Abstract
Oxidative stress and inflammation interact in the development of diabetic atherosclerosis. Intracellular hyperglycemia promotes production of mitochondrial reactive oxygen species (ROS), increased formation of intracellular advanced glycation end-products, activation of protein kinase C, and increased polyol pathway flux. ROS directly increase the expression of inflammatory and adhesion factors, formation of oxidized-low density lipoprotein, and insulin resistance. They activate the ubiquitin pathway, inhibit the activation of AMP-protein kinase and adiponectin, decrease endothelial nitric oxide synthase activity, all of which accelerate atherosclerosis. Changes in the composition of the gut microbiota and changes in microRNA expression that influence the regulation of target genes that occur in diabetes interact with increased ROS and inflammation to promote atherosclerosis. This review highlights the consequences of the sustained increase of ROS production and inflammation that influence the acceleration of atherosclerosis by diabetes. The potential contributions of changes in the gut microbiota and microRNA expression are discussed.
Abbreviations: ACC, acetyl-CoA carboxylase; AdipoR, adiponectin receptor; AGEs, advanced glycation end-products; AKR, aldo-keto reductase; AMPK, AMP-activated protein kinase; ApoE, apolipoprotein E; AR, aldose reductase; CAD, coronary artery disease; C/EBPα, CCAAT/enhancer binding protein α; CRP, C-reactive protein; CVD, cardiovascular disease; DAG, diacylglycerol; Drp-1, dynamic-related protein 1; eNOS, endothelial nitric oxide synthase; ERK1/2, extracellular regulating kinase 1/2; ER, endoplasmic reticulum; FOXO1, forkhead box O1; GAPDH, glyceraldehydes-3-phosphate dehydrogenase; GLP-1, glucagon-like peptide-1; Grxs, glutaredoxins; GSH, glutathione; H2S, hydrogen sulphide; HDL, high-density lipoprotein cholesterol; HIF-α, hypoxia inducible factor α; IKK, IkB kinase; ICAM-1, intercellular adhesion molecule-1; IκB, inhibitor of κB; IL-6, interleukin-6; iNOS, inducible NOS; JNK, c-Jun N-terminal kinase; KLF, Kruppel-like factors; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemotactic protein-1; MIP-1, macrophage inflammatory protein-1; miRNAs, microRNAs; miR-128-1, miRNA-128-1; MMPs, matrix metalloproteinases; NF-κB, nuclear factor-κB; NLRP3, NOD-like receptor family, pyrin domain-containing 3; nNOS, neuronal NOS; Nrf2, nuclear factor erythoid 2-related factor 2; ox-LDL, oxidized-low density lipoprotein; PARP, poly ADP-ribose polymerase; PGC-1α, PPARγ coactivator 1 α; PKC, protein kinase C; p90RSK, p90 ribosomal S6 kinase; PPAR, peroxisome proliferator activated receptor; RAGE, receptor for AGEs; ROS, reactive oxygen species; SIRT1, sirtuin 1; SREBP-1c, sterol regulatory-element-binding protein-1c; SR, scavenger receptor; SUMOylation, small ubiquitin-related modifier conjugation; TLR4, toll-like receptor 4; TMAO, trimethylamine N-oxide; TNF-α, tumor necrosis factor-α; T2DM, Type 2 diabetes mellitus; UPS, ubiquitin-proteasome system; VCAM-1, vascular cell adhesion molecule 1; VSMCs, vascular smooth muscle cells
Keywords: Atherosclerosis, Diabetes mellitus, Reactive oxygen species, Gut microbiota, MicroRNA
1. Introduction
The worldwide diabetes mellitus epidemic currently affects over 400 million adults, and the number is expected to rise to 550 million by the year 2030 [1]. Diabetes mellitus is characterized by hyperglycemia resulting from chronic and/or relative insulin insufficiency, and the type 2 form of the disease (T2DM) comprises approximately 85% of cases. Environmental influences such as excess intake of dietary calories and fat and a genetic component contribute to the development of T2DM [2], and the increase in prevalence has been accompanied by an increase in vascular disease. The microvascular complications of diabetes mellitus include retinopathy, nephropathy, and neuropathy. Macrovascular complications include premature atherosclerosis that can ultimately manifest as myocardial infarction, stroke, and peripheral vascular disease [3]. The progression of cardiovascular disease is accelerated, and the outcomes are worse, if diabetes mellitus is also present, and there is strong evidence that diabetes mellitus independently increases the risk of atherosclerosis. It also increases the pervasiveness of atherosclerosis, infiltration of inflammatory cells, and plaque necrosis [4].
Atherosclerosis is a complex process that is seen as an inflammation response to injury and involves the interaction of numerous cell types with formation of fatty streaks that progress to atheromatous plaques, plaque destabilization, and rupture. Endothelial dysfunction is an early event in the disturbance of vascular homeostasis, and it stimulates the production of proinflammatory cytokines such as interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1) and adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1) and E-selectin. These events promote monocyte and T-cell adhesion to the vascular endothelium and infiltration to form neointima lesions. Proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) promote formation of macrophages in the subendothelial space that internalize oxidized-low density lipoprotein (ox-LDL), form foam cells and contribute to fatty streak formation. Subsequent activation of matrix metalloproteinases, collagen degradation, migration and proliferation of vascular smooth muscle cells (VSMCs) and endothelial cells, all contribute to atherosclerotic plaque development [2], [5].
The presence and extent of oxidation products of lipids and proteins in vascular lesions and the relation to the severity of atherosclerotic disease was first described in the 1950s [6]. The role of hyperglycemia in promoting overproduction of ROS by the mitochondrial electron transport chain and the contribution of prolonged production of mitochondrial superoxide to development of diabetes-related vascular damage was proposed by Brownlee [7]. Excess superoxide leads to DNA strand breakage, activation of nuclear poly ADP ribose polymerase (PARP), which inhibits glyceraldehyde-3-phosphate dehydrogenase (GAPDH), shunting early glycolytic intermediates into pathogenic signaling pathways. The progression of atherosclerosis is then promoted by increased intracellular formation of advanced glycation end-products (AGEs), activation of protein kinase C (PKC), the polyol pathway, and the hexosamine pathway. These pathways may promote additional vascular ROS production that activate proinflammatory pathways associated with key molecular events in atherogenesis [2], [7], [8]. Excess ROS accelerate inflammation in atherosclerosis indirectly by increasing the formation of ox-LDL, promoting insulin resistance, activating ubiquitination-related pathways, and decreasing the activation of adiponectin, AMP-activated protein kinase (AMPK), and endothelial nitric oxide synthase (eNOS) [9].
Recent progress in understanding the involvement of oxidative stress and inflammation in the pathogenesis of diabetic atherosclerosis has been accompanied by investigations of the roles of micro(mi)RNAs and gut microbiota. The gut microbiome has endocrine functions, producing molecules that communicate with distal organs and influence metabolism-dependent pathways. Diabetes mellitus leads to microflora dysfunction that alters the production of metabolites. Increased uric acid results in generation of ROS and increased lipopolysaccharide (LPS) activates nuclear factor-κB (NF-κB) expression, both of which accelerate atherosclerosis progression [10], [11]. Diabetes mellitus has been associated with increased expression of some miRNAs, including miR-128-1, that can be detected in body fluids and may become markers of atherosclerosis or therapeutic targets. MiR-128-1 may modulate PKCβ interaction with ox-LDL to induce the overexpression of intercellular adhesion molecule (ICAM)-1 and activation of intracellular signaling to decrease AMPK antiatherogenic activity. Increased miR-200c expression promotes ROS production [12], [13]. The activities of the gut microbiota and miRNAs in diabetes mellitus and atherosclerosis are well investigated, but evidence linking them to the pathogenesis of diabetes-accelerated atherosclerosis is not extensive. This review will discuss the impact of diabetes mellitus on the acceleration of atherosclerosis via oxidative stress-induced inflammation, and changes in miRNAs and gut microbiota.
2. Hyperglycemia-induced excessive ROS production and accelerating atherosclerosis
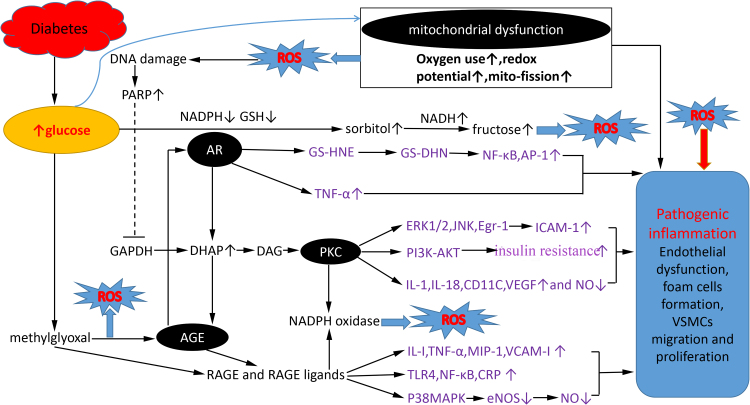
ROS can be generated in the vascular wall by NADPH oxidase, xanthine oxidase, the mitochondrial electron transport chain, and uncoupled eNOS [9]. Increased ROS in pancreatic β-cells suppress insulin generation by inhibiting insulin gene transcription factors such as pancreatic and duodenal homeobox factor-1 (PDX-1) or v-maf musculoaponeurotic fibrosarcoma oncogene homolog A [14]. In atherosclerosis, ROS promote endothelial dysfunction and expression of inflammation factors, such as MCP-1, ICAM-1 and IL-1, all of which in turn induce ROS production, leading to a vicious cycle. Endothelium-dependent dilation dysfunction is correlated with decreased NO production and increased formation and release of endothelin induced by ROS. ROS also facilitate foam cell formation and proliferation and migration of vascular muscle cells in vitro [2]. The four main regulatory pathways of ROS production and their links to diabetes mellitus and atherosclerosis are shown in Fig. 1.
Fig. 1.
Four hyperglycemia-induced pathogenic pathways which are related to overproduction of ROS and inflammation progression in atherosclerosis. Hyperglycemia causes mitochondrial overproduction of ROS via a greater oxygen use, high redox potential and mito-fission state. Increased ROS leads to nuclear DNA damage and activates nuclear PARP, which inhibits GAPDH activity, shunting early glycolytic intermediates into pathogenic signaling pathways, including activation of polyol pathway, PKC and AGE. These pathways amplify production of ROS and promote pathogenic inflammation progression in atherosclerosis. The polyol pathway generates ROS by consuming NADPH and GSH, and increasing subsequent NADH oxidation during the conversion of sorbitol to fructose. As a key rate-limiting enzyme of polyol pathway, AR promotes the expression of inflammatory cytokines such as TNF-α and NF-κB. Inhibition of GAPDH contributes to DHAP production and subsequent increase of PKC and AGE, both of which induce an increase in NADPH oxidase, inflammation factors expression and a decrease in eNOS activation. Moreover, PKC promotes insulin resistance by inhibiting downstream expression of PI3K-AKT. As a precursor of the majority of AGE adducts, methylglyoxal is increased in hyperglycemia, and then promote the expression of AGE, RAGE and RAGE ligands.
2.1. Mitochondrial dysfunction
During the production of ATP by oxidative phosphorylation in the mitochondrial electron transport chain, 1–5% of the O2 is reduced to superoxide O2– radicals, which can be converted by antioxidant enzymes to hydrogen peroxide, hydroxyl free radicals, or react with NO to form peroxynitrite [15], [16]. Excess ROS production or insufficient antioxidant activity lead to mitochondrial oxidative stress, which occurs in various pathologies [17].
Previous studies have revealed that diabetes mellitus and atherosclerosis share the same oxidative stress and mitochondrial pathologies [18]. Hyperglycemia increases ROS production by driving mitochondria toward increased oxygen use and increased redox potential [19] and by shifting O2 transport toward the respiratory chain complex II [7]. The conversion of ADP to ATP reduces membrane potential, and hyperglycemia increases ADP regeneration and consumption rates, resulting in decreased ATP formation, a continuing increase in membrane potential, and augmentation of ROS production [20]. Other effects of hyperglycemia that increase ROS production include an increased NADH/FADH2 ratio and increased mitochondrial fission with accumulation of fragmented mitochondria with impaired electron transport chain activity [21], [22]. Metformin slows the progression of diabetes mellitus-accelerated atherosclerosis by inhibiting mitochondrial fission in vascular endothelial cells via AMPK-mediated blockage of dynamic-related protein 1 (Drp-1) expression [23].
Hyperglycemia increases the mutation rate of nuclear DNA because exposure to oxygen radicals is increased [24]. The mechanism of ROS-induced breaks in nuclear DNA strands may include an increase in superoxide-related release of Fe2+ from ferritin and iron-sulfur cluster-containing proteins and increase of hydroxyl radicals that lead to bond cleavage [25]. The DNA fragments activate nuclear PARP, a DNA repair enzyme, which inhibits GAPDH, shunting early glycolytic intermediates into pathogenic signaling pathways. These events increase intracellular formation of the major AGEs and activate PKC and the polyol and hexosamine pathways, which then promote the progression of atherosclerosis [26].
Some evidence suggests that mitochondrial dysfunction pre-exists diabetes mellitus, as. increased ROS damages pancreatic β-cells and decreases peripheral tissue insulin sensitivity [14]. Elevated mitochondrial ROS is also associated with increased oxidation of LDL, vascular smooth muscle cell apoptosis, and endothelial cell dysfunction, all of which promote atherosclerosis [27]. Finally, mitochondrial dysfunction including changes in ATP production, and inflammation mediators may trigger pathogenic changes that promote diabetes mellitus and atherosclerosis [18].
