Abstract
Interleukin (IL)-12 and IL-23 that share subunit p40 are important cytokines in the pathogenesis of inflammatory bowel disease. We reported that mouse p40 peptide-based vaccines ameliorated intestinal inflammation in the prevention of trinitrobenzene sulfonic acid (TNBS)–induced murine colitis model. Here, we evaluated whether administration of the vaccine after establishment of colitis would be effective in modifying both TNBS-induced and dextran sulfate sodium (DSS)–induced chronic colitis and the underlying immune mechanisms. We further examined whether vaccination could exacerbate infections. Chronic colitis was developed by either intrarectally administrating TNBS or drinking 4% DSS water. Vaccination started after two TNBS administrations or 7 days of DSS treatment. Results showed that administrating p40 vaccine induced high tittered antibodies to IL-12 and IL-23, improved clinical scores, reduced intestinal inflammation and fibrosis, and down-regulated proinflammatory cytokine productions in colon tissue, compared with control mice. Furthermore, in lamina propria mononuclear cells and/or mesenteric lymph nodes, mice immunized with p40 peptide vaccine exhibited high ratios of Treg/Th1 and Treg/Th17 cells and increased IL-10 expression in CD11c+IL-10+cells. In mice infected with lung chlamydia, in which the protective role of Th1/Th17 is well documented, vaccine immunization did not increase lung bacterial burden. We conclude that p40 vaccine may provide a potential and safe approach for treatment of IBD.
Keywords: immunotherapy, IL-12/IL-23p40 vaccine, TNBS-induced colitis, and DSS-induced colitis
INTRODUCTION
Inflammatory bowel disease (IBD) refers to idiopathic intestinal inflammation, which is traditionally classified as Crohn’s disease (CD) and ulcerative colitis (UC).1, 2 Studies have demonstrated that Th1 and Th17 cells play important roles in the pathogenesis of Crohn’s disease.3–5 Interleukin-12 is required for an effective polarization of naïve T helper cells to the Th1 phenotype characterized by the expression of IFN-γ, while IL-23 stabilizes the proliferation of Th17 cells. Overproduced IL-12 and IL-23 have been found in the Crohn’s disease patients.4, 6 As IL-12 and IL-23 share p40 subunit, IL-12/IL-23p40 has been considered as the target for the treatment of Crohn’s disease.7 Monoclonal antibodies (mAb) against IL-12/IL-23p40 have been developed and tested in clinical studies.8–11 Briakinumab and Ustekinumab are human monoclonal antibodies against IL-12/IL-23p40, which induced clinical response and remission in a certain subtype of patients with CD.8–11 In a phase 2b trial, although the primary end point of clinical remission at week 6 was not met, Briakinumab numerically induced greater rates of remission and response in moderate to severe active CD patients at weeks 6, 12, or 24, when compared with placebo treatment.10 In a phase 3 randomized trial, Ustekinumab treatment induced significant clinical response and remission in moderate to severe CD patients refractory to prior TNF antagonists.11 This clinical improvement in mAb-treated patients may be associated with decreases in Th1-mediated inflammatory cytokines (IL-12, IFN-γ, and TNF) at the site of disease.8
As Crohn’s disease is a chronic inflammatory disease, long-term treatments are required. Monoclonal antibodies have a short half-life, and repeated injections are required to maintain their effects. Adverse reactions include high rates of acute infusion reactions and the development of antibodies to the infused mAb, which reduces the effectiveness of the treatment and occurs in 61 % of the patients receiving infliximab (an mAb to TNF) therapy,12, 13 and also occurs in patients receiving the mAb to IL-12/IL-23p40 treatment.8 To overcome these disadvantages, a new active immunization strategy employing vaccines that target over-expressed endogenous molecules is being investigated.14 This therapeutic vaccine induces relatively long-lasting antibody responses to the target cytokine. The vaccine strategy may provide an add-on supplement to the monoclonal antibody treatment, especially for the maintenance treatment of chronic inflammatory diseases.
We have successfully developed IL-12/IL-23p40 peptide-based vaccines which induce relatively long-lasting antibodies against IL-12, IL-23, and p40.15, 16 The antibodies induced by these vaccines are able to block the biological functions of IL-12 or IL-23 in vitro.15, 17 We have further demonstrated that immunization of mice with the p40 vaccine ameliorates TNBS-induced acute and chronic intestinal inflammation and fibrosis significantly, when the vaccine is given before the induction of the colitis.15, 16 However, the most important issue for a therapeutic strategy is whether this treatment is still effective when given after the disease has been established, especially on a chronic status. A potential concern with the p40 vaccine is that it may impede the normal functions of IL-12 and IL-23 in host defense against infections.
To address these, in the present study we administered the p40 peptide-based vaccine in mice with established TNBS-induced chronic colitis and evaluated clinical effectiveness and the immune mechanisms involved. As dextran sulfate sodium (DSS)–induced colitis represents human ulcerative colitis,18 we also evaluated the effectiveness of this vaccine in mice with established DSS-induced chronic colitis. Furthermore, using a mouse model of chlamydia lung infection in which IL-12 and IL-23 are elevated,19–21 we examined whether this vaccine immunization could exacerbate the severity of chlamydial lung infection.
MATHERAILS AND METHODS
Animals
Female BALB/c mice (7–8 weeks old), purchased from Charles River Laboratories (Saint-Constant, Quebec, Canada), were maintained at the Central Animal Care Services at the University of Manitoba. The animal procedures were performed in accordance with the animal management rules of the local authorities and approved by the University of Manitoba Animal Ethics Committee.
Protocols of Vaccine Immunization and Induction of Colitis
The IL-12/IL-23p40 vaccine, which consists of a peptide of 9 amino acids derived from mouse p40 unit and a carrier protein hepatitis B core antigen (HBcAg), was prepared and identified using the methods previously described.15 TNBS-induced chronic intestinal inflammation was developed by weekly intrarectal injection of TNBS 8 times (0.5–2.5 mg). Four days after the second injection of TNBS, mice were subcutaneously injected 3 times at a 2-week interval with vaccine, carrier (HBcAg), or saline (100 µg, 25 µg, and 25 µg in 200 µl, respectively) (n = 16). Mice were sacrificed 10 days after the last TNBS administration. The protocol is shown in Fig. 1A.
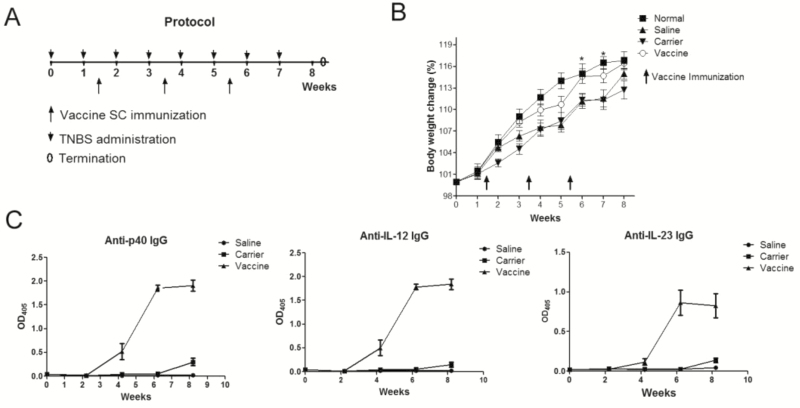
FIGURE 1.
