Significance
c-Myc is dynamically regulated by posttranslational modifications, including SUMOylation. However, how SUMOylation regulates c-Myc activity and stability remains elusive. In this study, we found that SENP1, a nuclear SUMO protease, deSUMOylates c-Myc, resulting in c-Myc stabilization and activation. We further show that c-Myc can be comodified by ubiquitination and SUMOylation and that this facilitates c-Myc targeting to the proteasome for degradation. DeSUMOylation of c-Myc by SENP1 suppressed c-Myc proteasome degradation and increased the level of transcriptionally active c-Myc, while knockdown of SENP1 reduced c-Myc levels and markedly suppressed cancer cell proliferation. We show that SENP1 is frequently overexpressed in human breast cancers. Thus, our study reveals SENP1 as a positive c-Myc regulator.
Keywords: c-Myc, SENP1, SUMOylation, deSUMOylation, ubiquitination
Abstract
Posttranslational modifications play a crucial role in the proper control of c-Myc protein stability and activity. c-Myc can be modified by small ubiquitin-like modifier (SUMO). However, how SUMOylation regulates c-Myc stability and activity remains to be elucidated. The deSUMOylation enzyme, SENP1, has recently been shown to have a prooncogenic role in cancer; however, mechanistic understanding of this is limited. Here we show that SENP1 is a c-Myc deSUMOylating enzyme. SENP1 interacts with and deSUMOylates c-Myc in cells and in vitro. Overexpression of wild-type SENP1, but not its catalytically inactive C603S mutant, markedly stabilizes c-Myc and increases its levels and activity. Knockdown of SENP1 reduces c-Myc levels, induces cell cycle arrest, and drastically suppresses cell proliferation. We further show that c-Myc can be comodified by both ubiquitination and SUMOylation. SENP1-mediated deSUMOylation reduces c-Myc polyubiquitination, suggesting that SUMOylation promotes c-Myc degradation through the proteasome system. Interestingly, SENP1-mediated deSUMOylation promotes the accumulation of monoubiquitinated c-Myc and its phosphorylation at serine 62 and threonine 58. SENP1 is frequently overexpressed, correlating with the high expression of c-Myc, in breast cancer tissues. Together, these results reveal that SENP1 is a crucial c-Myc deSUMOylating enzyme that positively regulates c-Myc’s stability and activity.
As a master transcription regulator, c-Myc regulates the expression of a large number of genes involved in the control of diverse cellular processes, including cell proliferation, differentiation, apoptosis, angiogenesis, metabolism, DNA replication, RNA processing, and ribosome biogenesis (1, 2). Properly regulated levels of c-Myc are essential for normal cell growth and proliferation, whereas deregulated c-Myc overexpression contributes to most human cancers (3). Thus, the level and activity of c-Myc must be tightly regulated in normal cells at multiple levels, including the regulation of c-Myc protein by posttranslational modifications (4, 5).
c-Myc protein stability is dynamically controlled by the ubiquitin (Ub)- proteasome system (4, 5). The growth-regulated turnover of c-Myc involves two conserved phosphorylation sites within Myc box I, threonine 58 (T58) and serine 62 (S62) (5, 6). c-Myc stability increases upon phosphorylation at S62 by ERK and/or CDK kinases in response to growth signals. Subsequent T58 phosphorylation by GSK3, following the cessation of growth signals, destabilizes c-Myc by recruiting the T58 phosphorylation-dependent ubiquitin ligase (E3) complex SCFFbw7 to mediate c-Myc ubiquitination and proteasome degradation (4, 6, 7). The importance of this signaling cascade leading to c-Myc degradation is highlighted by alterations of the pathway in cancers, such as mutations at or around T58 in a subset of Burkitt’s lymphomas (8), mutations, and deletions of FBW7 (7), as well as increased S62 phosphorylation (9). Likewise, c-Myc is regulated by a number of other ubiquitin ligases such as SCFSkp2, SCFβTRCP, and HUWE1 (4, 10), as well as deubiquitinating enzymes (DUBs), including the ubiquitin-specific protease (USP) family members USP28, USP36, USP37, USP13, and USP22 (10–14). Thus, c-Myc levels and activity are tightly controlled by a dynamic balance between ubiquitination and deubiquitination.
Recently, it has been shown that c-Myc is also regulated by SUMOylation (15–18), a posttranslational modification of proteins by small ubiquitin-like modifiers (SUMOs), including SUMO1, SUMO2, and SUMO3. While c-Myc lacks a sequence consensus SUMOylation lysine (Lys, K), it can be SUMOylated on at least 10 SUMO acceptor Ks, including K323 and K326 (15, 16, 18). However, mutating K323 and K326 or all of the 10 Ks did not abolish c-Myc SUMOylation and did not significantly alter its levels and activity (15, 16, 18). Thus, how SUMOylation regulates c-Myc stability and activity remains elusive. Further, SUMO modification is highly dynamic and the effects of c-Myc deSUMOylation remain unknown.
In an attempt to understand the deSUMOylation regulation of c-Myc, we identified SENP1 in a screen for deSUMOylating enzymes interacting with c-Myc. We confirmed that SENP1 directly binds to and deSUMOylates c-Myc in vitro and in cells. Interestingly, overexpression of wild-type (WT) SENP1, but not its catalytically inactive C603S mutant (SENP1C603S, Cys-603 mutated to Ser), stabilizes c-Myc and enhances c-Myc–driven transcription. Knockdown of SENP1 reduces c-Myc levels and drastically induces cell cycle arrest and suppresses cell proliferation. We showed that c-Myc is comodified by ubiquitin and SUMO, and c-Myc deSUMOylation by SENP1 reduces its polyubiquitination, indicating that SUMOylation promotes c-Myc degradation through the proteasome system. Consistent with this functional role, we find that SENP1 is frequently overexpressed in human breast cancer cell lines and primary breast cancer tissues, correlating with the up-regulation of c-Myc. Together, these results reveal that SENP1 is a crucial c-Myc deSUMOylating enzyme that positively regulates c-Myc stability and activity.