2.2. Increased intracellular formation of AGEs
AGEs are formed by a nonenzymatic reaction of ketones or aldehydes and the amino groups of proteins. Large amounts of ROS are generated during AGEs formation [7], and oxidized AGEs activate receptor for AGEs (RAGE) to stimulate NADPH oxidase-1, which contribute to ROS production in diabetes [28]. And AGEs are known to be expressed in both infiltrating monocytes/macrophages and in cells of vessel walls, and as post-translational modifications of proteins that activate proatherogenic processes including oxidative stress, inflammation and the rennin-angiotensin system [29]. Inhibition of AGE accumulation has been shown to slow the progression of established diabetes mellitus-associated atherosclerosis [30]. Methylglyoxal, which is formed by the nonenzymatic fragmentation of the glycolytic intermediate triose phosphate, is a precursor of the majority of AGE adducts in diabetes mellitus [31]. In nondiabetic apolipoprotein E (ApoE) null mice, increasing plasma methylglyoxal concentration to diabetic levels caused endothelial inflammation and atherogenesis similar to that seen in mice with diabetes mellitus [32]. Intracellular methylglyoxal has also been found to increase the expression of both RAGE and S100 calgranulins, its endogenous proinflammatory ligands [33].
AGEs can interact with two types of cell surface receptors, scavenger receptors involved in AGE capture, removal, and degradation and a pattern recognition receptor RAGE that initiates specific cellular signaling [34]. AGE-RAGE binding activates multiple signal transduction pathways that promote atherogenesis, including increased expression of VCAM-1, macrophage inflammatory protein-1 (MIP-1), MMP9, IL-1 and TNF-α that mediate leukocyte adhesion and vascular inflammatory reactions leading to mitochondrial dysfunction and cell death [35]. RAGE ligands in addition to AGEs, including S100 calgranulin family proteins and high mobility group protein B1 can activate innate immune system responses by toll-like receptor 4 (TLR4) signaling and increased NF-κB transcription factor activity [36]. Telmisartan has anti-inflammatory activity that decreases AGE-induced C-reactive protein (CRP) generation by inhibiting RAGE expression caused by activation of peroxisome proliferator activated receptor (PPAR)-γ activation [37]. Glucagon-like peptide-1 (GLP-1) inhibits RAGE mRNA transcription, leading to a decrease of ROS production and subsequent decrease of VCAM-1 expression [38]. Finally, AGEs dose-dependently activate oxidative stress-mediated P38 activation of mitogen-activated protein kinase (MAPK) signaling in endothelial cells, which enlarges the inhibition of NO synthesis caused by AGEs [39].
2.3. Activation of the polyol pathway
The polyol pathway includes a family of monomeric NADPH-dependent aldo-keto reductase (AKR) enzymes that catalyze the conversion of carbonyl compounds to sugar alcohols. In some cells, glucose is converted to sorbitol, and sorbitol is then converted to fructose by sorbitol dehydrogenase with NAD+ as a cofactor. The polyol pathway is involved in the pathogenesis of diabetic complications including cardiovascular disease [40], and studies in aldose reductase (AR)-deficient mice have shown that the polyol pathway contributes to diabetes mellitus-induced oxidative stress [41]. The generation of oxidative stress by the polyol pathway may begin with the conversion of glucose sorbitol, which consumes NADPH, a cofactor required to regenerate reduced glutathione (GSH), an important ROS scavenger. During the conversion of sorbitol to fructose by sorbitol dehydrogenase, the cofactor NAD+ is reduced to NADH and the subsequent oxidation of NADH by NADH oxidase leads to the production of superoxide ions disturbing the balance of antioxidation and oxidation [42]. In animal studies, AGEs have been found to promote AKR enzyme mRNA and protein expression, and in one such model, elevation of dihydroxy acetone phosphate (DHAP) resulted in a further increase of AGEs and an increase of diacylglycerol (DAG) that led to activation of the PKC pathway [43]. This is a vicious cycle that exists in diabetes mellitus and may be associated with oxidative stress. Another animal study showed that the EGF receptor-ERK/MAPK pathway activated AR gene expression under oxidative stress [44].
In ApoE knockout mice with diabetes mellitus, overexpression of human AR in endothelial cells accelerated atherosclerosis, which was prevented by pharmacological inhibition of the enzyme [45]. AR may also be able to accelerate the progression of atherogenesis and endothelial dysfunction by increasing the expression of inflammatory cytokines such as TNF-α in VSMCs [46]. AR activation of NF-κB and activator protein-1 (AP-1) also contributes to the synthesis and release of cytokines, chemokines, and other inflammatory markers, and AR inhibition was found to decrease NF-κB activity and reduce hyperglycemia-related inflammation [47], [48].
Studies have demonstrated that in addition to activation of the polyol pathway, inhibition of G6PD will also lead to a shunting of glucose into the hexosamine pathway [49], [50]. Hyperglycemia inhibits eNOS activity by activating hexosamine pathway via mitochondrial overproduction superoxide, which was accompanied by an increase in O-linked N-acetylglucosamine modification of eNOS and a decrease in O-linked serine phosphorylation at residue 1177 [51]. Hyperglycemia also decreases anti-inflammatory protein A20 expression via O-glucosamine-N-acetylation-dependent ubiquitination and proteasomal degradation in response to inflammatory stimuli in VSMCs and endothelial cells [52].
2.4. Activation of PKC
PKC is a serine-threonine protein kinase with 15 isoforms, and it is active in cellular signal transduction, regulates cell growth and proliferation, senescence, and apoptosis. Hyperglycemic activation of PKC signaling leading to increased ROS production has been associated with loss of the p47phox subunit of NADPH oxidase [53], and the induction of AGEs contributing to NADPH-related oxidative stress in cardiac vascular endothelial cells has also been associated with PKC activation [54]. Increased intracellular DAG has been reported to activate nine of the 15 PKC isoforms [55], [56]. AGEs, ROS, growth factors, and cytokines promote de novo synthesis of DAG from triose phosphate derived from glucose metabolism. Triose phosphate availability is increased by inhibition of the glycolytic enzyme GAPDH by an excess of ROS [7], [57]. These finding demonstrate the importance of ROS caused by PKC in diabetes mellitus.
PKC activation induces endothelial dysfunction, increases vascular permeability, and inhibits angiogenesis, all of which accelerate the progression of atherosclerosis. PKCβ activation in diabetic ApoE null mice increased the expression of inflammatory mediators and accelerated atherosclerosis. In that model animal model, PKCβ interacted with ox-LDL to induce the ICAM-1 expression by activating extracellular regulating kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and early growth response protein 1 signaling [58]. Transcription of the proinflammatory cytokines IL-1β, IL-18 and cluster of differentiation 11c were also increased [59]. The expression of inflammatory cytokines, macrophage infiltration of plaques, and lesion size were all reduced in diabetic ApoE−/− PKCβ-/-knockout mice [58]. PKCβ2 overexpression in vascular endothelium has been found to inhibit downstream expression of phosphatidylinositol 3-kinase (PI3K)-AKT, resulting in insulin resistance and to a decrease in eNOS activation [60]. The PKCβ-related effects can be blocked by the synthetic inhibitor LY379196 [61]. Other studies have shown that activation of PKC in VSMCs by hyperglycemia induces expression of the permeability enhancing factor VEGF and decreases NO generation. The evidence warrants further investigation of the PKCβ effects on PI3K/AKT signaling, Ras related C3 botulinum toxin substrate 1 (Rac1), P21 activated kinase 1 (PAK1), and the JNK/ERK/P38MAPK pathway. The harmful effects would include foam cell formation, increase endothelial inflammation and endothelial relaxation dysfunction [62], [63], [64].
3. Indirect role of ROS in atherosclerosis
3.1. Increased production of ox-LDL
T2DM is characterized by disorders of lipoprotein metabolism that are ultimately associated with plaque formation and ox-LDL accumulation during atherosclerosis [65], [66]. Inhibition of ROS production protects endothelial cells from ox-LDL accumulation and macrophage proliferation during plaque development. ROS interfere with endogenous antioxidants such as vitamin E and oxidize the polyunsaturated fatty acids on the surface of LDL particles causing them to rupture. Fatty acids are converted to reactive aldehydes by lipid peroxidase and they crosslink with apolipoprotein B to generate conjugated diolefines and ox-LDL [67], [68]. Ox-LDL are not recognized by LDL receptors, and they are more readily bound by the scavenger receptors of macrophages.
Ox-LDL induces endothelial cell apoptosis via both caspase dependent or independent pathways, promoting inclusion in atheromas and foam cell formation (Fig. 2) [69]. NO is an antioxidant, but combines with O2− to form peroxynitrite, which promote LDL oxidation plaque development and rupture [70]. Alteration of the structural integrity of vascular endothelium permits passage of ox-LDL into subendothelial tissues [71]. Ox-LDL stress was found to increase both miR-155 expression and autophagy in human endothelial cells, and was associated with inhibition of ras homolog enriched in brain (Rheb)-mediated mammalian target of rapamycin (mTOR)/P70S6kinase/eIF4E-binding protein (4-EBP) signaling (Fig. 2) [72]. The increase in NADPH oxidase activity in response to ox-LDL has been shown to lead to eNOS uncoupling and inhibition of NO production (Fig. 2). Ox-LDL-induced suppression of eNOS was inhibited by silencing the ox-LDL receptor-1 (LOX-1) [73].
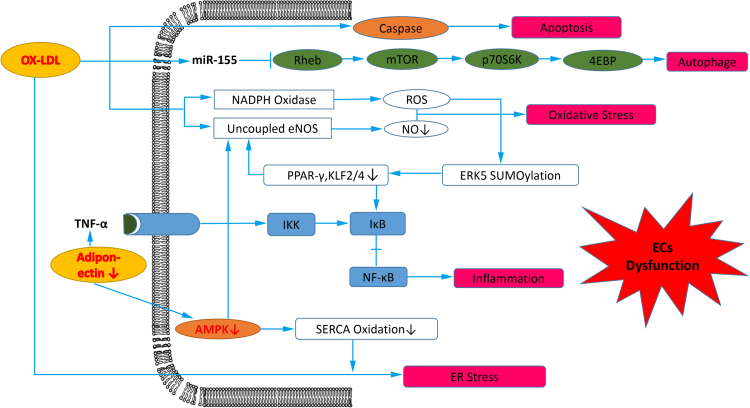
Fig. 2.
Downstream products of ROS promote endothelial cells (ECs) dysfunction in atherosclerosis. Ox-LDL induces ECs apoptosis via caspase dependent or independent pathway, and induces ECs autophage by repressing the Rheb-mediated mTOR/P70S6kinase/4EBP signaling pathway through increased miR-155 expression. Furthermore, ox-LDL induces oxidative stress in ECs through agumented NADPH oxidase and uncoupled eNOS. ERK5 SUMOylation induced by ROS leads to transrepression of atheroprotective genes PPARγ and KLF2/4-mediated eNOS expression, both of which increase NF-κB activation. Decreased adiponectin increase NF-κB activation, and decreased AMPK increase ER stress induced by ox-LDL via enhanced SERCA oxidation.
Ox-LDL induces the expression and release of inflammatory modulators, leading to promotion of monocyte migration, increased density of macrophage scavenger receptors, and increased uptake of ox-LDL during foam cells formation [74], [75] (Fig. 3). A previous study showed that the atherogenic effects of ox-LDL were mediated by the TLR4 pathway. Inhibition of TLR4 expression downregulated NF-κB activity, reduced MCP-1 and IL-8 expression in monocytes in response to ox-LDL, and slowed the progression of atherosclerosis [76]. However, study also demonstrate that ox-LDL and TLR4 activation to elicit an inflammatory response from human monocytes and macrophages was due to LPS contamination [77]. Ox-LDL were also shown to induce time- and dose-dependent downregulation of nuclear cAMP response element binding protein, which regulates VSMC quiescence [78]. Genetic deletion of lipoxygenases has been shown to decrease LDL oxidation in mouse models of atherosclerosis, and inactivation of paraoxonase 1 was found to indirectly inhibit LDL oxidation. Finally, the presence of anti-ox-LDL IgM antibodies was shown to be correlated with apoptosis, lipid metabolism, and inflammation [79].
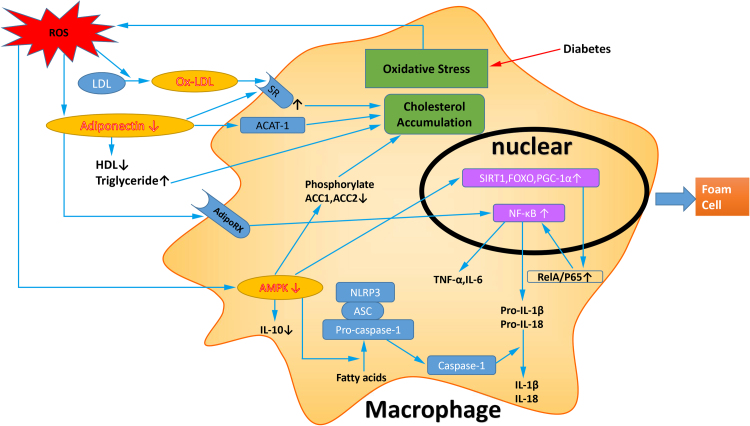
Fig. 3.
