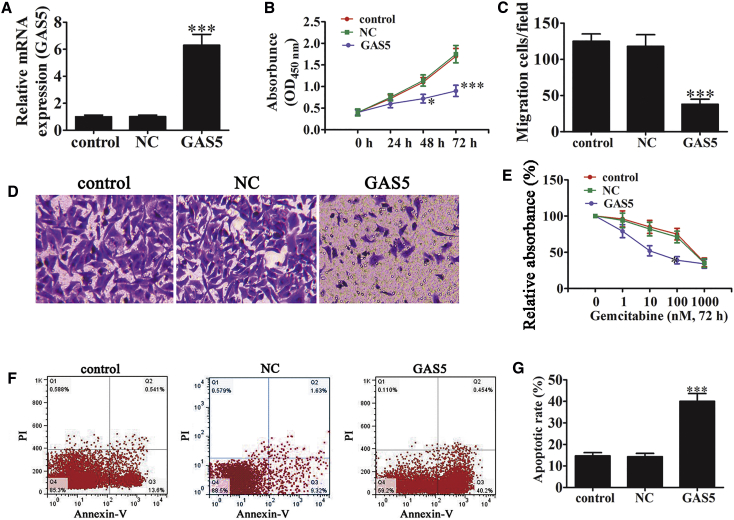
Overexpression of GAS5 Inhibits Cell Growth, Migration, and Chemotherapy Resistance In Vitro
(A) RT-PCR of GAS5 expression after transfection with a GAS5 overexpression vector. Data are means ± SD (n = 3). ***p < 0.001 versus control group. (B) Cell proliferation was examined by CCK8 assays. Data are means ± SD. *p < 0.05 and ***p < 0.001 versus control. (C) The relative migration cells were analyzed. (D) Transwell analyses showed the effects of GAS5 on PANC-1 cell migration (magnification, 200×). Data are means ± SD. ***p < 0.001 versus control. (E) Comparison of cell viability and IC50 of gemcitabine in PANC-1 cells with or without GAS5 overexpression. (F) Effect of GAS5 on gemcitabine-induced apoptosis detected using annexin V-PI double staining after exposure to 10 nM gemcitabine for 72 hr. (G) The relative apoptosis cells were calculated. Data are means ± SD. ***p < 0.001 versus control.
GAS5 overexpression significantly increased the inhibition of tumor growth by gemcitabine (Figures 2A and 2B). Immunohistochemistry (IHC) analysis confirmed that GAS5 overexpression suppressed Ki67 expression, which represents tumor proliferation ability (Figures 2C and 2D). Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) showed that GAS5 overexpression (Figure 2J) promoted gemcitabine-induced tumor cell apoptosis (Figures 2E and 2F). RT-PCR assays demonstrated that GAS5 overexpression inhibited miR-221 expression (Figure 2G), but it increased SOCS3 expression (Figure 2H). Live-imaging experiments showed that GAS5 overexpression suppressed metastasis of PANC-1 cells 5 weeks after tail intravenous injection (Figure 2I). Our study thus found that GAS5 overexpression inhibited xenograft tumor growth, metastasis, and gemcitabine resistance. This inhibitory effect may be involved in miR-221 and SOCS3 regulation.
Figure 2.
Overexpression of GAS5 Inhibits Xenograft Tumor Growth, Metastasis, and Gemcitabine Resistance
(A) Tumor growth curves of PANC-1 cells transfected with or without a GAS5 overexpression vector, treated with gemcitabine (125 mg/kg) or saline. Data are means ± SD (n = 5). ***p < 0.001. (B) Representative images of tumors from four groups at 5 weeks after subcutaneous transplantation or when mice were euthanatized. (C) Ki67 staining shows tumor growth. (D) The relative Ki67 expression was analyzed. Data are means ± SD (n = 5). ***p < 0.001 versus control; ###p < 0.001 versus gemcitabine treatment group. (E) The relative apoptosis rate was analyzed. (F) TUNEL analysis of four tumor xenografts groups. Data are means ± SD (n = 5). ***p < 0.001 versus control; ###p < 0.001 versus gemcitabine treatment group. (G and H) RT-PCR for miR-221 (G) and SOCS3 (H) in tumor tissue. Data are means ± SD (n = 5). ***p < 0.001 versus control group. (I) Live imaging of the effects of GAS5 overexpression on metastasis of PANC-1 cells 5 weeks after intravenous tail injection. (J) RT-PCR detection shows the GAS5 expression in tumor tissue. Data are means ± SD (n = 5). ***p < 0.001 versus control group.
Overexpression of GAS5 Inhibits Proliferation, Migration, and Chemotherapy Resistance by Suppressing the EMT and Tumor Stem Cell-like Properties
To further study the underlying mechanisms of GAS5 effects, PANC-1 cells were selected for in vitro experiments. GAS5 expression increased after transfection with a GAS5 overexpression vector (Figure 3A). Cell counting kit-8 (CCK8) analysis found that GAS5 overexpression significantly suppressed cell proliferation (Figure 3B). To determine the effect of GAS5 on metastasis, transwell migration assays were performed. GAS5 overexpression significantly suppressed migration compared with the control and NC groups (Figures 3C and 4D). To establish if GAS5 was involved in a chemo-modifying effect, PANC-1 cell lines with or without GAS5 overexpression were treated with 0–1,000 nM gemcitabine for 72 hr. Our data suggested that overexpression of GAS5 enhanced gemcitabine sensitivity, as shown by the reduced half-maximal inhibitory concentration (IC50) and increased numbers of apoptotic cells (Figures 3E–3G).
