Abstract
Brucellosis and Q fever impart high morbidity in humans and economic losses among livestock worldwide. However their prevalence is still not fully known in Thailand. We conducted a sero-survey of brucellosis and Q fever in beef, dairy cattle, goat, and sheep herds from Thai communities at the border with Cambodia, a cross-border trading center. Serum samples were tested for brucellosis and Q fever by antibody-based tests at the National Institute of Animal Health, Thailand. We surveyed a total of 520 individuals from 143 herds. Brucellosis herd-level seroprevalence for beef cattle and small ruminants (goats and sheep) was 2.6% (3/117) and 13.3% (2/15) respectively. Q fever herd-level seroprevalence for beef cattle, dairy cattle, and small ruminants was 4.3% (5/117), 27.3% (3/11) and 33.3% (5/15) respectively. This study identified a significant burden of brucellosis and Q fever among small ruminants and dairy cattle at the Thai-Cambodian border.
Keywords: Brucellosis, Q fever, Public health, Thailand, Zoonotic diseases
Highlights
-
•
Brucellosis and Q fever are present in livestock at the Thai-Cambodian border.
-
•
Brucellosis is highly prevalent in small ruminant flocks.
-
•
Q fever is highly prevalent in small ruminants and dairy cattle herds.
-
•
There is a need to strengthen the surveillance system in rural communities at border areas.
1. Introduction
Brucellosis and Q fever are major veterinary public health zoonoses associated with high morbidity in humans [1,2]. They are both considered re-emerging zoonoses in South-East Asia and present a health and economic threat linked to increasing animal trade and production [3,4]. Since the addition of Cambodia to the Association of South-East Asia Nations in 1999, its trade with Thailand has skyrocketed [5]. As a result, the need for appropriate disease surveillance is urgent in the province of Sa Kaeo, the entry point between Thailand and Cambodia.
In 2003, the Department of Livestock Development (DLD) started a national ‘brucellosis free’ campaign for dairy cows, goats, and sheep farms. No dairy herd was detected infected in Sa Kaeo province over the past 3 years (Ekgatat, unpublished data) and testing for small ruminants was not done systematically but rather conveniently. As for Q fever, no systematic and active data has recently been collected in livestock in Sa Kaeo province, and a DLD report from 2011 suggests the absence of Q fever in Thailand among livestock [6], despite human cases reported in 2012 [7]. There is thus no recent and precise estimate of prevalence for brucellosis and Q fever within each production type, and risk factors specific to Thailand are poorly understood.
Therefore, the aims of the study were to (1) determine the seroprevalence of brucellosis and Q fever at the herd level among cattle and small ruminant farms at the Thai-Cambodian border, and (2) identify possible risk factors associated with Brucella spp. and Coxiella burnetii seropositivity.
2. Materials and methods
2.1. Study design and study population
We conducted a cross-sectional study in Khlong Hat and Aranyaprathet districts in Sa Kaeo province, Thailand, which border Cambodia, and are major districts in terms of agriculture production.
The study unit was the herd, defined as animals from one unique production type (beef cattle, dairy cattle, goats, or sheep) owned by the same household and kept in the same location. Brucellosis in dairy cows was not a focus of our study since it was already being targeted by the DLD. All livestock in this area is of Thai origin but is sometimes sold to Cambodian farmers. The study was granted IACUC ethical approval.
