Significance
Inflammasomes are cytosolic protein complexes that detect the presence of pathogens and damages to elicit immune responses, and dysregulation in inflammasome signaling is associated with many human diseases. As the unified downstream effector of canonical inflammasomes, caspase-1 is recruited though CARD–CARD interactions with the adaptor proteins ASC or NLRC4. We have determined the cryo-EM structures of ASC CARD and NLRC4 CARD filaments. Using multidisciplinary methods, we reveal a common mechanism of caspase-1 CARD nucleation, assembly, and activation by equivalent assembly patterns in ASC and NLRC4. Collectively, our data provide insights into inflammasome assembly and activation and afford structural platforms for modulating these CARD–CARD interactions in potential therapeutic applications.
Keywords: ASC, NLRC4, inflammasome, caspase-1, CARD
Abstract
Canonical inflammasomes are cytosolic supramolecular complexes that activate caspase-1 upon sensing extrinsic microbial invasions and intrinsic sterile stress signals. During inflammasome assembly, adaptor proteins ASC and NLRC4 recruit caspase-1 through homotypic caspase recruitment domain (CARD) interactions, leading to caspase-1 dimerization and activation. Activated caspase-1 processes proinflammatory cytokines and Gasdermin D to induce cytokine maturation and pyroptotic cell death. Here, we present cryo-electron microscopy (cryo-EM) structures of NLRC4 CARD and ASC CARD filaments mediated by conserved three types of asymmetric interactions (types I, II, and III). We find that the CARDs of these two adaptor proteins share a similar assembly pattern, which matches that of the caspase-1 CARD filament whose structure we defined previously. These data indicate a unified mechanism for downstream caspase-1 recruitment through CARD–CARD interactions by both adaptors. Using structure modeling, we further show that full-length NLRC4 assembles via two separate symmetries at its CARD and its nucleotide-binding domain (NBD), respectively.
As the first line of defense, the innate immune system employs a variety of pattern recognition receptors (PRRs) to detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (1–3). So far, at least five families of PRRs have been characterized, including Toll-like receptors (TLRs), RIG-I–like receptors (RLRs), C-type lectin receptors (CLRs), AIM2-like receptors (ALRs), and nucleotide-binding domain (NBD) and leucine-rich repeat (LRR)–containing proteins (NLRs) (4). Of these, upon ligand stimulation, ALRs and some NLRs have been shown to form oligomeric supramolecular complexes known as canonical inflammasomes, which also contain adaptor proteins and caspase-1 (Casp-1) (2, 3) (Fig. 1A). Examples of canonical inflammasomes include, but are not limited to, the AIM2 inflammasome, the NLRP1 inflammasome, the NLRP3 inflammasome, and the NAIP inflammasomes (2).
Fig. 1.
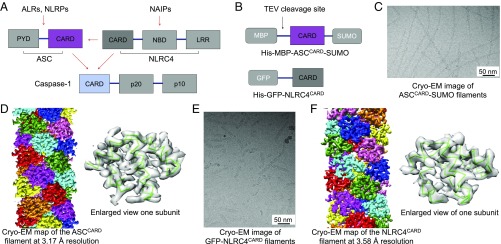
Cryo-EM structure determination of ASCCARD and NLRC4CARD filaments. (A) A brief schematic for ASC and NLRC4 recruitment of caspase-1. (B) ASC and NLRC4 CARD constructs used for EM studies. (C) An electron micrograph of ASCCARD filaments. (D) Side view of EM reconstruction fitted with the ASCCARD filament model with each subunit in a different color. One subunit is enlarged for closer view. (E) A micrograph of NLRC4CARD filaments. (F) Side view of EM reconstruction fitted with the NLRC4CARD filament model with each subunit in a different color. One subunit is enlarged for closer view.
Different inflammasomes are responsible for recognition of, and activation by, different ligands. For example, AIM2 recognizes double-stranded DNA in the cytosol (5–7); NLRP3 responds to K+ efflux that is in turn induced by multiple stimuli, such as extracellular ATP, uric acid crystals, and the bacterial toxin nigericin (8); and NAIP proteins detect flagellin and component proteins of the bacterial type III secretion system (9–12). Ligand binding activates these proteins to recruit adaptor proteins, such as ASC and NLRC4, which subsequently engage the downstream effector caspase-1. Most inflammasomes use the ASC adaptor, which possesses an N-terminal Pyrin domain (PYD) and a C-terminal caspase recruitment domain (CARD) (13) (Fig. 1A). The N-terminal PYD interacts with the PYD of the upstream sensors, and the C-terminal CARD recruits caspase-1 via homotypic CARD–CARD interactions (13, 14). In contrast, the NLRC4 adaptor exists only in NAIP inflammasomes, bridging an NAIP via its NBD and LRR and caspase-1 via its CARD upon ligand stimulation (15–19) (Fig. 1A). As the universal effector of canonical inflammasomes, caspase-1 is recruited and polymerized through its CARD to form filamentous structures, bringing the caspase catalytic domains into proximity and leading to its dimerization and activation (14). Activated caspase-1 processes cytokines pro–IL-1β and pro–IL-18 to their mature forms to elicit inflammatory responses and cleaves Gasdermin D to form pores that release the cytokines and cause pyroptotic cell death (20–25).
