Abstract
Pre-existing neutralizing antibody (NAb) against adeno-associated virus (AAV) commonly found in primates is a major host barrier that can severely compromise in vivo gene transfer by AAV vectors. To achieve proof-of-concept success in clinical development of recombinant AAV (rAAV)-based in vivo gene therapy, it is crucial to consider the potential interference of NAb and to enroll serologically compatible study subjects. In this study, we report a large AAV NAb dataset comprising multiple large animal species and AAV serotypes and compare two NAb assays based on in vitro or in vivo transduction inhibition, respectively. Together with previously published AAV seroepidemiology studies, these data can serve as a reference for selecting suitable serotypes, study subjects of large animal species, and potentially human patients for rAAV treatment. In addition, we modeled the intrathalamus rAAV9 delivery in the presence of circulating anti-AAV9 NAb generated by either pre-immunization or passive transfer of NAb-positive large animal serum to mice. The data showed that circulating NAb may not be the sole determinant to inhibit brain transduction. Other aspects of pre-existing AAV immunity following natural infection or rAAV administration may be further studied to establish a more accurate inclusion criterion for clinical studies employing intraparenchymal rAAV9 injections.
Keywords: adeno-associated virus, CNS gene transfer, gene therapy, large animals, neutralizing antibody
Introduction
Recombinant adeno-associated viruses (rAAVs) have been widely used as in vivo gene transfer vectors and extensively tested in various gene therapy applications. Translational studies revealed that, following in vivo delivery of rAAVs, the complex interactions between rAAV and the primate immune system represent a major hurdle toward efficacious and sustainable in vivo gene transfer.1, 2, 3
In humans, pre-existing neutralizing antibody (NAb) against assembled AAV capsid in the circulation is the first immunological barrier that can potentially compromise or abrogate transduction.4 Unfortunately, NAbs against AAV are widespread in general human populations.5 The presence of NAb is believed to stem from mother-to-child transmission and from natural AAV infection during childhood.6 The influence of NAb on rAAV-mediated in vivo gene delivery depends on several factors, such as route of administration, target tissue, NAb titer, and rAAV dose. Delivery to the bloodstream is most susceptible to NAb;7, 8 immune-privileged organs such as the brain and eyes are more resistant to NAb;9 NAb of a higher titer has a more profound inhibitory effect on transduction;10 increasing the rAAV dose can overcome NAb to some extent.11 In addition to humans, non-human primates (NHPs) are also natural hosts of AAV and therefore commonly possess AAV NAbs that interfere with rAAV-mediated translational gene therapy studies and basic research.
To circumvent the inhibitory effect of NAb, many approaches have been developed and demonstrated proof-of-concept efficacy, such as plasmapheresis12, 13 and using empty capsid decoys.14 However, these methods generally are not effective in tackling high-NAb titers, and their clinical impact on gene therapy is yet to be established. The simplest and most effective workaround is to screen subjects for AAV NAb and recruit suitable ones in a trial. Although it excludes a substantial number of patients from a clinical study, the enrollment criterion based on NAb titer is essential to demonstrate the efficacy of gene therapy for hemophilia B.15, 16 Currently, screening for NAb against the AAV serotype to be used in a gene-therapy study involving human subjects or large animals remains a common and effective practice. Therefore, accurate determination of the AAV NAb in study subjects is key to many rAAV gene transfer studies.
AAV NAb present in body fluid is usually determined by an in vitro transduction inhibition assay.5, 17 In this assay, a body fluid sample such as serum is serially diluted; each diluted sample is incubated with a certain serotype of rAAV expressing a reporter to allow for NAb-rAAV binding. Then the mixture is used to infect cell culture followed by the measurement of reporter expression as a gauge of transduction efficiency. The presence of NAb will inhibit transduction up to a certain dilution factor compared to proper controls. The titer of NAb can be defined as the lowest dilution factor that inhibits transduction by more than 50% or between this lowest dilution factor and the next one (e.g., this study). Alternatively, the dilution factor that inhibits transduction by 50% can be calculated by logistic regression and defined as the NAb titer. It should be noted that the in vitro AAV NAb assay involves several variables that are not standardized among different laboratories, such as incubation and culture conditions, reporter choice, cell type, and MOI. In addition, the reporter rAAVs are usually produced in-house and therefore subject to variations. Nevertheless, studies have shown that the assay remains robust and is resistant to some variables.18 At our facility, we have been using a standard operating procedure to perform in vitro NAb assays since 2008. A recent study showed that the NAb results obtained from our laboratory and another laboratory using a different protocol are highly consistent.19
An intrinsic limitation of the in vitro AAV NAb assay is the low sensitivity, due in part to the generally inefficient cell culture transduction by rAAVs. Therefore, a high MOI of reporter rAAV has to be used in the assay, which may artificially overcome the inhibitory effect of low-titer NAb and make a false-negative call. This caveat is especially important in scenarios that are prone to the inhibition by even low-titer NAb, such as systemic delivery to target the liver. To more closely mimic the neutralizing effect of NAb on in vivo rAAV transduction, an adoptive transfer method to detect NAb was developed.7, 10 In this assay, mice are pre-conditioned by receiving IV injection of a test sample, such as intravenous immunoglobulin (IVIg) or serum, followed by a second injection of a certain serotype of rAAV expressing a secretory reporter. The in vivo transduction is measured by quantifying reporter expression in the serum and compared to proper controls. Due to the high in vivo transduction capability of rAAV, a low dose of reporter rAAV is sufficient to yield robust expression and therefore allows for the detection of low-titer NAbs, i.e., at a higher sensitivity. The in vivo NAb assay was employed to screen for AAV8 sero-negative patients of hemophilia B and proved to be essential for the success of the gene therapy trial.15, 16 However, the in vivo NAb assay is far more cumbersome than the in vitro assay, especially when a large number of samples need to be screened.
