Abstract
We present a case of ovarian clear-cell carcinoma that was initially diagnosed as adenocarcinoma of lung origin. This is an instructive diagnostic pitfall for clinicians and pathologists because of the unusual clinical course, small biopsy material, and noteworthy immunophenotype of the carcinoma. Imaging analysis identified only lung and liver lesions. In addition, the biopsy specimen from the lung was TTF-1 negative and napsin A positive, which is still possible for cancer of lung origin. Postmortem examination found that the cancer should be classified as ovarian clear-cell carcinoma distinguished by positive staining for napsin A and paired-box gene 8 (PAX8). Although PAX8 may not be usually investigated when tumoral lesions are identified in only the lung and liver, it is important to keep the necessity of PAX8 in mind to excluding carcinoma of Müllerian, renal, or thyroid origin.
Keywords: Occult cancer, Ovarian clear-cell carcinoma, Adenocarcinoma of lung, Napsin A
1. Introduction
Occult carcinoma is a cancer with unidentified primary even though apparent metastatic lesions are identified. The condition often misleads clinicians and pathologists in identifying the primary lesion of the malignancy, hindering appropriate diagnosis and optimal treatment. In this report, we present an instructive case with unusual clinical course of ovarian clear-cell carcinoma (CCC) that could be clinically, histopathologically, and immunophenotypically misdiagnosed as primary adenocarcinoma of the lung.
2. Case report
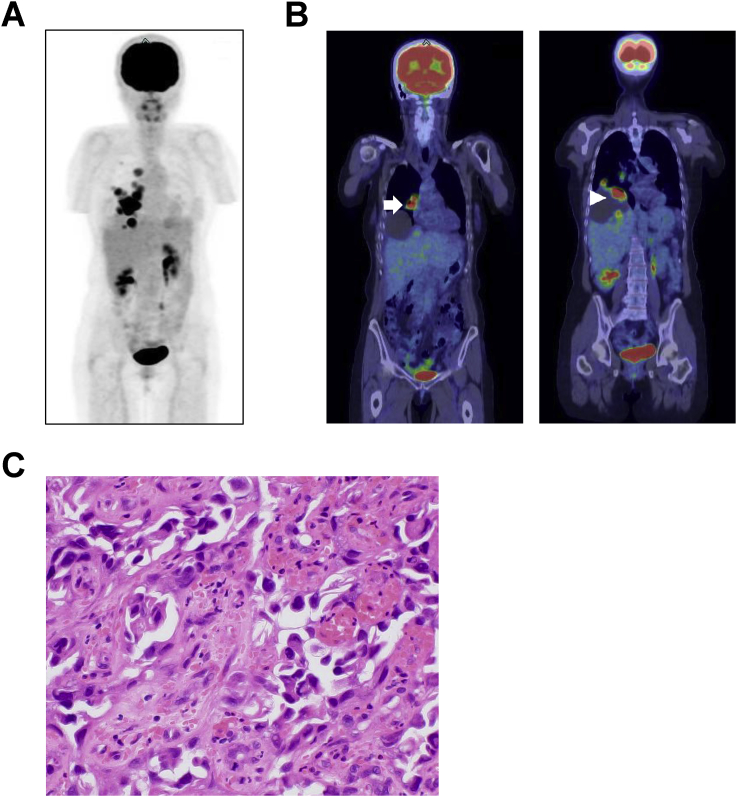
A 55-year-old woman with a history of endometrial cyst in the left ovary 24 years earlier had a respiratory illness. She visited our hospital and positron emission tomography and computed tomography (PET/CT) imaging found right lung and liver lesions (Fig. 1A and B). There was no lower abdominal mass suggesting gynecological malignancy. From the imaging analysis, the clinician suspected a primary lung carcinoma that had metastasized to the liver or vice versa. Circulating levels of cancer antigen 15-3 and cytokeratin fragments were higher than the reference ranges at 131.9 U/mL and 3.1 ng/mL, respectively. Neuron-specific enolase, carcinoembryonic antigen (CEA), protein induced by vitamin K absence-II, and α-fetoprotein were within the reference ranges. For identifying the primary lesion, transbronchial lung biopsy was performed. Histologically, atypical cells with large nuclei and amphophilic cytoplasm proliferated in a hobnail pattern (Fig. 1C) and were positive for cytokeratin (CK) 7 and napsin A (both cytoplasmic); CK20 (cytoplasmic) and thyroid transcription factor 1 (TTF-1) (nuclear) were negative (data not shown). The pathology report revealed that the histopathological findings and immunophenotype of the tumor were consistent with primary adenocarcinoma of the lung. After diagnosis, the patient underwent three courses of chemotherapy (carboplatin plus pemetrexed) for adenocarcinoma of the lung. The patients showed a partial response to chemotherapy based on the Response Evaluation Criteria in Solid Tumors guidelines. However, she declined further chemotherapy. Two months after the last chemotherapy session, she was admitted to our hospital with complaints of a feeling of abdominal fullness due to ascites. Her general condition gradually worsened within a month, and she eventually died.
Fig. 1.