As the first CARD filament structure in inflammasomes, Casp-1CARD revealed the molecular mechanism of its self-assembly, as well as regulation by CARD-only proteins INCA and ICERBERG (14). However, how the upstream adaptors nucleate Casp-1CARD filament assembly still remains elusive. By visualizing the structures of ASCCARD and NLRC4CARD filaments using cryo-EM, here, we reveal that ASC and NLRC4 adopt the same mechanism to nucleate the assembly and activation of caspase-1. The ASCCARD and NLRC4CARD filament structures show assembly patterns similar to that of Casp-1CARD, indicating that these adaptors template the polymerization of Casp-1CARD. Further structural analyses and biochemical assays show that ASC and NLRC4 utilize similar interfaces to recruit caspase-1 and confer a unidirectional polymerization of Casp-1CARD by charge and shape complementarity.
Results
Cryo-EM Reconstruction of the ASCCARD and NLRC4CARD Filament Structures.
Both ASCCARD and NLRC4CARD are capable of nucleating the assembly and activation of caspase-1 (Fig. 1A). To gain a mechanistic understanding of this process, we prepared ASCCARD and NLRC4CARD filaments for cryo-EM study. We found that His-MBP-ASCCARD-SUMO was purified as monomers over a gel filtration column and ASCCARD-SUMO formed filaments upon proteolytic removal of the His-MBP tag by the Tobacco Etch Virus (TEV) protease (Fig. 1 B and C). The averaged power spectrum showed a similar diffraction pattern to that of Casp-1CARD filaments (14). Based on possible indexing of the power spectrum, we adopted a calculated one-start helical symmetry with an azimuthal angle of −100.60° and an axial rise of 5.10 Å per subunit. The iterative helical real-space reconstruction (IHRSR) method (26) was used to generate an intermediate map, starting from a solid cylinder as the initial model. This map was then used as an initial model in RELION (27) for 3D classification (SI Appendix, Fig. S1) and refinement. The final volume contains mostly α-helices with the refined helical symmetry parameters of −100.58° rotation and 5.00 Å translation per subunit, respectively. Each subunit showed the typical six α-helices arranged in a W shape, which is a common feature of the death domain superfamily that includes the CARD (28) (Fig. 1D). The calculated power spectrum from the final volume corresponded well with the experimental power spectrum (SI Appendix, Fig. S2A). The NMR structure of ASCCARD (PDB ID code 2KN6) could be easily docked into the EM density (Fig. 1D and SI Appendix, Table S1). The model was manually adjusted in Coot (29), followed by refinement in Phenix (30). The amino acid sequence was unambiguously registered due to the clearly defined side chains. There is almost no density outside the filament, indicating that the C-terminal SUMO tag was largely disordered (Fig. 1D). The resolution of this reconstruction was measured at 3.2 Å using the gold standard Fourier shell correlation (FSC) in RELION (SI Appendix, Fig. S3).
In the case of the NLRC4CARD domain, we found that the construct of GFP-NLRC4CARD directly forms filaments suitable for structure determination (Fig. 1 B and E). As the cryo-EM images showed, NLRC4CARD filaments are generally shorter and wider than ASCCARD filaments, likely due to the effect of the larger GFP tag (Fig. 1 C and E). We employed a similar strategy as used for ASCCARD to determine the NLRC4CARD filament structure. The helical symmetry was first calculated from the averaged power spectrum (SI Appendix, Fig. S2B) as −100.50° in azimuthal angle and 5.10 Å in axial rise per subunit, which was refined to −100.48° and 4.93 Å, respectively. The GFP tag is largely disordered in that the final reconstruction contains only weak noisy densities in the periphery. We also calculated the power spectrum from the final volume, which matched well with the experimental power spectrum (SI Appendix, Fig. S2B). A homology structure model of NLRC4CARD derived from the Casp-1CARD structure (PDB ID code 5FNA) (14) was readily fitted into the cryo-EM density (SI Appendix, Table S2 and Fig. 1F). Similar to the case for ASCCARD, the obvious side chain densities of the NLRC4CARD map enabled manual model building in Coot (29), followed by refinement in Phenix (30). The resolution was measured at 3.6 Å using gold standard FSC (SI Appendix, Fig. S4).
Structure of the ASCCARD Filament.
The diameter of the ASCCARD filament is ∼8 nm, with a central hole of less than 1 nm (Fig. 2A). The filament structure assembles through a left-handed one-start helical symmetry, with about 3.6 subunits per turn (Fig. 2B). Like other filaments formed by members of the death domain superfamily, formation of the ASCCARD filament is mediated by three types of asymmetric interactions, namely type I, type II, and type III interactions (28) (Fig. 2B). In the filament architecture, the type III interaction is along the direction of the one-start helical strand while type I and type II interactions generate connections between the adjacent turns of the helical strands (Fig. 2B). The type I interaction is mostly composed of charge–charge interactions between residues located on helix α2 of one molecule and helices α1 and α4 of the partner molecule and is the most extensive in surface area among the three types of interactions. Possible residues involved in this interaction include R119, E130, D134, and R160 (Fig. 2C). These residues form several electrostatically complementary pairs. Unlike the type I interaction, the type II interaction in the ASCCARD filament is mainly contributed by hydrophobic residues, including W169 and Y187 (Fig. 2C). The type III interaction is also dominated by charge–charge interactions, with R160 of helix α4 and D143 and E144 of helix α3 forming charge complementary pairs at the interface (Fig. 2C).
