Significance
Feeding behavior changes drastically with infection and is a conserved behavioral response. However, the mechanistic aspects of how this behavior provides a survival advantage are poorly understood. Metabolic reprogramming is emerging as a mechanism by which disease tolerance is established and coordinated to the type of inflammatory insult to support tissue function. The type of metabolism required for survival of parasitic infections has not yet been explored. Here, we report that inhibition of glycolysis utilizing 2-deoxy glucose (2DG) prevented the development of cerebral malaria. The 2DG did not affect pathogen burden, degree of anemia, or cerebral inflammation. Instead, 2DG improved disease tolerance by affecting hemostasis and greatly diminished the formation of intracerebral microthrombi and hemorrhage.
Keywords: malaria, inflammation, metabolism
Abstract
Sickness behaviors are a conserved set of stereotypic responses to inflammatory diseases. We recently demonstrated that interfering with inflammation-induced anorexia led to metabolic changes that had profound effects on survival of acute inflammatory conditions. We found that different inflammatory states needed to be coordinated with corresponding metabolic programs to actuate tissue-protective mechanisms. Survival of viral inflammation required intact glucose utilization pathways, whereas survival of bacterial inflammation required alternative fuel substrates and ketogenic programs. We thus hypothesized that organismal metabolism would be important in other classes of infectious inflammation and sought to understand its role in the prototypic parasitic disease malaria. Utilizing the cerebral malaria model, Plasmodium berghei ANKA (PbA) infection in C57BL/6J male mice, we unexpectedly found that inhibition of glycolysis using 2-deoxy glucose (2DG) conferred protection from cerebral malaria. Unlike vehicle-treated animals, 2DG-treated animals did not develop cerebral malaria and survived until ultimately succumbing to fatal anemia. We did not find any differences in parasitemia or pathogen load in affected tissues. There were no differences in the kinetics of anemia. We also did not detect differences in immune infiltration in the brain or in blood–brain barrier permeability. Rather, on pathological analyses performed on the entire brain, we found that 2DG prevented the formation of thrombi and thrombotic complications. Using thromboelastography (TEG), we found that 2DG-treated animals formed clots that were significantly less strong and stable. Together, these data suggest that glucose metabolism is involved in inflammation-induced hemostasis and provide a potential therapeutic target in treatment of cerebral malaria.
Hypophagia or anorexia has been observed in a variety of organisms as a response to infectious or inflammatory challenge and is part of a larger set of stereotypic behaviors induced by inflammation collectively known as sickness behaviors (1). Available evidence suggests that the anorexia of infection is generally adaptive in a variety of organisms (2–4). However, how anorexia affects the course of infectious disease is largely unknown. Survival of infections depends on either disease resistance (control of pathogen burden) or disease tolerance (reducing the negative impact of infection on host fitness) (5–8). Disease resistance is a relatively well-understood function of the immune system, while disease tolerance remains poorly understood. Recent evidence indicates that anorexia can promote tolerance of infectious diseases (2, 9, 10).
One effect of anorexia is a metabolic switch from glucose utilization to alternative fuels, including fatty acids and ketone bodies. We recently found that the organismal metabolic state was critical in maintaining disease tolerance by providing the correct energy source that could support cellular stress adaptation and detoxification pathways (9). Furthermore, we found that different metabolic states were required for surviving different types of infections (9). When these metabolic states were decoupled from their corresponding inflammatory states, animals could no longer survive the challenge due to impaired disease tolerance. In our previous study, we primarily focused on bacterial and viral challenges. In these studies, we identified the brain as a critical and limiting organ for survival, while the systemic metabolic state was important in supporting stress adaptation pathways that preserved cerebral function. We thus asked what type of metabolic state was required in another class of infection in which the brain was a limiting organ for survival and chose to study the prototypic parasitic infectious model cerebral malaria.
Cerebral malaria is a clinical syndrome characterized by altered consciousness, seizure, and coma caused by infection with Plasmodium falciparum; it kills over 1 million people each year (mostly children under the age of 5 y in impoverished nations) despite effective antimalarial treatment (11). Our current understanding of cerebral malaria pathogenesis (gleaned from human autopsy series and advanced cerebral imaging) includes a role for microthrombi and hemorrhage, sequestration of infected red blood cells (iRBCs), neuroinflammation with a critical pathogenic role for CD8+ T cells (12), endothelial activation (13), increased permeability of the blood–brain barrier (BBB) (14), and excitotoxicity (15, 16). The mouse model of cerebral malaria, utilizing the introduction of Plasmodium berghei ANKA (PbA)-infected erythrocytes into C57BL/6J mice, has many features of human cerebral malaria, including both clinical features and pathological findings, and is widely used in understanding pathogenesis (17).
Much work has been done to understand the metabolic requirements of Plasmodium and Plasmodium iRBCs. These studies have elucidated unique metabolic programs utilized by the parasite, which has led to an understanding of how iRBC metabolism can be subverted to maintain the Plasmodium life cycle (18–20). Less is known about the effect of malaria on host metabolism or vice versa. Recent studies have shown that host metabolism may impact disease resistance. Zuzarte-Luís et al. (21) showed that preexposure to a high-fat diet reduced the malarial burden via a mechanism that involved antioxidants, while Mejia et al. (22) demonstrated that caloric restriction improved cerebral malaria via a mechanism that involved leptin. Gordon et al. (23) showed that manipulating the glutamine pathway also had major effects on parasite burden and on blunting the host immune response.