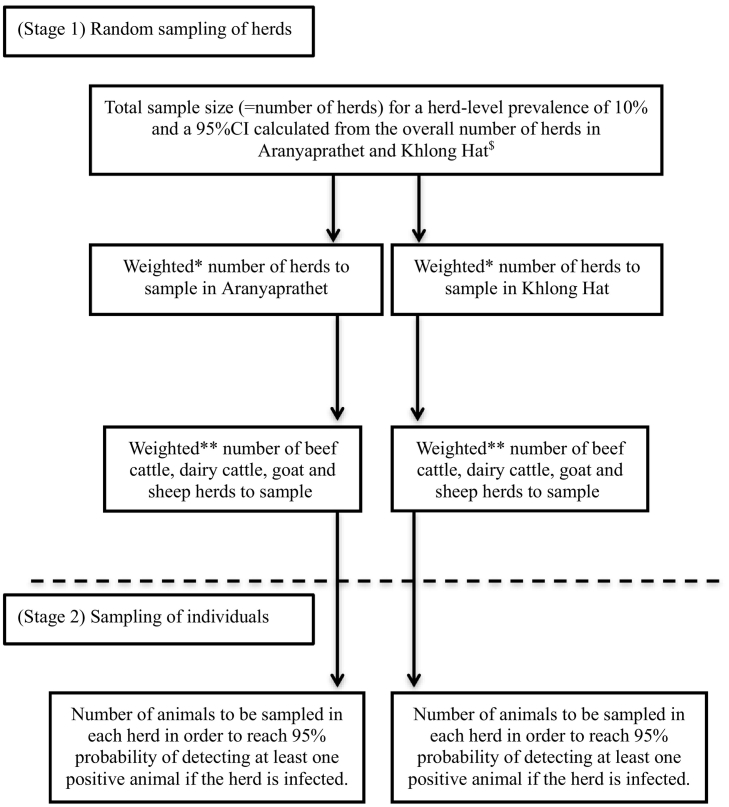
Data collection was based on a 2-stage random sampling design, at the herd-level and individual level. The sampling scheme is presented in Fig. 1. The herd-level sample size was calculated based on an expected herd-level prevalence for Q fever of 10% for all species [4, Ekgatat unpublished data]. The animal-level sample size was calculated with an adapted function from Musallam et al., 2015 for variable herd size [8].
Fig. 1.
Sampling scheme: two-step random sampling for sampling of herds and individuals by production type.
*Proportional to the ratio of the total number of herds in the district and the total number of herds in both district.
**Proportional to the number of herds from each production type in the district mentioned.
The figure presents the two stages of sampling used for data collection: sampling at the herd-level and sampling at the individual level.
2.2. Data collection
Herds were randomly selected in each province from an alphabetical list provided by the DLD. If an owner refused to participate, another herd was randomly selected. The inclusion criterion for herds was being within the administrative borders of the district. Exclusion criteria were refusing to participate and being unable to answer more than 50% of the questionnaire.
Within each selected herd, blood samples were collected from both male and female animals when possible. The individuals were selected conveniently. Inclusion criteria for individuals were being older than 6 months and not being vaccinated against brucellosis nor Q fever. Exclusion criteria were being in gestation and impossibility of drawing blood.
For each animal sampled, the age, sex, and body score of the animal were recorded. A survey of the farm was conducted to collect epidemiological information on the farmer's socio-economic status, the herd health history, the farm characteristics and location, and management practices.
2.3. Serological analyses
Sera were tested for Brucella spp. and Coxiella burnetii at the National Institute of Animal Health, Bangkok, Thailand. Testing for Brucella spp. consisted in parallel testing with the Rose Bengal Test (RBT) and the indirect Enzyme-Linked-Immunosorbent-Assay (iELISA IDEXX) with a sample-to-positive ratio cut-off of 80%, followed by the Complement Fixation Test (CFT APHA Scientific). Sera negative with both RBT and iELISA were considered negative for brucellosis. Sera positive for any of these tests were further tested by CFT with a cut-off of 20 ICFTU/ml. The RBT antigen was produced from B. abortus strain 99, Bureau of Veterinary Products. The antigen was tested with a secondary standard serum identical to the OIEISS one, and verified by ANSES [9,10].
Testing for Coxiella burnetii was done by iELISA (IDEXX) with a sample-to-positive ratio cut-off of 40% [11].
An animal with a positive serum was considered infected. A herd was considered positive for brucellosis or Q fever if at least one animal in the herd was seropositive for Brucella spp. or Coxiella burnetii, respectively.