Fig. 2.
Structural analysis of the ASCCARD filament structure. (A) Surface representation of ASCCARD filament structure, side view and top view. (B) Schematic diagram of the helical filament, with three neighboring subunits highlighted in green, magenta, and cyan. (C) Detailed type I, II, and III interfaces, respectively, of the ASCCARD filament structure. (D) Gel filtration profile of ASCCARD WT and mutants. Void fractions are from elution volumes 7 mL to 9 mL while less aggregated fractions are from elution volumes 14 mL to 17 mL. (E) WT and ASCCARD mutants overexpressed in HeLa cells examined by confocal microscopy. (Scale bar: 10 nm.)
To validate the importance of the interfacial residues identified by our structural analysis, we generated site-directed mutants on a construct of ASCCARD fused to GFP (GFP-ASCCARD). WT GFP-tagged ASCCARD primarily eluted at the void fraction on a gel filtration column (Fig. 2D). In contrast, R119D, N128A/E130R, and D134K of type I mutations completely abolished filament formation (Fig. 2D). The effectiveness of these charge-reversal mutations confirmed our observation that type I interaction is dominated by charge–charge interactions. In the case of type II interactions, W169G, Y187A, Y187K mutations almost completely disrupted filament formation (Fig. 2D). Additionally, mutation of Y187 to L or H partially disrupted filament formation, further showing the hydrophobic interaction of the type II interface (Fig. 2D). D143K/E144K and R160E of type III interactions almost completely disrupted filament formation (Fig. 2D). We further examined mCherry-tagged ASCCARD mutants using confocal microscopy in HeLa cells. In line with our in vitro biochemical data, WT ASCCARD formed filaments in cells while mutations that proved to be disruptive in vitro also abolished filament formation in cells (Fig. 2E). These mutagenesis studies strongly support the correctness of our structural model and analysis.
Structure of the NLRC4CARD Filament.
The NLRC4CARD filament has a very similar architecture to the ASCCARD filament, with a diameter of ∼8 nm and an even smaller central hole (Figs. 2A and 3A). Like the ASCCARD filament, each subunit of the NLRC4CARD filament interacts with its neighboring molecules through three types of interactions, of which the type III interaction mediates the intrastrand contact and type I and type II interactions mediate interstrand contacts (Fig. 3 A and B). The type I interaction is composed of helix α2 of one molecule and helices α1 and α4 of the other. Electrostatic complementary residues of R9, D25, D26, and R52 form charge–charge interactions at the interface (Fig. 3C). At the type II interface, K60/K61 and E47 interact with each other through charge complementarity (Fig. 3C). Like the type I interface, the type III interface is mainly composed of hydrophilic residues, including the charge pair consisting of K45 and E44 (Fig. 3C).
Fig. 3.
Structural analysis of the NLRC4CARD filament structure. (A) Surface representation of ASCCARD filament structure, side view and top view. (B) Schematic diagram of the helical filament, with three neighboring subunits highlighted in green, magenta, and cyan. (C) Detailed type I, II, and III interfaces, respectively, of the NLRC4CARD filament structure. (D) Gel filtration profile of NLRC4CARD WT and mutants. Void fractions are from elution volumes 7 mL to 9 mL while less aggregated fractions are from elution volumes 14 mL to 17 mL. (E) WT and NLRC4CARD mutants overexpressed in HeLa cells examined by confocal microscopy. (Scale bar: 10 nm.)
To further validate the NLRC4CARD filament model, we performed site-directed mutagenesis on the GFP tagged construct. While WT GFP-NLRC4CARD mainly eluted at the void fraction, mutations of residues on type I and type III interfaces (R52E on type Ia, D25K on type Ib, and E36R on type IIIa) effectively abolished its aggregation ability (Fig. 3D). R9E on type Ia and K60E/K61D double mutation on the type IIa interface led to partial disruption of filament formation (Fig. 3D). We also investigated eGFP-tagged NLRC4CARD mutants using confocal microscopy in HeLa cells. Consistent with our observation in vitro, WT NLRC4CARD formed filaments in cells, and surface mutants of all three types of interactions abolished or attenuated filament formation (Fig. 3E). Collectively, these mutagenesis data further support the helical assembly model of the NLRC4CARD filament.
The NLRC4CARD Filament Exists in the Full-Length NLRC4 Structure.