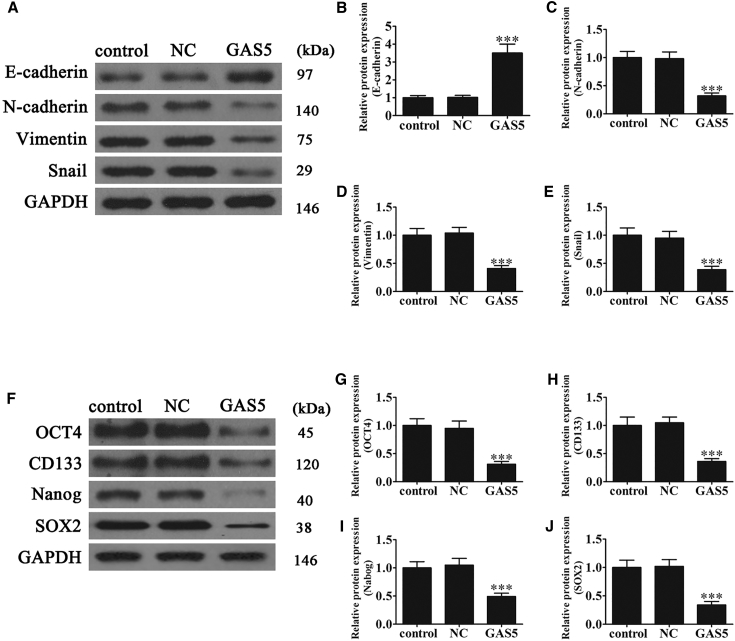
Figure 4.
Overexpression of GAS5 Inhibits Cell EMT and Stem Cell-like Properties
(A) Western blot detection shows the relative protein expression. The relative E-cadherin (B), N-cadherin (C), vimentin (D), and Snail (E) were analyzed. Data are means ± SD. ***p < 0.001 versus control. (F) Western blot detection shows the stemness marker protein. The relative protein expression of OCT4 (G), CD133 (H), Nanog (I), and SOX2 (J) were calculated. Data are means ± SD. ***p < 0.001 versus control.
Increasing evidence shows that the EMT and tumor stem cells are important in regulating proliferation, migration, and chemotherapy resistance.13, 26, 27, 28 To identify if GAS5 regulated PC proliferation, migration, and chemotherapy resistance through the EMT and/or tumor stem cells, PANC-1 cells were examined. Western blots showed that GAS5 overexpression suppressed N-cadherin, vimentin, and snail mesenchymal marker expression, but it increased E-cadherin epithelial marker expression (Figures 4A–4E). This result suggested that the expression of GAS5 inhibited the EMT. Western blots were then used to determine the relative levels of the stem cell-like markers OCT4, CD133, Nanog, and SOX2. GAS5-overexpressing cells expressed lower levels of OCT4, CD133, Nanog, and SOX2 compared with control cells (Figures 4F–4J). Our results also found that overexpression GAS5 decreased the EMT (Figure S1) and CSC (Figure S2) relative protein expression in Capan-2 cells. Collectively, these findings suggested that GAS5 repressed the EMT in PC cells and the development of stem cell-like phenotypes.
The Interaction of GAS5, miR-221, and SOCS3 in Pancreatic PANC-1 Cells
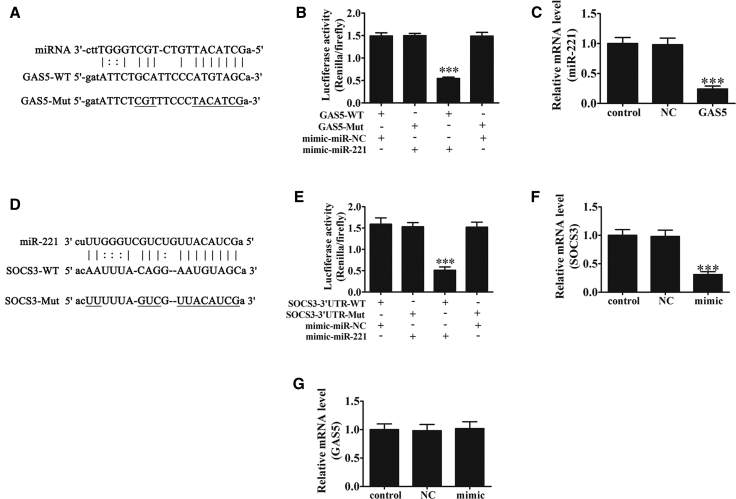
Bioinformative tool analysis (http://starbase.sysu.edu.cn/index.php) found that lncRNA GAS5 targeted miR-221. To determine if miR-221 was a possible target of GAS5, luciferase reporter analysis was employed. miR-221 was found to be a downstream binding target of GAS5 (Figure 5A). The results showed that GAS5 inhibited luciferase activity in wild-type cells but did not affect activity in mutated cell lines (Figure 5B). RT-PCR assays showed that GAS5 overexpression inhibited miR-221 expression (Figure 5C).
Figure 5.