2.4. Data management and statistical analyses
Goats and sheep were regrouped under the “small ruminants” denomination, to mirror the similarity in management practices. The prevalence for brucellosis and Q fever, and their 95% confidence interval (95%CI), were calculated at the herd level.
To analyze factors associated with brucellosis or Q fever, variables of interest were chosen based on biological plausibility and frequencies among the herds sampled. We explored the association between disease prevalence and farm-related activities such as mixing of livestock species, practices regarding the animals' placenta, use of manure, water source for the animals, presence of wild animals on the farm, and access of pets to the livestock area. We calculated the prevalence ratio and its 95%CI, as well as the associated p-value [12], for each of the selected variables in order to test for associations between exposure and positivity of the herd. We used a p-value cut-off of 0.05 for significance. The analysis was performed in R.
3. Results
Between June and August 2015, we contacted a total of 145 herds to offer them the opportunity to enroll in the study. Of these, two herds did not have any animals anymore. All other owners accepted to enroll in the study. A total of 143 herds were visited and included in the final analysis.
The overall herd-level prevalence was 4.8% (95%CI 2.3–9.5) and 11.4% (95%CI 7.3–17.3) for brucellosis and Q fever respectively. The prevalence by production type is presented in Table 1. Two small ruminant and one beef cattle herds were infected with both brucellosis and Q fever.
Table 1.
Estimated herd-level seroprevalence and 95% confidence intervals (95%CI) for brucellosis and Q fever by production type.
| Production type | Number of herds sampled | Number of individual samples | Number of positive herds - Seroprevalence (95%CI) |
|
|---|---|---|---|---|
| Q fever | Brucellosis | |||
| Overall | 143 | 530 | 13 11.4% (7.3–17.3) |
7 4.8% (2.3–9.5) |
| Beef cattle | 117 | 319 | 5 4.3% (1.8–9.6) |
3 2.6% (0.9–7.3) |
| Dairy cattle | 11 | 69 | 3 27.3% (9.7–56.6) |
– |
| Small ruminants | 15 | 142 | 5 33.3% (15.2–58.3) |
2 13.3% (3.7–37.9) |
| Goats | 12 | 114 | 4 33.3% (13.8–60.9) |
1 8.3% (1.5–35.4) |
| Sheep | 3 | 28 | 1 33.3% (6.15–79.2) |
1 33.3% (6.2–79.2) |
Casting of placenta in the field yielded high prevalence ratios for brucellosis in beef cattle (PR = 3.9 (95%CI 0.4–43.0), p = 0.26). Use of ground water source for animals yielded high prevalence ratios for Q fever in dairy cattle (PR = 2.2 (95%CI 0.9–5.9), p = 0.10). All other variables yielded prevalence ratios close to one and were not significantly associated with seropositivity of the herd.
4. Discussion
Our study shows a significant burden associated with brucellosis among small ruminants and Q fever among dairy cattle at the Thai-Cambodian border. Knowing the increasing movements of livestock and human workforce, associated with a change in animal production, brucellosis and Q fever have the potential to represent a public health threat across the Thai-Cambodian border.
The seroprevalence encountered for all species and diseases is concerning. Brucellosis prevalence was the most alarming in small ruminant herds and Q fever prevalence was highest for small ruminant and dairy cattle herds. Similar results have been reported in Malaysia, where the herd-prevalence of goat brucellosis has been estimated to be 7.0% [13,14] and Q fever has been shown to be present in goats and dairy cattle [15]. Data from 2012 to 2013 on Q fever seroprevalence in the provinces of Chiang Mai and Nakorn Ratchasima was recently published and confirmed that Q fever exists in Thailand, with a high prevalence in dairy cattle [16]. Dairy cattle and small ruminants should thus become a priority for future control programs in Thailand.
Although none of the explored practices were significant, our study suggests that non-destruction of the placenta and ground water consumption may increase the risk of transmission of brucellosis in beef cattle herds and Q fever within dairy cattle herds, respectively. Groundwater and pond water seem to be associated with higher risk of contamination with brucellosis for livestock and for exposed professions when compared to tap water consumption [17], potentially due to external contamination. Non-destruction of the placenta, on the other hand, is a well-known risk factor for herd contamination and human infection with both diseases [2,18].