Disease tolerance has also been shown to be an important strategy for the host response to malaria. Sickle cell trait, one of the most widely understood genetic mutations, which confers protection to the host, was demonstrated to enhance host tolerance to infectious burden. Ferreira et al. (24) recently showed that sickle cell trait did not affect parasite burden but, instead, decreased the amount of circulating free heme and blunting CD8+ T cell responses to the parasite, thereby suppressing pathogenesis of cerebral malaria. Cumnock et al. (25) recently demonstrated that survival in the Plasmodium chabaudi AJ model, where mice do not develop cerebral malaria but, instead, fatal anemia, was dependent on glucose metabolism, presumably because severe anemia reduced tissue oxygenation, thereby increasing the demand on processes that required glucose. They demonstrated that the dependence on glucose for mortality in this model was also altering disease tolerance since no changes in pathogen burden were observed.
In this study, we investigated how host metabolism affects tolerance of malaria infection using the cerebral malaria model PbA. We show here that inhibition of glycolysis using the competitive glucose analog 2-deoxy glucose (2DG) was protective in the PbA model of cerebral malaria. The 2DG analog did not affect parasitemia, the extent of anemia, the degree of cerebral edema, or neuroinflammation. Rather, 2DG had a major impact on hemostasis. The 2DG-treated animals had weaker clots that were more easily lysed. This correlated with reduced microthrombi and hemorrhage, which protected animals from cerebral malaria and dramatically extended survival.
Results
2DG Protects Mice from Cerebral Malaria.
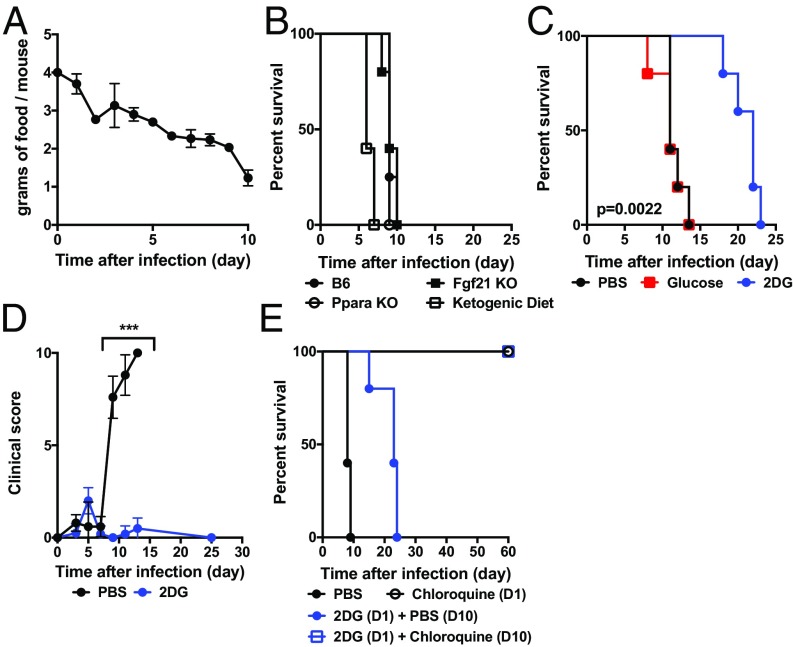
We observed that mice infected with PbA become hypophagic (Fig. 1A). To test if fasting metabolism was required for surviving malaria, we subjected animals deficient in the master regulator of ketogenesis Peroxisome Proliferator Activated Receptor-α (PPAR-α), mice deficient in the fasting hormone Fibroblast Growth Factor 21 (FGF21), or mice maintained on a ketogenic diet to cerebral malaria (Fig. 1B). We did not observe a difference in survival in PPAR-α– or FGF21-deficient mice, indicating that key components of fasting metabolism did not change susceptibility to cerebral malaria, but did note that mortality appeared to be enhanced by the ketogenic diet, although it did not reach statistical significance. In our previous study, we identified glucose utilization to be a key component in mediating disease tolerance in sepsis (9). To directly assess the role of glucose in cerebral malaria, we challenged mice infected with malaria with glucose and 2DG (Fig. 1C). We did not detect differences in glycemia, plasma free fatty acids, or ketone bodies subsequent to glucose or 2DG treatment (SI Appendix, Fig. S1). While glucose had no effect on mortality, 2DG prolonged survival by more than 10 d (Fig. 1C) and completely suppressed the clinical signs of cerebral malaria (Fig. 1D), although it was insufficient to rescue animals from mortality due to later complications of malaria, namely, persistent pathogen burden and severe anemia (Fig. 2). These findings are consistent with recent studies utilizing the P. chabaudi AJ model, where 2DG was likely exacerbating the damaging effects of severe anemia (25). We thus asked if the addition of the antimalarial antibiotherapy chloroquine would be sufficient to provide long-term survival (Fig. 1E). The addition of chloroquine after 2DG treatment was sufficient to fully rescue animals from cerebral malaria (Fig. 1E). Together, these data demonstrate that glucose metabolism plays an important role in the pathogenesis of cerebral malaria.