Pathogenic mechanisms induced by ROS to promote foam cells formation. In diabetes mellitus, increased ROS induce ox-LDL production and augment of SR density, promoting the cholesterol accumulation in macrophage. Decreased adiponectin induced by ROS also promotes cholesterol accumulation via increased expression of ACAT-1 and SR. Downregulated AMPK improve cholesterol accumulation in macrophage through loss of phosphorylating ACC1 at Ser79 and ACC2 at Ser221. In macrophage, the activity of NF-κB is increased by reduced adiponectin through AdipoRX and by decreased AMPK upregulating SIRT1, FOXO and PGC-1α. Decreased AMPK also increases NLRP3 activation in macrophage, and promote expression of inflammatory factors. These all contribute to the foam cells formation in diabetes mellitus-accelerated atherosclerosis. ASC, apoptosis-associated speck-like protein containing CARD.
3.2. Increased insulin resistance
Study discloses that diabetic rats countervail ROS stress by reprogramming the energy metabolic pathway from glycolysis into lipid oxidation to compensate the ATP requirement of the body, which causes insulin resistance [80]. The development of insulin resistance is dependent on not only genetic and environmental factors but also on immune-mediated mechanisms such as insulin or insulin receptor antibodies or autoimmune destruction of adipocytes [81], [82]. The involvement of ROS in insulin resistance may involve activation of redox-sensitive signaling pathways, such as p38MAPK, JNK, IkB kinase (IKK), and ERK, all of which increase serine phosphorylation of insulin receptor substrate (IRS) proteins and impaired insulin signaling [83], [84]. ROS interferes with PI3K/AKT signaling to decrease both NOS expression and glucose metabolism, but the MAPK pathway is stimulated. Insulin resistance results in compensated hyperinsulinemia. All these effects contribute to disruption of insulin-stimulated glucose metabolism and increased risk of cardiovascular disease (CVD) in type 2 diabetics mellitus [85]. A multi-center study reported an association between insulin resistance and multivessel coronary artery disease in nondiabetic survivors of myocardial infarction. The association was independent of other metabolic risk factors, and insulin resistance was associated with a higher risk of CVD in nondiabetic than in diabetic study participants [86], [87].
Insulin resistance has been correlated with atherosclerotic changes in endothelial cells, VSMCs, and macrophages [82], [88], [89]. It activates the MAPK pathway, increases production of inflammation cytokines, and promotes VSMCs proliferation and plaque formation [90]. The apoptosis of VSMCs and plaque rupture observed in an insulin resistance model were correlated with activation of the chemokine (C-X3-C motif) ligand 1 (CX3CL1)/chemokine (C-X3-C motif) receptor 1 (CX3CR1) axis [91]. Insulin resistance also leads to endoplasmic reticulum (ER) stress, macrophage apoptosis, and subsequently expansion of the arterial plaque cores [92]. Studies showed that increased insulin secretion in response to insulin resistance promotes atherosclerosis [93]. Insulin increases hepatic very low density lipoprotein synthesis by stimulating sterol regulatory-element-binding protein-1c (SREBP-1c), inhibiting acetyl-CoA carboxylase, and augmenting LDL-cholesterol transport in cultured arterial SMCs [94]. Insulin is also a growth factor that promotes collagen synthesis, arterial SMCs proliferation, and expression of multiple genes that modulate inflammation [95], [96]. In the presence of insulin resistance, plasma adiponectin concentration is decreased. Adiponectin is an insulin-sensitizing adipocytokine via AMPK and PPAR-α activation and has antiatherogenic and anti-inflammatory activity [97].
3.3. Decreased adiponectin
Adiponectin is a hormone secreted by adipose tissues that has anti-inflammatory and acts to increase insulin sensitivity fatty acid oxidation and glucose utilization, thereby suppressing atherosclerosis development [97]. Low serum adiponectin levels are associated with increased risk of T2DM and have been identified as a strong predictor of future development of diabetes [98]. PPAR-γ, CCAAT/enhancer binding protein α (C/EBPα) and sirtuin 1 (Sirt1)/forkhead box O1 (FOXO1) increase adiponectin gene transcription [99]; secretion is decreased in obesity-linked diseases, including insulin resistance/T2DM and atherosclerosis [100], [101]. ROS suppress expression of adiponectin mRNA in adipocytes through its effects on AKT and Janus kinase/signal transduction and activators of the (JAK/STAT) signaling pathway [102]. ER stress, JNK signaling, TNF-α acting through PKC and IL-6 acting through p42/44 MAPK downregulate adiponectin transcription [103], [104], [105], [106]. Adiponectin protects against oxidative stress in vascular endothelium by promoting NO synthesis via AMPK-mediated phosphorylation of eNOS at ser1177 and ser633 and suppressing the expression of gp91phox, a NADPH oxidase subunit, in an AMPK-independent manner [107], [108]. Adiponectin activity is determined by its binding to its AdipoR1 and AdipoR2 receptors. AdipoR1 binding induces the influx of extracellular Ca2+ and activation of Ca2+/calmodulin-dependent protein kinase and AMPK in skeletal muscle and liver, both of which have antiatherogenic activity [109]. AdipoR2 binding also increasing the expression of PPARα ligands that accelerate fatty acid oxidation and energy consumption [110]. AdipoR1/AMPK signaling upregulates SirT1, increases PPARγ coactivator (PGC-1α) expression, and decreases protein acetylation, and increases the number of mitochondria in myocytes. Consistent with those effects, decreased adiponectin and AdipoR1 may be causally associated with mitochondrial dysfunction and increased inflammation in diabetes mellitus [111]. SirT1 has also been reported to deacetylate PPARγ at Lys268 and Lys293, leading to upregulation of adiponectin and downregulation of proinflammatory cytokines, such as MCP-1 and IL-6 [112], [113].
Adiponectin downregulates the expression of adhesion molecules in the vascular endothelium. It decreases expression of ICAM-1, which promotes monocyte adhesion to the vascular endothelium, by inhibiting TNF-α mediated activation of NF-κB (Fig. 2) [114]. The suppression of NF-κB activation results in transrepression of NF-κB target genes, including COX2, which is thought to contribute to the antidiabetic and antiatherogenic activities of adiponectin [115]. Adiponectin reduces the expression of CRP mRNA in a dose-dependent manner by upregulating AMPK and downregulating NF-κB, and inhibits growth factor-induced VSMC proliferation by inhibiting MAPK [116]. Adiponectin inhibits foam cell formation by decreasing the expression of acyl-CoA:cholesterol acyltransferase 1 (ACAT-1) and macrophage scavenger receptor (SR)-A [117] (Fig. 3). It promotes AMPK-dependent polarization of macrophages toward an anti-inflammatory phenotype [118], and can increase macrophage IL-6 via activation of NF-κB through AdipoRX (Fig. 3). This leads to activation of STAT3 in hepatocytes, increased IRS-2 in the fasted state, and increased insulin sensitivity [119]. In an in vitro study, insulin reduced the expression of AdipoR1/2 via the PI3K/FOXO1-dependent signaling, and adiponectin increased high-density lipoprotein cholesterol and decreased triglyceride concentrations [120] (Fig. 3).
3.4. Decreased NO production
Hyperglycemia, ox-LDL, ROS, BH4 deficiency, l-arginine (an eNOS substrate) and eNOS S-glutathionylation, all lead to the uncoupling of eNOS [121], [122]. NOS has three isoforms. ENOS and neuronal (n)NOS are atheroprotective, but inducible (i)NOS is proatherogenic [123]. ENOS produces NO, which has endothelial vasoprotective activity [124], [125], and nNOS acts in the vascular wall and atherosclerotic plaques to promote vasodilation [126], [127]. INOS competes with eNOS for cofactor BH4 to reduce eNOS-mediated NO generation and facilitate the generation of peroxynitrite, a proatherosclerosis oxidant. Peroxynitrite also promotes eNOS uncoupling by transforming BH4 to BH2. In addition to decreasing NO production, peroxynitrite contributes to ROS production in diabetes mellitus [128], [129], [130].
Animal studies have demonstrated opposing activities of vascular ROS and NO in atherogenesis. NO protects against ROS-induced damage to macromolecules; ROS limits NO activity. ROS targets soluble guanylate cyclase, the intracellular receptor of NO, changing the β subunit Fe2+ to Fe3+, leading to loss of VSMC responsiveness to NO [131]. NO helps to maintain cardiovascular homeostasis, and the reduction of endogenous NO promotes atherosclerosis. The antiatherogenic effects of NO include inhibiting leukocyte and platelet adhesion to vascular wall, endothelial cell activation, macrophage infiltration and activation, VSMC proliferation and LDL oxidation [124], [132]. NO can also modulate inflammation in human vascular endothelial cells by activating PPAR-γ via a p38-MAPK-dependent mechanism [133].
Moreover, endothelium-derived NO appears to be involved in the enzymatic conversion of L-cysteine into hydrogen sulphide (H2S). Endogenous H2S is significantly impaired in the hyperglycemic condition in non-obese diabetic mice [134] and type 2 diabetes patients [135], the mechanism is associated with mitochondrial ROS overproduction increased H2S degradation, which induce damaged vasorelaxation and vascular inflammation. H2S replacement reduces DNA damage and activation of PARP to exhibit antioxidant properties, and protects hyperglycemic endothelial cells [136]. Furthermore, H2S attenuates diabetes-accelerated atherosclerosis, which may be associated with inhibition of oxidative stress via Keap1 sulfhydrylation at Cys151 to activate nuclear factor erythoid 2-related factor 2 (Nrf2) signaling and decrease endothelial dysfunction and foam cells formation. Nrf2 is an important cellular defense mechanism against oxidative stress as it induces a battery of antioxidative proteins, including heme oxygenase 1 [137].
3.5. Activation of the ubiquitin-proteasome system (UPS) and small ubiquitin-related modifier (SUMO) proteins
The UPS is a main protein degradation pathway in metabolism regulation. Recent evidence indicates that it takes part in the pathogenesis of CVD, ischemia-reperfusion injury, and atherosclerosis. Small ubiquitin-related modifier conjugation (SUMOylation) is similar to UPS and both depend on ubiquitin-activating enzyme E1, ubiquitin-conjugating enzyme E2, and ubiquitin protein ligase E3. Both UPS and SUMOylation begin with ATP-dependent formation of a covalent bond with ubiquitin catalyzed by E1 and transfer of ubiquitin to E2. E3 links ubiquitin to specific target proteins [138], [139]. SUMOylation regulates the interaction and location of proteins; UPS induces protein degradation [140]. SUMOylation and UPS are highly activated in diabetes mellitus by oxidative stress [141], and ubiquitin protein ligase E3 is involved in the development of insulin resistance. E3 ubiquitin ligase can directly degrade the insulin receptor, IRS and other insulin signaling molecules via UPS activity. It can also regulate insulin signaling indirectly by inducing production of proinflammatory mediators involved in the regulation of insulin signaling molecules, including TNF, IL-6, IL-4, IL-13, IL-1, MCP-1, and hypoxia-inducible factor α (HIF-α) [142].
Hyperglycemia triggers changes in the expression of genes in vascular endothelial cells that result in long-term upregulation of inflammation [143]. One mechanism involves increased inhibitor of κB (IκB) ubiquitination [144]. When bound to IκB, NF-κB is inactive. TNF can activate IKK, leading to IκB degradation via ubiquitination and the freeing of NF-κB, which translocates to the nucleus and transactivates proinflammatory genes [145]. IκBα can also be modified by SUMO1 to protect it from ubiquitination and degradation, thus limiting NF-κB activation [146]. Modification of IκBα by SUMO2/3 has the opposite effect and leads to NF-κB activation [147].
ERK5 is a MAPK with a protective role in endothelial homeostasis. It links diabetes mellitus and atherosclerosis by regulation of PPAR and Kruppel-like factors (KLF). In diabetes mellitus, ROS and AGEs stimulate ERK5 SUMOylation and inhibit ERK5 transcriptional activity in vascular endothelial cells. This leads to transrepression of atheroprotective genes by PPARγ and KLF2/4-mediated eNOS expression, both of which increase NF-κB activation and promote subsequent endothelial dysfunction and atherosclerosis [148] (Fig. 2). ERK5 SUMOylation is increased by activation of p90 ribosomal S6 kinase (p90RSK) that leads to phosphorylation of SUMO-specific proteases 2 at the T368 residue and loss of protease activity [149]. p90RSK binds to the C-terminus of ERK and phosphorylates it at the S496 residue, contributing to increased VCAM1 expression and reduced eNOS expression, in the presence of hyperglycemia. P90RSK also inhibits the transactivation of anti-inflammatory genes induced by ERK5 [150].
3.6. Decreased activation of AMPK
AMPK is a widely expressed serine/threonine protein kinase that functions as an intracellular energy sensor and participates in the regulation of cellular and whole body metabolism [151]. It forms heterotrimeric complexes, including an alpha (α1, α2) catalytic subunit and beta (β1, β2) and gamma (γ1, γ2, γ3) regulatory subunits. AMPK is activated physiologically by an increase of the cellular AMP/ATP ratio and pharmacologically by metformin, thiazolidinediones (TZDs) and statins [152]. AMPK activation has been found to occur in association with increased glucose uptake, fatty acid oxidation, mitochondrial biogenesis, and autophagy and with suppression of fatty acid and cholesterol synthesis [153]. Dysregulation of AMPK activation contributes to the onset and development of diabetes mellitus and atherosclerosis. AMPK activity is generally inhibited by activating transcription factor 3 as glucose concentration rise above the physiologically range in various cells, which is especially true in islet β-cells [154]. Glutaredoxins (Grxs) and ROS regulate AMPK activation. At low ROS levels, Grxs-mediated S-glutathionylation of the AMPK-α catalytic subunit activates AMPK to maintain the redox balance. Sustained high ROS levels result in loss of AMPK activity, which may be a cause of subsequent atherosclerosis [155].