The Relationships among GAS5, miR-221, and SOCS3 in Pancreatic PANC-1 Cells
(A) Complementary sequences between miR-221 and GAS5 mRNA were obtained using publicly available algorithms. The GAS5 mutant (MUT) is also shown. (B) Relative luciferase activity by luciferase reporter assays in PANC-1 cells co-transfected with wild-type GAS5 (GAS5-WT) or GAS5-MUT and miR-221 or miR negative control (NC). Data are means ± SD. ***p < 0.001 versus other groups. (C) Expression of miR-221 in PANC-1 cells transfected with GAS5 or NC by RT-PCR. Data are means ± SD. ***p < 0.001 versus control. (D) Complementary sequences between miR-221 and the 3′ UTR of SOCS3 mRNA were obtained using publicly available algorithms. The SOCS3 mutant is also shown. (E) Relative luciferase activity by luciferase reporter assays of PANC-1 cells co-transfected with SOCS3-WT or SOCS3-MUT and miR-221 or miR-NC. Data are means ± SD. ***p < 0.001 versus other groups. (F) SOCS3 expression in PANC-1 cells transfected with miR-221 mimics or NC by RT-PCR. Data are means ± SD. ***p < 0.001 versus control. (G) GAS5 expression in PANC-1 cells transfected with miR-221 mimics or NC by RT-PCR.
To further identify if SOCS3 was a possible target of miR-221, bioinformative analysis (https://www.genecards.org/) was used to find that miR-221 directed interactions with the 3′ UTR of SOCS3 and suppressed SOCS3 expression at the mRNA level (Figure 5D). Luciferase reporter analysis found that miR-221 inhibited luciferase activity in wild-type cells, but not in mutated cell lines (Figure 5E). Finally, RT-PCR assays showed that miR-221 overexpression inhibited SOCS3 expression (Figure 5F) but had no effect on GAS5 at the mRNA level (Figure 5G).
SOCS3 Overexpression Reverses miR-221 Overexpression-Induced Proliferation, Migration, EMT, Chemotherapy Resistance, and Stem Cell-like Properties in PANC-1 Cells
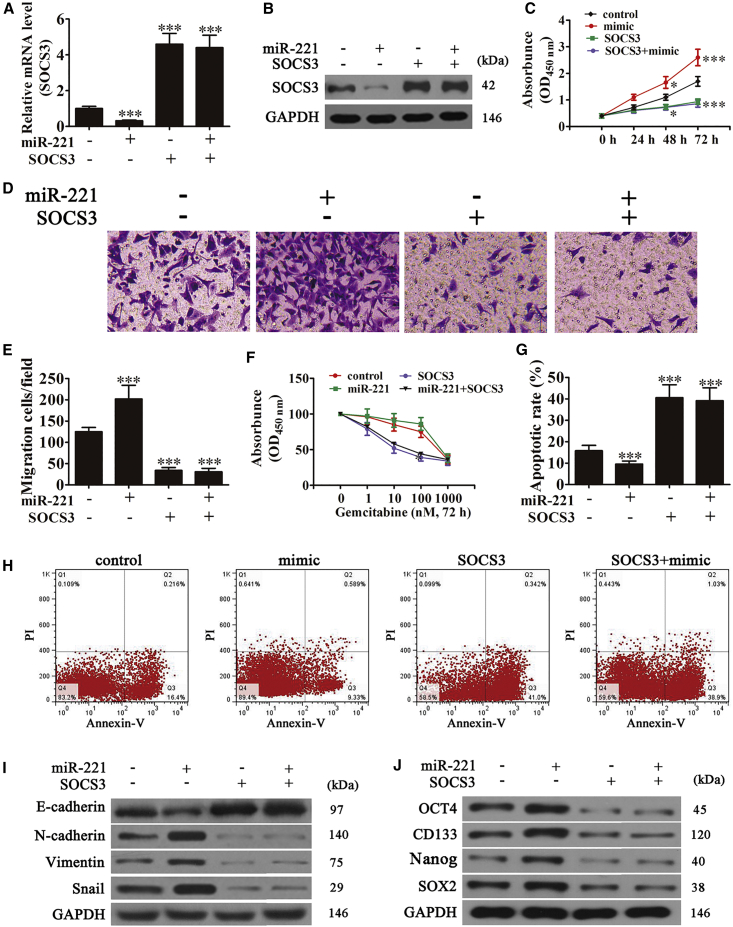
Studies have shown that SOCS3 is involved in different processes of tumorigenesis. High expression of SOCS3 was found in various cancers, including PC.29 To determine if SOCS3 was involved in miR-221-mediated tumor cell proliferation, migration, EMT, chemotherapy resistance, and stem cell-like properties, a SOCS3 overexpression vector was constructed and transfected into PANC-1 cells. RT-PCR assays and western blots showed that SOCS3 overexpression reversed miR-221-induced decreases in SOCS3 (Figures 6A and 6B). CCK8 and transwell migration assays were performed using PANC-1 cells, and they showed that cell proliferation and migration of SOCS3-transfected PANC-1 cells induced by miR-221 was significantly reversed (Figures 6B–6E).
Figure 6.
SOCS3 Overexpression Reverses miR-221 Overexpression-Induced Cell Proliferation, Migration, EMT, Chemotherapy Resistance, and Stem Cell-like Properties in Pancreatic PANC-1 Cells
(A and B) RT-PCR (A) and western blot (B) of SOCS3 after transfection with a SOCS3 overexpression vector or miR-221 mimic alone or combined. Data are means ± SD. ***p < 0.001 versus control. (C) Cell proliferation was examined by CCK8 assays. Data are means ± SD. *p < 0.05 and ***p < 0.001 versus control. (D) Transwell analyses of PANC-1 cell migration (magnification, 200×). (E) The relative migration cells were calculated. Data are means ± SD. ***p < 0.001 versus control. (F) Comparison of cell viability and IC50 of gemcitabine in PANC-1 cells. (G) The relative apoptosis was analyzed. (H) Effect of miR-221 and SOCS3 on gemcitabine-induced apoptosis detected using annexin V-PI double staining after treatment with 10 nM gemcitabine for 72 hr. Data are means ± SD. ***p < 0.001 versus control. (I) E-cadherin, N-cadherin, vimentin, and Snail1 proteins by western blots. (J) Stemness markers OCT4, CD133, Nanog, and SOX2 by western blots.
