Abstract
In the vascular wall, endothelial nitric oxide synthase (eNOS) produces NO to regulate peripheral vascular resistance, tissue perfusion, and blood pressure. In resistance arteries, eNOS couples with α-globin and, through chemical reactions, modulates NO diffusion needed for vascular smooth muscle relaxation. While α-globin protein alone is known to be unstable, the mechanisms that enable α-globin protein expression remain elusive. Here, Lechauve et al. report that arterial endothelium expresses α hemoglobin–stabilizing protein, which acts as a critical chaperone protein for α-globin expression and vascular function.
Nitric oxide signaling in the vessel wall
Nitric oxide (NO) is endogenously produced by the enzyme endothelial NO synthase (eNOS) and diffuses from the endothelium to the smooth muscles, where it relaxes the smooth muscles and regulates vascular tone, blood pressure, and tissue perfusion. In response to fluctuations in oxygen tension, arterioles possess the ability to modulate vasomotor tone through changes in NO signaling. In the vascular wall, diffusion of NO from endothelium to smooth muscles is in part controlled by chemical reactions with α-globin coupled with eNOS. In a reduced state, α-globin binds and reacts with and oxidizes NO in a near-diffusion–limited reaction to form nitrate, thus controlling NO levels. As a result, α-globin reduces the vasodilatory actions of NO on smooth muscles, thus preserving vascular tone. However, in the erythrocyte, α-globin is known to be unstable as a monomer and requires binding to β-globin or to the chaperone α-globin–stabilizing protein (AHSP). A role for AHSP in the endothelium, where α-globin, and not β-globin, is expressed, has not been studied. In this issue of the JCI, Lechauve et al. (1) suggest an essential role of AHSP as a critical chaperone for α-globin expression and vasoregulatory activity in endothelium.
Toning down endothelial cell NO signaling by α-globin
Hemoglobin A (HbA), traditionally regarded as the oxygen transporter in RBC, is composed of two α-globin and two β-globin proteins, with a coordinated heme iron in all globin proteins (four per HbA tetramer) that reversibly binds oxygen. It has long been considered that hemoglobin expression is exclusive to RBC; however, accumulating evidence continues to demonstrate that α-globin and β-globin are expressed outside of RBC in somatic cells such as neurons, endothelium, and epithelium (2–5). Accumulating evidence shows that somatic α-globin and β-globin expression is low compared with that in RBC and probably does not function in canonical oxygen storage or transport. Indeed, a number of nonerythrocyte globins, including cytoglobin, neuroglobin, globin X, and androglobin, are expressed at low concentrations in somatic cells. These highly conserved globin proteins are thought to evolve as oxygen sensors, nitrite reductases, and enzymatic scavengers of NO (6–9).
In the vascular wall, NO regulates a plethora of cardioprotective actions including regulation of the arterial vascular tone needed to control blood pressure and flow (10). Recently, expression of α-globin, but not β-globin, was discovered in arteriolar endothelium, concentrated at the myoendothelial junction (MEJ), a specialized cellular projection that adjoins endothelium and smooth muscle (3). In response to vasoconstriction and signals that arise from vascular smooth muscle, eNOS is activated at the MEJ to synthesize NO (11, 12). Released NO diffuses to vascular smooth muscles and activates soluble guanylyl cyclase to generate cyclic guanosine monophosphate (cGMP) needed to modulate vasoconstriction. To effectively balance this physiological process, α-globin serves as the rheostat to control NO diffusion (11). Toward this end, α-globin binds eNOS and uses its oxygenated, reduced heme iron (Fe2+) to react with and enzymatically degrade NO via the following deoxygenation reaction: reduced oxygenated α-globin (Fe2+ – O2) + NO → ferric oxidized α-globin (Fe3+) + NO3–.
Genetic knockdown of α-globin or disruption of the α-globin–eNOS complex using a peptide mimetic increases NO signaling, promoting vasodilation and lowering blood pressure (3, 13, 14). A challenge to this signaling paradigm is that α-globin alone is unstable and cytotoxic, particularly in its oxidized state (Fe3+), which forms via dioxygenation (15). In this issue of the JCI, Lechauve et al. provide new evidence demonstrating that AHSP and eNOS stabilize α-globin and promote vasoconstriction (1).