AMPK activity was reduced in response to chronic low-grade inflammation associated with T2DM, insulin resistance, and atherosclerosis caused by LPS, TNF-α, and a high-fat diet in a germ-free mouse model and in human adipose tissue [156], [157], [158]. AMPK is an inhibitor of acute proinflammatory responses [159] and regulates inflammation in atherosclerosis through phosphorylation of several key proteins that mediate downstream signaling pathways. AMPK can inhibit VSMC proliferation related to loss of cell cycle regulation by increasing P53 expression [160]. It can decrease TNF-α induced monocyte adhesion to human aortic endothelial cells [161]. AMPK has also been shown to protect against damage from ER stress by phosphorylating C/EBP-homologous protein and promoting its degradation in vivo [162]. Activation of AMPK has also been found to inhibit ER stress induced by ox-LDL and enhanced sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) oxidation, resulting in decreased endothelial dysfunction and atherosclerosis in an in vivo model [163]. AMPK stimulates release of NO in the vascular endothelial cells by phosphorylation of eNOS at Ser1177 and Ser633 [164] (Fig. 2).
AMPK phosphorylation of SREBP-1c at Ser372 suppresses SREBP-1c cleavage, translocation, and target gene expression in hepatocytes, leading to reduced lipogenesis and lipid accumulation when exposed to high glucose concentration [165]. Increased SREBP-1a expression in macrophages directly upregulates transcription of NLRP3 inflammasomes [166]. AMPK phosphorylation of acetyl-CoA carboxylase (ACC)1 at Ser79 and ACC2 at Ser221 inhibits macrophage cholesterol accumulation by promoting HDL-cholesterol efflux, leading to decreased formation of atherosclerosis plaques in ApoE-mice [167], [168] (Fig. 3) and in α1AMPK knockout mice on the LDL receptor knockout background [169]. AMPK was found to repress macrophage NF-κB expression by upregulating expression of Sirt1, FOXO family proteins, and PGC-1α, which then decreased activation of the RelA/p65 subunit of NF-κB [170] (Fig. 3). As with Sirt1 deacetylation of the P65 subunit of NF-κB subunit, activation of protein phosphatase 2A (PP2A) by AMPK decreases Ser536 phosphorylation of NF-κB p65, leading to the decreased expression of proinflammatory factors such as TNF-α, IL-1β and IL-6 in macrophages [171]. AMPK has also been found to upregulate the expression of the anti-inflammatory cytokine IL-10 in macrophages [172] (Fig. 3). Decreased AMPK expression was found to inhibit oxidation of fatty acids, with increased accumulation of fatty acids leading to ER stress that further activated the macrophage inflammatory response [173]. Other studies have shown that downregulation of macrophage AMPK mediated fatty acid activation of NLRP3 inflammasomes, with subsequent production of IL-1β and IL-18 [174], [175] (Fig. 3), and that AMPK activation suppressed ox-LDL-induced macrophage proliferation [176].
4. Gut microbiota in diabetes-accelerated atherosclerosis
The gut microbiota is a complex community of bacteria residing in the intestine, including more than 1014 species bacteria, archaea, viruses, fungi and protozoa [177], [178]. The majority are bacteria in the Phyla Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia [179], [180], [181]. The bacterial load in digestive system increases exponentially from the stomach, duodenum, jejunum, ileum, to the colon [182]. Insulin resistance and low-grade peripheral inflammation are more prevalent in patients with microflora dysfunction, and T2DM is characterized by altered gut microbiota, inflammation and gut barrier disruption [183], [184]. If the intestinal flora is disturbed, then LPS produced by gram-negative bacteria may lead to metabolic endotoxemia and low-level inflammation that can contribute to development of insulin resistance and T2DM. Fatty acids may trigger the binding of toxin, TLR4/CD14, and TLR2 to stimulate innate immune responses [185]. Recent evidence suggest that changes in the gut microbiota in response to metformin may mediate its treatment effects [186], [187]. Metformin induced marked alterations in the gut microbiota of treatment-naive T2DM patients, and the effect mediates the glucose-lowering effects of metformin. Transfer of fecal samples from those patients to germ-free mice improved their glucose tolerance, showing an improvement in glucose-lowering response [188]. The abundance of the gut microbiota also improves with metformin treatment in humans [189].
Diabetes mellitus and atherosclerosis are associated with some similar alterations in the composition of the gut microbiota composition and its metabolites, such as increased Lactobacillus, LPS and trimethylamine N-oxide (TMAO). The gut microbiota influences oxidative stress in the vascular wall and the progression of inflammation in atherosclerosis. An increase in the Escherichia coli population can increase production of uric acid, which contributes to the overproduction of oxygen free radicals, vascular endothelial dysfunction, and inflammation [190], [191]. Increased TMAO has been associated with glycemic control in T2DM patients [192], [193], [194]. In patients with atherosclerosis, choline produced by gut microbes was converted to trimethylamine and then to TMAO by flavin-containing monooxygenase 3. TMAO promoted CD36/SR-AI mRNA and protein expression in macrophages, increased foam cell formation, and enhanced aortic atherosclerotic plaque development [195]. LPS produced by increased gram-negative bacteria populations can be identified by TLR4 specificity, and activation of NF-κB by myeloid differentiation factor 88 (MyD88), resulting in increased expression of inflammatory factors such as IL-6 and TNF-α [196]. In addition, Helicobacter pylori may act directly on atherosclerotic plaques, as its DNA has been found in arterial plaques. It can induce the expression of TNF-α and IL-6 [197] (Fig. 4). Not all gut microbes promote atherosclerosis development. Increased probiotic bacteria such as Bifidobacterium or Lactobacillus can reduce inflammation and insulin resistance, and have been associated with decreased atherosclerosis [198], [199].
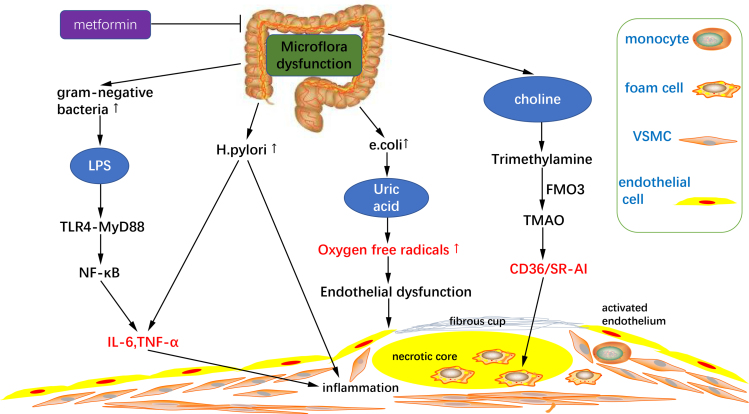
Fig. 4.
Microflora dysfunction contributes to atherosclerosis progression. Diabetes mellitus induce microflora dysfunction and subsequent inflammation progression. LPS produced by excessive gram-negative bacteria can activate NF-κB via TLR-4-MyD88 to increase the expression of inflammatory factors such as IL-6 and TNF-α. And increased Helicobacter pylori (H. pylori) can also induce the expression of TNF-α and IL-6. Increase of Escherichia coli (E. coli) promotes the production of uric acid, which contributes to the overproduction of oxygen free radicals, causing vascular endothelial dysfunction. Increased choline induces TMAO generation, promoting macrophage CD36/SR-AI mRNA and protein expression, increasing macrophage foam cells formation. FMO3, flavin-containing monooxygenase 3.
5. MicroRNAs in diabetes-accelerated atherosclerosis
MiRNAs are small noncoding RNAs with approximately 22 nucleotides. They downregulate target genes by post transcriptional mechanisms, and are involved in both physiological and pathological conditions [200]. MiRNAs thought to be involved in diabetes mellitus and in the progression of atherosclerosis are shown in Table 1. MiRNAs involved in the pathogenesis of diabetes mellitus include the let-7 family, miR-126, and miR-200, which influence pancreatic β-cell function and insulin resistance and have been directly associated with oxidative stress and inflammation [201]. The let-7 family members modulate inflammatory responses, are decreased in carotid plaques of humans with diabetes mellitus and in atherosclerosis that develops in animal diabetes models. Reduced let-7 miRNA expression via Lin28b, a negative regulator of Let-7 synthesis, was associated with in vitro PDGF and TNF-α treatment of VSMCs and endothelial cells. Restoration of let-7 expression inhibit the production of mediators in vascular inflammation, including IL-6, IL-1β, and NF-κB [202]. MiR-126 was significantly reduced in a group of Chinese patients at risk of atherosclerosis or with diabetes mellitus [203]. MiR-126 is an endothelium-specific microRNA that regulates vascular integrity and angiogenesis [204]. Upregulation of miR-126 activates endothelial cells and endothelial progenitor cells damaged by hypoxia leading to vascular repair [205]. The evidence shows that decreased miR-126 contributes to the development of atherosclerosis. ROS upregulates miR-200c via a p53-dependent mechanism. MiR-200c was found to directly target the 3′ untranslated regions of Sirt1, eNOS, and FOXO1, and to disturb the regulatory loops of these factors. This results in increased ROS production, decreased NO production and vascular dysfunction [200]. MiR-210 was found to target oxidative phosphorylation, affecting mitochondria complexes 1, 2, and 4, and decreasing mitochondrial ROS production. MiR-210 has also been found to negatively regulate the NF-κB pathway and inhibit the production of proinflammatory cytokines in macrophages [206].
Table 1.
MicroRNAs in diabetes joining in the inflammation progression in atherosclerosis. ABCA1, ATP binding casstte transporter A1; APC, adenomatous polyposis coli; ECs, endothelial cells; Egr2, early growth response protein 2; FAS, fatty acid synthetase; Grb 10, growth factor receptor-bound protein 10; NAMPT, nicotinamide phosphoribosyltransferse; PIK3R2, phosphstidylinositol-3-kinase regulatory subunit 2; SPRED1, sprouty-related, EVH1 domain containing protein 1; Suv39h1, suppressor of variegation 3–9 homolog 1; ZEB1, zinc finger E-box binding homeobox 1.
| Tissue | miRNA | Molecular targets | Functions | Refs. |
|---|---|---|---|---|
| Liver | miR-122↑ | HMGRC, FAS, SREBP-1 | Hypercholesterolemia, pro-atherogenic | [200], [201], [202] |
| Liver | miR-200c↑ | ? | [13] | |
| Liver | MIR-128-1↑ | LDL receptor and ABCA1 | Lipid metabolism and energy homeostasis dysregulation | [199] |
| Vascular | miR-128-1↑ | SIRT1 | AMPK related antiatherogenic function↓ | [12] |
| Vascular | miR-34a↑ | SIRT1, NAMPT | [196], [197] | |
| Vascular | miR-451↑ | LKB1 | [198] | |
| Vascular | miR-504↑ | Grb10, Egr2 | VSMCs dysfunction | [203] |
| Vascular | miR-125b, miR200 family↑ | Suv39h1 and ZEB1 | Triggers an inflammatory phenotype in VSMCs | [204], [205] |
| Vascular | miR-138↑ | SIRT1 | Regulates proliferation and migration in VSMCs | [206], [207] |
| Vascular | Let-7↓ | Inflammation modulators | Induces VSMCs and ECs activation | [190] |
| Vascular | miR-126↓ | PIK3R2, SPRED1 | Governs vascular integrity and angiogenesis | [191], [192], [193] |
| Vascular | miR-200c↑ | SIRT1, eNOS, FOXO1 | ROS production↑, NO availability↓ | [188], [194] |
| Vascular | miR-210↑ | components of the oxidative phosphorylation machinery, NF-κB | ROS↓, pro-inflammatory cytokines↓ | [188], [194] |
| Vascular | miR-210↑ | APC | stabilize carotid plaques | [209] |
MiR-34 was among the miRNAs upregulated in newly diagnosed diabetes mellitus patients, and was associated with AMPK activation [207]. Upregulation of miR-34a was related to insulin resistance and may be involved in accelerated diabetic atherosclerosis. MiR-34a directly decreased Sirt1 expression, NAD+-dependent lysine deacetylase, and targeted nicotinamide phosphoribosyl transferase, the rate-limiting enzyme in the salvage pathway of NAD+ biosynthesis [208]. As Sirt1-mediated deacetylation is essential for complete function of the AMPK–activating kinase liver kinase B1 (LKB1), AMPK-related antiatherogenic function was diminished [209]. In diabetic mellitus, overexpression of miR-451 decreases activation of AMPK by LKB1 via decrease of LKB1 scaffold protein calcium-binding protein expression. Consistent with this, the miR-144/451 cluster was downregulated by stretching of VSMCs, leading to phosphorylation of AMPK and subsequent contractile differentiation [210]. Insulin resistance was also associated with upregulation of miR-128-1, which is thought to regulate Sirt1 expression, and to influence expression of some components of AMPK [12].
MiR-128-1 is also linked to human dyslipidemia. Increased miR-128-1 dysregulated cholesterol lipid and energy hemostasis in an ApoE−/− mouse model [211]. MiR-200c has also been associated with hypercholesterolemia and is upregulated in diabetes mellitus [13]. MiR-122 participates in the pathogenesis of diabetes mellitus through is effects of pancreatic β-cell function and insulin resistance, and it has been implicated in the metabolism of hepatic cholesterol and fatty acids. In vivo studies have shown that miR-122 inhibition contributes to decreased plasma total cholesterol in both mice and nonhuman primates [212], [213], but was not associated with the development of incident CVD over 15 years of follow-up [214].