Upon activation by a ligand-bound NAIP protein (18, 19), NLRC4 with the N-terminal CARD deleted forms mainly 11- to 12-folded disk-shaped complexes with a central hole of ∼8 nm in diameter and an outer diameter of ∼30 nm (16, 17). The size of the hole is compatible with the ∼8-nm diameter of our NLRC4CARD filament structure. Similarly, a cryo-electron tomography (cryo-ET) study of the overexpressed, NAIP-activated full-length NLRC4 inflammasome showed a shallow, right-handed helical structure with a diameter of ∼28.0 nm, 11.6 subunits per turn, and a helical pitch of 6.5 nm (31). These two structures are related by a lock washer-like twist of the NLRC4NBD−LRR region. Unlike the CARD-deleted NLRC4 ring with a central hole, the full-length shallow NLRC4 helix contains a rod-shaped volume in the center that was designated as the CARD column (31). To gain better understanding on the full-length NLRC4 assembly, we docked the NLRC4CARD filament structure and the activated NLRC4NBD−LRR structure (PDB ID code 3JBL) (16, 17) into the cryo-ET map (Fig. 4A). Quite remarkably, at a 1:1 molecular ratio, the height of the central volume for the CARD helix matched well the height of the peripheral volume for the NBD-LRR helix (Fig. 4A). This observation is also explained by the similar rise per subunit for the NBD-LRR helix (∼5.7 ± 0.3 Å per subunit) (31) and the CARD helix (5.1 Å per subunit), despite the different numbers of subunits per turn.
Fig. 4.
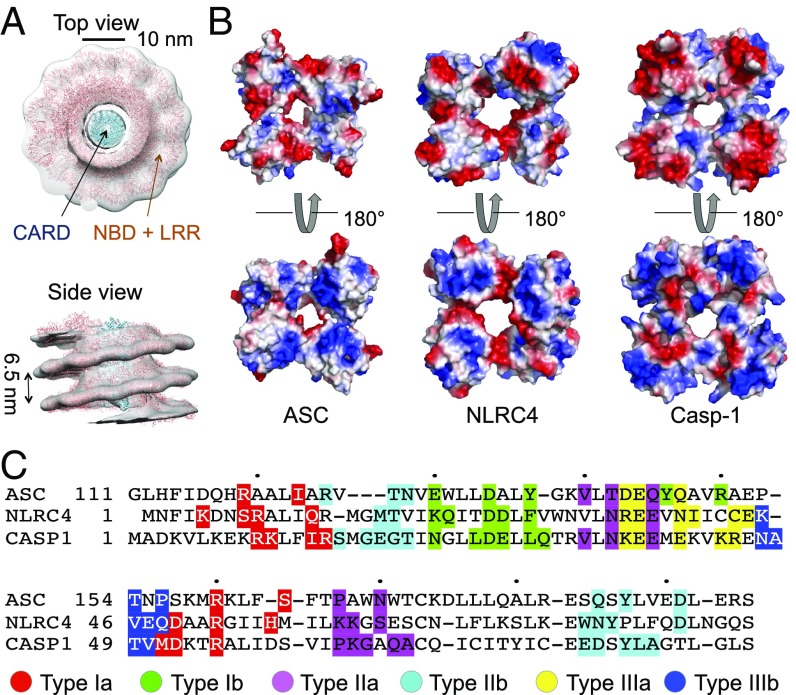
Structural comparison of ASC, NLRC4, and caspase-1 CARD filaments. (A) Fitting of NLRC4CARD filament structure (light blue, PDB ID code 6DRP) and NLRC4ΔCARD structure (pink, PDB ID code 3JBL) into the NLRC4 tomography map (EMDB 2901), top view and side view. The ratio of fitted NLRC4CARD and NLRC4ΔCARD subunits is 1:1. (B) Top and bottom view comparison of the electrostatic surface of one layer of ASC, NLRC4, and caspase-1 CARD filaments, respectively. (C) Multiple sequence alignment of ASC, NLRC4, and caspase-1 CARD domains. Different colors represent different types of interface (type Ia, red; type Ib, green; type IIa, purple; type IIb, cyan; type IIIa, yellow; type IIIb, blue).
It is intriguing that the helical architecture of full-length NLRC4 provides a fairly inefficient architecture for nucleating caspase-1 filament formation, and the longer the helical assembly, the less efficient this ability. This is because each helical assembly takes up many NLRC4 molecules, and yet only provides one nucleus to recruit and activate caspase-1. Structural analysis and experimental data show that only one end of the CARD filament is preferred for caspase-1 recruitment (see below). However, due to the low resolution of the CARD volume, direction of the CARD filament within the outer helix of NBD and LRR is ambiguous. We argue that, if NLRC4 indeed forms shallow helical oligomers at the endogenous expression level in cells, they may be very short helices to maximize caspase-1 activation.
Structure Comparison of ASCCARD, NLRC4CARD, and Casp-1CARD Filaments.
To gain deeper insights into these CARD filament assemblies, we compared the filament structures of ASCCARD and NLRC4CARD, as well as our previously published Casp-1CARD (14). All these filaments share a common overall architecture with similar helical parameters. In all cases, type III interactions mediate intrastrand assembly, and type I and II interactions mediate interstrand assembly (Figs. 2B and 3B). Upon closer examination of a single turn in these CARD filaments, we found that the top and bottom surfaces of each turn are largely charge complementary (Fig. 4B). Although the detailed features vary, they could be accounted for by the difference in the structures of the subunits. The similarity in the charge distribution patterns among ASC, NLRC4, and caspase-1 (Fig. 4 B and C) suggests a mechanism of caspase-1 recruitment by charge complementarity.
ASCCARD and NLRC4CARD Nucleate Casp-1CARD Filament Assembly Unidirectionally.