Circulating AAV NAb may also compromise transduction in the brain and eyes following a direct rAAV injection into these immune-privileged compartments.20, 21, 22 Previous studies revealed that such an inhibitory effect depends on several factors, such as the serotype,23 NAb titer,24, 25 and route of administration.21 Early studies mainly focused on AAV2 and AAV5 when evaluating the impact of circulating AAV NAb on brain transduction by a direct intraparenchymal injection. More recently, AAV9 and AAVrh.10 have been shown to more efficiently target CNS cells by a direct brain injection and therefore are suitable for gene therapy development for a range of CNS diseases such as Parkinson’s disease and lysosomal diseases with CNS involvement.26, 27
Here, we report a survey of circulating AAV NAbs in six different species of large animals that are commonly used in pre-clinical gene therapy development. In particular, the high-throughput in vitro NAb assay allowed us to analyze a large number of rhesus macaques (RMs) and cynomolgus macaques (CMs) and to test for multiple AAV serotypes. In addition, we also conducted in vivo NAb assay with a subset of serum samples, which showed higher sensitivity than the in vitro assay with the exceptions of some animal species. Lastly, we found that the circulating anti-AAV9 NAb up to 1:20–1:40 alone may not be sufficient to inhibit Th injection of rAAV9 in mice. Together, these results shed more light on screening and selecting suitable study subjects of large animal species and potentially humans for rAAV-mediated gene therapy.
Results
Survey of AAV NAbs in Large Animals
During the past few years, we performed an in vitro AAV NAb assay according to a standard operating procedure with serum samples from various large animal species, including RM, CM, marmoset, chimpanzee, dog, and sheep, generating a total of 2,555 assay data points. These serum samples were collected from naive animals that were screened for NAb titer for various purposes. In most cases, animals were screened to identify the ones with non-detectable or low levels of NAb for pre-clinical rAAV gene-therapy studies.
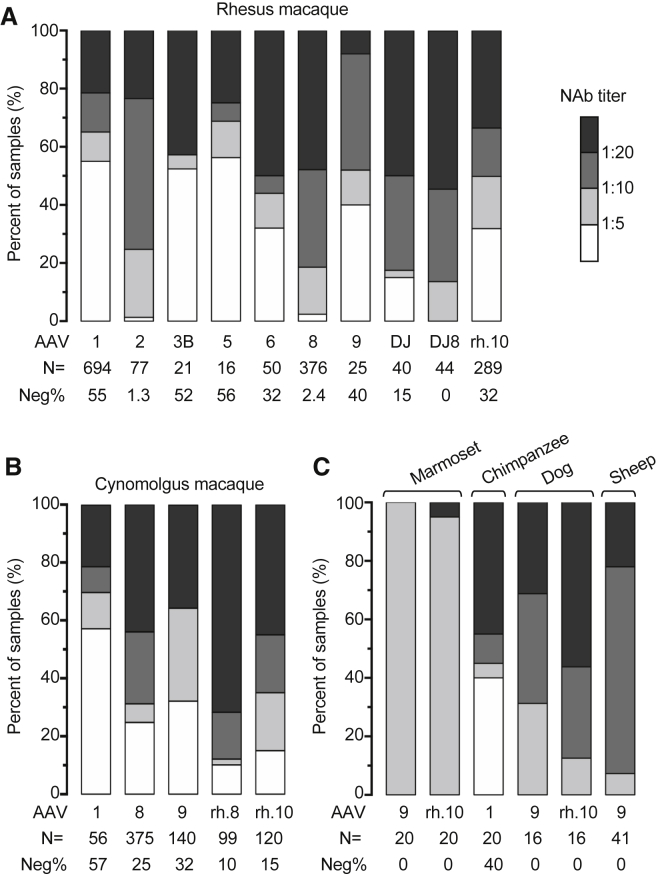
The results are summarized and presented in Figure 1. RM and CM account for the vast majority of donor animals (1,632 and 790 data points, respectively), reflecting their common use in translational gene therapy development. NAbs against a series of clinically relevant AAV serotypes were tested, including AAV1, 2, 3B, 5, 6, 8, 9, DJ, DJ8, rh.8, and rh.10, among which AAV1, 8, 9, rh.10 were most often tested. Anti-AAV1 NAb shows low prevalence in both RM and CM; sero-negative samples account for 55% and 57% in these species, respectively. Similarly, anti-AAV9 NAb shows low prevalence or tends to display low titer of less than 1:10 in these species. In contrast, both RM and CM tend to have high anti-AAV8 NAb titers of more than 1:10. Anti-AAVrh.10 NAb appears more prevalent in CM than in RM, mainly due to more CM samples containing titers of more than 1:20. Notably, RM samples have widespread anti-AAV2 NAb with only 1.3% sero-negative, but low prevalence of anti-AAV3B and AAV5 NAbs with more than 50% sero-negative despite small sample sizes. This NAb profile is similar to that of human populations previously reported.28 The sample sizes and serotypes tested for the other species are limited (Figure 1C). However, anti-AAV9 and rh.10 NAbs appear common in marmoset, dog, and sheep, but the titers are generally lower in marmoset than the other two species.
Figure 1.
Survey of AAV NAbs in Large Animals
Stacked histogram showing the distribution of (A) rhesus macaque, (B) cynomolgus macaque, (C) marmoset, chimpanzee, dog, and sheep serum samples harboring different levels of AAV NAbs as determined by an in vitro transduction inhibition assay (see Materials and Methods for assay details). Samples are categorized by the AAV NAb titer that is presented on a gray scale. Each stacked bar represents the test results for an individual AAV serotype. The AAV serotype, number of animals tested for each serotype (N), and percentage of NAb-negative samples (Neg%; defined as NAb titer <1:5) are labeled for each bar.