Our data also suggested that overexpression of SOCS3 enhanced gemcitabine sensitivity, as shown by reduced IC50s and increased numbers of apoptotic cells, even with miR-221 overexpression (Figures 6F–6H). Western blots showed that SOCS3 overexpression reversed miR-221-induced N-cadherin, vimentin, and Snail mesenchymal marker expression but decreased E-cadherin epithelial marker expression (Figure 6I). This result indicated that SOCS3 expression inhibited the EMT. Western blots also showed that SOCS3 overexpression reversed miR-221-induced OCT4, CD133, Nanog, and SOX2 expression (Figure 6J). Collectively, our findings suggested that SOCS3 inhibited cell proliferation, migration, and chemotherapy resistance of PC cells by repressing the EMT and the development of stem cell-like phenotypes. Taken together, the results showed that the antitumor effects of GAS5 on PC were related to miR-221/SOCS3-mediated EMT and stem cell-like phenotype inhibition.
Discussion
In previous studies, we found that toll-like receptor 2 (TLR2) has protumorigenic and prometastatic effects in intrahepatic cholangiocarcinoma through the upregulation of inflammatory cytokines induced by the activation of nuclear factor κB (NF-κB) signaling.30 The activation of the phosphatidylinositol 3-hydroxy kinase (PI3K)/protein kinase B (AKT)-ERK1/2-signaling pathways plays an important role in mediating CCL20-induced invasiveness in PC cells.31 In this study, we found that noncoding RNA (lncRNA GAS5) is involved in the progress of PC. Our results showed that the expression levels of GAS5 and SOCS3 were downregulated in both PC tissues and cell lines; however, the expression of miR-221 was increased. Upregulation of GAS5 promoted SOCS3 expression and suppressed PC growth, metastasis, and gemcitabine resistance by inhibiting the EMT and tumor stem cell accumulation in both in vivo and in vitro experiments.
Numerous studies have shown that lncRNAs play an important role in the regulation of cancer progression. In PC, several lncRNAs have been identified as either oncogenes, such as lncRNA H19, lncRNA NNT-AS1, and lncRNA SNHG15,32, 33, 34 or as tumor-suppressive lncRNAs, such as lncRNA MEG3 and lncRNA GAS5.35, 36 The function of GAS5 in PC has not been investigated. Consistently, our results showed that GAS5 was downregulated in PC tissues and cell lines, while overexpression of GAS5 suppressed cell proliferation, cell invasion, and migration. Further mechanistic studies demonstrated that GAS5 overexpression promoted gemcitabine-induced apoptosis. Taken together, our results suggest that GAS5 has tumor-suppressive effects in PC.
Bifluorescein experiments showed that miR-221 was the target of GAS5. GAS5 overexpression suppressed miR-221 expression, but miR-221 overexpression had no effect on GAS5 expression. Previous research found that miR-221 promoted oncogenic activity in gastric cancer.37 Expression of miR-221 was also shown to promote the proliferation of capan-2 pancreatic ductal adenocarcinoma cells by targeting PTEN-Akt.38 Downregulation of miR-221 enhanced tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sensitivity through DR5 upregulation in PC cells.39 Here we found that expression of miR-221 promoted PC cell proliferation, migration, and chemotherapy resistance by promoting the EMT and tumor stem cell accumulation. This suggests that miR-221 acts as an oncogene; our previous study found that miR-29a can promote tumor progression and invasion by downregulating tristetraprolin in PC.40
We also found that SOCS3 was a downstream target of miR-221; overexpression of miR-221 suppressed SOCS3 expression, while overexpression of SOCS3 reversed miR-221-induced proliferation, migration, and chemotherapy resistance of PC cells by inhibiting the EMT and tumor stem cell accumulation. Previous studies found that the expression of SOCS3 suppresses JAK2/STAT3-signaling activation.41 JAK2/STAT3 signaling is important in inducing CSC properties.42 Other studies found that SOCS3 overexpression inhibited advanced glycation end-product-induced EMT in proximal tubule epithelial cells.9 SOCS3 overexpression also inhibited the JAK2/STAT3-mediated EMT program in metastatic breast cancer.43
In conclusion, this study identified an interaction among GAS5, miR-221, and SOCS3 in PC. The results suggested that GAS5 positively regulated SOCS3 expression by acting as a competing endogenous RNA for miR-221 binding. Our study also found the downregulation of GAS5 and concomitant upregulation and downregulation of miR-221 and SOCS3, respectively. Our findings linking miR-221 to the regulation of miR-221 and SOCS3 expression in PC identify potential novel biomarkers and therapeutic targets, and they provide the basis for future studies aimed at exploring the effects of the modulation of the miR-221/SOCS3 axis as a potential therapeutic strategy in cancer.
Materials and Methods
Ethics Statement
Carcinomas and adjacent tissue from 60 patients with PC were collected from January 1, 2017 to September 31, 2017, at Tongren Hospital of Shanghai Jiaotong University School of Medicine, Shanghai, China. The study was approved by the Ethics Committee of Tongren Hospital of Shanghai Jiaotong University School of Medicine. All participants or their relatives were informed of the study and signed consent forms before inclusion.