Fig. 1.
Mice are protected from cerebral malaria by 2DG. (A) Food intake was measured in C57BL/6J males infected with PbA (three cages of five mice housed per cage). (B) Kaplan–Meier plot of mice of the indicated genotype or diet (n = 5 per group). B6, C57BL/6J; KO, knockout; Ppara, PPAR-α. (C) Kaplan–Meier plot of mice treated with 2DG (200 mg/kg per injection), glucose (1 mg/kg per injection), or PBS beginning 8 h after infection and then given twice daily (n = 10 per group; PBS vs. 2DG: P = 0.002). (D) Clinical score of PbA-infected mice treated with 2DG or PBS (n = 5 per group; ***P < 0.0001 on days 9, 11, and 13). (E) Kaplan–Meier plot of mice treated with 2DG, 2DG followed by chloroquine (1 mg/mouse/d) on day 10 (D10), or chloroquine alone starting from D1 (n = 5 per group; P < 0.001 2DG vs. 2DG + chloroquine).
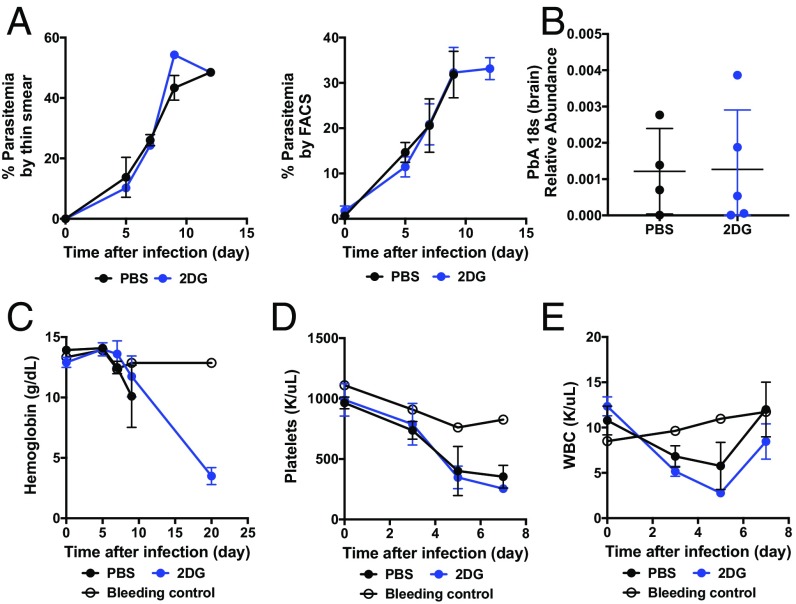
Fig. 2.
Parasitemia is not affected by 2DG. (A) Parasitemia was quantitated in PbA-infected animals treated with PBS or 2DG using thin smear and Wright–Giemsa stain (Left) and by flow cytometry utilizing GFP-labeled PbA (Right) (n = 5 per group). (B) Brains were harvested on day 10 after infection when vehicle-treated mice were demonstrating signs of cerebral malaria, and PbA at 18 s was measured using qRT-PCR (n > 4 per group). (C–E) Complete blood cell counts were obtained from bleeding controls (n = 3) and from PbA-infected animals treated with vehicle or 2DG (n = 5 per group).
2DG Treatment Does Not Impact Parasitemia or Tissue Parasite Burden.
Since 2DG protected mice from cerebral malaria, we investigated whether there were any differences in parasite burden. It has been previously shown that Plasmodium relies on host glucose stores for maintaining its life cycle within the host (26, 27). Various in vitro models have also shown that glycolysis inhibition was sufficient to attenuate the virulence of Plasmodium (28). We thus directly assessed the Plasmodium burden in mice treated with 2DG.
We did not observe any differences in parasitemia over time either when measured by thin-smear preparations (Fig. 2A) or when fluorescently labeled PbA was used and assessed by flow cytometry. Likewise, we could not detect differences in pathogen load in the brains of mice treated with 2DG compared with vehicle (Fig. 2B). Consistent with these findings, we did not detect differences in the kinetics of anemia during the entire viable period of mice given vehicle, and we did not observe any differences in peripheral leukocytes or platelets (Fig. 2 C–E). We found that mice infected with malaria treated with 2DG developed profound anemia (Fig. 2C), which was likely the cause of mortality. Together, these data indicated to us that pathogen control was not the mechanism by which 2DG was protecting mice from cerebral malaria.
2DG Treatment Does Not Impact Cerebral Edema or Neuroinflammation.
Cerebral malaria is known to cause major disruptions in BBB permeability, leading to increased cerebral edema. Neuroinflammation has also been implicated as a driver of pathogenesis in cerebral malaria and, in particular, the pathogenic role of CD8+ T cells (14, 29). Aerobic glycolysis is necessary for cellular activation and effector function in a wide variety of leukocytes, including CD8+ T cells (30, 31). We thus directly assessed whether 2DG impacted neuroinflammation.