Taken together, the survey of anti-AAV NAbs comprises several large-animal species and AAV serotypes with a large sample size in many cases. For RM and CM, two NHP species commonly used in translational gene therapy studies, they tend to have low prevalence and/or low titer of anti-AAV1 and AAV9 NAbs; the opposite is observed for anti-AAV8 NAb.
Correlation between In Vitro and In Vivo AAV NAb Assays
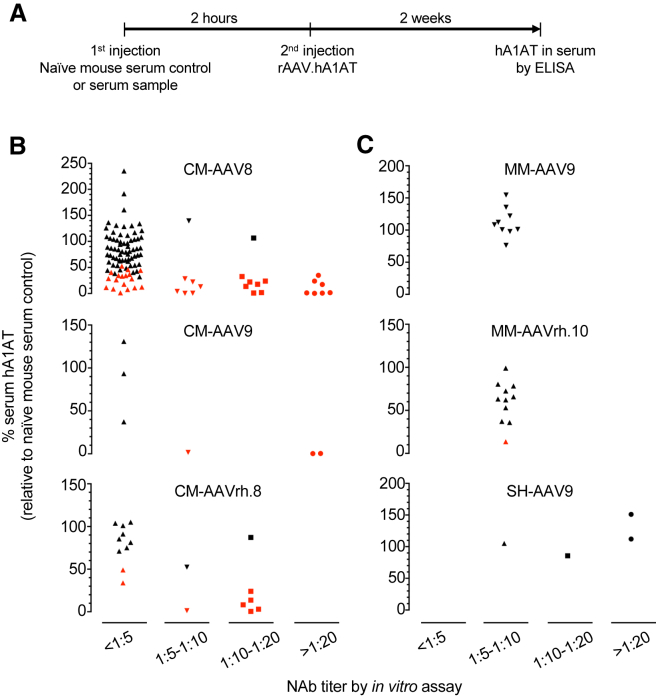
For a subset of serum samples that we tested by the in vitro NAb assay, we also performed an in vivo NAb assay that is based on adoptive transfer of serum sample to wild-type (WT) mice, and quantification of its inhibitory effect on in vivo transduction by rAAV of a certain serotype expressing human alpha 1-antitrypsin (hA1AT) as a secretory reporter. The hA1AT in the recipient mouse serum can be quantitatively measured to gauge transduction efficiency. The experimental workflow is illustrated in Figure 2A and detailed in the Materials and Methods.
Figure 2.
Correlation between In Vitro and In Vivo AAV NAb Assays
(A) Experimental workflow of the in vivo NAb assay (see Materials and Methods for assay details). (B and C) Dot plot of serum samples tested by both the in vitro and in vivo assays. Each dot represents the serum sample from an individual large animal. CM, Cynomolgus macaque; MM, marmoset; SH, sheep. Red dots, p < 0.05 compared against the naive mouse serum controls in the in vivo assay (normalized to 100% serum hA1AT; Student’s t test). Note that the majority of sero-positive (>1:5 by in vitro assay) sera of CM showed in vivo inhibition (B), but not the sero-positive sera of MM and SH (C).
Most samples tested in the in vivo NAb assay were sero-negative in the in vitro NAb assay (titer < 1:5) (Figure 2B), because the main purpose of performing the in vivo NAb assay was to identify the samples with a low NAb titer that was not detectable in the less-sensitive in vitro NAb assay. As expected, a small fraction of sero-negative samples determined by the in vitro NAb assay were able to significantly inhibit rAAV.hA1AT transduction in mice. In contrast, the majority of samples showing a high titer of more than 1:10 in the in vitro NAb assay were able to significantly inhibit rAAV.hA1AT transduction in mice (Figure 2B). Interestingly, almost all serum samples from marmoset and sheep were not able to inhibit in vivo transduction despite their NAb titers of more than 1:5 in the in vitro NAb assay (Figure 2C).
Overall, we found good correlation between the in vitro and in vivo NAb assays; most sero-negative samples determined by the in vitro assay did not significantly inhibit in vivo rAAV transduction, whereas most sero-positive samples determined by the in vitro assay did. The in vivo assay was able to identify the inhibitory sera that presumably contain an anti-AAV NAb titer below the detection limit of the in vitro assay (1:5 in this study).