Our results should be discussed in light of some limitations. The number of herds and animals selected was based on assumptions on Q fever seroprevalence, which turned out to be lower for brucellosis and for intra-herd seroprevalence. This resulted in a small sample size and wide confidence intervals, and limits the power of our study to determine risk factors for herd infection with Q fever and brucellosis. In addition, potential information bias could have resulted from the interviews. Based on the prevalence results and the potential risk factors highlighted in this study, further, larger studies in Thailand are needed, ideally testing for Q fever and brucellosis separately.
In conclusion, brucellosis and Q fever are present in livestock at the Thai-Cambodian border, and could represent a public health threat for both countries. Strengthening the surveillance system in rural communities is imperative in order to evaluate the transmission of the two diseases to humans and to focus our efforts on at-risk populations.
Disclosures
No competing financial interests exist. The authors report no conflict of interest. All authors have read and approved the final article.
Financial support
The project was funded by the Wilbur G Downs Fellowship, the Coca-Cola foundation grant, Yale South-East Asia Studies Council grant and USAID. The funding sources had no involvement in the study design, collection of data, analysis nor in the writing of the report or the decision to submit the article for publication.
Authors' contributions
S.C., E.W., M.E., A.I.K., and S.H. designed the research study. S.C. and E.W. acquired the data. M.E. contributed essential reagents or tools and performed laboratory testing. S.C. analyzed the data. A.I.K. and S.H. supervised the research. S.C., M.E., and S.H. wrote the paper. E.W. and A.I.K. revised the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
This project would not have been possible without the help of Ms. Paphanij Suangtho, Mrs. Arthicha Wongkumma, Ms. Apiladee Soonngam, Mrs. Punnarai Smithsuwan, Mr. Cherdchai Darajang and Dr. Pravit Choomkasien from the Bureau of Epidemiology, Ministry of Public Health, Thailand, Mr. Prawut Purisapun from Aranyaprathet District Livestock Office, Mr. Cherdchai Arsawaderose from Khlong Hat District Livestock Office, Mr. Manu Chaichana, artificial insemination technical officer for Aranyaprathet Office, Dr. Ampan Welutanti, Head of Sa Kaeo Provincial Livestock Office, Dr. Reka Kanitpun, head of Immunology section, NIAH and Ms. Kaewjaranai Youngkao from the Faculty of Veterinary Medicine, Khon Kaen University, Thailand. We also thank Jennifer Downs (Center for Global Health, Weill Cornell Medicine), Forrest Jones (Centers for Disease Control and Prevention), Emmanuelle Gilot-Fromont (Université de Lyon, VetagroSup), Maria-Halima Laaberki (Université de Lyon, VetagroSup), and Tristan Ferry (International Center for Research in Infectiology, Université Claude Bernard Lyon 1) for their feedback and insightful comments on this manuscript.