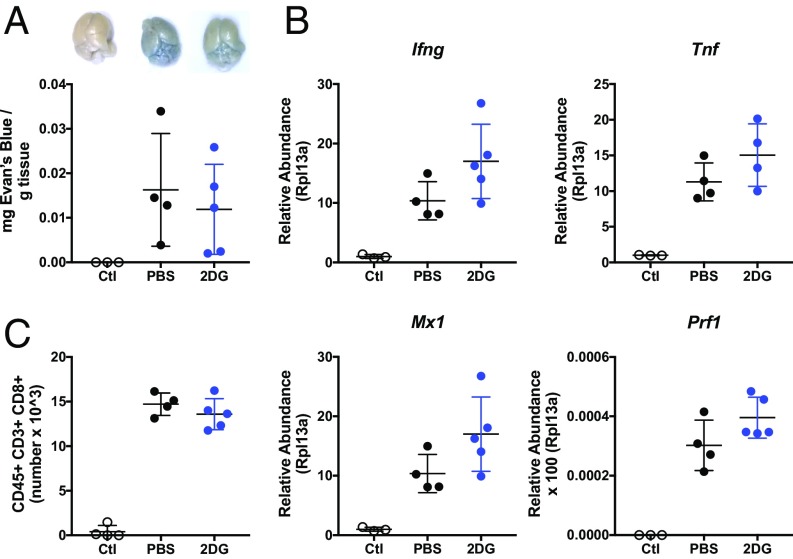
We found that the treatment with 2DG did not impact the degree of cerebral edema as assessed by Evan’s Blue extravasation (Fig. 3A). Since increased BBB permeability is thought to be a consequence of the vasodilatory properties of inflammatory mediators, we next examined whether there was any difference in cerebral inflammation. We could not detect any differences in the extent of inflammatory transcripts in the brains of mice treated with 2DG compared with vehicle (Fig. 3B). Moreover, flow cytometric analyses of whole brains did not reveal differences in immune infiltrates in the mice treated with 2DG compared with vehicle and, notably, in the number of infiltrating CD8+ T cells (Fig. 3C and SI Appendix, Fig. S2).
Fig. 3.
Neuroinflammation is not affected by 2DG. (A) Evans Blue stain extraction in sham-infected and PbA-infected mice treated with 2DG or vehicle on day 10 when vehicle-treated mice were exhibiting clinical symptoms with representative photographs (n > 3 per group). Ctl, control. (B) qRT-PCR analyses of IFN-γ (Ifng), TNF-α (Tnf), the type I IFN target gene MX1 (Mx1), and Perforin (Pfn1) on whole brain harvested on day 10 postinfection in vehicle and 2DG-treated mice (n > 4 per group). (C) Flow cytometric quantification of CD8+ T cells from whole brain harvested on day 10 (n > 4 per group).
These data indicated that the protective effect of 2DG against cerebral malaria was not due to dampening the immune response. Since we were unable to detect large differences in pathogen burden or extent of inflammation, we hypothesized that 2DG was exerting its protective effects via disease tolerance mechanisms.
2DG Treatment Decreases the Extent of Microthrombi and Stasis-Related Pathological Changes.
iRBC sequestration, microthrombi, and subsequent disruption in vascular flow and associated hemorrhage is a pathological hallmark of cerebral malaria and a proximal cause of cerebral dysfunction and death (32–35). Since mice treated with 2DG were not succumbing to cerebral malaria, we asked if 2DG was impacting hemostatic parameters.
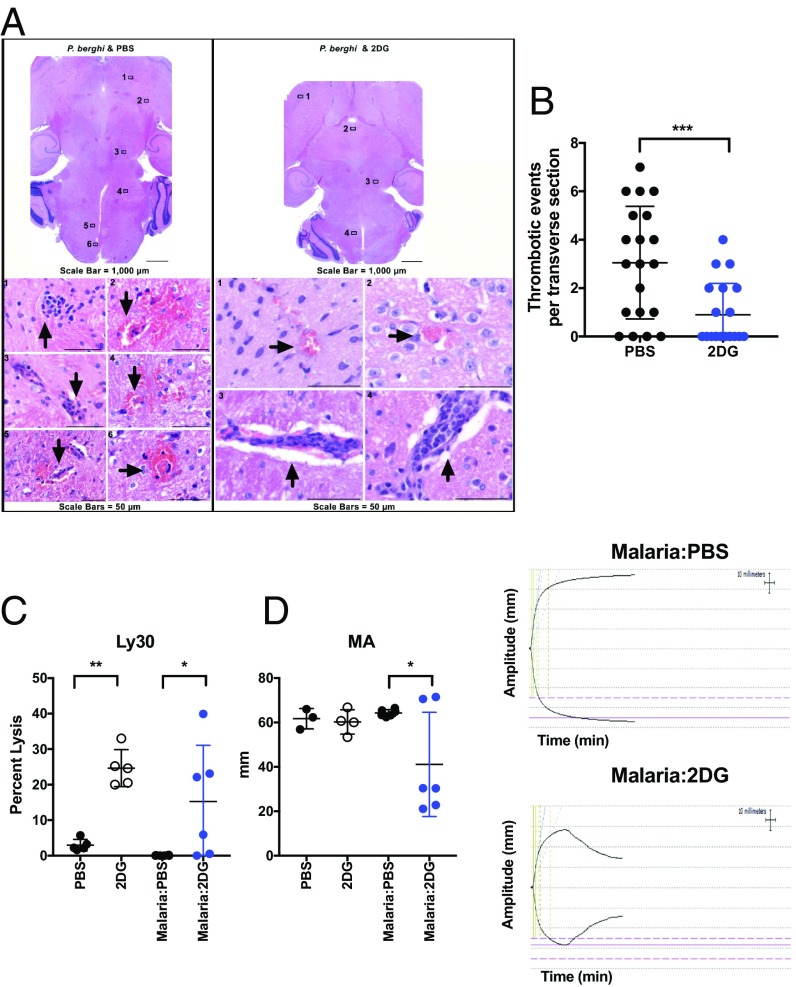
To test whether 2DG was affecting vascular events, we generated serial sections of the entire brains of animals infected with malaria treated with 2DG or vehicle. To our surprise, we found that 2DG dramatically decreased the number of microthrombi, sequestration, and associated hemorrhagic lesions when assessed in a blinded fashion by a pathologist (Fig. 4 A and B). Similar findings were observed in the liver (SI Appendix, Fig. S3A).