Pre-immunization with rAAV9 by IM Injection in Mice Compromises CNS Transduction by Brain rAAV9 Delivery
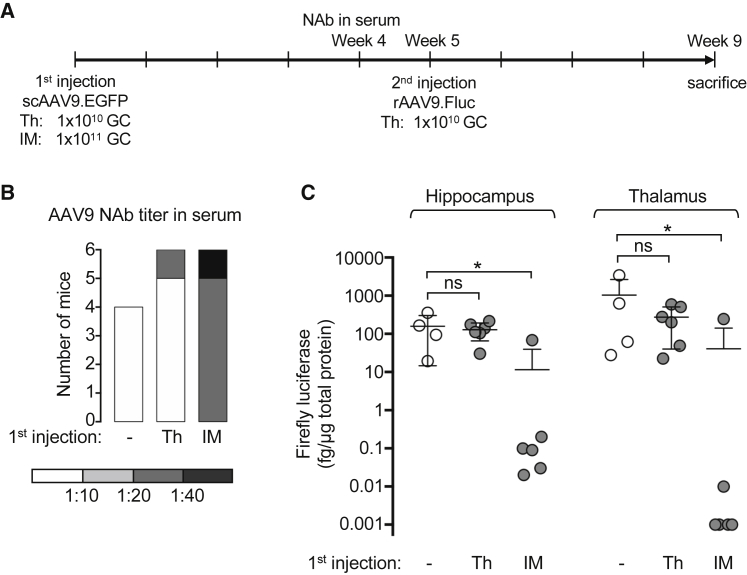
To model the effect of circulating NAb on direct brain gene delivery by rAAV9, we first pre-immunized mice with an AAV9 vector expressing EGFP (scAAV9.EGFP) by either Th injection or intramuscular (IM) injection. Four weeks later, anti-AAV9 NAb in the sera was determined by the in vitro NAb assay (Figures 3A and 3B). IM injection led to robust NAb formation of more than 1:20 in all mice (n = 6). In contrast, only one mouse receiving Th injection showed detectable NAb (n = 6). As expected, naive mice receiving no rAAV treatment were sero-negative (n = 4). Five weeks after the first injection, all mice received Th injection of an AAV9 vector expressing firefly luciferase (rAAV9.Fluc). For the mice that received the first Th injection, the second Th injection was performed at the contralateral hemisphere. Four weeks later, all mice were sacrificed and analyzed for Fluc expression in the tissue lysate of hippocampus and thalamus of the hemisphere receiving rAAV9.Fluc (Figures 3A and 3C). Compared with the mice receiving only the Th injection of rAAV9.Fluc, pre-treatment with a Th injection of scAAV9.EGFP did not change the transduction capability of the following rAAV9.Fluc in either brain region. In contrast, pre-immunization with scAAV9.EGFP by IM injection significantly reduced the transduction by rAAV9.Fluc in both brain regions, in most mice to several logs lower. These results indicate a correlation between the presence of systemic anti-AAV9 NAb induced by IM pre-immunization and the severely compromised brain transduction by a following direct intraparenchymal delivery of rAAV9. However, the IM pre-immunization regimen could trigger pleiotropic immune responses beyond generating circulating NAb.
Figure 3.
Pre-immunization with rAAV9 by IM Injection in Mice Compromises CNS Transduction by a Following Brain rAAV9 Delivery
(A) Experimental workflow. Wild-type C57BL/6 mice (male, 6–8 weeks old) received either intrathalamus (Th) or intramuscular (IM) delivery of scAAV9.EGFP as the first injection. Mice receiving no rAAV served as naive controls. Four weeks later, sera were collected to determine the anti-AAV9 NAb titer. One week later, all mice received rAAV9.Fluc by Th delivery. For the mice receiving Th delivery as the first injection, the second Th injection was at the contralateral hemisphere. Four weeks after the second injection, mice were sacrificed for gene expression analysis. GC, Genome copies. (B) Anti-AAV9 NAb titer in the sera collected 4 weeks after the first injection as determined by the in vitro assay. Samples are categorized by the AAV NAb titer that is presented on a gray scale. (C) Quantification of the firefly luciferase expressed in the hippocampus and thalamus of the hemisphere injected with rAAV.Fluc. Each dot represents an individual mouse. The means and SDs of each group (n = 4–6 mice) are shown. *p < 0.05; ns, not significant by Dunn’s test corrected for multiple comparisons within each tissue group.
Passive Transfer of NHP Serum Containing Anti-AAV9 NAb to Mouse Does Not Compromise Intracranial Gene Delivery by rAAV9
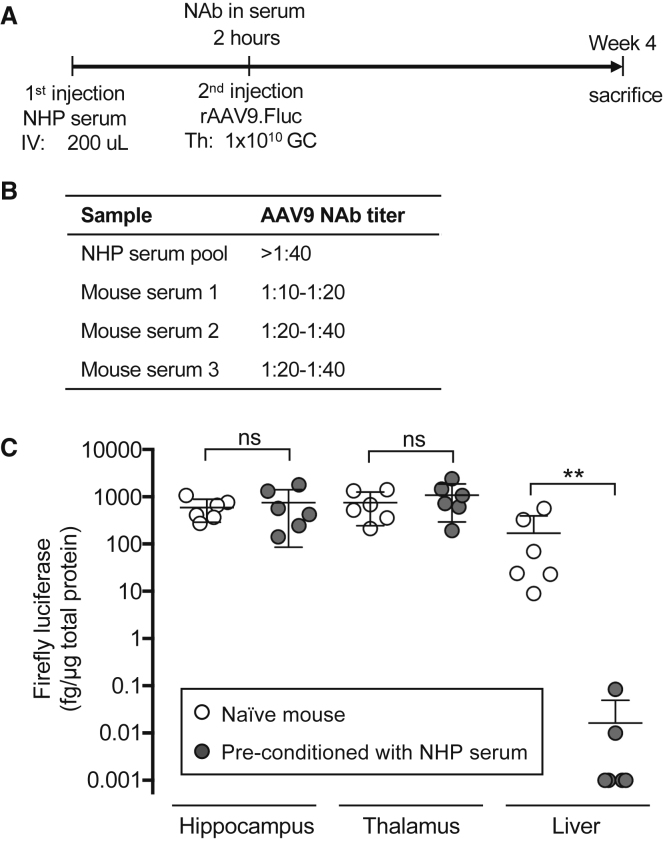
To further test whether the AAV9 NAb induced by the IM pre-immunization regimen was the sole cause of compromised brain transduction by brain rAAV9 delivery, we performed a passive transfer experiment (Figure 4A). We first pooled three cynomolgus monkey serum samples that were previously determined to be positive for AAV9 NAb, which allowed us to use the same NHP serum sample of sufficient amount to pre-condition multiple mice. In the pilot experiment, we determined that IV delivery of 200 μL of the NHP serum pool to mice generated robust AAV9 NAb in the mouse sera (Figure 4B). Next, we treated another batch of pre-conditioned mice or naive mice with Th delivery of rAAV9.Fluc. Four weeks later, mice were sacrificed, and we analyzed Fluc expression in the tissue lysate of hippocampus, thalamus, and liver. In contrast to the pre-immunization experiment (Figure 3C), passive transfer of AAV9 NAb-containing NHP serum did not compromise the intracranial gene delivery by rAAV9 (Figure 4C). In the naive mice not receiving NHP serum, the intracranial injection also led to liver transduction due to vector leakage out of the CNS compartment (Figure 4C). As expected, the liver transduction in the pre-conditioned mice was diminished (Figure 4C).