Results
SENP1 Interacts with c-Myc in Cells and in Vitro.
To examine whether c-Myc is regulated by deSUMOylation, we screened all known deSUMOylating enzymes for their ability to bind to c-Myc using coimmunoprecipitation (co-IP)–immunoblot (IB) assays and found that c-Myc interacted with SENP1, SENP3, and USPL1 (SI Appendix, Fig. S1 A and B). As USPL1 does not deSUMOylate c-Myc (SI Appendix, Fig. S1C) and SENP1 interaction with c-Myc is stronger than SENP3, we focused on SENP1 in this study. We confirmed that ectopic V5–c-Myc was coimmunoprecipitated with Flag-SENP1 (Fig. 1A) and V5-SENP1 was coimmunoprecipitated with Flag–c-Myc (Fig. 1B) using anti-Flag antibody when both proteins were coexpressed. Endogenous SENP1 was also coimmunoprecipitated with endogenous c-Myc by anti–c-Myc antibody, but not control IgG (Fig. 1C). Using GST-fusion protein–protein association assays, we showed that recombinant His–c-Myc protein purified from bacteria binds to purified GST-SENP1 protein in vitro (Fig. 1D). The c-Myc–SENP1 interaction does not require SENP1 catalytic activity, as c-Myc also interacts with the catalytically inactive SENP1C603S mutant (SI Appendix, Fig. S1D). Proximity ligation assay (PLA) with confocal microscopy further confirmed that endogenous SENP1 interacts with endogenous c-Myc in the nucleus and knockdown of SENP1 abolished the PLA signals (Fig. 1E). Together, these results demonstrated that SENP1 directly interacts with c-Myc in cells and in vitro.
Fig. 1.
SENP1 interacts with c-Myc in cells and in vitro. (A and B) Co-IP of ectopic SENP1 with ectopic Myc. H1299 cells transfected with V5–c-Myc and Flag-SENP1 (A) or V5-SENP1 and Flag–c-Myc (B) individually or together were assayed by co-IP. (C) Co-IP between endogenous SENP1 and c-Myc using 293 cell lysates. (D) SENP1 interacts with c-Myc in vitro. Purified GST or GST-SENP1 immobilized on glutathione beads was incubated with purified His-Myc. Bound proteins were assayed by IB with anti-Myc (Top). Coomassie staining of GST and GST-SENP1 proteins is shown (Bottom). (E) HeLa cells were stained with anti–c-Myc and anti-SENP1 antibodies followed by PLA. The PLA signals were overlaid with DAPI from confocal imaging.
The N-Terminal and C-Terminal Domains of both SENP1 and c-Myc Participate in Their Interaction.
To map the binding domain between SENP1 and c-Myc, we performed co-IP assays using deletion mutants of SENP1 and c-Myc. As shown in SI Appendix, Fig. S2A, either the N-terminal transactivation domain (TAD) or the C-terminal bHLH-LZ domain-containing fragments, but not the central region of c-Myc, interacted with SENP1. Similarly, we found that both the N-terminal (amino acids 1–180) and C-terminal (amino acids 420–644), but not the central region (amino acids 181–419) of SENP1 interacted with c-Myc (SI Appendix, Fig. S2B). The reciprocal domain binding was further verified by in vitro GST-pulldown assays using recombinant proteins (SI Appendix, Fig. S2 C and D). Thus, SENP1–c-Myc interaction involves two contacts between the two proteins (SI Appendix, Fig. S2 E and F).
SENP1 DeSUMOylates c-Myc in Cells and in Vitro.
Next, we examine whether SENP1 deSUMOylates c-Myc. In cell SUMOylation assays using nickel-nitrilotriacetic acid (Ni2+-NTA) purification showed that WT SENP1, but not SENP1C603S, significantly reduced the SUMOylation of exogenous c-Myc (SI Appendix, Fig. S3 A and B) and endogenous c-Myc (Fig. 2 A and B) modified by SUMO1 or SUMO2. To test whether SENP1 directly deSUMOylates c-Myc, we performed in vitro deSUMOylation assays. SUMOylated c-Myc was purified from 293 cells transfected with c-Myc and Flag-SUMO2 using affinity purification with anti-Flag (M2) agarose beads followed by Flag-peptide elution (14). As shown in Fig. 2C, purified recombinant WT SENP1, but not SENP1C603S, efficiently removed SUMO from the SUMOylated c-Myc. Thus, SENP1 directly deSUMOylates c-Myc in vitro.
Fig. 2.