Fig. 4.
Microthrombi formation and hemostasis are affected by 2DG. (A) Representative section of PbA-infected animals treated with PBS or 2DG harvested on day 10 postinfection. (B) Quantification of thrombotic areas in serial sections of whole brain obtained from three PbA-infected animals receiving PBS or 2DG harvested on day 10 postinfection. ***P < 0.0009. TEG analyses of freshly citrated whole blood in PbA or sham-infected animals treated with PBS or 2DG harvested on day 10 postinfection, percentage of clot lysed after 30 min (C; Ly30) and maximum amplitude (MA; D) (Left), with representative tracings (Right) (n > 3 mice per group; *P < 0.05, **P < 0.006 by ANOVA).
Since 2DG appeared to be limiting the development of pathological vascular occlusions, we asked if 2DG had an impact on coagulation globally. Coagulopathy has been widely reported in malaria (35–38). To this end, we subjected freshly citrated whole blood of animals with malaria treated with vehicle or 2DG to thromboelastography (TEG). TEG is a technique that measures global parameters of coagulation from initial clot formation to fibrinolysis and is often used in the clinical setting to quickly identify coagulopathies (39). In particular, TEG is a sensitive method of measuring viscoelastic strength and has been applied to the study of coagulopathy in malaria (40).
Using TEG, we found that 2DG decreased the clot strength as read out by maximum amplitude (MA, Fig. 4D), which corresponded to a decrease in fibrinolytic time as measured by the percentage of clot that has lysed after 30 min (Ly30, Fig. 4C). Interestingly, 2DG alone appeared to affect fibrinolytic time independent of the presence of malaria. We did not detect differences in primary clotting time as assessed by TEG (SI Appendix, Fig. S3B). The effects of 2DG in vivo could not be recapitulated by adding 2DG to ex vivo whole blood, indicating that the effects of 2DG were either indirect or required prolonged exposure in vivo (SI Appendix, Fig. S3C). Platelet counts were not different between groups (Fig. 2D). Since endothelial activation via the actions of von Willebrand factor (vWF) is one key aspect of initiating coagulation (41, 42), we assessed if 2DG was affecting endothelial vWF expression in situ (43). We were unable to detect differences in vWF staining in 2DG-treated versus vehicle-treated animals (SI Appendix, Fig. S4).
Taken together, our data demonstrate that glucose metabolism plays an important role in driving coagulopathy in cerebral malaria. Inhibition of glucose metabolism through the use of the glycolysis inhibitor 2DG protected animals from cerebral malaria not by enhancing pathogen clearance or by decreasing neuroinflammation but, instead, by enhancing host tolerance to malaria by decreasing the viscoelastic strength of malaria-induced thrombi, thereby limiting vascular hemostatic pathologies.
Discussion
Malarial infection induces loss of appetite in mice and humans. We and others demonstrated that the anorexia of inflammation is protective in a variety of infectious and inflammatory settings. One major effect of decreased appetite is reduced glucose intake and a reliance on alternative fuels generated by fasting metabolic programs. We thus hypothesized that limiting glucose utilization would be beneficial to the host in malarial infection. To test this, we utilized the competitive inhibitor of glycolysis 2DG in the PbA C57BL/6J murine model of cerebral malaria. We demonstrated that inhibition of glucose metabolism with 2DG prevented the development of cerebral malaria. Unexpectedly, 2DG did not affect pathogen burden, degree of neuroinflammation, BBB permeability, or anemia. Rather, 2DG reduced the burden of cerebral microthrombi, and hence prevented the development of cerebral malaria, but was unable to prevent mortality in malaria, which was likely mediated in the later stages by fatal anemia. Whole blood taken from infected animals treated with 2DG displayed decreased viscoelastic clot strength and hyperfibrinolysis compared with vehicle-treated controls. Together, our data implicate a role in glucose metabolism in mediating coagulopathy in maintaining disease tolerance to malaria.