Tumor Xenograft Formation and Metastasis Assays
The Animal Research Committee of Tongren Hospital of Shanghai Jiaotong University School of Medicine approved all experimental protocols and surgical procedures. In this study, 20 BALB/c nude mice, 4 weeks old and weighing 15–20 g, were used (SLARC, Shanghai, China). A total of 5 × 106 viable human pancreatic PANC-1 cancer cells, stably infected with lncRNA GAS5 overexpression or control vectors, was injected into the right flanks of the mice. When xenografts reached 0.5 cm in diameter (about 7 days after inoculation), the mice were randomly divided into a gemcitabine therapy group and a control group (n = 5), and they were given weekly intraperitoneal injections of either gemcitabine (125 mg/kg), as previously reported,44, 45 or saline (100 μL) as an NC. Tumor sizes were measured using a vernier caliper every 7 days beginning when the tumors reached 0.5 cm in diameter, and tumor volumes were calculated using the following formula: volume = 1/2 × length × width.2 At 35 days after implantation, mice were sacrificed for IHC and TUNEL.
For metastasis analysis, PANC-1 cells were transfected with luciferase expression vectors, and they were injected along with wild-type or GAS5-overexpressing cells into the mice’s tails intravenously (2 × 105 cells). After 35 days, metastasis of PANC-1 cells was analyzed by bioluminescence imaging after intravenous injection of luciferin into the tails (150 mg luciferin/kg body weight).
Cell Culture
The human pancreatic ductal epithelial cell line HPDE6-C7 was obtained from the American Type Culture Collection (Manassas, VA, USA). Human PC cell lines PANC-1, AsPC-1, Capan-2, SW1990, and BXPC-3 were all obtained from the laboratory of biliary-pancreatic surgery at Tongren Hospital. All cell lines were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS) in a humidified atmosphere of 5% CO2 at 37°C. PC cells were selected by continuous treatment of PANC-1 cells with 0–1,000 nM gemcitabine (Selleck, Houston, TX, USA) for 72 hr.
Cell Transfection
For miR-221 overexpression, an miR-221 mimic and corresponding NC (miR-NC) were purchased from GenePharma (Shanghai, China). PANC-1 cells were transfected with either the miR-221 mimic or miR-NC at a final concentration of 50 nM using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol. Cells were used for miR-221 expression analysis or other experiments after 48 hr of transfection.
For GAS5 overexpression, recombinant lentiviral overexpressing GAS5 (GenePharma) was used to increase GAS5 expression, with empty carrier recombinant lentiviral serving as an NC.
For SOCS3 downregulation experiments, small interfering RNA (siRNA) against SOCS3 and NC were obtained from GenePharma and used to increase SOCS3 expression.
Boyden Chamber Assays
Migration assays were performed using a Boyden chamber (8 μm; Corning, Corning, NY, USA) containing a polycarbonate membrane. Cells in serum-free medium (100 μL of 1 × 106 cells/mL) were added to the upper chamber, and 600 μL appropriate medium with 10% FBS was added to the lower chamber. Cells were incubated for 24 hr. Migratory cells from random regions of the lower surface were fixed and stained with crystal violet for 30 s at room temperature. Six random regions were photographed, and cells were counted to calculate the average number of migrated cells per plate.
Luciferase Reporter Assays
To construct luciferase reporter vectors, 3′ UTR fragments from SOCS3 or GAS5 cDNA containing the predicted miR-221-binding sites were amplified by PCR and subcloned downstream of the luciferase gene in the PYr-MirTarget luciferase vector (Ambion, Austin, TX, USA). The 3′ UTRs of SOCS3 or GAS5 with or without the mutant sequence were then amplified.
For luciferase assays, PANC-1 cells were cultured in 24-well plates and co-transfected with 50 ng vector containing firefly luciferase sequence with 25 ng miR-221 or control DNA, using Lipofectamine 2000 reagent. At 48 hr post-transfection, relative luciferase activity was calculated by normalizing firefly luminescence to Renilla luminescence using Dual-Luciferase Reporter Assays (Promega, Madison, WI, USA), according to the manufacturer’s instructions.
Cell Viability Assays
CCK8 (Sigma-Aldrich, St. Louis, MO, USA) was used to assess cell viability. PANC-1 cells (1 × 104) were seeded into 96-well plates and incubated overnight. Medium was removed and cells were washed three times with PBS. DMEM (90 μL; Gibco, Grand Island, NY, USA) and CCK8 reagent (10 μL) were added to each well and incubated for 1.5 hr at 37°C. A microplate reader was used to measure optical density at 450 nm.
Western Blots
Protein was extracted from tissues and cells using radio immunoprecipitation assay (RIPA) lysis buffer containing proteinase inhibitors (Sigma-Aldrich, St. Louis, MO, USA). Protein concentrations were determined using BCA Protein Assay kits (Vigorous Biotechnology Beijing, Beijing, China). Equal amounts of protein lysates (20 μg per lane) were resolved on 10% SDS-PAGE gels and electroblotted onto nitrocellulose membranes (Millipore, Madison, WI, USA). Membranes were blocked for 2 hr with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20, and they were incubated overnight at 4°C with primary antibodies. GAPDH was used as the internal control for protein loading. Membranes were incubated with secondary antibodies for 1 hr at room temperature. Immune complexes were detected by enhanced chemiluminescence (Cell Signaling Technology, Danvers, MA, USA). Integrated densities of bands were quantified by Quantity One software (Bio-Rad, Hercules, CA, USA).