Both ASC and NLRC4 are able to nucleate the assembly and activation of caspase-1 via homotypic CARD–CARD interactions. To recapitulate this process, we employed a fluorescence polymerization (FP) assay to reconstitute this process in vitro (Fig. 5 A–D). As controls, WT ASCCARD and NLRC4CARD efficiently promoted Casp-1CARD polymerization. As expected, mutants that disrupt ASCCARD and NLRC4CARD filament formation also failed to promote caspase-1 polymerization (Fig. 5 A–D). ASCCARD, NLRC4CARD, and Casp-1CARD filaments share a similar helical symmetry, which indicates a molecular templating mechanism for nucleation and assembly. In other words, ASCCARD or NLRC4CARD serves as a platform to promote the assembly of Casp-1CARD along its helical trajectory, a mechanism also found in other death domain family complexes (4).
Fig. 5.
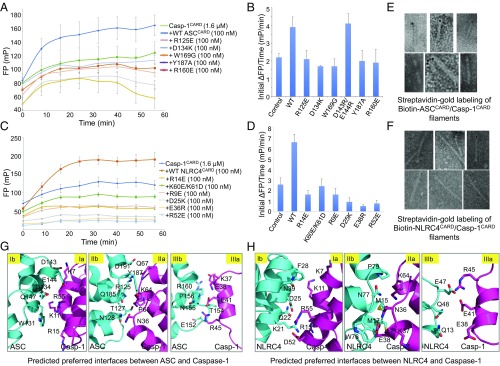
Recruitment of Casp-1CARD by ASCCARD and NLRC4CARD. (A) FP assay showing Casp-1CARD filament assembly nucleated by ASCCARD WT and mutants. (B) Initial polymerization rates of Casp-1CARD nucleated by ASCCARD WT and mutants. Error bars stand for fitting error. (C) FP assay showing Casp-1CARD assembly nucleated by NLRC4CARD WT and mutants. (D) Initial polymerization rate of Casp-1CARD nucleated by NLRC4CARD WT and mutants. Error bars stand for fitting error. (E) Gold labeling of ASC/Casp-1 CARD filament. (F) Gold labeling of NLRC4/Casp-1 CARD filament. (G) Predicted type I, type II, and type III interfaces of ASC recruitment of caspase-1. (H) Predicted type I, type II, and type III interfaces of NLRC4 recruitment of caspase-1. (Magnification: E and F, 30,000×.)
We further used nano-gold labeling experiments to localize ASC and NLRC4 in their complexes with Casp-1CARD. We expressed biotinylated ASCCARD and NLRC4CARD and used them to nucleate Casp-1CARD filaments. We then used 6-nm streptavidin-gold to label these filaments and visualized them by negative staining EM. The experiment showed that both ASC and NLRC4 were found at only one end of Casp-1CARD filaments (Fig. 5 E and F), suggesting unidirectional polymerization. However, in contrast to engagement of the FADD death effector domain (DED) unidirectionally to caspase-8 tandem DED (32), both of which are members of the death domain superfamily, all of the surfaces of ASCCARD and NLRC4CARD display charge complementarity with those of Casp-1CARD. Therefore, to analyze in more detail why caspase-1 is recruited only to one end of the helical platforms of ASC or NLRC4, we calculated the predicted buried surface areas between ASCCARD and Casp-1CARD and between NLRC4CARD and Casp-1CARD (SI Appendix, Table S3). The calculations showed that the buried interfaces are larger if both ASC and NLRC4 recruit Casp-1CARD from their type Ib, IIb, and IIIb surfaces (Fig. 5 G and H and SI Appendix, Fig. S5 and Table S3), suggesting a unified mechanism that ASC and NLRC4 use to recruit caspase-1 through CARD–CARD heterotypic interactions.
Discussion
Higher order assembly-mediated signal transduction has been proposed to be a general mechanism of innate immune signaling (33). With the structure elucidation of ASCCARD and NLRC4CARD filaments, we elaborated more details in the nucleation and polymerization of these higher order assemblies. First, ASCCARD and NLRC4CARD share a similar assembly pattern, with the type III interface forming intrastrand interactions, and the type I and II interfaces forming interstrand interactions. Second, interfaces of ASCCARD and NLRC4CARD are mainly composed of charge-complementary residues. A similar assembly pattern is also true in the case of Casp-1CARD filament (14). This observation indicates the possible recruitment of downstream Casp-1CARD through charge complementarity. Third, as upstream nucleators, both ASCCARD and NLRC4CARD must form oligomers to recruit downstream Casp-1CARD and promote its assembly. In summary, we showed a unified polymerization and nucleation process of ASC- and NLRC4-mediated caspse-1 assembly and activation.