Clinical and biopsy images of the patient. (A) PET maximum intensity projection image at the first visit. Abnormal fluorodeoxyglucose (FDG) uptake is observed in the right chest or upper abdomen. The kidneys, urinary bladder, and part of the left ureter are also imaged in the abdomen. (B) Coronal PET/CT images identifying a mass in the right lung with FDG accumulation (left panel; arrow) and a cystic liver lesion (right panel). Uptake is seen in the cystic lesion (arrowhead). (C) Histopathological biopsy image: atypical cells with large nuclei and amphophilic cytoplasm proliferating in a hobnail pattern. Original magnification: ×200.
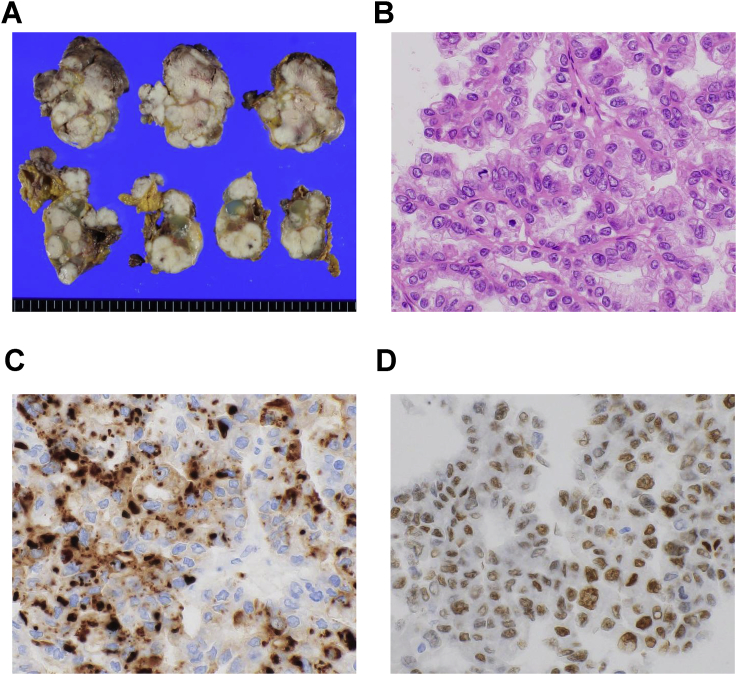
We performed an autopsy with the permission of the bereaved family. We found milky whitish cauliflower-like tumors, often with hemorrhage or mucin production, disseminated in the peritoneum, especially in the lower left abdomen (Fig. 2A). The size of the tumors varied from less than 1 cm to several centimeters. The left ovary was involved, and was not conspicuous. Lung and liver lesions were also found as identified on PET/CT imaging. Histologically, the masses comprised atypical cells with large nuclei and clear or pale eosinophilic cytoplasm forming papillary structures (Fig. 2B). The gross and microscopic appearance suggested ovarian CCC as a differential diagnosis. On immunohistochemistry (IHC), the tumor cells had identical immunophenotype to the lung biopsy specimen for CK7, CK20, TTF-1, and napsin A (Fig. 2C). In addition, nuclear staining for paired-box gene 8 (PAX8) was also positive, suggesting carcinoma of Müllerian origin (Fig. 2D). PAX8 expression in biopsy specimen was examined retrospectively by using IHC; the tumor cell nuclei were positive for PAX8, and negative for most markers of primary adenocarcinoma of lung. Taken these findings together, we finally classified the tumor as ovarian CCC with peritoneal dissemination and lung and liver metastasis. The right ovary and uterus were not infiltrated by the tumor on gross appearance although microscopic carcinoma was found in the right ovary. Ultimately, we could not conclude whether the left or right ovary was the primary site of this tumor.
Fig. 2.
Gross and microscopic morphology of the lesions in the peritoneum. (A) Cut surface of the left lower abdominal lesion. Milky whitish and multinodular mass is observed. (B) Histopathological image of representative lesion: atypical cells with large nuclei and clear or pale eosinophilic cytoplasm forming papillary structures. (C) and (D) IHC analysis of the tumor: Tumor cells are positive for (C) napsin A in the cytoplasm and (D) PAX8 in the nucleus. Original magnification: ×200.
3. Discussion
In this case, respiratory symptoms presumably due to metastasized lesions manifested initially; the typical symptoms of ovarian carcinoma, such as abdominal fullness and cancerous ascites, developed later. Initial PET/CT imaging found only lung and liver lesions but no abdominal lesion. However, at autopsy we clarified that the tumor was occult cancer of the ovary, which had metastasized to the lung and liver. This case highlights the diagnostic pitfall of PET/CT imaging and IHC using a small amount of biopsy specimen.