P40 peptide vaccine treatment induces high levels of specific antibody responses and ameliorates body weight loss after TNBS challenges. Mice were intrarectally administrated with TNBS once a week for 8 weeks to induce chronic colitis. Four days later after the second TNBS administration, mice were subcutaneously injected with p40 peptide vaccine, carrier, or saline 3 times at a 2-week interval (n = 16/group). Ten days after the last TNBS delivery, mice were sacrificed. A, protocol of TNBS-induced chronic colitis and vaccine immunization. B, body weight changes. C, antibody responses induced by IL-12/IL-23p40 vaccine.
DSS-induced chronic colitis was achieved by 4 cycles of DSS water treatment. In each cycle, mice received 4% DSS drinking water for 7 days, followed by normal drinking water for 7 days. After the first cycle, mice were subcutaneously injected 3 times with vaccine, carrier, or saline as performed previously (n = 16). Mice were sacrificed 10 days after the last DSS treatment. The clinical scoring of a disease activity index (DAI) for DSS-induced chronic colitis was based on stool consistency and bleeding.22 Stool consistency was categorized as follows: normal, 0; pasty, semiformed, 1; sticky, 2; sticky with some blood, 3; and completely liquid, bloody, or unable to defecate after 10 minutes, 4. Rectal bleeding was categorized as follows: no blood, 0; visible blood in rectum, 1; and visible blood on fur, 2.
Histological examination
Ten percent buffered formalin–fixed and paraffin-embedded colon sections were cut and stained with Haemotoxylin and Eosin for the evaluation of inflammation or Masson’s trichrome for the evaluation of collagens. Histological scoring was evaluated by a pathologist blinded to the source of treatment, based on the method previously described.23 During each histological examination, 3 different parameters were estimated: severity of inflammation, depth of injury, and crypt damage. All values were added to a sum; the maximum possible score was 10.
Soluble Collagen Assay
Colons were homogenized in 0.5M acetic acid containing 1 mg of pepsin (at a concentration of 10 mg of tissue/5 ml of acetic acid solution). The resulting mixture was then incubated and stirred for 24 hours at 4°C. Total soluble collagen content of the mixture was determined with a Sircol Collagen Assay Kit (Biocolor, Carrickfergus, County Antrim, United Kingdom).24 Acid soluble type 1 collagen supplied with the kit was used to generate a standard curve.
Measurements of Antibodies and Cytokines by Enzyme-linked Immunosorbent Assay (ELISA)
Frozen colonic samples were mechanically homogenized in buffer containing 1M Tris-HCl, 3M NaCl, and 10% Triton, supplemented with protease cocktail (Sigma-Aldrich). Samples were then frozen (−70°C) and thawed (37°C) 3 times, followed by centrifugation at 14,000 rpm for 30 minutes at 4°C. Supernatants were frozen at −70°C until assay.
Serum p40-, IL-12-, and IL-23-specific IgG levels were assayed by using ELISA techniques established in our laboratory.16, 17 The results were expressed using optical density at 405 nm (OD405). Serum-specific IgG titers were also assayed by ELISA using pooled sera and the results were expressed using “titer,” the reciprocal of the highest dilution in which the OD405 was twice that of the corresponding control serum when its OD405 was 0.10. Cytokine concentrations in colon tissues were measured by ELISA according to the manufacturer’s instructions. The kits for measuring IL-12p40, IL-17, and TNF-α were purchased from BD Bioscience and the kits for measuring IL-23 and IL-10 were purchased from eBioscience.
Intracellular Staining of Th1, Th17, and Treg Cells
To examine the percentages of Th1 and Th17 cells, 1 × 106 single-cell suspensions were cultured and stimulated for 7 hours with 50 ng/mL phorbol myristate acetate and 1 μg/mL ionomycin (Sigma-Aldrich), with brefeldin A added for the last 4 hours of culture. Cells were harvested, washed, and stained with anti-CD4 mAb (eBioscience). Surface-stained cells were fixed (2% paraformaldehyde) and resuspended in permeabilization buffer (0.5% saponin). This was followed by staining for intracellular IL-17 or IFN-γ (eBioscience). For Foxp3+ Treg staining, cells were fixed in eBioscience fixation and permeabilization buffer after the staining of surface molecules CD4 and CD25. Then the cells were permeabilized in eBioscience buffer and stained for Foxp3 according to the manufacturer’s instruction. Cells were analyzed using a FACSCanto II (BD Biosciences).
Chlamydial Infection and Quantitation of Chlamydial in Vivo Growth
To evaluate whether vaccine immunization increases the susceptibility to lung chlamydial infection, mice were first immunized with vaccine, carrier, or saline 3 times at 2-week intervals. Two weeks later, the mice were anesthetized and inoculated intranasally with 1 × 103 inclusion-forming units (IFU) of Chlamydia muridarum in 40 μl final volume of PBS. Eight days later, the mice were sacrificed. Body weight was monitored weekly from the beginning of the experiment. Serum p40-, IL-12-, and IL-23-specific IgG were assayed every 2 weeks to ensure the levels of specific antibody responses. Lung histology and quantification of the number of chlamydial were performed as described previously.25
Statistical Analysis
Values were expressed as mean ± standard error of mean (SEM). Differences between experimental groups were assessed by 1-way analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison tests (GraphPad Software, San Diego, California, USA). P values < 0.05 were considered statistically significant. In all figures, “*” represents P < 0.05; “**” represents P < 0.01; “***” represents P < 0.001.
RESULTS
P40 Peptide Vaccine Induces Specific IgG Antibodies and Increased Body Weight in TNBS-induced Chronic Colitis
The protocol was shown in Fig. 1A. After the first TNBS challenge, all mice showed obvious body weight loss at the first 2 days, and then gradually recovered in 1 week. After week 4, mice immunized with the p40 peptide vaccine showed significantly increased body weight when compared with mice receiving saline or carrier (Fig. 1B), as after 2 immunizations, the p40 peptide vaccine had induced relatively high levels of antibodies against IL-12, IL-23 and p40 (Fig. 1C). The titers of the serum pooled from p40 peptide vaccine immunized mice were 120,000 for p40-specific IgG, 70,000 for IL-12 specific IgG, and 10,000 for IL-23-specific IgG, which were similar to our previous reports.16 This explained why the body weight in p40 peptide vaccine group was obviously increased after week 4 when compared with carrier and saline groups, indicating that vaccine treatment could improve body weight loss in ongoing chronic colitis.