MiRNAs are also involved in VSMCs dysfunction. In diabetic mice, high glucose upregulated miR-504 and inflammatory genes, but downregulated Grb10, an miR-504 inhibitor. Overexpression of miR-504 in VSMCs inhibited contractile genes and enhanced ERK-1/2 activation, proliferation and migration [215]. MiR-125b and miR-200 family members were upregulated in VSMC from T2DM db/db model mice (db/dbVSMC) compared with control db/+ mice (db/+VSMC). The miRNAs triggered an inflammatory VSMC phenotype by targeting suppressor of variegation 3–9 homolog 1, a histone methyl transferase, and a Zinc-finger E-box binding homeobox 1, a transcription repressor [216], [217]. MiR-138 was also found to regulate proliferation and migration of VSMCs under diabetic conditions [218], [219]. MiR-210 was upregulated in the plasma and urine of a group of type 1 diabetes mellitus patients [220], and may contribute to stabilization of carotid plaques by inhibition of adenomatous polyposis coli expression, thereby affecting Wingless-related integration site (Wnt) signaling and regulating smooth muscle cell survival [221]. This indicates that miR-210 may be a therapeutic target for prevention of atherothrombotic vascular events.
6. Conclusion
Knowledge of the mechanisms responsible for accelerated atherosclerosis in diabetes mellitus patients has increased in recent years. The pathogenesis of diabetic atherosclerosis is complicated, involves not only hyperglycemia but also dyslipidemia, changes in secretion of hormone other than insulin, and a proinflammatory state. These factors influence the direct effects of elevated glucose on diabetic atherosclerosis, and may also influence the pathogenesis of diabetes mellitus. Oxidative stress induces inflammation; inflammation promotes ROS production. Oxidative stress and inflammation impair pancreatic β cell activity and insulin sensitivity. These effects form a vicious cycle that increases the complexity of the causes of diabetes-accelerated atherosclerosis. This review describes four pathways involved in ROS production, mitochondrial dysfunction, AGE formation, and activation of the polyol and PKC pathways. Intracellular hyperglycemia initially causes excessive ROS production in mitochondria and then increases ROS production by the other three pathways. These pathways directly contribute to expression of inflammatory factors and the subsequent progression of atherosclerosis. ROS itself and some downstream products, such as ox-LDL, AMPK, and insulin resistance, contribute to inflammation during the progression of atherosclerosis. Prevention of vascular oxidative stress may be a feasible therapeutic strategy. Studies in ApoE−/− mice reveal persistent aortic atherosclerotic plaque development despite normalization of blood glucose levels for at least 8 weeks, which is referred to as “hyperglycemic memory”. This may be a consequence of hyperglycemia-induced epigenetic changes and increased NF-κB subunit P65 expression, which can be prevented by normalizing ROS generation [2]. The development of novel treatments and prevention methods, and a deeper understanding of diabetes-accelerated atherosclerosis is required.
The influence of the intestinal microbiota in metabolic diseases, obesity and diabetes is widely accepted. Its effects in CVD are of ongoing interest. Similar changes occur in diabetes mellitus and atherosclerosis and may participate in mechanisms of diabetes-associated atherosclerosis. Gut microbiota dysregulation in diabetes mellitus can directly promote atherogenesis by changes in metabolites such as choline, oxidative stress, and inflammation. An increase of probiotic bacteria has an antiatherogenic effect by decreasing the production of endotoxins, enhancing intestinal barrier function, reducing inflammation, and improving insulin resistance. The identification of bacterial metabolites allows evaluation of microbial pathways as both mediators and potential pharmacological targets in cardiometabolic disease. Many studies indicate that diabetes mellitus is characterized by an imbalance of gut microbiota, and changes in the gut microbiota often appear before diabetes mellitus is diagnosed. However, the bacteria that are associated with progression of diabetes-accelerated atherosclerosis have not been identified. Further study of the casual relationship of gut microbiota and diabetes mellitus is warranted.
MiRNAs dysregulation contributes to diabetes mellitus and to the progression of atherosclerosis. MiRNAs are potential therapeutic targets, and the available clinical trial data shows the safety and efficacy of miRNA-based therapies. MiRNAs are easily synthesized, chemically modified to increase stability, and can be delivered to target tissues using specifically designed biomaterials. Many miRNAs dysregulation has been confirmed in atherosclerosis but not in diabetes mellitus. MiR-155 has been proposed as a therapeutic target for eNOS and impaired endothelial cell-dependent vasorelaxation. It also targets type 1 angiotensin 2 receptor (AT1R), regulating the expression of endothelial cell inflammatory molecules, such as VCAM-1 and MCP-1 [222]. MiR-33 is involved in the regulation of lipid metabolism, regulation of inflammatory genes, and oxidative stress [223]. Few data are available on changes in miR-155 or miR-33 expression in diabetes mellitus and it is not clear whether diabetes induces their dysregulation. Further study is required to clarify the involvement of miRNAs in diabetes-associated atherosclerosis.
Acknowledgements
We thank Dr. Zhangyong Song for critical reading of the article and helpful suggestions. We also thank International Science Editing for editing this manuscript. We apologized to those colleagues whose work is not specifically referenced, and gratefully acknowledge their contributions to our article.
Acknowledgments
Sources of funding
This study was partly supported by grants from National Undergraduate Innovation and Entrepreneurship Training Program (201710632002), Luzhou City Bureau of Science and Technology (2016LZXNYD-J26) and Scientific Research Fund of SiChuan Provincial Education Department (17ZA0426, 17ZA0433).
Disclosures
None.
Contributor Information
Huan Chen, Email: huanchen@swmu.edu.cn.
Xiang Xie, Email: xiangxie@swmu.edu.cn.
References
- 1.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 3.Nouwen A., Nefs G., Caramlau I., Connock M., Winkley K., Lloyd C.E., Peyrot M., Pouwer F. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care. 2011;34:752–762. doi: 10.2337/dc10-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross S., Gerstein H., Paré G. The genetic link between diabetes and atherosclerosis. Can. J. Cardiol. 2018;34:565–574. doi: 10.1016/j.cjca.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Russell R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Stocker R., Keaney J.F., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 8.Forrester Steven J., Kikuchi Daniel S., Hernandes Marina S., Xu Qian, Griendling Kathy K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017;120:713735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 10.Tang W.H., Kitai T., Hazen S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W., Gregory J.C., Org E. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N., Jiang S., Yang Y., Liu S., Ponnusamy M., Xin H., Yu T. Noncoding RNAs as therapeutic targets in atherosclerosis with diabetes mellitus. Cardiovasc. Ther. 2018;36:e12436. doi: 10.1111/1755-5922.12436. [DOI] [PubMed] [Google Scholar]
- 13.Magenta A., Ciarapica R., Capogrossi M.C. The emerging role of miR-200 family in cardiovascular diseases. Circ. Res. 2017;120:1399–1402. doi: 10.1161/CIRCRESAHA.116.310274. [DOI] [PubMed] [Google Scholar]
- 14.Zraika S., Hull R.L., Udayasankar J., Aston-Mourney K., Subramanian S.L., Kisilevsky R., Szarek W.A., Kahn S.E. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scialò F., Fernández-Ayala D.J., Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front. Physiol. 2017;8:428. doi: 10.3389/fphys.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stowe D.F., Camara A.K. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid. Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu E.P., Bennett M.R. The role of mitochondrial DNA damage in the development of atherosclerosis. Free Radic. Biol. Med. 2016;100:223–230. doi: 10.1016/j.freeradbiomed.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Bullon P., Newman H.N., Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontology 2000. 2014;64:139–153. doi: 10.1111/j.1600-0757.2012.00455.x. [DOI] [PubMed] [Google Scholar]
- 19.Anupam K., Kaushal J., Prabhakar N., Bhatnagar A. Effect of redox status of peripheral blood on immune signature of circulating regulatory and cytotoxic T cells in streptozotocin induced rodent model of type I diabetes. Immunobiology. 2018;223:586–597. doi: 10.1016/j.imbio.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Bayrami G., Alihemmati A., Karimi P., Javadi A., Keyhanmanesh R., Mohammadi M., Zadi-Heydarabad M., Badalzadeh R. Combination of vildagliptin and ischemic postconditioning in diabetic hearts as a working strategy to reduce myocardial reperfusion injury by restoring mitochondrial function and autophagic activity. Adv. Pharm. Bull. 2018;8:319–329. doi: 10.15171/apb.2018.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He C., Hart P.C., Germain D. SOD2 and the mitochondrial UPR: partners regulating cellular phenotypic transitions. Trends Biochem. Sci. 2016;41:568–577. doi: 10.1016/j.tibs.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jheng H.F., Tsai P.J., Guo S.M., Kuo L.H., Chang C.S., Su I.J., Chang C.R., Tsai Y.S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q., Zhang M., Torres G., Wu S., Ouyang C., Xie Z., Zou M.H. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission. Diabetes. 2017;66:193–205. doi: 10.2337/db16-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatsch E., De Carvalho J.A., Hausen B.S., Bollick Y.S., Torbitz V.D., Duarte T., Scolari R., Duarte M.M., Londero S.W., Vaucher R.A. Oxidative DNA damage is associated with inflammatory response, insulin resistance and microvascular complications in type 2 diabetes. Mutat. Res. 2015;782:17–22. doi: 10.1016/j.mrfmmm.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Giacco F., Du X., Carratú A., Gerfen G.J., D’Apolito M., Giardino I., Rasola A., Marin O., Divakaruni A.S., Murphy A.N., Shah M.S., Brownlee M. GLP-1 cleavage product reverses persistent ROS generation after transient hyperglycemia by disrupting an ROS-generating feedback loop. Diabetes. 2015;64:3273–3284. doi: 10.2337/db15-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah M.S., Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ. Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madamanchi N.R., Runge M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Jing J., Yu S., Song M., Tan H., Cui B., Huang L. Advanced glycation endproducts induce apoptosis of endothelial progenitor cells by activating receptor RAGE and NADPH oxidase/JNK signaling axis. Am. J. Transl. Res. 2016;8:2169–2178. [PMC free article] [PubMed] [Google Scholar]
- 29.Watson A.M., Soro-Paavonen A., Sheehy K., Li J., Calkin A.C., Koitka A., Rajan S.N., Brasacchio D., Allen T.J., Cooper M.E., Thomas M.C. Delayed intervention with AGE inhibitors attenuates the progression of diabetes-accelerated atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia. 2011;54:681–689. doi: 10.1007/s00125-010-2000-9. [DOI] [PubMed] [Google Scholar]
- 30.Menini S., Iacobini C., Ricci C., Blasetti Fantauzzi C., Pugliese G. Protection from diabetes-induced atherosclerosis and renal disease by D-carnosine-octylester: effects of early vs late inhibition of advanced glycation end-products in Apoe-null mice. Diabetologia. 2015;58:845–853. doi: 10.1007/s00125-014-3467-6. [DOI] [PubMed] [Google Scholar]
- 31.Fokkens B.T., Mulder D.J., Schalkwijk C.G., Scheijen J.L., Smit A.J., Los L.I. Vitreous advanced glycation endproducts and α-dicarbonyls in retinal detachment patients with type 2 diabetes mellitus and non-diabetic controls. PLoS One. 2017;12:e0173379. doi: 10.1371/journal.pone.0173379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tikellis C., Pickering R.J., Tsorotes D., Huet O., Cooper M.E., JandeleitDahm K., Thomas M.C. Dicarbonyl stress in the absence of hyperglycemia increases endothelial inflammation and atherogenesis similar to that observed in diabetes. Diabetes. 2014;63:3915–3925. doi: 10.2337/db13-0932. [DOI] [PubMed] [Google Scholar]
- 33.Yao D., Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byun K., Yoo Y., Son M., Lee J., Jeong G.B., Park Y.M., Salekdeh G.H., Lee B. Advanced glycation end-products produced systemically and by macrophages: a common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017;177:44–55. doi: 10.1016/j.pharmthera.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Bongarzone S., Savickas V., Luzi F. Targeting the receptor for advanced glycation endproducts (RAGE): a medicinal chemistry perspective. J. Med. Chem. 2017;60:7213–7232. doi: 10.1021/acs.jmedchem.7b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen T.B., Pantapalangkoor P., Yan J. Diabetes exacerbates infection via hyperinflammation by signaling through TLR4 and RAGE. MBio. 2017:8. doi: 10.1128/mBio.00818-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida T., Yamagishi S., Nakamura K., Matsui T., Imaizumi T., Takeuchi M., Koga H., Ueno T., Sata M. Telmisartan inhibits AGE-induced C-reactive protein production through downregulation of the receptor for AGE via peroxisome proliferator-activated receptor-gamma activation. Diabetologia. 2006;49:3094–3099. doi: 10.1007/s00125-006-0437-7. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi Y., Matsui T., Takeuchi M., Yamagishi S. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem. Biophys. Res. Commun. 2010;391:1405–1408. doi: 10.1016/j.bbrc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 39.Shen C., Li Q., Zhang Y.C., Ma G., Feng Y., Zhu Q., Dai Q., Chen Z., Yao Y., Chen L., Jiang Y., Liu N. Advanced glycation endproducts increase EPC apoptosis and decrease nitric oxide release via MAPK pathways. Biomed. Pharmacother. 2010;64:35–43. doi: 10.1016/j.biopha.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Ramasamy R., Goldberg I.J. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ. Res. 2010;106:1449–1458. doi: 10.1161/CIRCRESAHA.109.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho E.C., Lam K.S., Chen Y.S., Yip J.C., Arvindakshan M., Yamagishi S., Yagihashi S., Oates P.J., Ellery C.A., Chung S.S., Chung S.K. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006;55:1946–1953. doi: 10.2337/db05-1497. [DOI] [PubMed] [Google Scholar]