SENP1 deSUMOylates and stabilizes c-Myc and increases its activity. (A and B) SENP1 deSUMOylates c-Myc in cells. H1299 cells were transfected with His-SUMO1 (A) or His-SUMO2 (B) with or without Flag-SENP1 (WT or the C603S mutant) for 48 h and treated with MG132 for 6 h. The cells were subjected to pulldown (PD) using Ni2+-NTA bead under denaturing conditions, followed by IB. (C) SENP1 deSUMOylates c-Myc in vitro. SUMOylated c-Myc purified from 293 cells transfected with Flag-SUMO2 and c-Myc was incubated with recombinant WT GST-SENP1 or its C603S mutant, followed by IB. (D and E) SENP1 induces c-Myc levels in cells. HeLa cells transfected with increasing amounts of WT Flag-SENP1 (D) or with the C603S mutant (E), followed by IB. (F) SENP1 stabilizes c-Myc. HeLa cells transfected with control, WT SENP1, or its C603S mutant plasmid for 48 h were treated with 50 μg/mL cycloheximide (CHX). The cells were harvested at different time points and assayed by IB. (G) SENP1 increases c-Myc transactivation activity. H1299 cells transfected with luciferase reporter containing the E-box element from the E2F2 promoter in the presence of c-Myc and WT SENP1 or the C603S mutant, followed by luciferase activity measurement. *P < 0.05, compared with c-Myc only. (H) SENP1 stimulates c-Myc activity. U2OS-TO-Flag-SENP1WT or U2OS-TO-Flag-SENP1C603S cells were cultured in the absence or presence of doxycycline and assayed for gene expression by RT-qPCR. *P < 0.05, **P < 0.01, compared with the absence of doxycycline.
SENP1 Stabilizes c-Myc Protein and Increases Its Levels.
We then sought to test whether SENP1 regulates c-Myc levels. As shown in Fig. 2D, overexpression of SENP1 induced the levels of endogenous c-Myc in HeLa cells in a dose-dependent manner. This induction requires the deSUMOylating enzyme activity of SENP1, as the SENP1C603S mutant failed to induce c-Myc levels (Fig. 2E). Similarly, overexpression of WT SENP1, but not SENP1C603S, also induced the levels of exogenously expressed c-Myc (SI Appendix, Fig. S3 D and E). The induction involves stabilization of c-Myc, as overexpression of WT SENP1, but not SENP1C603S, prolonged the half-life of c-Myc protein (Fig. 2F), and neither overexpression (SI Appendix, Fig. S3 F and G) nor knockdown (SI Appendix, Fig. S3H) of SENP1 affected C-MYC mRNA levels. Consistently, induced expression of WT SENP1, but not SENP1C603S, in U2OS cells also induced the levels of endogenous c-Myc in multiple clones (SI Appendix, Fig. S3 I and J) and in a time-dependent manner in a representative clone (SI Appendix, Fig. S3K). Thus, the induction of c-Myc levels by SENP1 is robust in multiple cell types.
SENP1 Increases c-Myc Transactivation Activity.
To test whether stabilization of c-Myc by SENP1 also increases c-Myc activity, we performed luciferase reporter assays. As shown in Fig. 2G, WT SENP1, but not SENP1C603S, significantly increased the c-Myc–driven luciferase reporter activity. Furthermore, induced expression of WT SENP1, but not the SENP1C603S mutant, significantly induced the expression of tested Myc target genes, including nucleolin (C23), nucleostemin (GNL3), E2F2, 5S rRNA, and pre-rRNA genes, but not the actin and TBP genes (Fig. 2H). Consistently, WT SENP1, but not SENP1C603S, increased the binding of c-Myc to the promoters of C23 and GNL3 genes (SI Appendix, Fig. S3L). Thus, SENP1 induces c-Myc levels and promotes c-Myc transactivation activity.
DeSUMOylation of c-Myc by SENP1 Reduces c-Myc Polyubiquitination.
To understand how deSUMOylation of c-Myc increases its levels, we asked whether SENP1 regulates c-Myc ubiquitination and proteasome degradation. It has been shown that c-Myc can be comodified by both SUMOylation and ubiquitination and at least some of the comodified c-Myc is destined for proteasome degradation (15). Our sequential co-IP assays also revealed that c-Myc can be comodified by both SUMO and ubiquitin (SI Appendix, Fig. S4A). Proteasome inhibition by MG132 treatment significantly increased the levels of both SUMO1 modified c-Myc and SUMO2 modified c-Myc in HeLa (Fig. 3A), H1299 (SI Appendix, Fig. S4B), and U2OS (SI Appendix, Fig. S4C) cells as well as the global SUMOylation with endogenous SUMO1 and SUMO2 in these cells (SI Appendix, Fig. S4D). In line with this, proteasome inhibition increased endogenous SENP1–c-Myc interaction as determined by PLA (SI Appendix, Fig. S5A). As SUMO1 is regarded as a chain terminator (19, 20), the accumulation of SUMO1 modification observed above suggests that SUMO1 might form mixed SUMO chains with SUMO2. Indeed, proteins modified by endogenous SUMO1 were detected in the His-SUMO2 pulldown (lane 6, Fig. 3A) and endogenous SUMO2-modified species was detected in the His-SUMO1 pulldown in HeLa (lane 3, Fig. 3A), H1299 (SI Appendix, Fig. S4B), and U2OS (SI Appendix, Fig. S4C) cells, which is consistent with recent proteomic analysis of SUMO modifications (19, 21). These data suggest that SUMOylated c-Myc can be targeted for proteasome degradation possibly due to comodification with ubiquitin. Thus, we tested whether SENP1 stabilization of c-Myc is due to reduced ubiquitination following deSUMOylation. Indeed, overexpression of WT SENP1, but not the SENP1C603S mutant, reduced c-Myc polyubiquitination in cells treated with proteasome inhibitor MG132 (Fig. 3B). Interestingly, overexpression of WT SENP1, but not the SENP1C603S mutant, drastically increased the levels of monoubiquitinated c-Myc in HeLa (Fig. 3C) and H1299 (SI Appendix, Fig. S5B) cells in the absence of MG132 treatment, further supporting a cross-talk between SUMOylation and ubiquitination in the regulation of c-Myc. Together, these results suggest that SENP1 stabilizes c-Myc by suppressing c-Myc polyubiquitination following deSUMOylation.