IHC
Tumor tissue samples were fixed in 10% formalin solution and embedded in paraffin. Sections (5 μm) were stained with Ki67 or subjected to TUNEL to evaluate proliferation and apoptosis. Sections were examined using an Axiophot light microscope (Zeiss, Oberkochen, Germany) and photographed with a digital camera.
Flow Cytometry
Flow cytometry was used to determine the rate of apoptosis of PANC-1 cells. Apoptotic cells were differentiated from viable or necrotic cells by the combined application of annexin V (AV)-fluorescein isothiocyanate (FITC) and propidium iodide (PI) (Sigma-Aldrich, St. Louis, MO, USA). Cells were washed twice and adjusted to 1 × 106 cells/mL with cold D-Hanks buffer. AV-FITC (10 μL) and PI (10 μL) were added to 100-μL cell suspensions and incubated for 15 min at room temperature in the dark before 400 μL binding buffer was added. Without washing, samples were analyzed using flow cytometry. Experiments were performed in triplicate.
RNA and miRNA Extraction and RT-PCR
Total RNA was isolated from PANC-1 cells or tumor tissue using TRIzol reagent (Takara, Osaka, Japan). First-strand cDNA was synthesized using PrimeScript RT Master Mix (Perfect Real Time) kits (Takara) and used for RT-PCR, with forward and reverse primers and Power SYBR Green PCR Master Mix (Life Technologies, New York, USA). GAPDH or U6 was the internal control. Relative expression of target genes was determined using the 2−ΔΔCt method.
Statistical Analysis
Continuous variables were expressed as means ± SD. One-way ANOVA was performed for multiple comparisons using GraphPad Prism software, version 5.0 (GraphPad, La Jolla, CA, USA); p values ≤ 0.05 indicated a statistically significant difference.
Author Contributions
M.S. and A.M. designed the experiment, conducted experiments, acquired and analyzed data, and wrote and edited the manuscript; B.L. provided tissue samples; S.W. analyzed the histological slides; J.M. conceived the study, designed experiments, provided reagents, analyzed and critically evaluated the data, and edited the manuscript; Z.X., L.W., and F.Z. conceived the study, designed experiments, provided samples and reagents, and wrote and edited the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81702942 to B.L. and 81702872 to S.Y.).
Footnotes
Supplemental Information includes two figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.09.026.
Contributor Information
Mingyi Shang, Email: smy3058@shtrhospital.com.
Aiwu Mao, Email: maw2856@shtrhospital.com.
Supplemental Information
References
- 1.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A., Jemal A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Swaroop Vege S. Continuing Medical Education Questions: April 2017: A Multidisciplinary Approach to Pancreas Cancer in 2016: A Review. Am. J. Gastroenterol. 2017;112:555. doi: 10.1038/ajg.2016.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z., Li Y., Ahmad A., Banerjee S., Azmi A.S., Kong D., Sarkar F.H. Pancreatic cancer: understanding and overcoming chemoresistance. Nat. Rev. Gastroenterol. Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]
- 4.Li Y., VandenBoom T.G., 2nd, Kong D., Wang Z., Ali S., Philip P.A., Sarkar F.H. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai M.H., Xu X.G., Yan S.L., Sun Z., Ying Y., Wang B.K., Tu Y.X. Depletion of HDAC1, 7 and 8 by Histone Deacetylase Inhibition Confers Elimination of Pancreatic Cancer Stem Cells in Combination with Gemcitabine. Sci. Rep. 2018;8:1621. doi: 10.1038/s41598-018-20004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Dong P., Wang W., Huang M., Tian B. Gemcitabine treatment causes resistance and malignancy of pancreatic cancer stem-like cells via induction of lncRNA HOTAIR. Exp. Ther. Med. 2017;14:4773–4780. doi: 10.3892/etm.2017.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., Wang Y., Sun D., Bu J., Ren F., Liu B., Zhang S., Xu Z., Pang S., Xu S. miR-455-5p promotes cell growth and invasion by targeting SOCO3 in non-small cell lung cancer. Oncotarget. 2017;8:114956–114965. doi: 10.18632/oncotarget.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabrera Ortega A.A., Gonçalves Vde.P., Guimarães M.R., Rossa Junior C., Spolidorio L.C. Overexpression of Bcl-2, SOCS 1, 3 and Cdh 1, 2 are associated with the early neoplasic changes in modified 4-nitroquinoline 1-oxide-induced murine oral cancer model. J. Oral Pathol. Med. 2016;45:573–580. doi: 10.1111/jop.12413. [DOI] [PubMed] [Google Scholar]