P40 Peptide Vaccine Ameliorates Intestinal Inflammation in TNBS-induced Chronic Colitis
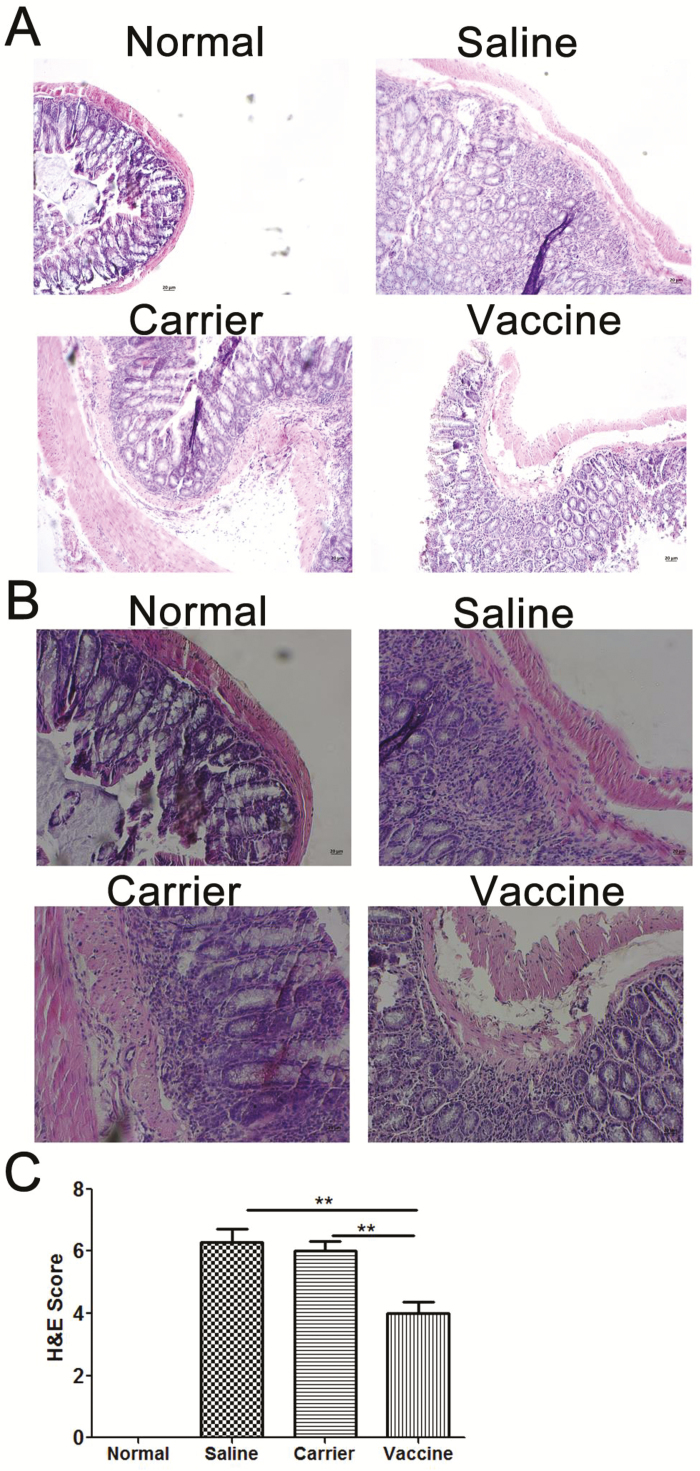
In addition to the improvement of body weight loss, p40 peptide vaccine treatment also ameliorated TNBS-induced chronic intestinal inflammation. Histological analysis revealed that colons from saline and carrier groups exhibited obvious inflammation, including inflammatory cell infiltration, goblet cell reduction, and distorted architecture (Fig. 2A, B), which is similar to what we have found in the prevention study.16 In contrast, colon tissues of vaccine-treated mice showed much less inflammation than controls. The vaccine-induced improvement of colon inflammation was further confirmed by semiquantitative analysis in which treated mice had significantly lower inflammatory scores than those in saline and carrier groups (P < 0.01) (Fig. 2C).
FIGURE 2.
P40 peptide vaccine treatment ameliorates intestinal inflammation in established TNBS-induced chronic colitis. Colonic specimens were formalin-fixed and embedded in paraffin blocks, and then 6-μm sections were stained with hematoxylin and eosin (H&E). A, Representative H&E staining histological images of colon samples (original magnification x 100). B, Representative H&E staining histological images of colon samples (original magnification x 200). C, Semiquantitative analysis of histological inflammation. H&E scores 0–10 were given for each mouse. (**, P < 0.01).
P40 Peptide Vaccine Treatment Inhibits Fibrosis in TNBS-induced Chronic Colitis
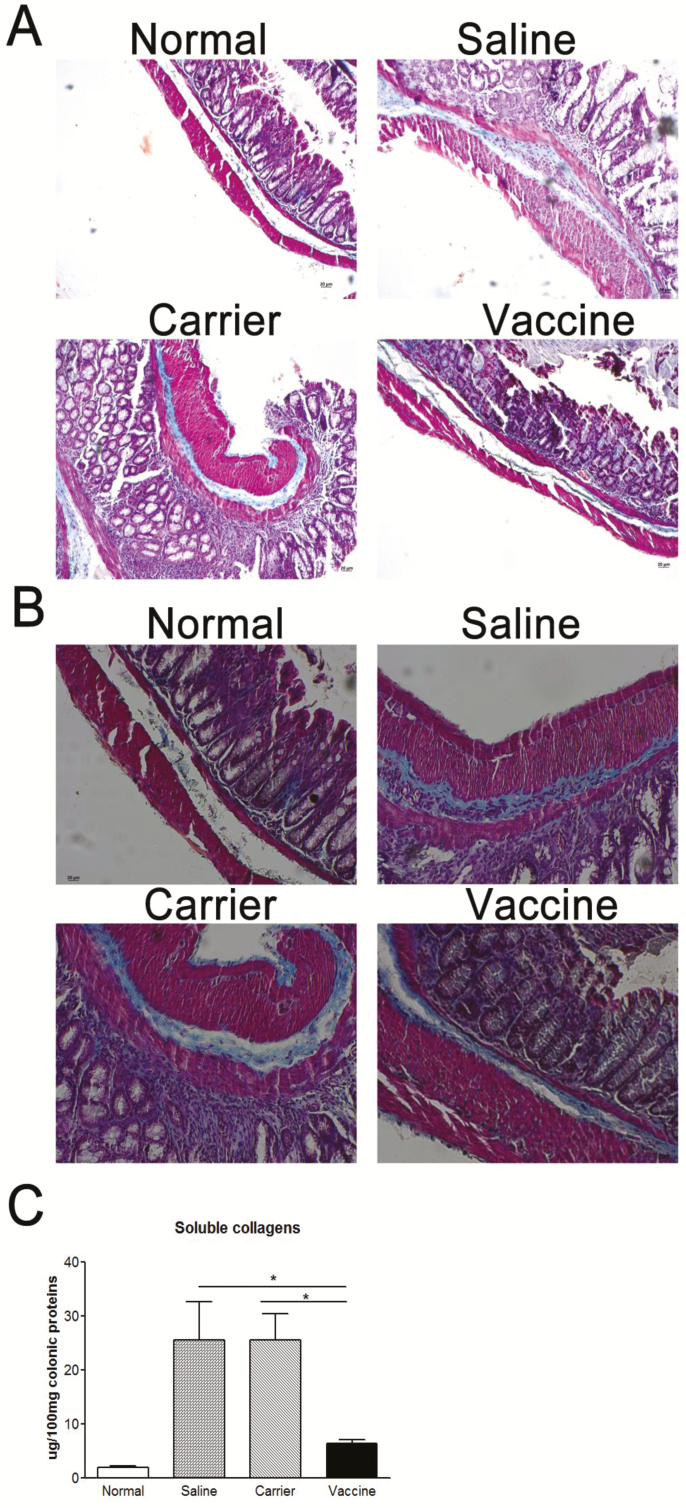
Development of colon fibrosis is one of the major characteristics of chronic colitis. As shown in Fig. 3A and 3B, compared with the normal group, the amount of collagen (the blue color) is increased in subepithelium, deeper layers of the colonic lamina propria, and the muscular layer in the saline and carrier groups. But no significant increase in collagen deposition was observed in p40 peptide vaccine–immunized mice. The reduction in collagen deposition was further confirmed by quantitative measurement of soluble collagen. The amount of soluble collagen in vaccine-treated mice was significantly lower than those in the saline and carrier groups (P < 0.05), which was reduced almost to the normal level (Fig. 3C), confirming that p40 peptide vaccine immunization could intervene the development of colon fibrosis in chronic colitis.