Fig. 3.
SENP1 inhibits c-Myc polyubiquitination and degradation. (A) Proteasome inhibitor treatment leads to accumulation of SUMOylated c-Myc with SUMO1 and SUMO2. HeLa cells transfected with His-SUMO1 or His-SUMO2 were treated with MG132 or DMSO for 6 h. The cells were subjected to Ni2+-NTA pulldown (PD) under denaturing conditions followed by IB. The SUMOylated c-Myc species are indicated. (B and C) SENP1 reduces polyubiquitination of c-Myc, but increases monoubiquitinated c-Myc. HeLa cells transfected with His-Ub and Flag-SENP1 as indicated and treated with MG132 (B) or without MG132 (C). The cells were subjected to Ni2+-NTA PD followed by IB. The polyubiquitinated (B) and monoubiquitinated (C) c-Myc species are indicated. (D) Co-IP of SENP1 with Fbw7α in H1299 cells transfected with V5-SENP1 with or without Flag-Fbw7α using anti-Flag antibody. (E) SENP1 inhibits c-Myc degradation mediated by Fbw7α. HeLa cells transfected with the indicated plasmids were assayed by IB. (F) SENP1 induces c-Myc levels in Fbw7+/+, but not Fbw7−/−, HCT116 cells. Cells transfected with control or Flag-SENP1 were assayed by IB. (G) Knockdown of SENP1 reduces c-Myc levels in Fbw7+/+, but not Fbw7−/−, HCT116 cells. The cells were infected with scrambled (scr) or SENP1 shRNA lentiviruses followed by IB. (H) SENP1 inhibits Fbw7-mediated ubiquitination of c-Myc. HeLa cells transfected with the indicated plasmids were treated with MG132 before harvesting. The cells were subjected to Ni2+-NTA PD under denaturing conditions followed by IB.
SENP1 Suppresses FBW7-Mediated c-Myc Degradation.
To further examine the role of SENP1-mediated deSUMOylation in inhibiting c-Myc degradation, we tested its effect on Fbw7, as SENP1 interacts with Fbw7 (Fig. 3D). We found that overexpression of WT SENP1, but not SENP1C603S, abrogated the degradation of c-Myc by either Fbw7α (Fig. 3E) or Fbw7γ (SI Appendix, Fig. S5C). Also, overexpression of SENP1 increased the levels of c-Myc in Fbw7+/+, but not Fbw7-deficient (Fbw7−/−), HCT116 cells (Fig. 3F), whereas knockdown of SENP1 reduced the levels of c-Myc less efficiently in Fbw7−/− HCT116 cells compared with Fbw7+/+ HCT116 cells (Fig. 3G and SI Appendix, Fig. S5D). Further, overexpression of WT SENP1, but not SENP1C603S, suppressed the c-Myc ubiquitination by Fbw7α (Fig. 3H). The SENP1 regulation of c-Myc does not depend on the c-Myc DUB USP28 (13), as knockdown of USP28 did not abrogate the induction of c-Myc by SENP1 (SI Appendix, Fig. S5E). Also, overexpression of SENP1 does not affect the levels of USP28 (SI Appendix, Fig. S5 F and G). These results demonstrate that SENP1-mediated deSUMOylation abrogates c-Myc degradation by reversing or suppressing c-Myc ubiquitination by Fbw7.
SENP1-Stabilized c-Myc Is Phosphorylated.
We next examined whether SENP1 stabilization of c-Myc affects its phosphorylation. We found that overexpression of WT SENP1, but not SENP1C603S, significantly increased c-Myc phosphorylation at S62 and T58 (Fig. 4A and SI Appendix, Fig. S6A), whereas knockdown of SENP1 reduced c-Myc phosphorylation at both S62 and T58 (Fig. 4B and SI Appendix, Fig. S6B) to a greater extent compared with total c-Myc. Nuclear fractionation assays showed that S62 and T58 phosphorylated c-Myc is enriched by SENP1 expression in both the chromatin and nuclear soluble fractions (Fig. 4C). We also observed nuclear interaction between endogenous SENP1 and pS62–c-Myc by PLA (Fig. 4D) with enrichment in the nuclear periphery, consistent with a prior study showing the nuclear periphery localization of pS62–c-Myc (22), and the interaction is increased by MG132 treatment (SI Appendix, Fig. S6C). The specificity of the phospho-specific antibodies was confirmed using IP-Western blot (SI Appendix, Fig. S6 D and E). Together, these results suggest that SENP1 inhibition of c-Myc polyubiquitination backs up the degradation process on and off chromatin, stabilizing c-Myc in a double T58 and S62 phosphorylated state. Interestingly, c-Myc–SENP1 interaction and deSUMOylation of c-Myc and its increased expression do not depend on the phosphorylation, as the T58A, S62A, and T58A/S62A double mutant (TSA) of c-Myc were all able to interact with SENP1 (SI Appendix, Fig. S6F) and were stabilized by WT SENP1, but not the SENP1C603S mutant (SI Appendix, Fig. S6G). Both T58A and S62A can be SUMOylated in cells (SI Appendix, Fig. S6H) and deSUMOylated by WT SENP1, but not the SENP1C603S mutant (SI Appendix, Fig. S6 I and J). Thus, SENP1 regulates c-Myc subsequent to but not dependent on its S62 and T58 phosphorylation.
Fig. 4.