- 9.Yu L., Zhang Y., Zhang H., Li Y. SOCS3 overexpression inhibits advanced glycation end product-induced EMT in proximal tubule epithelial cells. Exp. Ther. Med. 2017;13:3109–3115. doi: 10.3892/etm.2017.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ru P., Steele R., Hsueh E.C., Ray R.B. Anti-miR-203 Upregulates SOCS3 Expression in Breast Cancer Cells and Enhances Cisplatin Chemosensitivity. Genes Cancer. 2011;2:720–727. doi: 10.1177/1947601911425832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhammad N., Bhattacharya S., Steele R., Ray R.B. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget. 2016;7:58595–58605. doi: 10.18632/oncotarget.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Z., Chen C., Zhou Q., Wang Y., Zhao Y., Zhao X., Li W., Zheng S., Ye H., Wang L. LncRNA HOTTIP modulates cancer stem cell properties in human pancreatic cancer by regulating HOXA9. Cancer Lett. 2017;410:68–81. doi: 10.1016/j.canlet.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K., Toden S., Ravindranathan P., Han H., Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis. 2017;38:1036–1046. doi: 10.1093/carcin/bgx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C., Wang J., Yuan X., Qian W., Zhang B., Shi M., Xie J., Shen B., Xu H., Hou Z., Chen H. Long noncoding RNA uc.345 promotes tumorigenesis of pancreatic cancer by upregulation of hnRNPL expression. Oncotarget. 2016;7:71556–71566. doi: 10.18632/oncotarget.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye K., Wang S., Zhang H., Han H., Ma B., Nan W. Long Noncoding RNA GAS5 Suppresses Cell Growth and Epithelial-Mesenchymal Transition in Osteosarcoma by Regulating the miR-221/ARHI Pathway. J. Cell. Biochem. 2017;118:4772–4781. doi: 10.1002/jcb.26145. [DOI] [PubMed] [Google Scholar]
- 16.Yan H., Li Q., Wu J., Hu W., Jiang J., Shi L., Yang X., Zhu D., Ji M., Wu C. MiR-629 promotes human pancreatic cancer progression by targeting FOXO3. Cell Death Dis. 2017;8:e3154. doi: 10.1038/cddis.2017.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhary A.K., Mondal G., Kumar V., Kattel K., Mahato R.I. Chemosensitization and inhibition of pancreatic cancer stem cell proliferation by overexpression of microRNA-205. Cancer Lett. 2017;402:1–8. doi: 10.1016/j.canlet.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao D., Li T., Ye C., Zeng L., Li H., Pu X., Ding C., He Z., Huang G.L. miR-221 inhibits autophagy and targets TP53INP1 in colorectal cancer cells. Exp. Ther. Med. 2018;15:1712–1717. doi: 10.3892/etm.2017.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng L., Lei Q., Wang Y., Wang Z., Xie G., Zhong X., Wang Y., Chen N., Qiu Y., Pu T. Downregulation of miR-221-3p and upregulation of its target gene PARP1 are prognostic biomarkers for triple negative breast cancer patients and associated with poor prognosis. Oncotarget. 2017;8:108712–108725. doi: 10.18632/oncotarget.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakka M., Allen-Rhoades W., Li Y., Kelly A.J., Shen J., Taylor A.M., Barkauskas D.A., Yustein J.T., Andrulis I.L., Wunder J.S., TARGET osteosarcoma consortium Biomarker significance of plasma and tumor miR-21, miR-221, and miR-106a in osteosarcoma. Oncotarget. 2017;8:96738–96752. doi: 10.18632/oncotarget.18236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Zhang H., Li Y., Zhao C., Fan Y., Liu J., Li X., Liu H., Chen J. MiR-182 inhibits the epithelial to mesenchymal transition and metastasis of lung cancer cells by targeting the Met gene. Mol. Carcinog. 2018;57:125–136. doi: 10.1002/mc.22741. [DOI] [PubMed] [Google Scholar]
- 22.Fujisaka Y., Iwata T., Tamai K., Nakamura M., Mochizuki M., Shibuya R., Yamaguchi K., Shimosegawa T., Satoh K. Long non-coding RNA HOTAIR up-regulates chemokine (C-C motif) ligand 2 and promotes proliferation of macrophages and myeloid-derived suppressor cells in hepatocellular carcinoma cell lines. Oncol. Lett. 2018;15:509–514. doi: 10.3892/ol.2017.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Gu J., Lu H. The GAS5/miR-222 Axis Regulates Proliferation of Gastric Cancer Cells Through the PTEN/Akt/mTOR Pathway. Dig. Dis. Sci. 2017;62:3426–3437. doi: 10.1007/s10620-017-4831-4. [DOI] [PubMed] [Google Scholar]
- 24.Wei W.F., Zhou C.F., Wu X.G., He L.N., Wu L.F., Chen X.J., Yan R.M., Zhong M., Yu Y.H., Liang L., Wang W. MicroRNA-221-3p, a TWIST2 target, promotes cervical cancer metastasis by directly targeting THBS2. Cell Death Dis. 2017;8:3220. doi: 10.1038/s41419-017-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Zhu Y., Li T., Chen G., Yan G., Zhang X., Wan Y., Li Q., Zhu B., Zhuo W. Identification of a serum microRNA expression signature for detection of lung cancer, involving miR-23b, miR-221, miR-148b and miR-423-3p. Lung Cancer. 2017;114:6–11. doi: 10.1016/j.lungcan.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Yan T., Li H.Y., Wu J.S., Niu Q., Duan W.H., Han Q.Z., Ji W.M., Zhang T., Lv W. Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol. Lett. 2017;14:5400–5408. doi: 10.3892/ol.2017.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaianigo N., Melisi D., Carbone C. EMT and Treatment Resistance in Pancreatic Cancer. Cancers (Basel) 2017;9:E122. doi: 10.3390/cancers9090122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review) Int. J. Oncol. 2017;51:1357–1369. doi: 10.3892/ijo.2017.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L., Hu B., Ni J., Wu J., Jiang W., Chen C., Yang L., Zeng Y., Wan R., Hu G., Wang X. Transcriptional repression of SOCS3 mediated by IL-6/STAT3 signaling via DNMT1 promotes pancreatic cancer growth and metastasis. J. Exp. Clin. Cancer Res. 2016;35:27. doi: 10.1186/s13046-016-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Liu B., Yan S., Jia Y., Ma J., Wu S., Xu Y., Shang M., Mao A. TLR2 promotes human intrahepatic cholangiocarcinoma cell migration and invasion by modulating NF-κB pathway-mediated inflammatory responses. FEBS J. 2016;283:3839–3850. doi: 10.1111/febs.13894. [DOI] [PubMed] [Google Scholar]