- 42.Tang W.H., Martin K.A., Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012:1–8. doi: 10.3389/fphar.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qingyuang D., Tao G. Advances in research on the role of oxidative stress in diabetes and atherosclerosis. Adv. Cardiovasc. Dis. 2013;34:664–668. [Google Scholar]
- 44.Nishinaka T., Yabe-Nishimura C. EGF receptor-ERK pathway is the major signaling pathway that mediates upregulation of aldose reductase expression under oxidative stress. Free Radic. Biol. Med. 2001;31:205–216. doi: 10.1016/s0891-5849(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 45.Vedantham S., Noh H., Ananthakrishnan R. Human aldose reductase expression accelerates atherosclerosis in diabetic apolipoprotein E-/- mice. Arterioscler. Thromb. Vasc. Biol. 2011;31:1805–1813. doi: 10.1161/ATVBAHA.111.226902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy A.B., Ramana K.V., Srivastava S., Bhatnagar A., Srivastava S.K. Aldose reductase regulates high glucose-induced ectodomain shedding of tumor necrosis factor (TNF)-alpha via protein kinase C-delta and TNF-alpha converting enzyme in vascular smooth muscle cells. Endocrinology. 2009;150:63–74. doi: 10.1210/en.2008-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yadav U.C., Ramana K.V., Srivastava S.K. Aldose reductase inhibition suppresses airway inflammation. Chem. Biol. Interact. 2011;191:339–345. doi: 10.1016/j.cbi.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solinas G., Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–2611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- 49.Hecker P.A., Mapanga R.F., Kimar C.P. Effects of glucose-6-phosphate dehydrogenase deficiency on the metabolic and cardiac responses to obesogenic or high-fructose diets. Am. J. Physiol. Endocrinol. Metab. 2012;303:E959–E972. doi: 10.1152/ajpendo.00202.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaskin R.S., Estwick D., Peddi R. G6PD deficiency: its role in the high prevalence of hypertension and diabetes mellitus. Ethn. Dis. 2001;11:749–754. [PubMed] [Google Scholar]
- 51.Li H., Förstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013;13:161–167. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Li H., Horke S., Förstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 2013;34:313–319. doi: 10.1016/j.tips.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Qiang L.U., Hua-birr S.U., WANG Lei, He-ping W.U. Effects of PKC/NADPH oxidase on ROS in HUVEs cultured with high glucose. Prog. Modern Biomed. 2015;15 (5843–5845+5849) [Google Scholar]
- 54.Cheng Yong-xia, Gui-bo Liu, Yan Bin. Effects of PKC/NADPH oxidase on ages-induced eNOS uncoupling in rat cardiac microvascular endothelial cells. Basic Clin. Med. 2013;33:82–87. [Google Scholar]
- 55.Isakov N. Protein kinase C (PKC) isoforms in cancer, tumor promotion and tumor suppression. Semin. Cancer Biol. 2018;48:36–52. doi: 10.1016/j.semcancer.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Land M., Rubin C.S. A calcium- and diacylglycerol-stimulated protein kinase C (PKC), Caenorhabditis elegans PKC-2, links thermal signals to learned behavior by acting in sensory neurons and intestinal cells. Mol. Cell. Biol. 2017:37. doi: 10.1128/MCB.00192-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brinkmann C., Schwinger R.H., Brixius K. Physical activity and endothelial dysfunction in type 2 diabetic patients: the role of nitric oxide and oxidative stress. Wien. Med. Wochenschr. 2011;161:305–314. doi: 10.1007/s10354-011-0868-8. [DOI] [PubMed] [Google Scholar]
- 58.Kong L., Shen X., Lin L., Leitges M., Rosario R., Zou Y.S., Yan S.F. PKCβ promotes vascular inflammation and acceleration of atherosclerosis in diabetic ApoE null mice. Arterioscler. Thromb. Vasc. Biol. 2013;33:1779–1787. doi: 10.1161/ATVBAHA.112.301113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durpès M.C., Morin C., Paquin-Veillet J., Beland R., Paré M., Guimond M.O., Rekhter M., King G.L., Geraldes P. PKC-β activation inhibits IL-18- binding protein causing endothelial dysfunction and diabetic atherosclerosis. Cardiovasc. Res. 2015;106:303–313. doi: 10.1093/cvr/cvv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Q., Park K., Li C., Rask-Madsen C., Mima A., Qi W., Mizutani K., Huang P., King G.L. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-β isoform in the endothelium. Circ. Res. 2013;113:418–427. doi: 10.1161/CIRCRESAHA.113.301074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tabit C.E., Shenouda S.M., Holbrook M. Protein kinase c-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harja E., Chang J.S., Lu Y., Leitges M., Zou Y.S., Schmidt A.M., Yan S.F. Mice deficient in PKC beta and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009;23:1081–1091. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verrier E., Wang L., Wadham C., Albanese N., Hahn C., Gamble J.R., Chatterjee V.K., Vadas M.A., Xia P. PPARγ agonists ameliorate endothelial cell activation via inhibition of diacylglycerol-protein kinase C signaling pathway: role of diacylglycerol kinase. Circ. Res. 2004;94:1515–1522. doi: 10.1161/01.RES.0000130527.92537.06. [DOI] [PubMed] [Google Scholar]
- 64.Rask-Madsen Christan, Li Qian, Freund Bryn. Loss of insulin signaling in vascular endothelial cells accelerate atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giacco F., Du X., D'Agati V.D., Milne R., Sui G., Geoffrion M., Brownlee M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes. 2014;63:291–299. doi: 10.2337/db13-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furukawa S., Suzuki H., Fujihara K., Kobayashi K., Iwasaki H., Sugano Y., Yatoh S., Sekiya M., Yahagi N., Shimano H. Malondialdehyde-modified LDL-related variables are associated with diabetic kidney disease in type 2 diabetes. Diabetes Res. Clin. Pract. 2018;141:237–243. doi: 10.1016/j.diabres.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 67.Skilton M.R. Intrauterine risk factors for precocious atherosclerosis. Pediatrics. 2008;121:570–574. doi: 10.1542/peds.2007-1801. [DOI] [PubMed] [Google Scholar]
- 68.Oxidized phospholipids as a new landmark in atherosclerosis, Prog. Lipid Res., vol. 37, 1998, pp. 181–207. [DOI] [PubMed]
- 69.Kaplan M., Aviram M., Hayek T. Oxidative stress and macrophage foam cell formation during diabetes mellitus-induced atherogenesis: role of insulin therapy. Pharmacol. Ther. 2012;136:175–185. doi: 10.1016/j.pharmthera.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida H., Kisugi R. Mechanisms of LDL oxidation. Clin. Chim. Acta. 2010;411:1875–1882. doi: 10.1016/j.cca.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 71.Hayek T., Kaplan M., Kerry R., Aviram M. Macrophage NADPH oxidase activation, impaired cholesterol fluxes, and increased cholesterol biosynthesis in diabetic mice: a stimulatory role for D-glucose. Atherosclerosis. 2007;195:277–286. doi: 10.1016/j.atherosclerosis.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 72.Lv J., Yang L., Guo R., Shi Y., Zhang Z., Ye J. Ox-LDL-induced microRNA-155 promotes autophagy in human endothelial cells via repressing the Rheb/mTOR pathway. Cell. Physiol. Biochem. 2017;43:1436–1448. doi: 10.1159/000481875. [DOI] [PubMed] [Google Scholar]
- 73.Xu L., Wang S., Li B., Sun A., Zou Y., Ge J. A protective role of ciglitazone in ox-LDL-induced rat microvascular endothelial cells via modulating PPARγ-dependent AMPK/eNOS pathway. J. Cell. Mol. Med. 2015;19:92–102. doi: 10.1111/jcmm.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rać M.E., Safranow K., Rać M., Kurzawski G., Krzystolik A., Sagasz-Tysiewicz D., Jakubowska K., Poncyljusz W., Chlubek D. CD36 gene is associated with thickness of atheromatous plaque and ankle-brachial index in patients with early coronary artery disease. Kardiol. Pol. 2012;70:918–923. [PubMed] [Google Scholar]
- 75.Sawada H., Saito Y., Noguchi N. Enhanced CD36 expression changes the role of Nrf2 activation from anti-atherogenic to pro-atherogenic in apoE-deficient mice. Atherosclerosis. 2012;225:83–90. doi: 10.1016/j.atherosclerosis.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 76.Geng H., Wang A., Rong G., Zhu B., Deng Y. The effects of ox-LDL in human atherosclerosis may be mediated in part via the toll-like receptor 4 pathway. Mol. Cell. Biochem. 2010;342:201–206. doi: 10.1007/s11010-010-0484-8. [DOI] [PubMed] [Google Scholar]
- 77.Kannan Y., Sundaram K., Aluganti Narasimhulu C., Parthasarathy S., Wewers M.D. Oxidatively modified low density lipoprotein (LDL) inhibits TLR2 and TLR4 cytokine responses in human monocytes but not in macrophages. J. Biol. Chem. 2012;287:23479–23488. doi: 10.1074/jbc.M111.320960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schauer I.E., Knaub L.A., Lloyd M., Watson P.A., Gliwa C., Lewis K.E., Chait A., Klemm D.J., Gunter J.M., Bouchard R., McDonald T.O. CREB downregulation in vascular disease: a common response to cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2010;30:733–741. doi: 10.1161/ATVBAHA.109.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernal-Lopez M.R., Garrido-Sanchez L., Gomez-Carrillo V., Gallego-Perales J.L., Llorente-Cortes V., Calleja F., Gomez-Huelgas R. Antioxidized LDL antibodies are associated with different metabolic pathways in patients with atherosclerotic plaque and type 2 diabetes. Diabetes Care. 2013;36:1006–1011. doi: 10.2337/dc12-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dong K., Ni H., Wu M., Tang Z., Halim M., Shi D. ROS-mediated glucose metabolic reprogram induces insulin resistance in type 2 diabetes. Biochem. Biophys. Res. Commun. 2016;476:204–211. doi: 10.1016/j.bbrc.2016.05.087. [DOI] [PubMed] [Google Scholar]
- 81.Savage D.B., Semple R.K., Clatworthy M.R. Complement abnormalities in acquired lipodystrophy revisited. J. Clin. Endocrinol. Metab. 2009;94:10–16. doi: 10.1210/jc.2008-1703. [DOI] [PubMed] [Google Scholar]
- 82.Pae M., Baek Y., Lee S., Wu D. Loss of ovarian function in association with a high-fat diet promotes insulin resistance and disturbs adipose tissue immune homeostasis. J. Nutr. Biochem. 2018;57:93–102. doi: 10.1016/j.jnutbio.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 83.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 84.Nishikawa T., Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2007;9:343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 85.DeFronzo R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kannan L., Chernoff A. Insulin resistance and atherosclerosis: is it time to measure HOMA-IR to predict coronary artery disease? N. Am. J. Med. Sci. 2013;5:615–616. doi: 10.4103/1947-2714.120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vafaeimanesh J., Parham M., Norouzi S., Hamednasimi P., Bagherzadeh M. Insulin resistance and coronary artery disease in non-diabetic patients: is there any correlation? Casp. J. Intern. Med. 2018;9:121–126. doi: 10.22088/cjim.9.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lalić K., Nedeljković M., Jotić A., Babić R., Rajković N., Popović L., Lukić L., Miličić T., Singh Lukač S., Stošić L., Maćešić M. Endothelial dysfunction of coronary arteries in subjects without diabetes: an association with both insulin resistance and impaired insulin secretion response. Diabetes Res. Clin. Pract. 2018;139:179–187. doi: 10.1016/j.diabres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Nishizawa T., Bornfeldt K.E. Diabetic vascular disease and the potential role of macrophage glucose metabolism. Ann. Med. 2012;44:555–563. doi: 10.3109/07853890.2011.585346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xian H.M., Che H., Qin Y., Yang F., Meng S.Y., Li X.G., Bai Y.L., Wang L.H. Coriolus versicolor aqueous extract ameliorates insulin resistance with PI3K/Akt and p38 MAPK signaling pathways involved in diabetic skeletal muscle. Phytother. Res. 2018;32(551–560) doi: 10.1002/ptr.6007. [DOI] [PubMed] [Google Scholar]
- 91.Martínez-Hervás S., Vinué A., Núñez L., Andrés-Blasco I., Piqueras L., Real J.T., Ascaso J.F., Burks D.J., Sanz M.J., González-Navarro H. Insulin resistance aggravates atherosclerosis by reducing vascular smooth muscle cell survival and increasing CX3CL1/CX3CR1 axis. Cardiovasc. Res. 2014;103:324–336. doi: 10.1093/cvr/cvu115. [DOI] [PubMed] [Google Scholar]
- 92.Tsukano H., Gotoh T., Endo M., Miyata K., Tazume H., Kadomatsu T., Yano M., Iwawaki T., Kohno K., Araki K., Mizuta H., Oike Y. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2010;30:1925–1932. doi: 10.1161/ATVBAHA.110.206094. [DOI] [PubMed] [Google Scholar]
- 93.Kashyap S.R., Defronzo R.A. The insulin resistance syndrome: physiological considerations. Diabetes Vasc. Dis. Res. 2007;4:13–19. doi: 10.3132/dvdr.2007.001. [DOI] [PubMed] [Google Scholar]