SENP1 induces c-Myc phosphorylation. (A) Overexpression of WT SENP1, but not the C603S mutant, increases c-Myc phosphorylation. HeLa cells transfected with the indicated plasmids were assayed by IB. Quantification is shown in SI Appendix, Fig. S6A. (B) Knockdown of SENP1 reduces c-Myc phosphorylation. HeLa cells infected with scrambled or SENP1 shRNA lentiviruses were assayed by IB. Quantification is shown in SI Appendix, Fig. S6B. (C) SENP1 is associated with chromatin. Nuclei isolated from HeLa cells transfected with the indicated plasmids were fractionated to chromatin and soluble fractions, followed by IB. (D) Binding of SENP1 with pS62–c-Myc in cells determined by PLA. HeLa cells were stained with anti-SENP1 and anti–pS62-Myc antibodies followed by PLA. The PLA signals are shown in the nucleus with enrichment in the nuclear periphery.
Knockdown of SENP1 Reduces the Levels of c-Myc and Suppresses Cell Proliferation and Transformation.
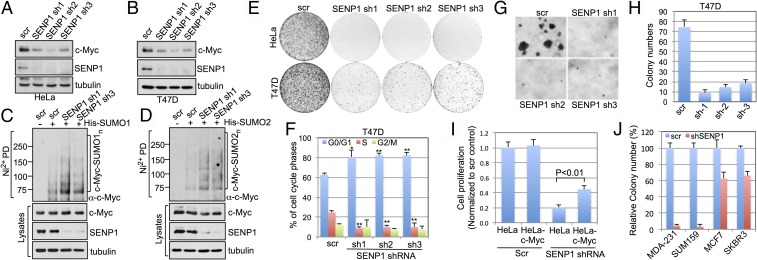
To examine biologic effects of the regulation of c-Myc by endogenous SENP1, we performed SENP1 knockdown assays. Lentiviral-mediated knockdown of SENP1 by three individual shRNAs significantly reduced the levels of endogenous c-Myc protein in HeLa (Fig. 5A) and breast cancer T47D (Fig. 5B) cells. Consistently, knockdown of SENP1 significantly increased both SUMO1-modified (Fig. 5C) and SUMO2-modified (Fig. 5D) c-Myc. Knockdown of SENP1 suppressed cell proliferation as determined by colony formation assays in HeLa, T47D (Fig. 5E), and H1299 cells (SI Appendix, Fig. S7 A and B) as well as cell viability assays (SI Appendix, Fig. S7C). Consistently, knockdown of SENP1 induced cell cycle arrest at G1 phase (Fig. 5F). Treatment of cells with nocodazole to induce G2/M arrest did not abrogate c-Myc reduction upon SENP1 knockdown (SI Appendix, Fig. S7D). Also, knockdown of SENP1 abolished c-Myc induction following serum stimulation (SI Appendix, Fig. S7E). These results demonstrate that endogenous SENP1 plays a critical role in c-Myc stabilization in response to growth signals and that c-Myc reduction upon SENP1 knockdown is not due to G1 arrest. The above effects are SENP1 specific, as knockdown of SENP1 did not reduce the levels of other SENP proteins in both HeLa and T47D cells (SI Appendix, Fig. S7 F and G). Further, knockdown of SENP1 significantly suppressed anchorage-independent growth of T47D cells in soft agar (Fig. 5 G and H), suggesting that SENP1 may play a key role in cell transformation. Of note, overexpression of c-Myc partially rescued the cell growth inhibition by SENP1 knockdown (Fig. 5I) and knockdown of SENP1 suppresses cell proliferation (SI Appendix, Fig. S8A) and anchorage-independent growth (Fig. 5J and SI Appendix, Fig. S8B) of Myc-dependent breast cancer MDA-MB-231 and SUM159 cell lines more significantly than c-Myc–independent MCF7 and SKBR3 cell lines (23). Together, these results suggest that endogenous SENP1 regulates c-Myc levels and this correlates with changes in c-Myc–dependent cell proliferation and transformation.
Fig. 5.
Knockdown of SENP1 reduces c-Myc levels and suppresses cell proliferation. (A and B) Knockdown of SENP1 reduces c-Myc levels. HeLa (A) or T47D (B) cells were infected with scrambled or SENP1 shRNA-encoding lentiviruses, followed by IB. (C and D) Knockdown of SENP1 increases c-Myc SUMOylation. H1299 cells transfected with His-SUMO1 (C) or His-SUMO2 (D) were infected with scrambled or SENP1 shRNA lentiviruses. The cells were assayed by Ni2+-NTA pulldown (PD) under denaturing conditions followed by IB. (E) Knockdown of SENP1 suppresses cell proliferation. HeLa or breast cancer T47D cells infected with control or SENP1 shRNA lentiviruses were cultured for up to 3 wk. The colonies were visualized by staining with crystal violet. (F) Knockdown of SENP1 induces G1 cell cycle arrest. T47D cells were infected with control or SENP1 shRNA lentiviruses followed by cell cycle analysis using propidium iodide staining. The mean percentage of cells in different cell cycle phases obtained from three independent experiments is shown. *P < 0.05, **P < 0.01, compared with the scrambled control. (G and H) Soft agar colony formation assays. T47D cells were infected with control or SENP1 shRNA lentiviruses, followed by colony formation assay in soft agar. Representative images are shown in G and average colony numbers were shown in H. (I) Overexpression of c-Myc partially alleviates the growth inhibition by SENP1 knockdown. Control or c-Myc–expressing HeLa cells were infected with scrambled or SENP1 shRNA lentiviruses, followed by MTT cell proliferation assays. (J) Knockdown of SENP1 inhibits anchorage-independent growth of Myc-sensitive breast cancer cells (SUM159 and MDA-MB-231) more efficiently than that of Myc-insensitive breast cancer cells (MCF7 and SKBR3). Shown is relative inhibition of colony formation in soft agar compared SENP1 shRNA infected cells to scramble control (J).