- 31.Liu B., Jia Y., Ma J., Wu S., Jiang H., Cao Y., Sun X., Yin X., Yan S., Shang M., Mao A. Tumor-associated macrophage-derived CCL20 enhances the growth and metastasis of pancreatic cancer. Acta Biochim. Biophys. Sin. (Shanghai) 2016;48:1067–1074. doi: 10.1093/abbs/gmw101. [DOI] [PubMed] [Google Scholar]
- 32.Ma L., Tian X., Guo H., Zhang Z., Du C., Wang F., Xie X., Gao H., Zhuang Y., Kornmann M. Long noncoding RNA H19 derived miR-675 regulates cell proliferation by down-regulating E2F-1 in human pancreatic ductal adenocarcinoma. J. Cancer. 2018;9:389–399. doi: 10.7150/jca.21347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Y.B., Jiang Q., Yang M.Y., Zhou J.X., Zhang Q. Long noncoding RNA NNT-AS1 promotes hepatocellular carcinoma progression and metastasis through miR-363/CDK6 axis. Oncotarget. 2017;8:88804–88814. doi: 10.18632/oncotarget.21321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Z., Huang H., Wang J., Zhou Y., Pu F., Zhao Q., Peng P., Hui B., Ji H., Wang K. Long non-coding RNA SNHG15 inhibits P15 and KLF2 expression to promote pancreatic cancer proliferation through EZH2-mediated H3K27me3. Oncotarget. 2017;8:84153–84167. doi: 10.18632/oncotarget.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma L., Wang F., Du C., Zhang Z., Guo H., Xie X., Gao H., Zhuang Y., Kornmann M., Gao H. Long non-coding RNA MEG3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncol. Rep. 2018;39:1132–1140. doi: 10.3892/or.2018.6178. [DOI] [PubMed] [Google Scholar]
- 36.Gao Z.Q., Wang J.F., Chen D.H., Ma X.S., Wu Y., Tang Z., Dang X.W. Long non-coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR-32-5p/PTEN axis. Cell Biosci. 2017;7:66. doi: 10.1186/s13578-017-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma M., Chen S., Liu Z., Xie H., Deng H., Shang S., Wang X., Xia M., Zuo C. miRNA-221 of exosomes originating from bone marrow mesenchymal stem cells promotes oncogenic activity in gastric cancer. OncoTargets Ther. 2017;10:4161–4171. doi: 10.2147/OTT.S143315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang W., Yang Y., Xia L., Yang Y., Wang F., Song M., Chen X., Liu J., Song Y., Zhao Y., Yang C. MiR-221 Promotes Capan-2 Pancreatic Ductal Adenocarcinoma Cells Proliferation by Targeting PTEN-Akt. Cell. Physiol. Biochem. 2016;38:2366–2374. doi: 10.1159/000445589. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka R., Tomosugi M., Horinaka M., Sowa Y., Sakai T. Metformin Causes G1-Phase Arrest via Down-Regulation of MiR-221 and Enhances TRAIL Sensitivity through DR5 Up-Regulation in Pancreatic Cancer Cells. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0125779. e0125779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun X.J., Liu B.Y., Yan S., Jiang T.H., Cheng H.Q., Jiang H.S., Cao Y., Mao A.W. MicroRNA-29a Promotes Pancreatic Cancer Growth by Inhibiting Tristetraprolin. Cell. Physiol. Biochem. 2015;37:707–718. doi: 10.1159/000430389. [DOI] [PubMed] [Google Scholar]
- 41.Eid R.A., Alkhateeb M.A., Eleawa S., Al-Hashem F.H., Al-Shraim M., El-Kott A.F., Zaki M.S.A., Dallak M.A., Aldera H. Cardioprotective effect of ghrelin against myocardial infarction-induced left ventricular injury via inhibition of SOCS3 and activation of JAK2/STAT3 signaling. Basic Res. Cardiol. 2018;113:13. doi: 10.1007/s00395-018-0671-4. [DOI] [PubMed] [Google Scholar]
- 42.Wu L., Guo L., Liang Y., Liu X., Jiang L., Wang L. Curcumin suppresses stem-like traits of lung cancer cells via inhibiting the JAK2/STAT3 signaling pathway. Oncol. Rep. 2015;34:3311–3317. doi: 10.3892/or.2015.4279. [DOI] [PubMed] [Google Scholar]
- 43.Kim M.S., Jeong J., Seo J., Kim H.S., Kim S.J., Jin W. Dysregulated JAK2 expression by TrkC promotes metastasis potential, and EMT program of metastatic breast cancer. Sci. Rep. 2016;6:33899. doi: 10.1038/srep33899. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Yu G., Jia B., Cheng Y., Zhou L., Qian B., Liu Z., Wang Y. MicroRNA-429 sensitizes pancreatic cancer cells to gemcitabine through regulation of PDCD4. Am. J. Transl. Res. 2017;9:5048–5055. [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong J., Wang D., Wei A., Ke N., Wang Y., Tang J., He S., Hu W., Liu X. MicroRNA-410-3p attenuates gemcitabine resistance in pancreatic ductal adenocarcinoma by inhibiting HMGB1-mediated autophagy. Oncotarget. 2017;8:107500–107512. doi: 10.18632/oncotarget.22494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.