- 94.Wang C., Li Y., Hao M., Li W. Astragaloside IV inhibits triglyceride accumulation in insulin-resistant hepG2 cells via AMPK-induced SREBP-1c phosphorylation. Front. Pharmacol. 2018;9:345. doi: 10.3389/fphar.2018.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coletta D., Balas B., Chavez A.O., Baig M., Abdul-Ghani M., Kashyap S.R., Folli F., Tripathy D., Mandarino L.J., Cornell J.E. Effect of acute physiological hyperinsulinemia on gene expression in human skeletal muscle in vivo. Am. J. Physiol. Endo Metab. 2008;294:E910–E917. doi: 10.1152/ajpendo.00607.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guan H.P., Chen G. Factors affecting insulin-regulated hepatic gene expression. Prog. Mol. Biol. Transl. Sci. 2014;121:165–215. doi: 10.1016/B978-0-12-800101-1.00006-5. [DOI] [PubMed] [Google Scholar]
- 97.Matsuda M., Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 2014;15:1–10. doi: 10.1007/s11154-013-9271-7. [DOI] [PubMed] [Google Scholar]
- 98.Diwan A.G., Kuvalekar A.A., Dharamsi S., Vora A.M., Nikam V.A., Ghadge A.A. Correlation of serum adiponectin and leptin levels in obesity and type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2018;22:93–99. doi: 10.4103/ijem.IJEM_491_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamauchi T., Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Yamauchi T., Iwabu M., Okada-Iwabu M., Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best. Pract. Res. Clin. Endocrinol. Metab. 2014;28:15–23. doi: 10.1016/j.beem.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010;86:131–141. doi: 10.2183/pjab.86.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen B., Wei J., Wang W., Cui G., Zhao Y., Zhu X., Zhu M., Guo W., Yu J. Identification of signaling pathways involved in aberrant production of adipokines in adipocytes undergoing oxidative stress. Arch. Med. Res. 2009;40:241–248. doi: 10.1016/j.arcmed.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 103.Han K.L., Choi J.S., Lee J.Y., Song J., Joe M.K., Jung M.H., Hwang J.K. Therapeutic potential of peroxisome proliferators—activated receptor-alpha/gamma dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes. 2008;57:737–745. doi: 10.2337/db07-0972. [DOI] [PubMed] [Google Scholar]
- 104.Lim J.Y., Kim W.H., Park S.I. GO6976 prevents TNF-alpha-induced suppression of adiponectin expression in 3T3-L1 adipocytes: putative involvement of protein kinase C. FEBS Lett. 2008;582:3473–3478. doi: 10.1016/j.febslet.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 105.Kim K.Y., Kim J.K., Jeon J.H., Yoon S.R., Choi I., Yang Y. C-Jun Nterminal kinase is involved in the suppression of adiponectin expression by TNF-alpha in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2005;327:460–467. doi: 10.1016/j.bbrc.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 106.Fasshauer M., Kralisch S., Klier M., Lossner U., Bluher M., Klein J., Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 107.Cheng K.K., Lam K.S., Wang Y., Huang Y., Carling D., Wu D., Wong C., Xu A. Erratum. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 2007;56:1387–1394. Diabetes. 2016;65:3218. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 108.Tao L., Gao E., Jiao X., Yuan Y., Li S., Christopher T.A., Lopez B.L., Koch W., Chan L., Goldstein B.J., Ma X.L. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 109.Iwabu M., Yamauchi T., Okada-Iwabu M. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 110.Yamauchi T., Nio Y., Maki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 111.Iwabu M., Yamauchi T., Okada-Iwabu M. Adiponectin and AdipoR1 regulate PGC-1 alpha and mitochondria by Ca2þ and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 112.Qiang L., Wang L., Kon N., Zhao W., Lee S., Zhang Y., Rosenbaum M., Zhao Y., Gu W., Farmer S.R., Accili D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of PPARγ. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li C.C., Liu C., Fu M., Hu K.Q., Aizawa K., Takahashi S., Hiroyuki S., Cheng J., von Lintig J., Wang X.D. Tomato powder inhibits hepatic steatosis and inflammation potentially through restoring SIRT1 activity and adiponectin function independent of carotenoid cleavage enzymes in mice. Mol. Nutr. Food Res. 2018;62:e1700738. doi: 10.1002/mnfr.201700738. [DOI] [PubMed] [Google Scholar]
- 114.Chen Y., Zheng Y., Liu L., Lin C., Liao C., Xin L., Zhong S., Cheng Q., Zhang L. Adiponectin inhibits TNF-α-activated PAI-1 expression via the cAMP-PKA-AMPK-NF-κB axis in human umbilical vein endothelial cells. Cell. Physiol. Biochem. 2017;42:2342–2352. doi: 10.1159/000480006. [DOI] [PubMed] [Google Scholar]
- 115.Chandrasekar B., Boylston W.H., Venkatachalam K., Webster N.J., Prabhu S.D., Valente A.J. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-kappaB/PTEN suppression. J. Biol. Chem. 2008;283:24889–24898. doi: 10.1074/jbc.M804236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arita Y., Kihara S., Ouchi N. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 117.Ouchi N., Kihara S., Arita Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 118.Ohashi K., Parker J.L., Ouchi N., Higuchi A., Vita J.A., Gokce N., Pedersen A., Kalthoff C., Tullin S., Sams A., Summer R. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Awazawa M., Ueki K., Inabe K. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011;13:401–412. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 120.Ryo M., Nakamura T., Kihara S., Kumada M., Shibazaki S., Takahashi M., Nagai M., Matsuzawa Y., Funahashi T. Adiponectin as a biomarker of the metabolic syndrome. Circ. J. 2004;68:975–981. doi: 10.1253/circj.68.975. [DOI] [PubMed] [Google Scholar]
- 121.Du X.L., Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shrikhande G.V., Scali S.T., da Silva C.G., Damrauer S.M., Csizmadia E., Putheti P., Matthey M., Arjoon R., Patel R., Siracuse J.J. O-glycosylation regulates ubiquitination and degradation of the anti-inflammatory protein A20 to accelerate atherosclerosis in diabetic ApoE-null mice. PLoS One. 2010;5:e14240. doi: 10.1371/journal.pone.0014240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu V.W., Huang P.L. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li H., Förstermann U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr. Pharm. Des. 2009;15:3133–3145. doi: 10.2174/138161209789058002. [DOI] [PubMed] [Google Scholar]
- 125.Förstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. (837a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schödel J., Padmapriya P., Marx A., Huang P.L., Ertl G., Kuhlencordt P.J. Expression of neuronal nitric oxide synthase splice variants in atherosclerotic plaques of apoE knockout mice. Atherosclerosis. 2009;206:383–389. doi: 10.1016/j.atherosclerosis.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Capettini L.S., Cortes S.F., Silva J.F., Alvarez-Leite J.I., Lemos V.S. Decreased production of neuronal NOS-derived hydrogen peroxide contributes to endothelial dysfunction in atherosclerosis. Br. J. Pharmacol. 2011;164:1738–1748. doi: 10.1111/j.1476-5381.2011.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lind M., Hayes A., Caprnda M., Petrovic D., Rodrigo L., Kruzliak P., Zulli A. Inducible nitric oxide synthase: good or bad? Biomed. Pharmacother. 2017;93:370–375. doi: 10.1016/j.biopha.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 129.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA. 2018;115:5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liaudet L., Rosenblatt-Velin N., Pacher P. Role of peroxynitrite in the cardiovascular dysfunction of septic shock. Curr. Vasc. Pharmacol. 2013;11:196–207. [PubMed] [Google Scholar]
- 131.Chen J.Y., Ye Z.X., Wang X.F., Chang J., Yang M.W., Zhong H.H., Hong F.F., Yang S.L. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018;97:423–428. doi: 10.1016/j.biopha.2017.10.122. [DOI] [PubMed] [Google Scholar]
- 132.Chen J.Y., Ye Z.X., Wang X.F., Chang J., Yang M.W., Zhong H.H., Hong F.F., Yang S.L. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018;97:423–428. doi: 10.1016/j.biopha.2017.10.122. [DOI] [PubMed] [Google Scholar]
- 133.Ptasinska A., Wang S., Zhang J., Wesley R.A., Danner R.L. Nitric oxide activation of peroxisome proliferator-activated receptor gamma through a p38 MAPK signaling pathway. FASEB J. 2007;21:950–961. doi: 10.1096/fj.06-6822com. [DOI] [PubMed] [Google Scholar]
- 134.Brancaleone V., Roviezzo F., Vellecco V., De Gruttola L., Bucci M., Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br. J. Pharmacol. 2008;155:673–680. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jain S.K., Bull R., Rains J.L., Bass P.F., Levine S.N., Reddy S., McVie R., Bocchini J.A. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid. Redox Signal. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Suzuki K., Olah G., Modis K. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. USA. 2011;108:13829–13834. doi: 10.1073/pnas.1105121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xie L., Gu Y., Wen M. Hydrogen sulfide induces Keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes. 2016;65:3171–3184. doi: 10.2337/db16-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mansour M.A. Ubiquitination: friend and foe in cancer. Int. J. Biochem. Cell Biol. 2018;101:80–93. doi: 10.1016/j.biocel.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 139.He Z., Huang T., Ao K., Yan X., Huang Y. Sumoylation, phosphorylation, and acetylation fine-tune the turnover of plant immunity components mediated by ubiquitination. Front. Plant Sci. 2017;8:1682. doi: 10.3389/fpls.2017.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao C., Huang W., Kanasaki K., Xu Y. The role of ubiquitination and sumoylation in diabetic nephropathy. Biomed. Res. Int. 2014 doi: 10.1155/2014/160692. (2014:160692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marfella R., D'Amico M., Di Filippo C., Siniscalchi M., Sasso F.C., Ferraraccio F., Rossi F., Paolisso G. The possible role of the ubiquitin proteasome system in the development of atherosclerosis in diabetes. Cardiovasc. Diabetol. 2007;6:35. doi: 10.1186/1475-2840-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang F., Lerman A., Herrmann J. Dysfunction of the ubiquitin-proteasome system in atherosclerotic cardiovascular disease. Am. J. Cardiovasc. Dis. 2015;5:83–100. [PMC free article] [PubMed] [Google Scholar]
- 143.Suryavanshi S.V., Kulkarni Y.A. NF-κβ: a potential target in the management of vascular complications of diabetes. Front. Pharmacol. 2017;8:798. doi: 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang W., Xu L., Zhou X., Gao C., Yang M., Chen G., Zhu J., Jiang L., Gan H., Gou F., Feng H., Peng J., Xu Y. High glucose induces activation of nf-kappab inflammatory signaling through ikappabalpha sumoylation in rat mesangial cells. Biochem. Biophys. Res. Commun. 2013;438:568–574. doi: 10.1016/j.bbrc.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 145.Xiao L., Liu Y., Wang N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H317–H325. doi: 10.1152/ajpheart.00182.2013. [DOI] [PubMed] [Google Scholar]
- 146.Liu Q., Li J., Khoury J., Colgan S.P., Ibla J.C. Adenosine signaling mediates SUMO-1 modification of IkappaBalpha during hypoxia and reoxygenation. J. Biol. Chem. 2009;284:13686–13695. doi: 10.1074/jbc.M809275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Culver C., Sundqvist A., Mudie S., Melvin A., Xirodimas D., Rocha S. Mechanism of hypoxia-induced NF-KappaB. Mol. Cell. Biol. 2010;30:4901–4921. doi: 10.1128/MCB.00409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chang E., Abe J. Kinase-SUMO networks in diabetes-mediated cardiovascular disease. Metabolism. 2016;65:623–633. doi: 10.1016/j.metabol.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Heo K.S., Le N.T., Cushman H.J., Giancursio C.J., Chang E., Woo C.H., Sullivan M.A., Taunton J., Yeh E.T., Fujiwara K., Abe J. Disturbed flow-activated p90rsk kinase accelerates atherosclerosis by inhibiting senp2 function. J. Clin. Invest. 2015;125:1299–1310. doi: 10.1172/JCI76453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Le N.T., Heo K.S., Takei Y. A crucial role for p90rsk-mediated reduction of erk5 transcriptional activity in endothelial dysfunction and atherosclerosis. Circulation. 2013;127:486–499. doi: 10.1161/CIRCULATIONAHA.112.116988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hardie D.G. Adenosine monophosphate-activated protein kinase: a central regulator of metabolism with roles in diabetes, cancer, and viral infection. Cold Spring Harb. Symp. Quant. Biol. 2011;76:155–164. doi: 10.1101/sqb.2011.76.010819. [DOI] [PubMed] [Google Scholar]
- 152.Xiao B., Sanders M.J., Underwood E., Heath R., Mayer F.V., Carmena D., Jing C., Walker P.A., Eccleston J.F., Haire L.F. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Almabrouk T.A., Ewart M.A., Salt I.P., Kennedy S. Perivascular fat, AMP-activated protein kinase and vascular diseases. Br. J. Pharmacol. 2014;171:595–617. doi: 10.1111/bph.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim J.Y., Park K.J., Kim G.H. In vivo activating transcription factor 3 silencing ameliorates the AMPK compensatory effects for ER stress-mediated β-cell dysfunction during the progression of type-2 diabetes. Cell Signal. 2013;25:2348–2361. doi: 10.1016/j.cellsig.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 155.Dong K., Wu M., Liu X., Huang Y., Zhang D., Wang Y., Yan L.J., Shi D. Glutaredoxins concomitant with optimal ROS activate AMPK through S-glutathionylation to improve glucose metabolism in type 2 diabetes. Free Radic. Biol. Med. 2016;101:334–347. doi: 10.1016/j.freeradbiomed.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 156.Mottillo E.P., Desjardins E.M., Crane J.D. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through Brown and Beige adipose tissue function. Cell Metab. 2016;24:118–129. doi: 10.1016/j.cmet.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gauthier M.S., O'Brien E.L., Bigornia S., Mott M., Cacicedo J.M., Xu X.J., Gokce N., Apovian C., Ruderman N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem. Biophys. Res. Commun. 2011;404:382–387. doi: 10.1016/j.bbrc.2010.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ganeshan K., Chawla A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dashwood M.R., Dooley A., Shi-Wen X., Abraham D.J., Souza D.S. Does periadventitial fat-derived nitric oxide play a role in improved saphenous vein graft patency in patients undergoing coronary artery bypass surgery? J. Vasc. Res. 2007;44:175–181. doi: 10.1159/000099833. [DOI] [PubMed] [Google Scholar]