SENP1 Is Overexpressed in Breast Cancers.
To test whether SENP1 regulation of c-Myc is implicated in human cancers, we examined its expression in a panel of breast cancer cell lines. RT-qPCR assay showed that SENP1 mRNA is overexpressed in half of the tested breast cancer cell lines relative to nontransformed MCF10A cells (Fig. 6A). Consistently, SENP1 protein is also overexpressed in most of the tested breast cancer cell lines (Fig. 6B). We further examined SENP1 and c-Myc expression in breast cancer tissue arrays by immunohistochemistry (IHC) and found that SENP1 expression is elevated in two-thirds of the breast cancer tissues (Fig. 6 C and D). Importantly, the overexpression of SENP1 is significantly correlated with the increase of c-Myc levels in these tissues (Fig. 6D). Thus, by deSUMOylating and stabilizing c-Myc, SENP1 may contribute to c-Myc–driven tumorigenesis.
Fig. 6.
SENP1 is overexpressed in breast cancer cells. (A and B) SENP1 is overexpressed in breast cancer cell lines. The expression of SENP1 mRNA (A) and protein (B) was examined in a panel of breast cancer cell lines by RT-qPCR and IB, respectively, compared with the immortalized mammary epithelia MCF10A cells. (C and D) SENP1 is overexpressed in primary breast cancer tissues. Serial sections of breast cancer tissue arrays were examined by IHC using anti-SENP1 and anti–c-Myc antibodies. Representative images for the high or low expression of SENP1 and c-Myc are shown in C and correlation between SENP1 and c-Myc expression was determine by χ2 test (D).
Discussion
In this study, we report that SENP1 is a c-Myc deSUMOylating enzyme that deSUMOylates c-Myc and leads to c-Myc stabilization and activation. Treatment of cells with proteasome inhibitor markedly increases the SUMOylated species of c-Myc. We show that c-Myc can be comodified by both ubiquitin and SUMO. These results suggest that SUMOylated c-Myc can be targeted to the proteasome for degradation. Indeed, overexpression of SENP1 inhibited c-Myc polyubiquitination, possibly due to the removal of ubiquitin along with SUMO from c-Myc that is comodified by both SUMO and Ub at the same Lys residues. Alternatively, SUMOylation may prime c-Myc for ubiquitination. Therefore, SUMOylation interplays with ubiquitination to modulate c-Myc protein stability. Intriguingly, we observed that SENP1-mediated deSUMOylation results in an increase of the monoubiquitinated form of c-Myc. This unexpected finding could be explained by SUMO attachment to monoubiquitinated c-Myc, via SUMOylation of ubiquitin, to form mixed SUMO-ubiquitin chains so that deSUMOylation by SENP1 results in single ubiquitin conjugated to c-Myc, which is supported by recent proteomic analyses that identified SUMO conjugation to multiple lysines of ubiquitin (19, 24). One possibility is that the monoubiquitination stabilizes c-Myc, whereas the subsequent SUMO and ubiquitin mixed modifications target c-Myc for degradation. Thus, our results not only support the cross-talk between SUMOylation and ubiquitination, which has increasingly emerged (19, 24, 25), but also explain why SUMOylation sites at c-Myc are promiscuous, as SUMO can be conjugated to ubiquitin. Supporting the role of SENP1 in cross-regulating c-Myc ubiquitination, we show that SENP1 suppresses Fbw7-mediated c-Myc ubiquitination dependent on its deSUMOylating enzyme activity. Thus, SENP1 regulates growth signal-regulated c-Myc turnover via counteracting Fbw7, consistent with its role in cell growth and proliferation (Fig. 5). It is interesting to examine whether SENP1 suppresses Fbw7’s ubiquitin E3 activity by directly binding to Fbw7 and/or deSUMOylating Fbw7 and how Fbw7 contributes to the SUMO-ubiquitin comodification of c-Myc. In addition, SENP1 also stabilizes the c-MycT58A mutant, suggesting that SUMOylation may enable the T58A mutant to be targeted by Fbw7. Alternatively, SENP1 may also deSUMOylate c-Myc ubiquitinated by other ubiquitin E3s. Of note, SENP1 has been shown to possess specificity toward SUMO1 and is required for deSUMOylating SUMO1-modified proteins during mouse development (26). Here we show that SENP1 strongly deSUMOylates both SUMO1-modified (Fig. 2A) and SUMO2-modified (Fig. 2B) c-Myc, suggesting that SENP1 also directly acts on SUMO2-modified c-Myc, consistent with the previously reported SENP1 activity toward SUMO2/3 modifications (27, 28). Alternatively, SENP1 may remove SUMO1 from c-Myc comodified with both SUMO2 and SUMO1 (Fig. 3A and SI Appendix, Fig. S4 B and C). In fact, proteasome inhibition accumulates global SUMO1-modified proteins in various cells (SI Appendix, Fig. S4D), suggesting that comodification of proteins with both SUMO1 and SUMO2 may be common in cells (19, 21). Nevertheless, our results clearly show that SENP1 deSUMOylation prevents c-Myc from proteasome degradation.