In complex systemic infections such as malaria, organismal survival is a direct consequence of critical organ failure. The limiting organ varies among different infections, and different organs can be limiting during different stages of the infectious course. For example, in pneumonia syndromes with sepsis pathology, failure in pulmonary function is limiting for the organism. In the case of pneumonia, the use of a mechanical ventilator to support oxygenation and ventilation can sustain the organism despite respiratory failure. These and other “organ-supporting” modalities, such as hemodialysis in the case of renal failure or the use of adrenergic support in the case of cardiovascular collapse, are examples of therapies directed at enhancing disease tolerance. In the case of pneumonia, supporting the lung function using mechanical ventilation allows time for the organism to revert to a homeostatic state unless sepsis progression causes another limiting organ to fail, as would be the case with the development of disseminated intravascular coagulopathy and cerebral failure from stroke. In the case of cerebral malaria, intracerebral coagulopathy is limiting for the organism. In our experiments, 2DG was sufficient to rescue mice from cerebral malaria, but they ultimately succumbed to fatal anemia in the absence of pathogen clearance. Our findings are consistent with those of Cumnock et al. (25), who showed that 2DG may be detrimental in severe anemia. Indeed, we were able to rescue 2DG-treated animals that survived cerebral malaria by subsequent treatment with chloroquine, suggesting that any therapeutic use of 2DG for treating cerebral malaria will need to be combined with chemotherapeutic drugs targeting the parasite and that the use of 2DG must be carefully timed to the stage of the disease.
There are now a couple of examples where disease tolerance is mechanistically linked to metabolism (9, 10). Given the diversity of infectious etiologies, each producing different physiological states in which different organs may be limiting for the organism at different times during the disease course, it is not surprising that there are a variety of disease tolerance mechanisms. Adjustment of metabolic programs is an important aspect of disease tolerance, since intrinsic cellular stress adaptation programs, and thus tissue function, require specific metabolic fuels. This is best studied in immunometabolism, where it is clear that cellular effector functions are tied to specific metabolic pathways that are required for effector function (44, 45).
We were surprised that 2DG did not alter pathogen burden or neuroinflammation in our study. Glucose metabolism has been extensively studied in lymphocytes and, in particular, T cells. Inhibition of glycolysis has been shown to be critical in activation and effector function of CD8+ T cells (46), which are critical in the pathogenesis of cerebral malaria. Glucose utilization has also been studied in the parasite. Since Plasmodium relies on a constant supply of host glucose for growth, the effect on inhibiting glycolysis has been studied in parasite growth in vitro. In these studies, 2DG and hexokinase inhibitors were shown to inhibit parasite growth (28). However, in systemic infections like malaria, where metabolic fuels are finite and limited, especially in the setting of anorexia, substrate utilization is a far more complex and dynamic process, a complexity that is difficult to model in in vitro systems. While we were unable to detect differences in parasite burdens between groups, it is possible that Plasmodium virulence was reduced by 2DG, thereby lessening the severity of the disease, as was observed in the case of Salmonella, where infection-induced anorexia was observed to impact Salmonella virulence and transmission (47).
While cellular metabolic requirements have been well studied in leukocytes, less work has been done on platelets and in the coagulation cascade. Previous work in porcine systems demonstrated a role in platelet metabolism and coagulation (48). In these studies, which also utilized TEG, disruption of ATP production in platelets reduced clot stability and induced hyperfibrinolysis, which is similar to what we observe in our system. We were unable to recapitulate the effects of 2DG administered in vivo when we added 2DG to ex vivo whole blood of animals. This suggested that the coagulation changes occurred as a consequence of prolonged exposure to 2DG and/or to secondary effects of 2DG on factors important in clot strength. The precise mechanism by which inhibition of glucose metabolism impacts fibrinolysis and clot strength remains unknown.
In summary, we demonstrate a role for 2DG in protecting animals from cerebral malaria. The 2DG did not appear to affect pathogen burden or degree of neuroinflammation, and thus appeared to act in a manner that enhanced disease tolerance by affecting hemostasis and preventing the formation of fatal cerebral microthrombi. Although our study was not designed to be a therapeutic study, the combined observations that glycolysis may be important for parasite replication, immune-mediated inflammation, and hemostasis suggest there may be a therapeutic role for 2DG in the treatment of human cerebral malaria, which remains a major clinical problem in the developing world.
Methods
Animals and Malaria Infection.
Only male mice 8–10 wk of age were used. C57BL/6J and PPAR-α knockout mice were purchased from The Jackson Laboratories. FGF21 knockout mice were a generous gift from David J. Mangelsdorf, University of Texas Southwestern Medical Center, Dallas. Mice were infected i.p. with PbA by injecting 1 × 106 PbA iRBCs obtained from infected C57BL6/J mice. PbA and fluorescent PbA (GFP) were a generous gift from Richard Bucala, Yale University School of Medicine, New Haven, CT. Parasitemia was monitored using thin preparation smears and also by flow cytometry as described below. Mice were clinically scored as previously described (49). All animal experiments were performed according to institutional regulations upon review and approval of Yale University’s Institutional Animal Care and Use Committee.