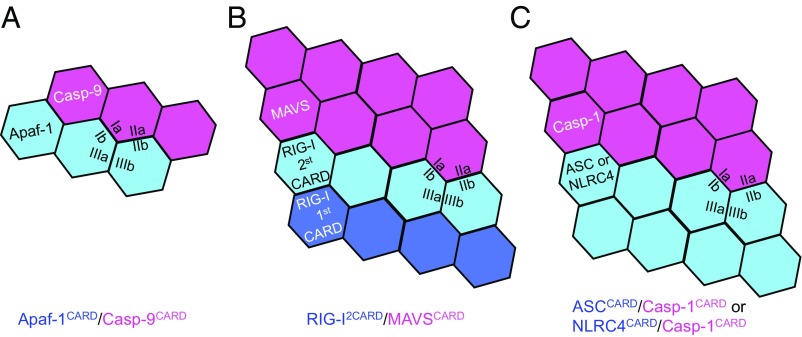
On the other hand, previous structural studies of heterooligomeric CARD complexes showed that the upstream molecules always use one unique side to form a structural platform for the recruitment of downstream molecules. We compared the proposed ASC/Casp-1 and NLRC4/Casp-1 CARD heterocomplexes with Apaf-1/Casp-9 and RIG-I/MAVS CARD heterocomplexes. These three systems all adopt helical assembly but display distinct features (Fig. 6). In the Apaf-1/Casp-9 complex core, three Apaf-1 CARDs form one turn to recruit three Casp-9 CARDs; due to the special assembly mode within the apoptosome, the complex assembly is not infinite but limited at up to a 4:4 complex (34–37). All of the subunits in one turn use the type III interface, and the type I and type II interactions are responsible for contacts between Apaf-1 and Casp-9. Different from the Apaf-1/Casp-9 complex, the RIG-I tandem CARDs (2CARD) forms a limited tetramer to mediate infinite MAVS filament assembly (38). In this tetramer, the RIG-I 2CARD subunits form type II interactions within subunits, and type I and III interactions between subunits. The second CARDs in the tetramer nucleate MAVS filament formation using the type I and type II interactions. Here, we show a different assembly pattern. Both ASC and NLRC4 CARDs are able to self-assemble into filaments for downstream Casp-1CARD recruitment, with the type III interface mediating interactions between neighboring subunits in one turn and type I and type II interfaces dominating the interactions of subunits between turns. The recruitment specificity between ASC and caspase-1 and between NLRC4 and caspase-1 comes from both charge and shape complementarity, which is the case for the formation of many heterocomplexes in the death domain superfamily, including the Myddosome (39). Our study provides examples for CARD assembly-mediated signal transduction.
Fig. 6.
A schematic for the assembly of different CARD complexes. (A) The Apaf-1/Casp-9 CARD assembly. (B) The RIG-I/MAVS CARD assembly. (C) The ASC/Casp-1 or NLRC4/Casp-1 CARD assembly.
Materials and Methods
Protein Expression and Purification.
To generate monomeric ASCCARD, the CARD domain of human ASC (A107-S195) was cloned into an engineered HIS-MBP-SUMO sandwich-tagged vector. This construct was transformed into BL21(DE3) cells and expressed overnight using 0.4 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) induction at 18 °C. The cells were harvested and lysed by sonication in lysis buffer containing 20 mM Hepes, pH 8.0, 200 mM NaCl, 5 mM imidazole, 5 mM β-mercaptoethanol, and 10% glycerol. Cell lysate was then centrifuged, and the supernatant containing monomeric ASCCARD was incubated with nickel-nitrilotriacetic acid (Ni-NTA) affinity resin for 1 h at 4 °C, washed in lysis buffer containing 20 mM imidazole, and eluted with lysis buffer containing 300 mM imidazole. The eluate was subsequently loaded onto a Superdex 200 10/300 GL column preequilibrated with 20 mM Hepes, pH 8.0, 150 mM NaCl, and 2 mM DTT. Peak fractions of monomeric ASCCARD were collected and treated by TEV to remove N-terminal His-MBP tag. The ASCCARD filament was formed directly after cleavage. The sample was then incubated with amylose resin to get rid of excess His-MBP. To assess the effects of the interfacial residues in filament assembly, we generated the His-GFP–tagged ASCCARD, which formed filament and eluted in the void fractions of a gel filtration column. All mutants in this construct were introduced using the QuikChange mutagenesis protocol and purified in a similar method.
The CARD domain of human NLRC4 (M1-S120) was cloned into an engineered pET28a vector, with an N-terminal His-GFP tag. This construct was transformed into BL21(DE3) cells and expressed overnight using 0.4 mM IPTG induction at 18 °C. Similar to the purification of ASCCARD, NLRC4CARD was purified by Ni-NTA affinity chromatography followed by gel filtration. Void fractions of NLRC4CARD filament were collected. All mutants in this construct were introduced using the QuikChange mutagenesis protocol and purified in a similar method.
Cryo-EM Data Collection and Processing.
For cryo-EM sample preparation, 3 μL of filament sample was applied to glow discharged holey carbon Quantifoil grids (R1.2/1.3) and plunge-frozen into liquid ethane using a Vitrobot Mark IV (FEI). Movie mode micrographs were collected at the National Cancer Institute Cryo-Electron Microscopy Facility on a 300-keV FEI Titan Krios electron microscope equipped with a K2 summit direct electron detector, under superresolution counting mode and pixel size 0.66 Å. Each movie stack contained 40 subframes, and the exposure time for each frame was 300 ms. This resulted in an accumulated exposure time of 12 s, and dose per exposure was ∼41 electrons per Å2. All subframes in each movie stack were subjected to drift correction and dose weighting and then added up to a single image with MotionCor2 (40). Coordinates used for filament extraction were generated using the program e2helixboxer within EMAN2 (41). RELION (27) was used for all of the following data-processing steps, except that a starting model was produced by the SPIDER (42) software package and IHRSR (26), with a separate dataset collected on a Tecnai Arctica electron microscope.