FIGURE 3.
P40 peptide vaccine treatment reduces colon fibrosis in established TNBS-induced chronic colitis. A, Representative colon sections stained with Masson’s trichrome showing collagen deposition (the blue color) (original magnification x 100). B, Representative colon sections stained with Masson’s trichrome showing collagen deposition (original magnification x 200). C, Quantitation of soluble collagens in the colon tissue, measured by a Sircol assay. (*, P < 0.05).
P40 Peptide Vaccine Down-regulates Colon Cytokine Expression in TNBS-induced Chronic Colitis
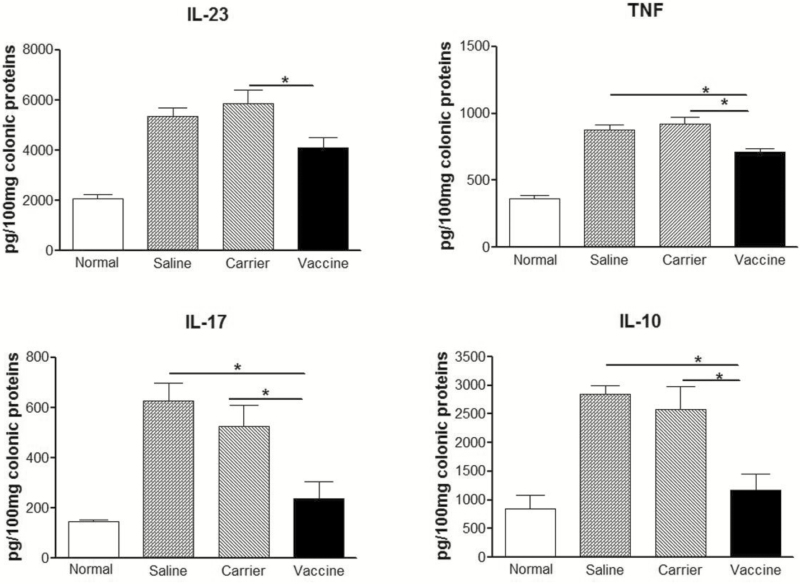
After administrations of TNBS, the cytokine levels of IL-23, IL-17, TNF and IL-10 in colon tissue of saline and carrier groups were significantly higher than those in normal controls (Fig. 4). As expected, these cytokine levels were significantly decreased in p40 peptide vaccine–treated mice (P < 0.05), indicating that vaccine treatment is effective in down-regulating cytokine production in the colonic tissue of chronic colitis.
FIGURE 4.
P40 peptide vaccine treatment down-regulates colon cytokine production in established TNBS-induced chronic colitis. Frozen colonic samples were mechanically homogenized in buffer supplemented with protease cocktail. Samples were centrifuged to get the supernatants. The concentrations of IL-23, IL-17, TNF, and IL-10 in the supernatants were determined by ELISA. (*, P < 0.05).
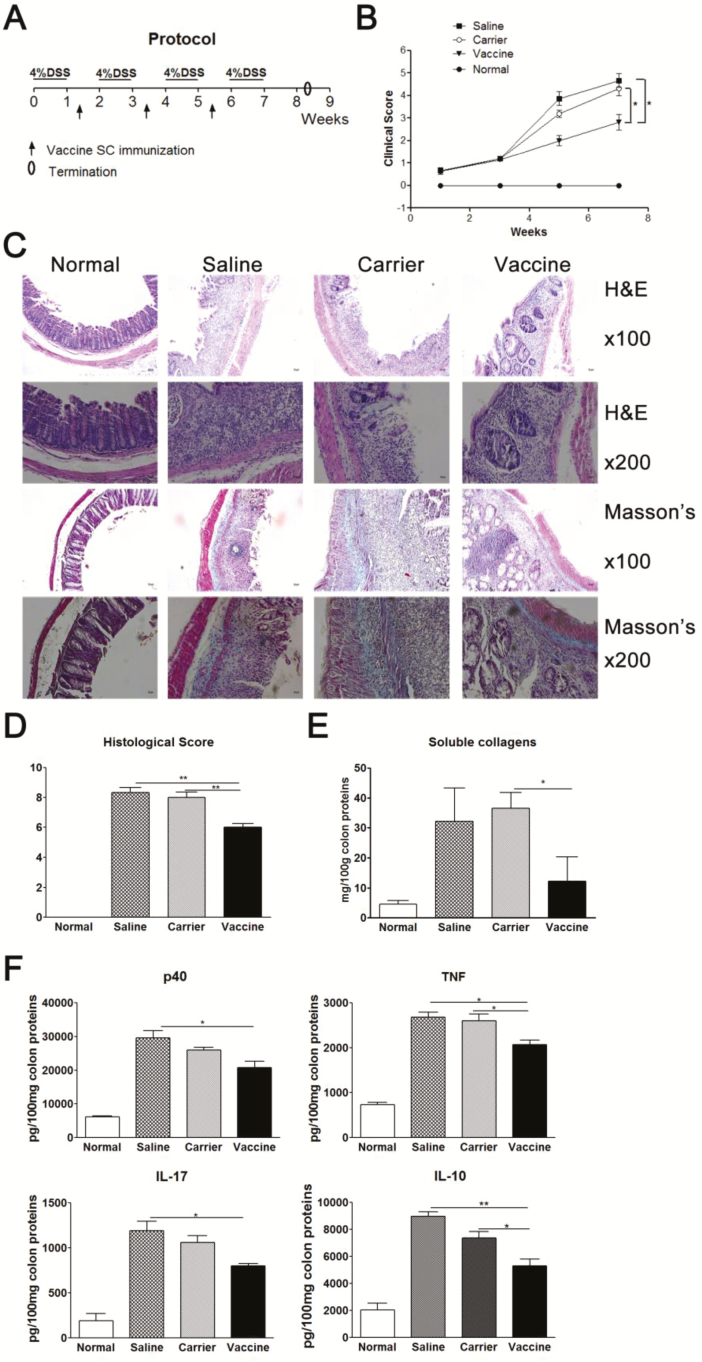
P40 Peptide Vaccine Decreases Clinical Scores and Colon Inflammation in DSS-induced Chronic Colitis
TNBS-induced colitis represents Crohn’s disease, while DSS-induced colitis is similar to ulcerative colitis. To confirm the therapeutic role of the p40 vaccine, DSS-induced chronic colitis was used to evaluate the effectiveness of the p40 vaccine strategy. The protocol of DSS-induced chronic colitis and vaccine immunization was shown in Fig. 5A. Mice first received 4% (wt/vol) DSS drinking water for 7 days to induce intestinal inflammation. After the intestinal inflammation developed, on day 10 mice started to receive injections of the vaccine, vaccine carrier, or saline three times at a 2-week interval. Our results showed that after DSS challenges, the clinical scores were significantly increased in the saline and carrier groups, compared with normal mice (Fig. 5B). But in p40 peptide vaccine–treated mice, the clinical score was significantly lower than in the saline and carrier control groups (P < 0.05). Vaccine treatment also significantly improved colon inflammatory scores (H&E staining, P < 0.01) and soluble collagen deposition (P < 0.05), compared with control groups (Fig. 5C–E). The collagen levels in vaccine-treated mice were close to those of normal mice. The levels of cytokines (TNF, IL-17, IL-12/IL-23p40, and IL-10) in colon tissue were also significantly reduced in vaccine-treated mice (Fig. 5F). These results have demonstrated that administration of the p40 vaccine to ongoing chronic colon inflammation is also effective in a different chronic murine colitis model: DSS-induced colitis.