References
- 1.Franco M.P., Mulder M., Gilman R.H., Smits H.L. Human brucellosis. Lancet Infect. Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 2.Porter S.R., Czaplicki G., Mainil J., Guattéo R., Saegerman C. Q fever: current state of knowledge and perspectives of research of a neglected zoonosis. Int. J. Microbiol. 2011:248418–248422. doi: 10.1155/2011/248418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suputtamongkol Y., Rolain J.M., Losuwanaruk K., Niwatayakul K., Suthinont C., Chierakul W. Q fever in Thailand. Emerg. Infect. Dis. 2015;9:1186–1188. doi: 10.3201/eid0909.030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manosuthi W., Thummakul T., Vibhagool A., Vorachit M., Malathum K. Case report: brucellosis: a re-emerging disease in Thailand. SE Asian J. Trop. Med. Public Health. 2004;35:109–112. [PubMed] [Google Scholar]
- 5.Chemsripong S. INTECH Open Access Publisher; 2012. The Extent of Intra Industry Trade Between Thailand and ASEAN Economic Community (AEC) [Google Scholar]
- 6.Department of Livestock Development (DLD) Animal Health in Thailand 2011. 2012. http://cdn.aphca.org/dmdocuments/PAP_12_Thailand%20Animal%20Health%202011_DLD.pdf Available at. (Accessed March 14, 2015)
- 7.Pachirat O., Fournier P.E., Pussadhamma B., Taksinachanekij S., Lulitanond V., Baggett H.C. The first reported cases of Q fever endocarditis in Thailand. Infect. Dis. Rep. 2012;4:e7. doi: 10.4081/idr.2012.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musallam I.I., Abo-Shehada M., Omar M., Guitian J. Cross-sectional study of brucellosis in Jordan: prevalence, risk factors and spatial distribution in small ruminants and cattle. Prev. Vet. Med. 2015;118:387–396. doi: 10.1016/j.prevetmed.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Ekgatat M., Kanitpun R., Khunchit P., Arampong W., Raksajit S., Thammasart S. Comparison of serological tests for antibody detection against Brucella melitensis infection in goats. Kasetsart Veterinarians. 2010;20:19–26. [Google Scholar]
- 10.Wongkasemjit S., Kanitpun R., Ekgatat M. National Institute of Animal Health; Bangkok, Thailand: 2014. Comparison of Serological Tests for Antibody Detection Against Brucella abortus Infection in Cattle and Buffaloes, Thailand-Japan Animal Health Research. The 3rd Thailand - Japan Joint Conference on Animal Health 2014, July 16–18.http://niah.dld.go.th/th/images/stories/213/icon/xTJJC2014_proceeding.compressed.pdf Available at. [Google Scholar]
- 11.Horigan M.W., Bell M.M., Pollard T.R., Sayers A.R., Pritchard G.C. Q fever diagnosis in domestic ruminants: comparison between complement fixation and commercial enzyme-linked immunosorbent assays. J. Vet. Diagn. Investig. 2011;23:924–931. doi: 10.1177/1040638711416971. [DOI] [PubMed] [Google Scholar]
- 12.Altman D.G., Bland J.M. How to obtain the p value from a confidence interval. BMJ. 2011;343:d2304. doi: 10.1136/bmj.d2304. [DOI] [PubMed] [Google Scholar]
- 13.Anka M.S., Hassan L., Adzhar A., Khairani-Bejo S., Bin Mohamad R., Zainalet M.A. Bovine brucellosis trends in Malaysia between 2000 and 2008. BMC Vet. Res. 2013;9:230. doi: 10.1186/1746-6148-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bamaiyi P.H., Hassan L., Khairani-Bejo S., Zainal-Abidin M., Ramlan M., Adzhard A. The prevalence and distribution of Brucella melitensis in goats in Malaysia from 2000 to 2009. Prev. Vet. Med. 2015;119:232–236. doi: 10.1016/j.prevetmed.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Bina Rai S., Kamaludin F., Chow T.S., Yoon C.K. First documented zoonotic case of Q fever in penang, Malaysia. OSIR. 2011;4:1–5. [Google Scholar]
- 16.Doung-Ngern P., Chuxnum T., Pangjai D., Opaschaitat P., Kittiwan N., Rodtian P. Seroprevalence of coxiella burnetii antibodies among ruminants and occupationally exposed people in Thailand, 2012-2013. Am. J. Trop. Med. Hyg. 2017;96:786–790. doi: 10.4269/ajtmh.16-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Talafhah A.H., Lafi S.Q., Al-Tarazi Y. Epidemiology of ovine brucellosis in awassi sheep in northern Jordan. Prev. Vet. Med. 2003;60:297–306. doi: 10.1016/s0167-5877(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 18.Díaz Aparicio E. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev. Sci. Tech. Off. Int. Epiz. 2013;32:53–60. [PubMed] [Google Scholar]