For each dataset, symmetry information was obtained by trial-and-error based on the averaged power spectrum. For the ASCCARD filament, −100.60° and 5.10 Å were found to give a stable reconstruction and lead to recognizable secondary structures. Then, 264,167 particles were first extracted in RELION (27), with a shift of two asymmetric units for each segment box. After two rounds of 2D classification and one round of 3D classification, 226,603 particles remained for the final refinement. The refined helical symmetry was −100.58° and 5.00 Å. Postprocessing in RELION (27) resulted in a 3.17-Å reconstruction. For the NLRC4CARD filament, −100.50° and 5.10 Å were found to give a stable reconstruction and lead to recognizable secondary structures. Then, 400,565 particles were first extracted in RELION, with a shift of one asymmetric unit for each segment box. After two rounds of 2D classification and one round of 3D classification, 199,312 particles remained for the final refinement. The refined helical symmetry was 100.48° and 4.93 Å. Postprocessing in RELION (27) resulted in a 3.58-Å reconstruction. In both cases, 3D classification only revealed negligible differences in helical symmetries, indicating mainly rigid assembly of both filaments (SI Appendix, Fig. S1).
Model Building and Refinement.
The ASCCARD monomer structure was derived from the NMR structure of ASC (PDB ID code 2KN6) (43). The NLRC4CARD monomer structure was modeled with the SWISS-MODEL server (44) using a Casp-1CARD subunit in its filament structure (14). For each structure, a filament model containing eight subunits was fitted in the EM density by manual adjustment in Coot (29) and subsequent refinement in Phenix (45). An EM map of full-length NLRC4 was downloaded from EMDB (ID 2901). Monomeric NACHT-LRR was downloaded from PDB (PDB ID code 5AJ2) and manually fitted in the EM map in UCSF Chimera (46). Helical symmetry was then imposed to generate a model with 33 NACHT-LRR molecules, and each molecule was fitted into the EM map separately. An NLRC4CARD filament model containing 33 molecules was manually fitted in the central rod-like density in UCSF Chimera (46).
Fluorescence Polarization Assay.
A C-terminal “LPETG” motif was added to a native N-terminal MBP-tagged ASC-CARD construct for sortase labeling (47) and a fluorescence polarization (FP) assay. For labeling, 30 μM of a freshly purified protein substrate with the “LPETG” motif was incubated with 5 μM calcium-independent sortase and 500 μM tetramethylrhodamine (TAMRA)-conjugated triglycine nucleophile (GGG-TAMRA) overnight at 4 °C. The mixture was then passed through a size-exclusion column to remove excess nucleophile. Labeled proteins were diluted to an appropriate concentration to perform FP assays on a SpectraMax M5e plate reader. Each experiment was repeated three times. Polarization values were averaged and plotted in Excel.
Cellular Imaging.
ASC-mCherry and NLRC4-eGFP constructs were transfected into HeLa cells using standard protocols. The cells were fixed and stained by Hoechst 24 h posttransfection and then examined by confocal microscopy.
Nano-Gold Labeling.
ASCCARD and NLRC4CARD were cloned into the pDW363 biotinylation vector to be expressed as N-terminal biotin acceptor peptide-tagged recombinant proteins, which become biotinylated by the BirA enzyme encoded on the pDW363 vector when expressed in Escherichia coli (48). The pDW363 vector containing either ASCCARD or NLRC4CARD was cotransformed into E. coli BL21(DE3) with a pET28a vector containing His-GFP–tagged caspase-1 CARD for coexpression. The binary complexes of ASCCARD/Casp-1CARD and NLRC4CARD/Casp-1CARD were purified by Ni-NTA affinity and gel filtration, similar to the purification of His-GFP-NLRC4CARD described above. Streptavidin-gold conjugate (6-nm-diameter gold; Electron Microscopy Sciences) was employed for labeling the biotinylated binary complexes. A carbon-coated copper EM grid was incubated with 5 μL of sample for 1 min at room temperature, followed by blotting with filter paper to remove excess samples. Then, the grid was rinsed for 1 min using 25 μL of incubation buffer (20 mM Hepes, pH 7.5, 150 mM NaCl, 1 mM Tris(2-carboxyethyl)phosphine, and 0.1% gelatin) three times. The grid was incubated with 25 μL of 6-nm streptavidin-gold conjugate diluted in incubation buffer for 30 min at room temperature. The grid was washed three times with incubation buffer and stained by 1% uranyl formate for examination by electron microscopy.
Supplementary Material
Acknowledgments
This work was supported by US National Institutes of Health Grants HD087988 and AI124491 (to H.W.), by Harvard Digestive and Disease Center Grant HDDC P30 DK034854 (to T.-M.F.), and by the National Cancer Institute’s National Cryo-EM Facility at the Frederick National Laboratory for Cancer Research.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.wwpdb.org [PDB ID codes 6DRN (ASCCARD filaments) and 6DRP (NLRC4CARD filaments)], and the cryo-EM reconstructions have been deposited in the EM Data Bank, www.emdatabank.org [ID codes EMD-8902 (ASCCARD filaments) and EMD-8903 (NLRC4CARD filaments)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810524115/-/DCSupplemental.