Treatments and Ketogenic Diet.
The 2DG (Sigma–Aldrich) was reconstituted in water and injected i.p. twice a day starting 8 h postinfection (200 mg/kg twice daily). The d-(+) Glucose (Sigma–Aldrich) was reconstituted in water and injected i.p. twice a day starting 8 h postinfection (1 mg/kg twice daily). The ketogenic diet was purchased from Envigo. Chloroquine (Sigma–Aldrich) was reconstituted in PBS and administered i.p. at a rate of 1 mg per mouse daily.
Quantification of Parasitemia and Complete Blood Count.
Thin preparations were prepared using Wright–Giemsa stain (Sigma–Aldrich). Briefly, blood was collected from the retroorbital plexus, and a thin smear was prepared, subsequently fixed, and stained with methanol and Wright–Giemsa stain. Slides were blinded and given to a single observer, who quantitated the number of infected RBCs per high-power (40× magnification) field over five fields. Fluorescent PbA was utilized and analyzed by flow cytometry. Ter119 and CD45 (BD Pharmingen) were utilized, and CD45− Ter119+ GFP+ cells were expressed as a percentage of CD45− Ter119+ cells. For complete blood cell counts, 100 μL of whole blood was collected into EDTA (Gibco), and a Hemavet HV950FS (Drew Scientific) was utilized to analyze peripheral blood composition.
Quantification of Parasite Load in Brain.
Mice were anesthetized with ketamine/xylazine and perfused with cold PBS. Brains were removed and immediately placed into RNA Bee RNA isolation reagent (Tel Test, Inc.) and disrupted by bead homogenization in Lysing Matrix D tubes using a FastPrep-24 5G homogenizer (MP Biomedicals). The RNeasy Kit (Qiagen) was used to extract RNA per the manufacturer’s protocol. cDNA synthesis was performed using MMLV Reverse Transcriptase (Clontech) with oligo(dT) primers. Quantitative RT-PCR (qRT-PCR) reactions were performed on either a CFX96 Real-Time System or CFX384 Real-Time System (Bio-Rad) using PerfeCTa SYBR Green SuperMix (Quanta Biosciences). Transcript levels were normalized to Rpl13a using the delta-delta cycle threshold method. Primer sequences used in qRT-PCR can be found in SI Appendix, Table S1.
BBB Permeability Measurement.
Evan’s Blue (Sigma–Aldrich) was injected i.v. at 20 mg/kg via the retroorbital plexus. Mice were then anesthetized and perfused as described above. N, N-dimethylformamide was used to extract Evan’s Blue and was quantified using a SpectraMax spectrophotometer (Molecular Devices) set at 620 nm excitation and 695 nm emission.
Flow Cytometry.
Leukocytes in the brain were quantified by flow cytometry. Mice were anesthetized and perfused as described above. Brains were removed and then chopped with a razor blade until fine and digested in 1 mg/mL collagenase IV (Worthington) for 30 min at 37 °C at constant agitation (200 rpm) in a C-24 Incubator Shaker (New Brunswick). The homogenate was then filtered through 70 μM nylon mesh and a 90–60–40% discontinuous Percoll gradient was underlayered. Cells were centrifuged for 20 min at 1,000 × g. Cells at the 40–60% interface were collected. The fraction was washed in PBS with 1% FCS (Gibco) and counted. The following antibodies were utilized for FACS analyses: CD45 BUV395 (Becton Dickinson), CD8 phycoerythrin (Biolegend), CD4 APC-Cy7 (Becton Dickinson), CD3 Pacific Blue (Becton Dickinson), NK1.1 PeCy7 (Becton Dickinson), CD11b APC (eBioscience), Gr1 FITC (Becton Dickinson), and ethidium monoazide bromide (Thermo Fisher Scientific).
TEG.
TEG was performed on whole blood that was collected from the retroorbital plexus into pediatric citrated tubes (BD Pharmingen), gently agitated continuously, and run on a TEG 5000 system (Haemonetics) within 30 min of collection. The machine was quality-controlled before each run as per the manufacturer’s protocol. Twenty microliters of 0.2 M CaCl2 was loaded into each cup. Citrated samples were added to a vial of kaolin and filled to 1 mL, and 340 μL of sample from the kaolin vial was added to the cup and run within 30 s. TEG 5000 software generated all tracings and calculated all values as per manufacturer’s protocol.
Blood Glucose, Plasma Ketone Bodies, and Plasma Free Fatty Acids.
Glycemia was measured by whole-blood collection via the retroorbital plexus and assessed using a glucometer (OneTouch). Plasma was separated using lithium heparin-coated microcentrifuge tubes (BD Diagnostics). Plasma free fatty acid was measured using a kit according to the manufacturer’s instructions (Wako Diagnostics). Plasma β-hydroxybutyrate was measured using a kit per the manufacturer’s instructions (Cayman Chemical).
Histology.