Although SENP1 deSUMOylation of c-Myc does not require c-Myc phosphorylation at T58 and S62, SENP1 overexpression promotes c-Myc phosphorylation at the two residues. The increased S62 phosphorylation and T58 phosphorylation could occur due to back up of the degradation process and the reversal of its mixed SUMO and ubiquitin modifications before proteasome degradation. pS62–c-Myc has been shown to localize at the nuclear periphery (22), where SENP1 is also enriched at the nuclear pores (29, 30), and is associated with proliferative gene activation. Consistently, we observed that SENP1 interaction with pS62-Myc is enriched in the nuclear periphery (Fig. 4D), suggesting that SENP1 may regulate c-Myc activity by deSUMOylating c-Myc on chromatin at both the nuclear interior and nuclear pore. Future studies are warranted to delineate the specific role for SENP1-mediated c-Myc deSUMOylation at the nuclear pore in c-Myc transactivation and transformation. It is also important to test whether SENP1 has an additional role in transcription by controlling chromatin SUMOylation.
Interestingly, PIAS1 has recently been shown as a SUMO ligase for c-Myc that mediates c-Myc SUMOylation, mainly at K51 and K52, and also stabilizes c-Myc (17). This SUMOylation is thought to create a docking site for JNK1 to phosphorylate c-Myc at S62. Consequently, PIAS1 overexpression enhances c-Myc transactivation activity and promotes c-Myc–driven tumorigenesis (17). These seemingly conflicting results highlight the critical role for properly regulating the balance of SUMOylation in c-Myc turnover and function. Either increasing or abrogating c-Myc SUMOylation would disrupt such a balance and affect c-Myc stabilization. The dynamic nature of c-Myc posttranslational modifications has also been seen in its ubiquitination, which can either positively or negatively regulate c-Myc stability and either increase or reduce c-Myc activity in a ligase-dependent manner (4, 10). It is possible that SUMOylation of c-Myc at different SUMO sites by different SUMO ligases may differentially regulate c-Myc stabilization and activity. Alternatively, SUMO regulation of c-Myc may be cell-context dependent. Different expression levels of SUMO ligases and deSUMOylating enzymes may have differential effects on c-Myc stability and activity. It is worth noting that SENP7 neither binds to (SI Appendix, Fig. S1A) nor deSUMOylates (SI Appendix, Fig. S1 E and F) c-Myc, indicating that a previously reported effect of SENP7 knockdown on c-Myc SUMOylation (15) may be indirect or cell-type dependent. Adding to the complexity of the SUMO regulation of c-Myc, a recent report showed that RNF4, a SUMO-targeted ubiquitin ligase (STUbL) that targets SUMO2-modified PML for ubiquitination and proteasome degradation, increases c-Myc stability by promoting K11- and K33-linked polyubiquitination, which does not depend on c-Myc SUMOylation, but requires preexisting c-Myc S62 phosphorylation (31). These results strongly argue that SUMOylation dynamically regulates c-Myc activity and levels in a cell context-dependent manner, similar to c-Myc ubiquitination.
Functionally, we show that SENP1 is essential for cancer cell growth and proliferation. Knockdown of SENP1 drastically induced cell cycle arrest and suppressed cell proliferation in several tested human cancer cell lines. These effects depend, at least partially, on its regulation of c-Myc, as overexpression of c-Myc partially rescued the cell growth inhibition by SENP1 knockdown (Fig. 5I) and SENP1 knockdown inhibits cell proliferation and transformation activity more efficiently in Myc-sensitive breast cancer cell lines than in Myc-insensitive cell lines (Fig. 5J). Consistently, SENP1 expression is frequently increased in human breast cancer cell lines and primary tissues, which correlates with the overexpression of c-Myc. Thus, SENP1 may contribute to tumorigenesis by stabilizing c-Myc. Also, SENP1 deSUMOylates PIN1, a key c-Myc regulator, and increases PIN1’s stability and oncogenic activity (32), providing further evidence for an oncogenic role for SENP1. It would be interesting to analyze whether this SENP1 overexpression is associated with patient survival and treatment outcomes, particularly in different breast cancer subtypes.
Together, our results identify SENP1 as a positive regulator for c-Myc and highlight the importance of dynamic and tightly controlled SUMO modifications in the control of c-Myc stability and activity. It remains to be fully elucidated how the SUMO pathway interplays with the ubiquitin-proteasome system to coregulate c-Myc. Future studies are also critically needed to investigate the role of SENP1–c-Myc regulation in vivo in various tissues and biological contexts.
Materials and Methods
Cell lines were cultured as described in SI Appendix, SI Materials and Methods. Flag-tagged WT SENP1 was obtained from Addgene (25). Flag-SENP1C603S mutant was generated by site-directed mutagenesis. A detailed description of all other plasmids, antibodies, ubiquitination and SUMOylation assays, PLA, IB, co-IP, ChIP-qPCR, RT-qPCR, cell proliferation, flow cytometry, IHC staining, GST-fusion protein association, and soft agar assays can be found in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank members of the M.-S.D. and R.C.S. laboratories for active discussion. This work was supported by NIH/National Cancer Institute Grants R01 CA186241 (to M.-S.D. and R.C.S.), R01 CA160474 (to M.-S.D.), and R01 CA 100855 (to R.C.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802932115/-/DCSupplemental.