Figure 4.
Passive Transfer of NHP Serum Containing Anti-AAV9 NAb to Mouse Does Not Compromise Intracranial Gene Delivery by rAAV9
(A) Experimental workflow. Wild-type C57BL/6 mice (male, 6–8 weeks old) received either no NHP serum (naive) or intravascular (IV) delivery of NHP serum pool containing anti-AAV9 NAb as the first injection. In the first set of experiments, sera were collected after 2 hr to determine the anti-AAV9 NAb titer. In the second set of experiments, another batch of mice were pre-conditioned by the same NHP serum pool and then received rAAV9.Fluc by Th delivery. Four weeks after the Th injection, mice were sacrificed for gene expression analysis. GC, Genome copies. (B) Anti-AAV9 NAb titer in the NHP serum pool and sera from three pre-conditioned mice collected in the first set of experiment as determined by the in vitro assay. (C) Quantification of the firefly luciferase expressed in the hippocampus and thalamus of the hemisphere injected with rAAV.Fluc and the liver from mice in the second set of experiments. Each dot represents an individual mouse. The means and SDs of each group (n = 6 mice) are shown. **p < 0.01; ns, not significant by non-parametric Mann-Whitney test.
Discussion
The inhibitory effect of circulating AAV NAb on gene-therapy efficacy is evident in clinical studies. The lack of effective methods to circumvent neutralization necessitates the exclusion of sero-positive patients from some clinical trials. The same issue exists when performing a pre-clinical toxicology study and/or gene-transfer efficiency study in large-animal species such as NHPs. Previous studies surveyed NAbs against several AAV serotypes in general human populations,5, 28 specific patient populations,29, 30, 31, 32 and several animal species.33, 34 Collectively, these studies provide a guidance for selecting AAV serotype vectors to maximize the serologically compatible study subjects. Here, we greatly extend this effort by reporting a large AAV NAb dataset consisting of multiple animal species and AAV serotypes. The vast majority of data points concern RMs and CMs, two species commonly used in rAAV gene therapy development. These data can be informative for the selection of AAV serotypes and animal species for testing a gene-therapy application. For example, similar to humans,28 RMs appear to have limited AAV5 NAb prevalence (Figure 1A), justifying their use in testing rAAV5-mediated gene transfer. For testing applications using rAAV8, CMs may be preferred over RMs, because AAV8 NAb is more prevalent in the latter species (Figure 1A). It should be noted that some studies may allow for NAb titers up to a certain degree or require sero-positive subjects, such as testing strategies to circumvent the NAb barrier. The NAb dataset reported here will also be informative in such cases.
By comparing the results of in vitro and in vivo NAb assays for a large number of serum samples, we confirmed previous findings that both assays can consistently distinguish sero-positive and sero-negative subjects in most cases.10, 35 However, due to the low sensitivity of the in vitro NAb assay, it may not identify subjects that harbor low-titer NAbs (Figure 2B). Based on this notion, the more rigorous in vivo NAb assay was used to screen patients for the hemophilia B trial that employed an AAV8 vector.15, 16 Overall, our results support a two-step AAV NAb screening strategy: performing the high-throughput in vitro assay first, followed by the in vivo assay to identify subjects with low-titer NAbs if necessary. The unexpected discrepancy between the two assays with marmoset and sheep sera may indicate that the current in vitro assay may overestimate their neutralizing effects and that assay optimization is necessary. Some non-neutralizing serum factors may exist to alter rAAV transduction. For example, previous studies showed that certain serum factors can impact rAAV transduction in a serotype- and species-dependent manner.34
The immune-privileged CNS compartment is not completely resistant to pre-existing AAV immunity during rAAV gene delivery. Multiple routes of rAAV administration can target the CNS. Although a convenient systemic delivery of certain AAV serotype vectors, such as AAV9 and AAVrh.10, can transduce widespread CNS regions, this route of administration faces the barrier of circulating NAbs and demands a large quantity of vectors. Nevertheless, it is practical and effective in treating infant patients of spinal muscular atrophy;36 children at this age usually have limited AAV NAbs and need a relatively small amount of rAAV due to their low body weight. rAAV delivery to the cerebrospinal fluid (CSF) is a viable option to achieve global CNS gene transfer with a greatly reduced vector load.26 Importantly, studies in NHPs demonstrated that intra-CSF rAAV9 delivery to target the CNS is resistant to NAb in the bloodstream to a degree.9, 20 To target CNS diseases that afflict a limited brain region such as Parkinson’s disease, a direct intraparenchymal rAAV injection to the disease foci remains an attractive approach and is under extensive clinical evaluation,26 in part due to the robust local transduction and very small amount of rAAV needed. Early studies mainly evaluated AAV2 and AAV5 vectors for such applications including the impact of anti-AAV2 and -AAV5 NAbs. These studies suggested that brain transduction by intraparenchymal rAAV delivery can be severely compromised in the presence of circulating NAbs.24, 37 Recently, other AAV serotype vectors were shown to more efficiently target CNS cells by intraparenchymal delivery.27
To examine whether a highly CNS-tropic serotype such as AAV9 may overcome NAb, we modeled intraparenchymal rAAV9 delivery in the presence of anti-AAV9 NAb generated by either pre-immunization or passive transfer of NHP serum to mice. Our results showed that circulating anti-AAV9 NAb up to 1:20–1:40 alone is not sufficient to compromise intracranial rAAV9 gene delivery. The inhibitory effect by the pre-immunization regimen may be due to pleiotropic immune responses induced by the IM route of administration. Therefore, our results suggest that assaying pre-existing NAb alone may not be informative in studies employing intraparenchymal rAAV9 injections. The effects of other aspects of pre-existing AAV immunity on brain intraparenchymal rAAV gene delivery remain to be studied. Notably, the immune responses triggered by natural infection and rAAV administration are different.38 Therefore, studying the pre-existing AAV immunity in these scenarios may provide insights for different applications including rAAV re-administration. It will be also informative to determine whether the NAb titer can serve as a surrogate to gauge overall pre-existing AAV immunity and thus a convenient marker to select proper subjects.