- 161.Ewart M.A., Kohlhaas C.F., Salt I.P. Inhibition of tumor necrosis factor alpha-stimulated monocyte adhesion to human aortic endothelial cells by AMP-activated protein kinase. Arterioscler. Thromb. Vasc. Biol. 2008;28:2255–2257. doi: 10.1161/ATVBAHA.108.175919. [DOI] [PubMed] [Google Scholar]
- 162.Dai X., Ding Y., Liu Z., Zhang W., Zou M.H. Phosphorylation of CHOP (C/EBP homologous protein) by the AMP-activated protein kinase alpha 1 in macrophages promotes CHOP degradation and reduces injury-induced neointimal disruption in vivo. Circ. Res. 2016;119:1089–1100. doi: 10.1161/CIRCRESAHA.116.309463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dong Y., Zhang M., Wang S., Liang B., Zhao Z., Liu C., Wu M., Choi H.C., Lyons T.J., Zou M.H. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 2010;59:1386–1396. doi: 10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen Z., Peng I.C., Sun W., Su M.I., Hsu P.H., Fu Y., Zhu Y., DeFea K., Pan S., Tsai M.D., Shyy J.Y. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ. Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li Y., Xu S., Mihaylova M.M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Im S.S., Yousef L., Blaschitz C., Liu J.Z., Edwards R.A., Young S.G., Raffatellu M., Osborne T.F. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13:540–549. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fullerton M.D., Ford R.J., McGregor C.P., LeBlond N.D., Snider S.A., Stypa S.A., Day E.A., Lhoták Š., Schertzer J.D., Austin R.C., Kemp B.E., Steinberg G.R. Salicylate improves macrophage cholesterol homeostasis via activation of AMPK. J. Lipid Res. 2015;56:1025–1033. doi: 10.1194/jlr.M058875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Galic S., Fullerton M.D., Schertzer J.D., Sikkema S., Marcinko K., Walkley C.R., IzonD, Honeyman J., Chen Z.P., van Denderen B.J., Kemp B.E., Steinberg G.R. Hematopoietic AMPK b1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Invest. 2011;121:4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao Q., Cui X., Wu R., Zha L., Wang X., Parks J.S., Yu L., Shi H., Xue B. Myeloid deletion of α1AMPK exacerbates atherosclerosis in LDL receptor knockout (LDLRKO) mice. Diabetes. 2016;65:1565–1576. doi: 10.2337/db15-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Salminen A., Hyttinen J.M., Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen B., Li J., Zhu H. AMP-activated protein kinase attenuates oxLDL uptake in macrophages through PP2A/NF-κB/LOX-1 pathway. Vasc. Pharmacol. 2016;85:1–10. doi: 10.1016/j.vph.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 172.Yang Z., Kahn B.B., Shi H., Xue B.Z. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Erbay E., Babaev V.R., Mayers J.R., Makowski L., Charles K.N., Snitow M.E., Fazio S., Wiest M.M., Watkins S.M., Linton M.F., Hotamisligil G.S. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T., Brickey W.J., Ting J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Steinberg G.R., Schertzer J.D. AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: implications for diabetes and cardiovascular disease. Immunol. Cell Biol. 2014;92:340–345. doi: 10.1038/icb.2014.11. [DOI] [PubMed] [Google Scholar]
- 176.164Ishii N., Matsumura T., Kinoshita H., Motoshima H., Kojima K., Tsutsumi A., Kawasaki S., Yano M., Senokuchi T., Asano T., Nishikawa T., Araki E. Activation of AMP-activated protein kinase suppresses oxidized low-density lipoprotein-induced macrophage proliferation. J. Biol. Chem. 2009;284:34561–34569. doi: 10.1074/jbc.M109.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rajilić-Stojanović M., Heilig H.G., Tims S., Zoetendal E.G., de Vos W.M. Long-term monitoring of the human intestinal microbiota composition. Lancet. 2010;376(9750):1393–1400. doi: 10.1111/1462-2920.12023. [DOI] [PubMed] [Google Scholar]
- 178.Palm N.W., de Zoete M.R., Flavell R.A. Immune-microbiota interactions in health and disease. Clin. Immunol. 2015;159(2):122–127. doi: 10.1016/j.clim.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tremaroli V., Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 180.Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J.T. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Koren O., Goodrich J.K., Cullender T.C., Spor A., Laitinen K., Backhed H.K., Gonzalez A., Werner J.J., Angenent L.T., Knight R. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen J., Li Y., Tian Y., Huang C., Li D., Zhong Q., Ma X. Interaction between microbes and host intestinal health: modulation by dietary nutrients and gut-brain-endocrine-immune axis. Curr. Protein Pept. Sci. 2015;16:592–603. doi: 10.2174/1389203716666150630135720. [DOI] [PubMed] [Google Scholar]
- 183.Wu G.D., Chen J., Hoffmann C. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muegge B.D., Kuczynski J., Knights D., Clemente J.C., Gonzalez A., Fontana L., Henrissat B., Knight R., Gordon J.I. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang F., Zhang C., Zeng Q. Gut microbiota and immunopathogenesis of diabetes mellitus type 1 and 2. Front. Biosci. (Landmark Ed.) 2016;21:900–906. doi: 10.2741/4427. [DOI] [PubMed] [Google Scholar]
- 186.Lee H., Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014;80:5935–5943. doi: 10.1128/AEM.01357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S., Prifti E., Vieira-Silva S., Gudmundsdottir V. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Buford T.W. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5:80. doi: 10.1186/s40168-017-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Montandon S.A., Jornayvaz F.R. Effects of antidiabetic drugs on gut microbiota composition. Genes. 2017:8. doi: 10.3390/genes8100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chistiakov D.A., Bobryshev Y.V., Kozarov E., Sobenin I.A., Orekhov A.N. Role of gut microbiota in the modulation of atherosclerosis-associated immune response. Front. Microbiol. 2015;6:671. doi: 10.3389/fmicb.2015.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kang D.H., Ha S.K. Uric acid puzzle: dual role as anti-oxidant and pro-oxidant. Electrolyte Blood Press. 2014;12:1–6. doi: 10.5049/EBP.2014.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamashiro K., Tanaka R., Urabe T., Ueno Y., Yamashiro Y., Nomoto K., Takahashi T., Tsuji H., Asahara T., Hattori N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12:e0171521. doi: 10.1371/journal.pone.0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang Z., Klipfell E., Bennett B.J. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Qin J., Li Y., Cai Z. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 195.Holmes E., Li J.V., Marchesi J.R., Nicholson J.K. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;16:559–564. doi: 10.1016/j.cmet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 196.Singh V., Yeoh B.S., Vijay-Kumar M. Gut microbiome as a novel cardiovascular therapeutic target. Curr. Opin. Pharmacol. 2016;27:8–12. doi: 10.1016/j.coph.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sharma V., Aggrawal A. Helicobacter pylori: does it add to risk of coronary artery disease. World J. Cardiol. 2015;7:19–25. doi: 10.4330/wjc.v7.i1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang Y., Ames N.P., Tun H.M., Tosh S.M., Jones P.J., Khafipour E. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front. Microbiol. 2016;7:129. doi: 10.3389/fmicb.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Glaus S.P. Will gut microbiota help design the next generation of GLP-1-based therapies for type 2 diabetes? Cell Metab. 2017;26:6–7. doi: 10.1016/j.cmet.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 200.Magenta A., Ciarapica R., Capogrossi M.C. The emerging role of miR-200 family in cardiovascular diseases. Circ. Res. 2017;120:1399–1402. doi: 10.1161/CIRCRESAHA.116.310274. [DOI] [PubMed] [Google Scholar]
- 201.Lucia C., Komici K., Borghetti G., Femminella G.D., Bencivenga L., Cannavo A., Corbi G., Ferrara N., Houser S.R. MicroRNA in cardiovascular aging and age-related cardiovascular diseases. Front. Med. 2017;4:74. doi: 10.3389/fmed.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Brennan E., Wang B., McClelland A. Protective effect of let-7 miRNA family in regulating inflammation in diabetes-associated atherosclerosis. Diabetes. 2017;66:2266–2277. doi: 10.2337/db16-1405. [DOI] [PubMed] [Google Scholar]
- 203.Zhang T., Lv C., Li L., Chen S., Liu S., Wang C., Su B. Plasma miR-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individuals. Biomed. Res. Int. 2013;2013:761617. doi: 10.1155/2013/761617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., Richardson J.A., Bassel-Duby R., Olson E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. The role of miR-126 in embryonic angiogenesis, adult vascular homeostasis, and vascular repair and its alterations in atherosclerotic disease. J. Mol. Cell. Cardiol. 2016;97:47–55. doi: 10.1016/j.yjmcc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 206.Fuschi P., Maimone B., Gaetano C., Martelli F. Noncoding RNAs in the vascular system response to oxidative stress. Antioxid. Redox Signal. 2017 doi: 10.1089/ars.2017.7229. [DOI] [PubMed] [Google Scholar]
- 207.Kong L., Zhu J., Han W., Jiang X., Xu M., Zhao Y., Dong Q., Pang Z., Guan Q., Gao L., Zhao J., Zhao L. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48:61–69. doi: 10.1007/s00592-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 208.Choi S.E., Fu T., Seok S., Kim D.H., Yu E., Lee K.W., Kang Y., Li X., Kemper B., Kemper J.K. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;12:1062–1072. doi: 10.1111/acel.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kuwabara Y., Horie T., Baba O., Watanabe S., Nishiga M., Usami S., Izuhara M., Nakao T., Nishino T., Otsu K., Kita T., Kimura T., Ono K. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and highfat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ. Res. 2015;116:279–288. doi: 10.1161/CIRCRESAHA.116.304707. [DOI] [PubMed] [Google Scholar]
- 210.Turczyńska K.M., Bhattachariya A., Säll J., Göransson O., Swärd K., Hellstrand P., Albinsson S. Stretch-sensitive down-regulation of the miR-144/451 cluster in vascular smooth muscle and its role in AMP-activated protein kinase signaling. PLoS One. 2013;8:e65135. doi: 10.1371/journal.pone.0065135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wagschal A., Najafi-Shoushtari S.H., Wang L. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat. Med. 2015;21:1290–1297. doi: 10.1038/nm.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chung H.H. New insights for controversial issues of miR-122 in hepatic lipid metabolism. Gastroenterology. 2018;154:1552–1553. doi: 10.1053/j.gastro.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 213.Elmén J., Lindow M., Schütz S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 214.Willeit P., Philipp S., Alexander R. Circulating microRNA-122 is associated with the risk of new-onset metabolic syndrome and type-2-diabetes. Diabetes. 2017;66:347–357. doi: 10.2337/db16-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Reddy M.A., Das S., Zhuo C., Jin W., Wang M., Lanting L., Natarajan R. Regulation of vascular smooth muscle cell dysfunction under diabetic conditions by miR-504. Arterioscler. Thromb. Vasc. Biol. 2016;36:864–873. doi: 10.1161/ATVBAHA.115.306770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reddy M.A., Jin W., Villeneuve L., Wang M., Lanting L., Todorov I., Kato M., Natarajan R. Pro-inflammatory role of microrna-200 in vascular smooth muscle cells from diabetic mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:721–729. doi: 10.1161/ATVBAHA.111.241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Villeneuve L.M., Kato M., Reddy M.A., Wang M., Lanting L., Natarajan R. Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes. 2010;59:2904–2915. doi: 10.2337/db10-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen T.C., Sung M.L., Kuo H.C., Chien S.J., Yen C.K., Chen C.N. Differential regulation of human aortic smooth muscle cell proliferation by monocytederived macrophages from diabetic patients. PLoS One. 2014;9:e113752. doi: 10.1371/journal.pone.0113752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xu J., Li L., Yun H.F., Han Y.S. MiR-138 promotes smooth muscle cells proliferation and migration in db/db mice through down-regulation of SIRT1. Biochem. Biophys. Res. Commun. 2015;463:1159–1164. doi: 10.1016/j.bbrc.2015.06.076. [DOI] [PubMed] [Google Scholar]
- 220.Osipova J., Fischer D.C., Dangwal S., Volkmann I., Widera C., Schwarz K., Lorenzen J.M., Schreiver C., Jacoby U., Heimhalt M., Thum T., Haffner D. Diabetes-associated microRNAs in pediatric patients with type 1 diabetes mellitus: a cross-sectional cohort study. J. Clin. Endocrinol. Metab. 2014;99(9):E1661–E1665. doi: 10.1210/jc.2013-3868. [DOI] [PubMed] [Google Scholar]
- 221.Eken S.M., Jin H., Chernogubova E. MicroRNA-210 enhances fibrous cap stability in advanced atherosclerotic lesions. Circ. Res. 2017;120:633–644. doi: 10.1161/CIRCRESAHA.116.309318. [DOI] [PubMed] [Google Scholar]
- 222.Zhu N., Zhang D., Chen S., Liu X., Lin L., Huang X., Guo Z., Liu J., Wang Y., Yuan W., Qin Y. Endothelial enriched microRNAs regulate angiotensin II‐induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 223.Ouimet M., Ediriweera H.N., Gundra U.M. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J. Clin. Invest. 2015;125:4334–4348. doi: 10.1172/JCI81676. [DOI] [PMC free article] [PubMed] [Google Scholar]