AHSP and eNOS stabilize α-globin protein
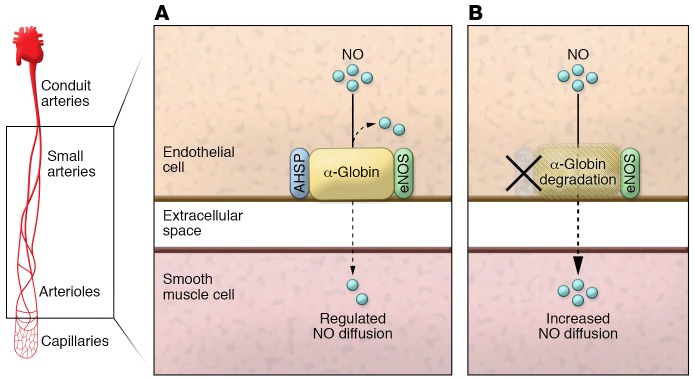
In RBC precursors, AHSP is a chaperone protein that binds nascent α-globin and enables its folding and heme insertion during hemoglobin heterotetramer assembly with β-globin (15). Previous studies revealed that genetic deletion of the AHSP gene in RBC results in a modest insufficiency in hemoglobin heterotetramer production (15). Like RBC, endothelial cells also express AHSP (3); however, the functional role of AHSP in endothelium remains obscure. Using AHSP-KO mice, Lechauve et al. demonstrated that α-globin protein levels are decreased by 70%, leading to a concomitant attenuation in blood pressure and an abrogated response to the vasoconstrictor phenylephrine (1). In parallel studies, HBA1-KO mice transplanted with WT bone marrow also showed decreased blood pressure and blunted responses to phenylephrine. Collectively, these data provide additional support for the fundamental role of AHSP in promoting endothelial α-globin stability, NO signaling, and vascular tone (Figure 1).
Figure 1. Role of AHSP in the regulation of NO.
(A) In resistance arteries and arterioles, a complex between α-globin, eNOS, and AHSP forms to stabilize and regulate NO diffusion from endothelium to smooth muscle to control vascular tone. (B) Loss of AHSP causes a decrease in α-globin expression, resulting in a concomitant increase in NO diffusion.
The authors also include intriguing cell culture studies showing that, in addition to AHSP, eNOS can also stabilize α-globin expression. These data raise several questions about the physiological sequence of events that facilitate the stabilization, positioning, and coupling of α-globin and eNOS at the MEJ to control NO signaling. Previous work using vascular cell culture to model the MEJ showed AHSP to be expressed at the apical endothelial cell monolayer, but not at the MEJ (3). Therefore, if the MEJ is the primary site where α-globin controls NO diffusion, what is the role of AHSP in this regulatory process? One possibility is that AHSP serves a role in endothelium that is similar to its role in RBC, where AHSP enables folding and heme insertion of α-globin before routing it to eNOS for assembly of the α-globin–eNOS complex. Once the α-globin–eNOS complex is formed, the stabilized α-globin–eNOS complex is trafficked to the MEJ, where it serves to control NO diffusion and signaling. It is also possible that oxygen levels could modulate eNOS activity, α-globin tertiary structure, and α-globin–eNOS coupling. As such, further studies are necessary to define the sequence of events including whether oxygen regulates AHSP-α–globin binding, α-globin–eNOS binding, or both.
α-Globin heme redox cycling: can eNOS do it all?