In summary, the large-animal AAV NAb dataset reported here may further facilitate the selection of serotype and animal species for a rAAV-mediated gene-transfer study. Our data suggest that the more convenient in vitro NAb assay is sufficient in most cases. The more sensitive in vivo NAb assay may be necessary to better identify subjects with low-titer NAbs; these subjects may not be suitable for applications that are prone to NAbs, such as systemic rAAV delivery. In studies involving brain intraparenchymal rAAV gene delivery, screening for NAbs may not provide a definitive inclusion criterion. Further studies are required to determine whether other immunological markers can be informative and whether NAb titer can serve as a surrogate for this purpose.
Materials and Methods
Serum Sample Collection from Large Animals
Sera were collected from large animals by various parties, including academic laboratories and pharmaceutical companies, shipped to the University of Massachusetts Medical School Viral Vector Core on dry ice and stored under −80°C until assay. For confidentiality reasons, the detailed information of the donor animals was not available in most cases, such as geographic location, age, sex, and housing conditions.
Use of Mice
WT C57BL/6 male mice (6–8 weeks old) were used in all experiments. Mice were obtained by in-house breeding and kept with a 12-hr light cycle. Intravascular delivery was performed by tail-vein injection. Th delivery was performed using a stereotaxic instrument (Stoelting) on anesthetized mice. One microliter of rAAV preparation was delivered using a 33G Hamilton syringe (model #1701, Hamilton) attached to an automatic infusion pump over 5 min. The injection coordinates were X = ±1.5 mm; Y = −2.0 mm (Bregma was set as X = 0, Y = 0); Z = −3.5 mm (skull surface was set as Z = 0). IM delivery was performed by direct injection of 10 μL of rAAV preparation into the left tibialis anterior muscle. All animal procedures were approved by the Institutional Animal Care and Use Committee at University of Massachusetts Medical School.
rAAV Production
All rAAV genome constructs carry the ubiquitous cytomegalovirus enhancer-chicken beta-actin promoter driving the expression of a transgene of human alpha-1-antitrypsin (hA1AT), EGFP, Fluc, or β-galactosidase (LacZ). All rAAVs were generated by triple-transfection of a cis-plasmid containing the rAAV genome construct flanked by AAV2 inverted terminal repeats, a trans-plasmid containing the AAV2 rep gene and serotype-specific cap gene, and a helper plasmid containing certain adenovirus genes in HEK293 cells. The hA1AT, Fluc, and LacZ vectors were packaged as single-stranded genomes, and the EGFP vector was packaged as self-complementary genome. rAAV particles were purified from cell lysate and culture media by two rounds to cesium chloride sedimentation and dialysis. The genome titer of rAAV preparation was determined by Taqman real-time qPCR. The purity was assessed by SDS-PAGE followed by silver staining to visualize the viral protein (VP)1, VP2, and VP3.
In Vitro AAV- NAb Assay
Serum sample was heat-inactivated under 56°C for 35 min and 1:5 diluted in Dulbecco's Modified Eagle's Medium (DMEM) followed by 2-fold serial dilutions. Each diluted serum sample was incubated with 1.2 × 109 genome copies (GC) of rAAV.LacZ in a total volume of 120 μL DMEM under 37°C/5% CO2 for 1 hr. Naive mouse serum (Sigma-Aldrich, cat. #S-3509) was prepared in the same manner as negative control. Huh7 cells grown in a 96-well plate were first infected with WT adenovirus serotype 5 (100 viral particles per cell). Three to four hours later, 100 μL of pre-incubated rAAV-serum mixture containing 1 × 109 GC of rAAV.LacZ was added to each well of Huh7 cell culture and incubated under 37°C/5% CO2 for 1 hr. Triplicate wells were included for each dilution of a sample. Cells were then cultured with 5% fetal bovine serum under 37°C/5% CO2 overnight. β-galactosidase activity in cell lysate was measured using the Galacto-Star One-Step β-galactosidase Reporter Gene Assay System (Thermo Fisher Scientific, cat. #T1014), in which the β-galactosidase activity correlates with luminescence readings. Because the presence of mouse serum enhances the transduction of certain AAV serotypes in a dose-dependent manner, the luminescence readings from the naive mouse serum controls at each dilution were normalized to 100%. The transduction inhibition effect of a test sample at each dilution was calculated by comparing with the naive mouse serum control at the same dilution. The NAb titer was defined as a range between the lowest dilution factor that yielded more than 50% transduction inhibition and the next dilution factor that could not inhibit transduction by more than 50%. For example, a serum sample inhibited transduction by 60% at 1:5 dilution but inhibited transduction by only 30% at 1:10 dilution; the NAb titer was deemed 1:5–1:10. If the least diluted (1:5) serum sample could not inhibit transduction by more than 50%, the NAb titer of the sample was deemed <1:5. If the most diluted (1:20) serum sample could inhibit transduction by more than 50%, the NAb titer of the sample was deemed >1:20. Due to the limited amount of mouse serum (Figure 3), the starting dilution was 1:10.