FIGURE 5.
P40 peptide vaccine treatment improves clinical score and colonic inflammation in established DSS-induced chronic colitis. Mice were given 4% DSS drinking water for 7 days followed by 7 days of regular water for 4 cycles to induce chronic colitis. After the first DSS treatment, on day 10 mice were subcutaneously injected with p40 peptide vaccine, carrier, or saline 3 times at a 2-week interval (n = 16/group). Mice were sacrificed 10 days after the last DSS drinking water. A, Protocol. B, Clinical score. C, Representative figures of H&E staining and Masson’s trichrome staining. D, Colon inflammatory score (H&E staining). E, Soluble collagen production in colon tissue. F, The expression levels of cytokines in colon tissue.
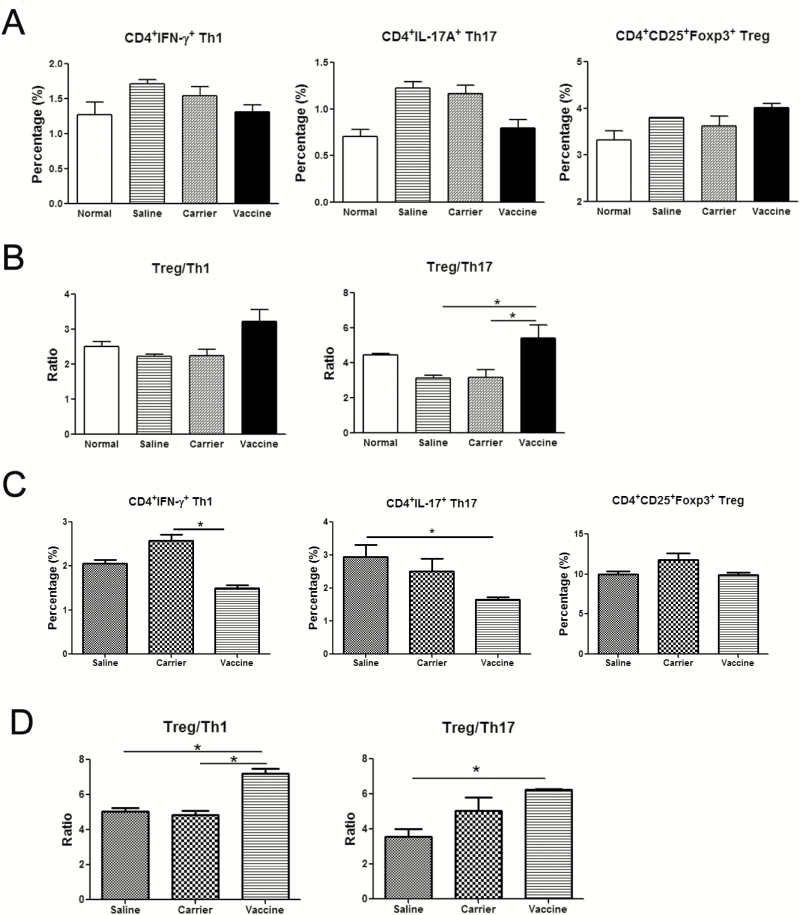
The Effects of P40 Peptide Vaccine on the Percentages of Th1, Th17, and Treg Cells in TNBS-induced Colitis
Crohn’s disease is usually described as a Th1 and Th17 cell–mediated disease, in which Th1 and Th17 cells are increased.4 As IL-12 and IL-23 play important roles in the differentiation of Th1 and Th17 cells, respectively, IL-12/IL-23p40 vaccine might exert its effects through inhibiting these 2 pathways. As indicated in Fig. 6A, the percentages of CD4+IFN-γ+ Th1 and CD4+IL-17+ Th17 cells are increased in mesenteric lymph nodes (MLNs) after TNBS challenges when compared with normal controls. However, p40 peptide vaccine treatment could down-regulate the percentages of Th1 and Th17 cells. In Crohn’s disease, regulatory T cells (Treg) are dysfunctional due to either the defective number or their faulty suppressive function-or both.26 The percentage of CD4+CD25+Foxp3+ Treg in MLNs was also analyzed. Our results showed that vaccine treatment could slightly increase the percentage of Treg, compared with those in saline and carrier groups (P>0.05, Fig. 6A). Although the changes did not reach statistically significant differences, further analysis of the ratios of Treg to Th17 and Treg to Th1 in MLNs showed a significant increase in the p40 peptide vaccine–treated group when compared with the saline and carrier groups (Fig. 6B). Similarly, after p40 peptide vaccine treatment, the percentages of Th1 and Th17 in lamina propria mononuclear cells (LPMCs) were decreased when compared with the saline or carrier group, but there were no significant changes on the percentage of Tregs in LPMCs among these 3 groups (Fig. 6C). The ratios of Treg to Th17 and Treg to Th1 in LPMCs were increased in the vaccine-treated group (Fig. 6D). Taken together, these results indicated that p40 peptide vaccine treatment could rebalance Th1, Th17, and Treg responses in murine colitis.
FIGURE 6.
p40 peptide vaccine treatment rebalances Th1/Th17/Treg responses in TNBS-induced chronic colitis. After stimulation with PMA and inomycin, lymphocytes from mesenteric lymph nodes (MLNs) or lamina propria mononuclear cells (LPMCs) were stained with anti-CD4, anti-IFN-γ ,and anti-IL-17 to detect the percentages of CD4+IFN-γ+Th1 and CD4+IL-17+Th17 cells by flow cytometry. CD4+CD25+Foxp3+ Treg cells were stained according to the manufacturer’s manual. A, The percentages of Th1, Th17, and Tregs in CD4+T cells of MLNs. B, The ratios of Tregs to Th1 and Tregs to Th17 in MLNs. C, The percentages of Th1, Th17, and Tregs in CD4+T cells of LPMCs. D, The ratios of Tregs to Th1 and Tregs to Th17 in LPMCs. (*, P < 0.05).
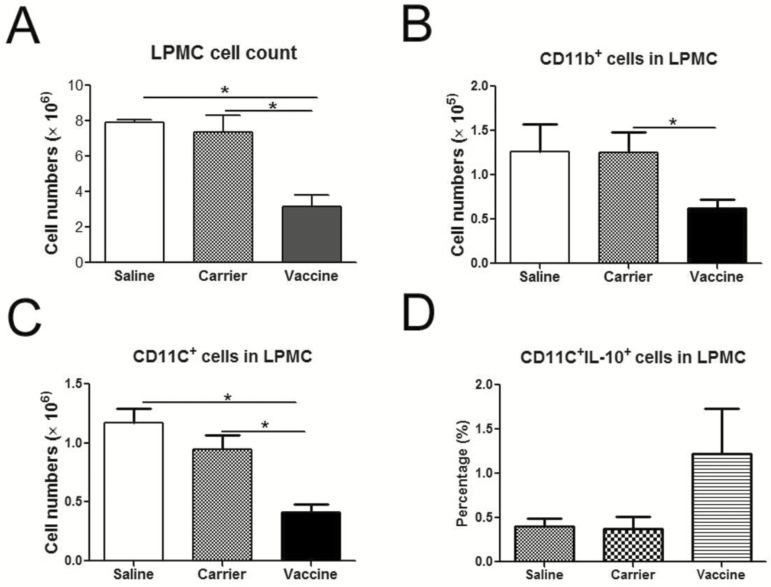
P40 Vaccine Promotes the Production of IL-10 by CD11c+ Cells in LPMCs of TNBS-induced Colitis
IL-12 and IL-23 are mainly produced by activated professional antigen presenting cells (APCs), such as dendritic cells (DCs) and macrophages. IL-12, IL-23, and p40 can also influence the function of these professional APCs, for example, promoting their migration and lipopolysaccharides (LPS)-induced DC maturation.27,28 Here we evaluated the influences of vaccine treatment on CD11b+ cells and CD11c+ cells in LPMCs, which included macrophages and DCs. Compared with the saline and control groups, the total number of LPMCs was significantly decreased in the vaccine treatment group (Fig. 7A, P < 0.05). The numbers of CD11b+ cells (Fig. 7B) and CD11c+ cells (Fig. 7C) in LPMCs were also significantly decreased after vaccine treatment (P < 0.05). To our surprise, the percentage of CD11c+IL-10+ cells in LPMCs was increased after vaccine treatment (Fig. 7D). These results indicated that p40 peptide vaccine treatment decreased the infiltration of CD11b+ cells and CD11c+ cells into local inflamed tissue but increased the production of IL-10 by CD11c+ cells.