References
- 1.von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE. Recognition of bacteria by inflammasomes. Annu Rev Immunol. 2013;31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 2.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Lu A, Wu H. Structural mechanisms of inflammasome assembly. FEBS J. 2015;282:435–444. doi: 10.1111/febs.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin Q, Fu TM, Li J, Wu H. Structural biology of innate immunity. Annu Rev Immunol. 2015;33:393–416. doi: 10.1146/annurev-immunol-032414-112258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bürckstümmer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 10.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rayamajhi M, Zak DE, Chavarria-Smith J, Vance RE, Miao EA. Cutting edge: Mouse NAIP1 detects the type III secretion system needle protein. J Immunol. 2013;191:3986–3989. doi: 10.4049/jimmunol.1301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci USA. 2013;110:14408–14413. doi: 10.1073/pnas.1306376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu A, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu A, et al. Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nat Struct Mol Biol. 2016;23:416–425. doi: 10.1038/nsmb.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Shao F. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol Rev. 2015;265:85–102. doi: 10.1111/imr.12293. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, et al. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 2015;350:404–409. doi: 10.1126/science.aac5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Z, et al. Structural and biochemical basis for induced self-propagation of NLRC4. Science. 2015;350:399–404. doi: 10.1126/science.aac5489. [DOI] [PubMed] [Google Scholar]
- 18.Tenthorey JL, et al. The structural basis of flagellin detection by NAIP5: A strategy to limit pathogen immune evasion. Science. 2017;358:888–893. doi: 10.1126/science.aao1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, et al. Structural basis for specific flagellin recognition by the NLR protein NAIP5. Cell Res. 2018;28:35–47. doi: 10.1038/cr.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sborgi L, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aglietti RA, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci USA. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan J, Xia S, Liu X, Lieberman J, Wu H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature. 2018;557:62–67. doi: 10.1038/s41586-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egelman EH. The iterative helical real space reconstruction method: Surmounting the problems posed by real polymers. J Struct Biol. 2007;157:83–94. doi: 10.1016/j.jsb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Scheres SH. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrao R, Wu H. Helical assembly in the death domain (DD) superfamily. Curr Opin Struct Biol. 2012;22:241–247. doi: 10.1016/j.sbi.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 30.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diebolder CA, Halff EF, Koster AJ, Huizinga EG, Koning RI. Cryoelectron tomography of the NAIP5/NLRC4 inflammasome: Implications for NLR activation. Structure. 2015;23:2349–2357. doi: 10.1016/j.str.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Fu TM, et al. Cryo-EM structure of caspase-8 tandem DED filament reveals assembly and regulation mechanisms of the death-inducing signaling complex. Mol Cell. 2016;64:236–250. doi: 10.1016/j.molcel.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu H. Higher-order assemblies in a new paradigm of signal transduction. Cell. 2013;153:287–292. doi: 10.1016/j.cell.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, et al. Mechanistic insights into caspase-9 activation by the structure of the apoptosome holoenzyme. Proc Natl Acad Sci USA. 2017;114:1542–1547. doi: 10.1073/pnas.1620626114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su TW, et al. Structural insights into DD-fold assembly and caspase-9 activation by the Apaf-1 apoptosome. Structure. 2017;25:407–420. doi: 10.1016/j.str.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Cheng TC, Hong C, Akey IV, Yuan S, Akey CW. A near atomic structure of the active human apoptosome. eLife. 2016;5:e17755. doi: 10.7554/eLife.17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Qiao Q, Wu H. Understanding CARD tricks in apoptosomes. Structure. 2017;25:575–577. doi: 10.1016/j.str.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu B, et al. Molecular imprinting as a signal-activation mechanism of the viral RNA sensor RIG-I. Mol Cell. 2014;55:511–523. doi: 10.1016/j.molcel.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng SQ, et al. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang G, et al. EMAN2: An extensible image processing suite for electron microscopy. J Struct Biol. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Shaikh TR, et al. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat Protoc. 2008;3:1941–1974. doi: 10.1038/nprot.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Alba E. Structure and interdomain dynamics of apoptosis-associated speck-like protein containing a CARD (ASC) J Biol Chem. 2009;284:32932–32941. doi: 10.1074/jbc.M109.024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biasini M, et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiMaio F, et al. Atomic-accuracy models from 4.5-Å cryo-electron microscopy data with density-guided iterative local refinement. Nat Methods. 2015;12:361–365. doi: 10.1038/nmeth.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettersen EF, et al. UCSF Chimera–A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 47.Hirakawa H, Ishikawa S, Nagamune T. Design of Ca2+-independent Staphylococcus aureus sortase A mutants. Biotechnol Bioeng. 2012;109:2955–2961. doi: 10.1002/bit.24585. [DOI] [PubMed] [Google Scholar]
- 48.Tsao KL, DeBarbieri B, Michel H, Waugh DS. A versatile plasmid expression vector for the production of biotinylated proteins by site-specific, enzymatic modification in Escherichia coli. Gene. 1996;169:59–64. doi: 10.1016/0378-1119(95)00762-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.