In Vivo AAV NAb Assay
200 μL of heat-inactivated (56°C for 35 min), undiluted serum was injected to each mouse through the tail vein. Either immune-compromised7 or immune-competent mice10 can be used in this assay, and we used the immune-competent C57BL/6 mice for convenience. Three to four mice were treated for each test serum sample. Mice receiving naive mouse serum (Sigma-Aldrich, cat. #S-3509) served as baseline control. Two hours later, rAAV.hA1AT was injected through the tail vein; the dose was dependent on the AAV serotype. Two weeks later, blood was collected from the facial vein and serum was isolated using a serum separator (BD, cat. #365967) and stored under −80°C until assay. Serum hA1AT was determined using an enzyme-linked immunosorbent assay (ELISA). The serum hA1AT from the mice receiving naive mouse serum was normalized to 100% in each assay, and the transduction inhibition in the presence of passively transferred test serum was calculated and statistically compared with the baseline control by Student’s t test.
Firefly Luciferase (Fluc) Assay
Mice were transcardially perfused with ice-cold PBS. The whole brain was quickly dissected and cut in halves longitudinally on an ice-cold metal block. The hippocampus and thalamus of the hemisphere receiving rAAV9.Fluc were carefully dissected into a pre-chilled 2-mL Eppendorf tube and stored under −80°C until Fluc assay. Frozen tissue was homogenized in 200 μL of 1× Passive Lysis Buffer (Promega, cat. #1941) with protease inhibitor (Roche, cat. #4693159001) using TissueLyzer II (QIAGEN). Cleared lysate was quantified for total protein concentration using the BCA assay (Pierce, cat. #23225). Fluc activity in tissue lysate was quantified using the Luciferase Assay System (Promega, cat. #E1501) and compared against a standard curve generated using purified recombinant Fluc (Promega, cat. #E1701). The amount of Fluc in tissue lysate was calculated as femtograms (fg) of Fluc per μg of total protein.
Statistical Analysis
Comparison between two groups was analyzed by Student t test (two-tailed). Comparison among three groups was analyzed by one-way ANOVA, and the following pairwise comparisons were corrected for multiple comparisons. All statistical analysis was performed using the software Prism 7.
Author Contributions
Conceptualization, N.A., G.G.; Methodology, D.W., L.Z.; Investigation, D.W., M.L., L.Z., J.L., K.T., L.R., R.H., R.P.M., C.F., T.K.; Resources, C.F., T.K., M.S.-E., T.R.F., N.A., and G.G.; Writing – Original Draft, D.W.; Writing – Review & Editing, G.G.; Visualization, D.W., L.Z.; Supervision, N.A., G.G.; Project Administration: D.W., L.Z.; Funding Acquisition, N.A. and G.G.
Conflicts of Interest
G.G. is a scientific co-founder of Voyager Therapeutics and holds equity in the company. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics and other biopharmaceutical companies.
Acknowledgments
This work was funded by the CHDI Foundation.
Contributor Information
Neil Aronin, Email: guangping.gao@umassmed.edu.
Guangping Gao, Email: neil.aronin@umassmed.edu.
References
- 1.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 2.Vandamme C., Adjali O., Mingozzi F. Unraveling the Complex Story of Immune Responses to AAV Vectors Trial After Trial. Hum. Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mingozzi F., High K.A. Overcoming the Host Immune Response to Adeno-Associated Virus Gene Delivery Vectors: The Race Between Clearance, Tolerance, Neutralization, and Escape. Annu. Rev. Virol. 2017;4:511–534. doi: 10.1146/annurev-virology-101416-041936. [DOI] [PubMed] [Google Scholar]
- 4.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calcedo R., Morizono H., Wang L., McCarter R., He J., Jones D., Batshaw M.L., Wilson J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scallan C.D., Jiang H., Liu T., Patarroyo-White S., Sommer J.M., Zhou S., Couto L.B., Pierce G.F. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 8.Jiang H., Couto L.B., Patarroyo-White S., Liu T., Nagy D., Vargas J.A., Zhou S., Scallan C.D., Sommer J., Vijay S. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray S.J., Nagabhushan Kalburgi S., McCown T.J., Jude Samulski R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013;20:450–459. doi: 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Calcedo R., Bell P., Lin J., Grant R.L., Siegel D.L., Wilson J.M. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum. Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurlbut G.D., Ziegler R.J., Nietupski J.B., Foley J.W., Woodworth L.A., Meyers E., Bercury S.D., Pande N.N., Souza D.W., Bree M.P. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol. Ther. 2010;18:1983–1994. doi: 10.1038/mt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monteilhet V., Saheb S., Boutin S., Leborgne C., Veron P., Montus M.F., Moullier P., Benveniste O., Masurier C. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol. Ther. 2011;19:2084–2091. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chicoine L.G., Montgomery C.L., Bremer W.G., Shontz K.M., Griffin D.A., Heller K.N., Lewis S., Malik V., Grose W.E., Shilling C.J. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol. Ther. 2014;22:338–347. doi: 10.1038/mt.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mingozzi F., Anguela X.M., Pavani G., Chen Y., Davidson R.J., Hui D.J., Yazicioglu M., Elkouby L., Hinderer C.J., Faella A. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 2013;5:194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meliani A., Leborgne C., Triffault S., Jeanson-Leh L., Veron P., Mingozzi F. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum. Gene Ther. Methods. 2015;26:45–53. doi: 10.1089/hgtb.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M., Crosby A., Hastie E., Samulski J.J., McPhee S., Joshua G., Samulski R.J., Li C. Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther. 2015;22:984–992. doi: 10.1038/gt.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellsworth J.L., O’Callaghan M., Rubin H., Seymour A. Low Seroprevalence of Neutralizing Antibodies Targeting Two Clade F AAV in Humans. Hum. Gene Ther. Clin. Dev. 2018 doi: 10.1089/humc.2017.239. Published online February 27 2018. [DOI] [PubMed] [Google Scholar]