FIGURE 7.
The influence of p40 peptide vaccine treatment on DCs and macrophages in LPMCs. Colonic LPMCSs were isolated, and the total cell numbers of LPMCSs were counted. LPMCs were then stimulated with PMA and inomycin for 6 hours and then stained with anti-CD11b, anti-CD11c, and anti-IL-10 antibodies and analyzed by flow cytometry. A, The total cell numbers of LPMCs. B, The cell numbers of CD11b+ macrophages in LPMCs. C, The cell numbers of CD11c+ DCs in LPMCs. D, The percentages of CD11c+IL-10+ cells in LPMCs. (*, P < 0.05).
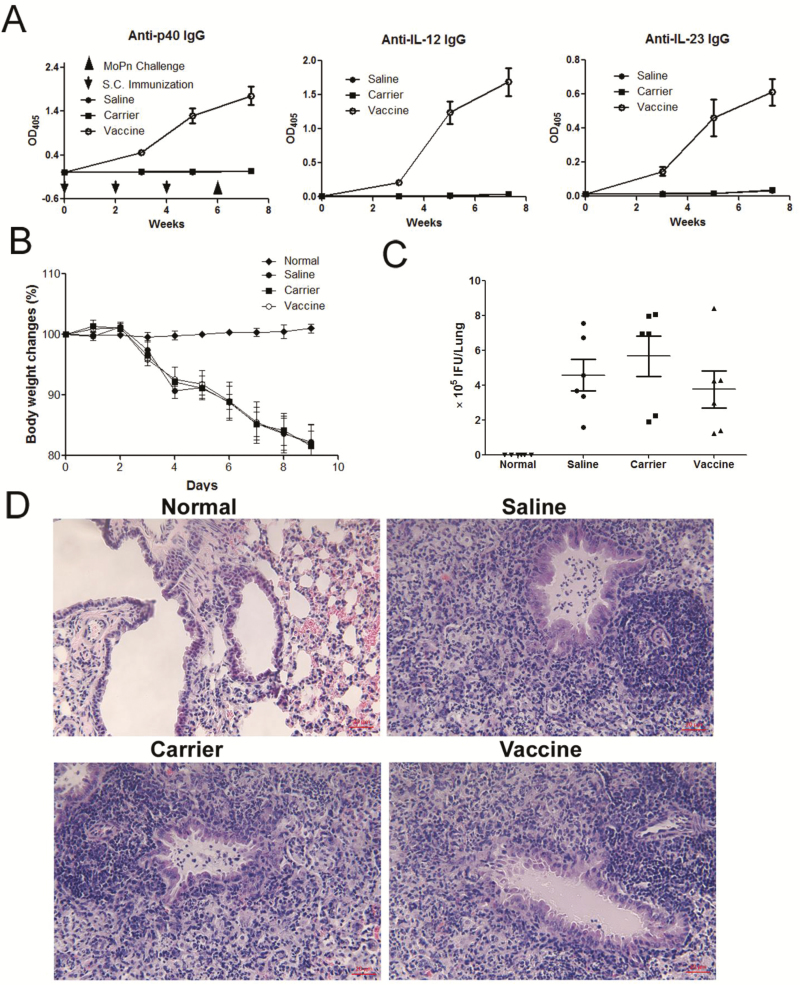
P40 Vaccine Does Not Increase the Susceptibility to Chlamydia Infection
As IL-12 and IL-23 play important roles in the host defense against infections, there is a possibility that employment of IL-12/IL-23p40 vaccine might increase the susceptibility to infections. To address this concern, we tested the effects of p40 vaccine treatment on a mouse model of chlamydial lung infection, in which Th1 and Th17 cells and IL-12 and IL-23 production are vital for the defense against infection.20,29,30 Mice were first immunized with p40 peptide vaccine 3 times to induce antibody responses. After establishing high levels of antibodies against IL-12, IL-23, and p40 (Fig. 8A), at week 6 mice were intranasally administered with Chlamydia muridarum to induce lung infection. All groups of mice receiving the infection demonstrated obvious body weight loss, but there was no significant difference among the saline, carrier, and vaccine groups (Fig. 8B). More importantly, the p40 peptide vaccine–treated group did not show any difference in bacterial burden, when compared with the saline and carrier groups (Fig. 8C) (P > 0.05). Lung histological examination further confirmed that there was no significant difference in inflammation among the saline, carrier, and vaccine groups (Fig. 8D). Taken together, our results suggested that mice immunized with IL-12/IL-23p40 vaccine did not increase in susceptibility to or the disease process of chlamydia lung infection.
FIGURE 8.
P40 peptide vaccination does not increase the susceptibility to Chlamydia muridarum lung infection. Mice were immunized with p40 peptide vaccine, carrier, or saline 3 times at a 2-week interval (n = 6/group). Two weeks after the last immunization, the mice were challenged with Chlamydia muridarum to induce lung infection and monitored body weight changes. Nine days following infection, mice were sacrificed to analyze. A, The levels of specific antibodies against IL-12, IL-23 and p40 induced by the vaccine. B, Body weight changes. C, The burden of chlamydial growth in vivo in the lung tissue. D, H&E staining and light microscopy of lung sections. Original magnification x 200.
DISCUSSION
Our previous studies have confirmed that vaccination of mice with IL-12/IL-23p40 vaccine could prevent the development of TNBS-induced acute and chronic colitis.16 In the present study, we evaluated whether administration of this vaccine after the colitis has been established would still be effective in TNBS-induced chronic murine colitis and whether this vaccine strategy would also be effective in DSS-induced chronic murine colitis, a model representing human ulcerative colitis. Our results revealed that after TNBS- or DSS-induced murine colitis has been developed, p40 peptide–vaccinated mice had significant decreases in clinical score, intestinal inflammation and fibrosis, and levels of p40, IL-23, IL-17, and TNF in colon tissue, compared with the saline and carrier groups.
Crohn’s disease is usually believed to be caused by an overly aggressive Th1 immune response and an excessive IL-23/Th17 pathway activation by bacterial antigens in genetically predisposed individuals.31–35 IL-12, vital for Th1 differentiation to produce IFN-γ, shares IL-12p40 subunit with another cytokine, IL-23. IL-23 stabilizes the proliferation of Th17 cells and promotes the production of IL-17.35, 36 Therefore, blocking IL-12/IL-23p40 might suppress the differentiation of both Th1 and Th17 cells. Our results have shown that Th1 and Th17 cells are increased in murine colitis, confirming previous findings that Th1 and Th17 cells are involved in the pathogenesis of Crohn’s disease. Similar to the results of our previously reported preventive study in TNBS-induced colitis,16 in the present intervention study, mice immunized with IL-12/IL-23p40 vaccine also had decreased percentages of Th1 and Th17 cells in MLN, with significant improvement of body weight loss and reduction of colon inflammation and soluble collagen amounts, which became close to the levels in normal mice.