Prior work identified that cytochrome b5 reductase 3 (Cyb5R3), also known as methemoglobin reductase, serves as the primary reductase controlling heme iron redox cycling (Fe3+ → Fe2+) of α-globin, as well as other nonerythroid globins (3). The reduced form of α-globin is required for oxygen binding and inhibitory NO deoxygenation reactions. Knockdown of Cyb5R3 in endothelium of isolated thoracodorsal arteries resulted in blunted vasoconstriction due to a proposed increase in NO concentration (3). Subsequent studies demonstrated that acute pharmacological inhibition of Cyb5R3 resulted in lowering of systemic blood pressure in spontaneously hypertensive rats and impaired responsiveness to infusion of the vasoconstrictors norepinephrine and angiotensin-2 (16). These data support a model whereby Cyb5R3 expression modulates α-globin heme redox cycling to the reduced form necessary for NO inactivation reactions. In the current study, Lechauve et al. provide new evidence using in vitro recombinant protein experiments and show that the reductase domain of eNOS can also reduce α-globin. They suggest that eNOS reduces the α-globin–(Fe3+)–AHSP complex faster than the previously reported Cyb5R3 or the Cyb5R3-Cyb5 system. Although these studies are provocative and biochemically favor involvement of the eNOS reductase domain in reducing α-globin–(Fe3+)–AHSP (17), the physiological relevance for this mechanism remains uncertain. In the endothelium, activation of eNOS by Ca2+/calmodulin aligns the oxygenase domain with the reductase domain and permits electron shuttling to heme for l-arginine oxidation (18). The current studies excluded key components of eNOS such as a functioning oxygenase domain, the critical cofactor calmodulin for reductive activity, and the NO substrate l-arginine. Without these factors, the eNOS reductase domain would favor a fully reduced flavin with a low redox potential and high reductive capacity to reduce the α-globin–(Fe3+)–AHSP complex. It is questionable whether the fully reduced flavoproteins FAD or FMN are at a sufficiently significant concentration to reduce the α-globin–AHSP complex in vivo. Further studies are needed to test the rate of reduction in the presence of known eNOS cofactors and substrates (18, 19).
Outlook and future directions
While biochemical evidence supports the localization and conservation of α-globin and AHSP in both human and mouse endothelium, it remains to be determined whether this mechanism operates in the human microcirculation. A recent analysis of α-thalassemia in Kenyan adolescents showed no reported blood pressure abnormalities, challenging the purported roles of α-globin in blood pressure control (20). While many complex mechanisms may compensate for the blood pressure control in these individuals in the steady state, it is possible that stress-specific conditions may increase endothelial cell α-globin expression and modify NO signaling. A recent study showed that in the setting of pulmonary hypertension, α-globin expression increases and may contribute to the low NO signaling state routinely observed in pulmonary arteries (14). In addition, conditions that increase the expression of Kruppel-like factors (KLFs) such as KLF-2 or KLF-4, which are also known to increase α-globin expression, may also participate in human vascular disease and NO control (21). Collectively, these data continue to highlight the importance of α-globin in endothelium and a fine-tuned control of NO regulation in the arteriolar vascular wall.
Acknowledgments
Funding support was provided by NIH grants R01 HL128304 and R01 133864 (to ACS) and R01 HL098032, R01 HL125886, and P01 HL103455 (to MTG); the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (to ACS and MTG); and Bayer Pharmaceuticals (to ACS).
Version 1. 10/08/2018
Electronic publication
Version 2. 11/01/2018
Print issue publication
Footnotes
Conflict of interest: MTG is a co-inventor on pending patent applications and planned patents directed to the use of recombinant neuroglobin and heme-based molecules as antidotes for CO poisoning, which have recently been licensed by Globin Solutions Inc. MTG is a shareholder in, advisor to, and director of Globin Solutions Inc. Additionally, and unrelated to CO poisoning, MTG is a co-inventor on patents directed to the use of nitrite salts in cardiovascular diseases, which have been licensed by United Therapeutics and Hope Pharmaceuticals, and is a co-investigator in a research collaboration with Bayer Pharmaceuticals to evaluate riociguate as a treatment for patients with SCD. Patent numbers: US9114109 (Five-coordinate neuroglobin and use thereof as a blood substitute) and US8927030/US9675637/US 9700578 (Use of nitrite salts for the treatment of cardiovascular conditions).
Reference information: J Clin Invest. 2018;128(11):4755–4757. https://doi.org/10.1172/JCI124302.