- 20.Samaranch L., Salegio E.A., San Sebastian W., Kells A.P., Foust K.D., Bringas J.R., Lamarre C., Forsayeth J., Kaspar B.K., Bankiewicz K.S. Adeno-Associated Virus Serotype 9 Transduction in the Central Nervous System of Nonhuman Primates. Hum. Gene Ther. 2012;23:382–389. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotterman M.A., Yin L., Strazzeri J.M., Flannery J.G., Merigan W.H., Schaffer D.V. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22:116–126. doi: 10.1038/gt.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janelidze S., Nordström U., Kügler S., Brundin P. Pre-existing immunity to adeno-associated virus (AAV)2 limits transgene expression following intracerebral AAV2-based gene delivery in a 6-hydroxydopamine model of Parkinson’s disease. J. Gene Med. 2014;16:300–308. doi: 10.1002/jgm.2779. [DOI] [PubMed] [Google Scholar]
- 23.Peden C.S., Burger C., Muzyczka N., Mandel R.J. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanftner L.M., Suzuki B.M., Doroudchi M.M., Feng L., McClelland A., Forsayeth J.R., Cunningham J. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol. Ther. 2004;9:403–409. doi: 10.1016/j.ymthe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Treleaven C.M., Tamsett T.J., Bu J., Fidler J.A., Sardi S.P., Hurlbut G.D., Woodworth L.A., Cheng S.H., Passini M.A., Shihabuddin L.S., Dodge J.C. Gene transfer to the CNS is efficacious in immune-primed mice harboring physiologically relevant titers of anti-AAV antibodies. Mol. Ther. 2012;20:1713–1723. doi: 10.1038/mt.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hocquemiller M., Giersch L., Audrain M., Parker S., Cartier N. Adeno-Associated Virus-Based Gene Therapy for CNS Diseases. Hum. Gene Ther. 2016;27:478–496. doi: 10.1089/hum.2016.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sondhi D., Johnson L., Purpura K., Monette S., Souweidane M.M., Kaplitt M.G., Kosofsky B., Yohay K., Ballon D., Dyke J. Long-term expression and safety of administration of AAVrh.10hCLN2 to the brain of rats and nonhuman primates for the treatment of late infantile neuronal ceroid lipofuscinosis. Hum. Gene Ther. Methods. 2012;23:324–335. doi: 10.1089/hgtb.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg B., Butler J., Felker G.M., Ponikowski P., Voors A.A., Pogoda J.M., Provost R., Guerrero J., Hajjar R.J., Zsebo K.M. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther. 2016;23:313–319. doi: 10.1038/gt.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrington E.A., Sloan J.L., Manoli I., Chandler R.J., Schneider M., McGuire P.J., Calcedo R., Wilson J.M., Venditti C.P. Neutralizing Antibodies Against Adeno-Associated Viral Capsids in Patients with mut Methylmalonic Acidemia. Hum. Gene Ther. 2016;27:345–353. doi: 10.1089/hum.2015.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rincon M.Y., Prada C.E., Lopez M., Castillo V., Echeverria L.E., Serrano N. Determination of Anti-Adeno-Associated Viral Vector Neutralizing Antibodies in Patients With Heart Failure in the Cardiovascular Foundation of Colombia (ANVIAS): Study Protocol. JMIR Res. Protoc. 2016;5:e102. doi: 10.2196/resprot.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corden A., Handelman B., Yin H., Cotrim A., Alevizos I., Chiorini J.A. Neutralizing antibodies against adeno-associated viruses in Sjögren’s patients: implications for gene therapy. Gene Ther. 2017;24:241–244. doi: 10.1038/gt.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calcedo R., Franco J., Qin Q., Richardson D.W., Mason J.B., Boyd S., Wilson J.M. Preexisting Neutralizing Antibodies to Adeno-Associated Virus Capsids in Large Animals Other Than Monkeys May Confound In Vivo Gene Therapy Studies. Hum. Gene Ther. Methods. 2015;26:103–105. doi: 10.1089/hgtb.2015.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapti K., Louis-Jeune V., Kohlbrenner E., Ishikawa K., Ladage D., Zolotukhin S., Hajjar R.J., Weber T. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol. Ther. 2012;20:73–83. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun L., Tu L., Gao G., Sun X., Duan J., Lu Y. Assessment of a passive immunity mouse model to quantitatively analyze the impact of neutralizing antibodies on adeno-associated virus-mediated gene transfer. J. Immunol. Methods. 2013;387:114–120. doi: 10.1016/j.jim.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 37.Peden C.S., Manfredsson F.P., Reimsnider S.K., Poirier A.E., Burger C., Muzyczka N., Mandel R.J. Striatal readministration of rAAV vectors reveals an immune response against AAV2 capsids that can be circumvented. Mol. Ther. 2009;17:524–537. doi: 10.1038/mt.2008.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calcedo R., Wilson J.M. AAV Natural Infection Induces Broad Cross-Neutralizing Antibody Responses to Multiple AAV Serotypes in Chimpanzees. Hum. Gene Ther. Clin. Dev. 2016;27:79–82. doi: 10.1089/humc.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]