Impaired balances of Th1/Treg and Th17/Treg responses are also reported in IBD.37 The dysfunction of Tregs in IBD is usually believed to be due to defective number of Tregs or their suppressive function, resulting in uncontrolled intestinal inflammation. Recently, IL-23 has been found to restrain the activity of Treg to drive T-cell-dependent colitis,38 and the homodimer of p40 is found to suppress the expression of CD25 and Foxp3.39 Therefore, targeting IL-12/IL-23p40 using vaccine strategy may promote Treg expansion. Our results indicated that the percentages of CD4+CD25+Foxp3+ Treg in MLNs were slightly increased in the saline and carrier groups after TNBS challenges (no significant difference) and obviously increased in vaccine-treated mice when compared with the normal group (Fig. 6A). The ratios of Treg to Th1 and Treg to Th17 decreased in the saline and carrier groups, especially the ratio of Treg to Th17 (Fig. 6B), which corresponds with the findings reported by Eastaff-Leung et al, in which a significant decrease of Treg/Th17 ratio is observed in the peripheral blood mononuclear cells of IBD patients.37 However, p40 peptide vaccine treatment increases the ratios of Treg to Th1 and Treg to Th17 in contrast to those of the saline and carrier groups (Fig. 6B, D). This data indicates that IL-12/IL-23p40 vaccine–mediated improvement may be achieved via rebalancing Th1/Th17/Treg responses.
Studies already showed that intestinal macrophages and dendritic cells play critical roles in maintaining intestinal homeostasis and are also drivers of the pathology associated with IBD through inappropriate responses to enteric microbial stimuli, inefficient clearance of microbes from host tissues, and impaired transition from pro-inflammatory responses to anti-inflammatory responses that promote resolution.45 The effects of IL-12/IL-23p40 vaccine on macrophages and DCs were explored in the present study. The p40 subunit contributes to both the IL-12 and IL-23 heterodimers. However, p40 can also be secreted as a monomer p40 or a homodimer p80.40, 41 P40 is secreted at a 50-fold higher rate than IL-12 in a murine shock model and at a 10–20-fold excess in stimulated human peripheral blood cells.28 Natural p80 exists at 20%–40% of the total p40 in the serum in the murine model of systemic infection.28 Studies have shown that p80 can act as a macrophage chemoattractant and an inducer of DC migration.42, 43 Recombinant murine p80 can act as the chemoattractant for both rat and mouse macrophages over a dose range of 10–1000 ng/ml.42 And Rusell et al have demonstrated that intratracheal delivery of recombinant murine p80-not recombinant murine IL-12 or p40-promotes the recruitment of macrophages into the airway.44 It has also been reported that migration of DCs from the lung to the draining lymph nodes after exposure to Mycobacterium tuberculosis (Mtb) is defective in mice lacking p40, not in mice lacking IL-12p35. The delivery of p80 to p40-deficient DCs restores Mtb-induced DC migration and the ability of p40-deficient DCs to activate naïve T cells.43 Our results showed that IL-12/IL-23p40 vaccine treatment significantly decreased the total cell numbers and the numbers of macrophages and DCs in LPMCs, suggesting that the infiltration of macrophages and DCs into the inflamed colon tissue was inhibited via blocking the functions of IL-12/IL-23p40 or p80. Our results also showed that IL-10 production was increased in CD11c+ DCs in p40 peptide vaccine–treated mice, suggesting that promoting IL-10 production from CD11c+ DCs may be another mechanism involved in p40 peptide vaccine–mediated improvement. In a future study, we will systemically explore whether IL-12/IL-23p40 peptide vaccine treatment will influence the functions of intestinal macrophages and DCs in the response to enteric microbial stimuli, clearance of microbes, and transition of pro-inflammation or anti-inflammation.
A potential concern with cytokine vaccines is that the long-lasting auto-antibodies against cytokines might increase the susceptibility to certain infections, as IL-12 and IL-23 also plays an important role in the host defense against infections, such as chlamydia infection. The levels of IL-12 and IL-23 are increased in chlamydia infection.21 Neutralization of IL-12 inhibits chlamydia-specific delayed-type hypersensitivity, which is required for chlamydia clearance, delaying chlamydia clearance.30 Recent evidence shows that Th17/IL-17 also contributes to the protection against chlamydial lung infection.29, 46 Therefore, we explored the effects of IL-12/IL-23p40 vaccine on the Chlamydia muridarum lung infection in the present study. Our results showed that immunization of IL-12/IL-23p40 vaccine did not exaggerate this infection or disease process because the body weight loss, Chlamydia muridarum burden, and lung inflammation are comparable among saline, carrier and p40 peptide vaccine–treated groups (Fig. 8), indicating that immunization with IL-12/IL-23p40 vaccine may not increase the susceptibility to Chlamydia muridarum infections. Since host defense against chlamydial lung infection typically replies on Th1/Th17 responses, the finding is very encouraging. It might suggest that the degree of inhibition on Th1 and Th17 responses induced by the p40 peptide vaccine is beneficial for controlling the chronic inflammation in inflammatory bowel diseases but not detrimental to host defense against infections. It should be noted, however, that this conclusion cannot be drawn just from one or even several types of infections. Further exploration of the effect of this p40 peptide vaccine on different types of infection, especially in the gut infection models such as Citrobacter rodentium GI infection47 and measurements of relevant immune responses, should be warranted before it is used as a therapeutic strategy.
In summary, our results have demonstrated that administration of the IL-12/IL-23p40 vaccine is effective in ameliorating ongoing TNBS-induced chronic intestinal inflammation and fibrosis through rebalancing Th1/Th17/Treg responses and increasing IL-10 production from CD11c+ DC cells in LPMCs. Furthermore, we have also evaluated the p40 vaccination in a different colitis model and found that this strategy is effective in the intervention of DSS-induced chronic colitis. Encouragingly, this p40 peptide vaccine strategy did not increase the susceptibility and pathogenesis of Chlamydia muridarum lung infection, in which Th1 and Th17 responses play a critical role for host defense. Therefore, the IL-12/IL-23p40 vaccine may provide a potential and safe approach for the long-term treatment of inflammatory bowel disease and other disorders where IL-12 and IL-23 are important pathogenic cytokines.
Conflicts of interest: The authors declare no competing financial interests.
Author contributions: QG and ZP designed the project; QG, CR, and SW performed the experiments; GQ performed histological analyses. XY provided consultation on immunology and experimental design of lung chlamydia infection. RJW and CNB provided consultation on immunology and inflammatory bowel disease. QG analyzed the data, wrote the manuscript and finally approved it. RJW, XY, and ZP critically reviewed the manuscript.
Supported by: This research was supported by grants from the Canadian Institutes of Health Research (CIHR) (CIHR/ROP-92387 to ZP and CIHR/MOP-130423 to XY) and conducted using facilities of the Children Hospital Research Institute of Manitoba (CHRIM). QG was the recipient of the CIHR Frederick Banting and Charles Best Canada Graduate Scholarship and the CHRIM Graduate Studentship.