References
- 1.Lechauve C, et al. Endothelial cell α-globin and its molecular chaperone α-hemoglobin–stabilizing protein regulate arteriolar contractility. J Clin Invest. 2018;128(11):5073–5082. doi: 10.1172/JCI99933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahaman MM, Straub AC. The emerging roles of somatic globins in cardiovascular redox biology and beyond. Redox Biol. 2013;1:405–410. doi: 10.1016/j.redox.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straub AC, et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. 2012;491(7424):473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schelshorn DW, et al. Expression of hemoglobin in rodent neurons. J Cereb Blood Flow Metab. 2009;29(3):585–595. doi: 10.1038/jcbfm.2008.152. [DOI] [PubMed] [Google Scholar]
- 5.Bhaskaran M, Chen H, Chen Z, Liu L. Hemoglobin is expressed in alveolar epithelial type II cells. Biochem Biophys Res Commun. 2005;333(4):1348–1352. doi: 10.1016/j.bbrc.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 7.Tiso M, et al. Human neuroglobin functions as a redox-regulated nitrite reductase. J Biol Chem. 2011;286(20):18277–18289. doi: 10.1074/jbc.M110.159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, et al. Cytoglobin regulates blood pressure and vascular tone through nitric oxide metabolism in the vascular wall. Nat Commun. 2017;8:14807. doi: 10.1038/ncomms14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinogradov SN, Moens L. Diversity of globin function: enzymatic, transport, storage, and sensing. J Biol Chem. 2008;283(14):8773–8777. doi: 10.1074/jbc.R700029200. [DOI] [PubMed] [Google Scholar]
- 10.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 11.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci U S A. 1997;94(12):6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biwer LA, Taddeo EP, Kenwood BM, Hoehn KL, Straub AC, Isakson BE. Two functionally distinct pools of eNOS in endothelium are facilitated by myoendothelial junction lipid composition. Biochim Biophys Acta. 2016;1861(7):671–679. doi: 10.1016/j.bbalip.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straub AC, et al. Hemoglobin α/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction. Arterioscler Thromb Vasc Biol. 2014;34(12):2594–2600. doi: 10.1161/ATVBAHA.114.303974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez RA, et al. Targeting pulmonary endothelial hemoglobin α improves nitric oxide signaling and reverses pulmonary artery endothelial dysfunction. Am J Respir Cell Mol Biol. 2017;57(6):733–744. doi: 10.1165/rcmb.2016-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kihm AJ, et al. An abundant erythroid protein that stabilizes free alpha-haemoglobin. Nature. 2002;417(6890):758–763. doi: 10.1038/nature00803. [DOI] [PubMed] [Google Scholar]
- 16.Rahaman MM, et al. Structure guided chemical modifications of propylthiouracil reveal novel small molecule inhibitors of cytochrome b5 reductase 3 that increase nitric oxide bioavailability. J Biol Chem. 2015;290(27):16861–16872. doi: 10.1074/jbc.M114.629964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao YT, et al. Thermodynamics of oxidation-reduction reactions in mammalian nitric-oxide synthase isoforms. J Biol Chem. 2004;279(18):18759–18766. doi: 10.1074/jbc.M308936200. [DOI] [PubMed] [Google Scholar]
- 18.Roman LJ, Martásek P, Masters BS. Intrinsic and extrinsic modulation of nitric oxide synthase activity. Chem Rev. 2002;102(4):1179–1190. doi: 10.1021/cr000661e. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Soud HM, Stuehr DJ. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci U S A. 1993;90(22):10769–10772. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etyang AO, et al. Blood pressure and arterial stiffness in Kenyan adolescents with α+thalassemia. J Am Heart Assoc. 2017;6(4):e005613. doi: 10.1161/JAHA.117.005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sangwung P, et al. Regulation of endothelial hemoglobin alpha expression by Kruppel-like factors. Vasc Med. 2017;22(5):363–369. doi: 10.1177/1358863X17722211. [DOI] [PMC free article] [PubMed] [Google Scholar]