References
- 1.Kress TR, Sabò A, Amati B. MYC: Connecting selective transcriptional control to global RNA production. Nat Rev Cancer. 2015;15:593–607. doi: 10.1038/nrc3984. [DOI] [PubMed] [Google Scholar]
- 2.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 3.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 4.Farrell AS, Sears RC. MYC degradation. Cold Spring Harb Perspect Med. 2014;4:a014365. doi: 10.1101/cshperspect.a014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hann SR. Role of post-translational modifications in regulating c-Myc proteolysis, transcriptional activity and biological function. Semin Cancer Biol. 2006;16:288–302. doi: 10.1016/j.semcancer.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Sears RC. The life cycle of c-Myc: From synthesis to degradation. Cell Cycle. 2004;3:1133–1137. [PubMed] [Google Scholar]
- 7.Welcker M, Clurman BE. FBW7 ubiquitin ligase: A tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 8.Bahram F, von der Lehr N, Cetinkaya C, Larsson LG. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood. 2000;95:2104–2110. [PubMed] [Google Scholar]
- 9.Zhang X, et al. Mechanistic insight into Myc stabilization in breast cancer involving aberrant Axin1 expression. Proc Natl Acad Sci USA. 2012;109:2790–2795. doi: 10.1073/pnas.1100764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun XX, Sears RC, Dai MS. Deubiquitinating c-Myc: USP36 steps up in the nucleolus. Cell Cycle. 2015;14:3786–3793. doi: 10.1080/15384101.2015.1093713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang X, et al. Deubiquitinase USP13 maintains glioblastoma stem cells by antagonizing FBXL14-mediated Myc ubiquitination. J Exp Med. 2017;214:245–267. doi: 10.1084/jem.20151673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D, et al. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J Cell Physiol. 2017;232:3664–3676. doi: 10.1002/jcp.25841. [DOI] [PubMed] [Google Scholar]
- 13.Popov N, et al. The ubiquitin-specific protease USP28 is required for MYC stability. Nat Cell Biol. 2007;9:765–774. doi: 10.1038/ncb1601. [DOI] [PubMed] [Google Scholar]
- 14.Sun XX, et al. The nucleolar ubiquitin-specific protease USP36 deubiquitinates and stabilizes c-Myc. Proc Natl Acad Sci USA. 2015;112:3734–3739. doi: 10.1073/pnas.1411713112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-Prieto R, Cuijpers SA, Kumar R, Hendriks IA, Vertegaal AC. c-Myc is targeted to the proteasome for degradation in a SUMOylation-dependent manner, regulated by PIAS1, SENP7 and RNF4. Cell Cycle. 2015;14:1859–1872. doi: 10.1080/15384101.2015.1040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalkat M, et al. Identification of c-MYC SUMOylation by mass spectrometry. PLoS One. 2014;9:e115337. doi: 10.1371/journal.pone.0115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabellino A, et al. PIAS1 promotes lymphomagenesis through MYC upregulation. Cell Rep. 2016;15:2266–2278. doi: 10.1016/j.celrep.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabò A, Doni M, Amati B. SUMOylation of Myc-family proteins. PLoS One. 2014;9:e91072. doi: 10.1371/journal.pone.0091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendriks IA, Vertegaal AC. A comprehensive compilation of SUMO proteomics. Nat Rev Mol Cell Biol. 2016;17:581–595. doi: 10.1038/nrm.2016.81. [DOI] [PubMed] [Google Scholar]
- 20.Matic I, et al. In vivo identification of human small ubiquitin-like modifier polymerization sites by high accuracy mass spectrometry and an in vitro to in vivo strategy. Mol Cell Proteomics. 2008;7:132–144. doi: 10.1074/mcp.M700173-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendriks IA, et al. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat Struct Mol Biol. 2014;21:927–936. doi: 10.1038/nsmb.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myant K, et al. Serine 62-phosphorylated MYC associates with nuclear lamins and its regulation by CIP2A is essential for regenerative proliferation. Cell Rep. 2015;12:1019–1031. doi: 10.1016/j.celrep.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler JD, et al. A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science. 2012;335:348–353. doi: 10.1126/science.1212728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamoliatte F, McManus FP, Maarifi G, Chelbi-Alix MK, Thibault P. Uncovering the SUMOylation and ubiquitylation crosstalk in human cells using sequential peptide immunopurification. Nat Commun. 2017;8:14109. doi: 10.1038/ncomms14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma P, Yamada S, Lualdi M, Dasso M, Kuehn MR. Senp1 is essential for desumoylating Sumo1-modified proteins but dispensable for Sumo2 and Sumo3 deconjugation in the mouse embryo. Cell Rep. 2013;3:1640–1650. doi: 10.1016/j.celrep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickey CM, Wilson NR, Hochstrasser M. Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol. 2012;13:755–766. doi: 10.1038/nrm3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendes AV, Grou CP, Azevedo JE, Pinto MP. Evaluation of the activity and substrate specificity of the human SENP family of SUMO proteases. Biochim Biophys Acta. 2016;1863:139–147. doi: 10.1016/j.bbamcr.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Chow KH, Elgort S, Dasso M, Powers MA, Ullman KS. The SUMO proteases SENP1 and SENP2 play a critical role in nucleoporin homeostasis and nuclear pore complex function. Mol Biol Cell. 2014;25:160–168. doi: 10.1091/mbc.E13-05-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duheron V, Nilles N, Pecenko S, Martinelli V, Fahrenkrog B. Localisation of Nup153 and SENP1 to nuclear pore complexes is required for 53BP1-mediated DNA double-strand break repair. J Cell Sci. 2017;130:2306–2316. doi: 10.1242/jcs.198390. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JJ, et al. RNF4-dependent oncogene activation by protein stabilization. Cell Rep. 2016;16:3388–3400. doi: 10.1016/j.celrep.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CH, et al. SENP1 deSUMOylates and regulates Pin1 protein activity and cellular function. Cancer Res. 2013;73:3951–3962. doi: 10.1158/0008-5472.CAN-12-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.