REFERENCES
- 1. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D’Haens GR, Sartor RB, Silverberg MS et al. Future directions in inflammatory bowel disease management. J Crohns Colitis. 2014;8:726–34. [DOI] [PubMed] [Google Scholar]
- 3. Sarra M, Pallone F, Macdonald TT et al. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–13. [DOI] [PubMed] [Google Scholar]
- 4. Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- 6. Dryden GW., Jr Overview of biologic therapy for Crohn’s disease. Expert Opin Biol Ther. 2009;9:967–74. [DOI] [PubMed] [Google Scholar]
- 7. Guan Q, Zhang J. Recent advances: the imbalance of cytokines in the pathogenesis of inflammatory bowel disease. Mediators Inflamm. 2017;2017:4810258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mannon PJ, Fuss IJ, Mayer L et al. ; Anti-IL-12 Crohn’s Disease Study Group Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004;351:2069–79. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Feagan BG, Fedorak RN et al. ; Ustekinumab Crohn’s Disease Study Group A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–41. [DOI] [PubMed] [Google Scholar]
- 10. Panaccione R, Sandborn WJ, Gordon GL et al. Briakinumab for treatment of Crohn’s disease: results of a randomized trial. Inflamm Bowel Dis. 2015;21:1329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandborn W, Gasink C, Blank M et al. O-001 a multicenter, double-blind, placebo-controlled Phase3 Study of Ustekinumab, a human IL-12/23P40 mAB, in moderate-service Crohn’s disease refractory to anti-TFNalpha: UNITI-1. Inflamm Bowel Dis. 2016;22(Suppl 1):S1. [Google Scholar]
- 12. Baert F, De Vos M, Louis E et al. ; Belgian IBD Research Group Immunogenicity of infliximab: how to handle the problem?Acta Gastroenterol Belg. 2007;70:163–70. [PubMed] [Google Scholar]
- 13. Orlando A, Mocciaro F, Civitavecchia G et al. Minimizing infliximab toxicity in the treatment of inflammatory bowel disease. Dig Liver Dis. 2008;40(Suppl 2):S236–S46. [DOI] [PubMed] [Google Scholar]
- 14. Delavallée L, Assier E, Denys A et al. Vaccination with cytokines in autoimmune diseases. Ann Med. 2008;40:343–51. [DOI] [PubMed] [Google Scholar]
- 15. Guan Q, Ma Y, Hillman CL et al. Development of recombinant vaccines against IL-12/IL-23 p40 and in vivo evaluation of their effects in the downregulation of intestinal inflammation in murine colitis. Vaccine. 2009;27:7096–104. [DOI] [PubMed] [Google Scholar]
- 16. Guan Q, Ma Y, Hillman CL et al. Targeting IL-12/IL-23 by employing a p40 peptide-based vaccine ameliorates TNBS-induced acute and chronic murine colitis. Mol Med. 2011;17:646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guan Q, Ma Y, Aboud L et al. Targeting IL-23 by employing a p40 peptide-based vaccine ameliorates murine allergic skin and airway inflammation. Clin Exp Allergy. 2012;42:1397–405. [DOI] [PubMed] [Google Scholar]
- 18. Mizoguchi A, Mizoguchi E. Animal models of IBD: linkage to human disease. Curr Opin Pharmacol. 2010;10:578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu H, Zhong G. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live chlamydia trachomatis infection. Infect Immun. 1999;67:1763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jupelli M, Selby DM, Guentzel MN et al. The contribution of interleukin-12/interferon-gamma axis in protection against neonatal pulmonary chlamydia muridarum challenge. J Interferon Cytokine Res. 2010;30:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shavlakadze N, Gorgoshidze B. IL-17/il-23 and Chlamydia trachomatis. Georgian Med News. 2010:45–51. [PubMed] [Google Scholar]
- 22. Troy AE, Zaph C, Du Y et al. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J Immunol. 2009;183:2037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moriyama I, Ishihara S, Rumi MA et al. Decoy oligodeoxynucleotide targeting activator protein-1 (AP-1) attenuates intestinal inflammation in murine experimental colitis. Lab Invest. 2008;88:652–63. [DOI] [PubMed] [Google Scholar]
- 24. Fichtner-Feigl S, Fuss IJ, Young CA et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–70. [DOI] [PubMed] [Google Scholar]
- 25. Bilenki L, Wang S, Yang J et al. Adoptive transfer of CD8ALPHA+ dendritic cells (DC) isolated from mice infected with chlamydia muridarum are more potent in inducing protective immunity than CD8ALPHA- DC. J Immunol. 2006;177:7067–75. [DOI] [PubMed] [Google Scholar]
- 26. Hardenberg G, Steiner TS, Levings MK. Environmental influences on T regulatory cells in inflammatory bowel disease. Semin Immunol. 2011;23:130–8. [DOI] [PubMed] [Google Scholar]
- 27. Bastos KR, de Deus Vieira de Moraes L, Zago CA et al. Analysis of the activation profile of dendritic cells derived from the bone marrow of interleukin-12/interleukin-23-deficient mice. Immunology. 2005;114:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–8. [DOI] [PubMed] [Google Scholar]
- 29. Bai H, Cheng J, Gao X et al. IL-17/th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol. 2009;183:5886–95. [DOI] [PubMed] [Google Scholar]
- 30. Perry LL, Feilzer K, Caldwell HD. Immunity to chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–52. [PubMed] [Google Scholar]
- 31. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. [DOI] [PubMed] [Google Scholar]
- 32. Monteleone G, Fina D, Caruso R et al. New mediators of immunity and inflammation in inflammatory bowel disease. Curr Opin Gastroenterol. 2006;22:361–4. [DOI] [PubMed] [Google Scholar]
- 33. Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2008;14:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pizarro TT, Cominelli F. Cytokine therapy for Crohn’s disease: advances in translational research. Annu Rev Med. 2007;58:433–44. [DOI] [PubMed] [Google Scholar]
- 35. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. [DOI] [PubMed] [Google Scholar]
- 36. Croxford AL, Kulig P, Becher B. IL-12-and IL-23 in health and disease. Cytokine Growth Factor Rev. 2014;25:415–21. [DOI] [PubMed] [Google Scholar]
- 37. Eastaff-Leung N, Mabarrack N, Barbour A et al. Foxp3+ regulatory T cells, th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–9. [DOI] [PubMed] [Google Scholar]
- 38. Izcue A, Hue S, Buonocore S et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brahmachari S, Pahan K. Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide. J Immunol. 2009;183:2045–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gillessen S, Carvajal D, Ling P et al. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–6. [DOI] [PubMed] [Google Scholar]
- 41. Gately MK, Carvajal DM, Connaughton SE et al. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann N Y Acad Sci. 1996;795:1–12. [DOI] [PubMed] [Google Scholar]
- 42. Ha SJ, Lee CH, Lee SB et al. A novel function of IL-12p40 as a chemotactic molecule for macrophages. J Immunol. 1999;163:2902–8. [PubMed] [Google Scholar]
- 43. Khader SA, Partida-Sanchez S, Bell G et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Russell TD, Yan Q, Fan G et al. IL-12 p40 homodimer-dependent macrophage chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta 1. J Immunol. 2003;171:6866–74. [DOI] [PubMed] [Google Scholar]
- 45. Steinbach EC, Plevy SE. The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm Bowel Dis. 2014;20:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X, Gao L, Lei L et al. A myd88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen chlamydia muridarum. J Immunol. 2009;183:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gibson DL, Ma C, Bergstrom KS et al. Myd88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:618–31. [DOI] [PubMed] [Google Scholar]