IHC for a combination of multiple markers is essential in making accurate diagnosis of malignancy. Multivesicular cytoplasmic staining of napsin A, an aspartyl protease processing pulmonary surfactant protein B in type II alveolar cells, is a recent defined as more sensitive marker than TTF-1 in IHC using formalin fixed paraffin embedded specimen for primary lung adenocarcinoma [1]. Nuclear staining for TTF-1 is still a reliable diagnostic marker for adenocarcinoma of lung or thyroid carcinoma, although 25% of lung adenocarcinoma is reported to be negative for it [2,3]. Therefore, pathologists typically use IHC for TTF-1 together with napsin A. The lung tissue biopsied in this case was histologically adenocarcinoma. Because primary adenocarcinoma of the lung presents a multifarious histopathological image, it is difficult for many pathologist to distinguish a primary lung adenocarcinoma from lung metastasis on morphological analysis alone. Although the results of the lung biopsy showing positive napsin A and negative TTF-1 staining were not completely typical, these results were still possible for primary adenocarcinoma of the lung. However, recent reports have shown that 83%–100% of ovarian CCC expressed napsin A, but no other types of ovarian carcinoma did, as assessed by IHC [4,5]. Therefore, it is possible for TTF-1 negative tumor cells, which are simultaneously napsin A positive, to be lung adenocarcinoma or ovarian CCC.
Many cases of ovarian CCC may be found at the early stage [6], although lung or liver metastasis of this carcinoma is not unusual. In contrast, lung adenocarcinoma rarely metastasizes to the ovary or disseminates in the peritoneum; there are only a few reports in the literature [7]. Immunophenotype investigating CK7 and CK20, a standard approach for estimating primary lesion of cancer, are common feature of both adenocarcinoma of lung and ovarian CCC. A previous report suggested TTF-1, CEA, cancer antigen 125, and octamer-binding transcription factor 4 would be useful diagnostic markers in distinguishing between lung adenocarcinoma and ovarian CCC [7]. With these insights, this case taught us that PAX8 should be added to the IHC panel for distinguishing the primary site when the tumor cells are TTF-1 negative and also napsin A positive in female patients. Positive staining for PAX8 in the nucleus is a useful IHC marker implying renal, thyroid, or Müllerian carcinoma including ovarian CCC [8]. By contrast, lung adenocarcinoma should not express PAX8 [9,10]. Although PAX8 may not be usually investigated when tumoral lesions are only identified in the lung and liver, it is important to keep in mind the necessity of PAX8 for excluding carcinoma of Müllerian, renal, or thyroid origin.
In conclusion, the present case highlights a diagnostic pitfall when the primary lesion of a tumor is not identified, even though visible metastatic lesions are detected. Systemic examination of patients, clear dialogue between clinicians and pathologists, and both specialties keeping in mind this potential diagnostic pitfall will provide patients proper evaluation and better treatment of the disease.
Disclosures
The authors have no financial conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2018.10.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Turner B.M., Cagle P.T., Sainz I.M., Fukuoka J., Shen S.S., Jagirdar J., Napsin A. A new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: evaluation of 1674 cases by tissue microarray. Arch. Pathol. Lab. Med. 2012;136:163–171. doi: 10.5858/arpa.2011-0320-OA. [DOI] [PubMed] [Google Scholar]
- 2.Stenhouse G., Fyfe N., King G., Chapman A., Kerr K.M. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J. Clin. Pathol. 2004;57:383–387. doi: 10.1136/jcp.2003.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufmann O., Dietel M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology. 2000;36:8–16. doi: 10.1046/j.1365-2559.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 4.Rekhi B., Deodhar K.K., Menon S. Napsin A and WT 1 are useful immunohistochemical markers for differentiating clear cell carcinoma ovary from high-grade serous carcinoma. APMIS. 2018;126:45–55. doi: 10.1111/apm.12784. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita Y., Nagasaka T., Naiki-Ito A. Napsin A is a specific marker for ovarian clear cell adenocarcinoma. Mod. Pathol. 2015;28:111–117. doi: 10.1038/modpathol.2014.61. [DOI] [PubMed] [Google Scholar]
- 6.Lengyel E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howell N.R., Zheng W., Cheng L. Carcinomas of ovary and lung with clear cell features: can immunohistochemistry help in differential diagnosis? Int. J. Gynecol. Pathol. 2007;26:134–140. doi: 10.1097/01.pgp.0000233166.56385.d0. [DOI] [PubMed] [Google Scholar]
- 8.Ozcan A., Liles N., Coffey D., Shen S.S., Truong L.D. PAX2 and PAX8 expression in primary and metastatic mullerian epithelial tumors: a comprehensive comparison. Am. J. Surg. Pathol. 2011;35:1837–1847. doi: 10.1097/PAS.0b013e31822d787c. [DOI] [PubMed] [Google Scholar]
- 9.Ye J., Hameed O., Findeis-Hosey J.J. Diagnostic utility of PAX8, TTF-1 and napsin A for discriminating metastatic carcinoma from primary adenocarcinoma of the lung. Biotech. Histochem. 2012;87:30–34. doi: 10.3109/10520295.2011.591838. [DOI] [PubMed] [Google Scholar]
- 10.El-Maqsoud N.M., Tawfiek E.R., Abdelmeged A., Rahman M.F., Moustafa A.A. The diagnostic utility of the triple markers Napsin A, TTF-1, and PAX8 in differentiating between primary and metastatic lung carcinomas. Tumour Biol. 2016;37:3123–3134. doi: 10.1007/s13277-015-3964-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.