All mice were anesthetized utilizing ketamine/xylazine and perfused with PBS and then 10% neutral buffered formalin. Tissues were then immersion-fixed in 10% neutral buffered formalin. The brain was paraffin-embedded, and 50 μM sections were cut transversely every 100 μM throughout the entire brain and stained for hematoxylin and eosin by routine methods. Livers were trimmed, embedded, cut, and stained with hematoxylin and eosin by standard methods. Tissue staining for vWF and fibrinogen was done using standard methods. Tissues were evaluated by a veterinarian (C.J.B.) trained in veterinary pathology with extensive mouse experience, who was blinded to experimental groups. Digital light microscopic images were acquired using a Zeiss Axio Imager A1 microscope, an AxioCam MRc5 camera, and AxioVision 4.8.3.0 imaging software (Carl Zeiss MicroImaging, Inc.). The resulting images were optimized using Adobe Photoshop 13.0.1x 64.
Quantification and Statistical Analyses.
Statistical information is indicated in the main text or figure legends. Statistical analyses were performed using Prism 6.0 (GraphPad Software, Inc.). Where appropriate, the Student’s t test or ANOVA was used. The log-rank Mantel–Cox test was used to compare Kaplan–Meier curves. A P value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank members of the R.M. laboratory for helpful discussions, Alvaro Baeza Garcia from the Bucala laboratory for technical assistance and discussion, Rick Bucala for the kind gift of PbA and PbA-GFP, the Department of Laboratory Medicine for assistance with TEG, and David Mangelsdorf for making FGF21 knockout mice available to us. Preparation of the histology sections was performed by Michael Schadt in Yale Mouse Research Pathology (Department of Comparative Medicine), and the immunohistochemistry was performed by Amos Brooks in Research Histology (Department of Pathology). This study was supported by the Howard Hughes Medical Institute, Else Kröner Fresenius Foundation, and Blavatnik Family Foundation. A.W. was supported by NIH Grants T32 AR07107-39 and K08 AI128745. S.C.H. was supported by NIH Grant K08 DK110424 and the American Society of Nephrology Carl W. Gottschalk Research Scholar Grant. H.H.L. was supported by the Gruber Science Fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1806376115/-/DCSupplemental.
References
- 1.Adamo SA. Parasitic suppression of feeding in the tobacco hornworm, Manduca sexta: Parallels with feeding depression after an immune challenge. Arch Insect Biochem Physiol. 2005;60:185–197. doi: 10.1002/arch.20068. [DOI] [PubMed] [Google Scholar]
- 2.Ayres JS, Schneider DS. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 2009;7:e1000150. doi: 10.1371/journal.pbio.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray MJ, Murray AB. Anorexia of infection as a mechanism of host defense. Am J Clin Nutr. 1979;32:593–596. doi: 10.1093/ajcn/32.3.593. [DOI] [PubMed] [Google Scholar]
- 4.Feingold KR, et al. FGF21 is increased by inflammatory stimuli and protects leptin-deficient ob/ob mice from the toxicity of sepsis. Endocrinology. 2012;153:2689–2700. doi: 10.1210/en.2011-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–294. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- 6.Schneider DS, Ayres JS. Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soares MP, Gozzelino R, Weis S. Tissue damage control in disease tolerance. Trends Immunol. 2014;35:483–494. doi: 10.1016/j.it.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang A, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016;166:1512–1525.e12. doi: 10.1016/j.cell.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weis S, et al. Metabolic adaptation establishes disease tolerance to sepsis. Cell. 2017;169:1263–1275.e14. doi: 10.1016/j.cell.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . World Malaria Report. WHO; Geneva: 2016. [Google Scholar]
- 12.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Higgins S, Liles WC, Kain KC. Endothelial activation and dysregulation in malaria: A potential target for novel therapeutics. Curr Opin Hematol. 2011;18:177–185. doi: 10.1097/MOH.0b013e328345a4cf. [DOI] [PubMed] [Google Scholar]
- 14.Belnoue E, et al. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol. 2002;169:6369–6375. doi: 10.4049/jimmunol.169.11.6369. [DOI] [PubMed] [Google Scholar]
- 15.Miranda AS, et al. Increased levels of glutamate in the central nervous system are associated with behavioral symptoms in experimental malaria. Braz J Med Biol Res. 2010;43:1173–1177. doi: 10.1590/s0100-879x2010007500130. [DOI] [PubMed] [Google Scholar]
- 16.Dobbie M, Crawley J, Waruiru C, Marsh K, Surtees R. Cerebrospinal fluid studies in children with cerebral malaria: An excitotoxic mechanism? Am J Trop Med Hyg. 2000;62:284–290. doi: 10.4269/ajtmh.2000.62.284. [DOI] [PubMed] [Google Scholar]
- 17.Craig AG, et al. participants of the Hinxton Retreat meeting on Animal Models for Research on Severe Malaria The role of animal models for research on severe malaria. PLoS Pathog. 2012;8:e1002401. doi: 10.1371/journal.ppat.1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke H, et al. Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell Rep. 2015;11:164–174. doi: 10.1016/j.celrep.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olszewski KL, et al. Host-parasite interactions revealed by Plasmodium falciparum metabolomics. Cell Host Microbe. 2009;5:191–199. doi: 10.1016/j.chom.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava A, et al. Stage-specific changes in Plasmodium metabolism required for differentiation and adaptation to different host and vector environments. PLoS Pathog. 2016;12:e1006094. doi: 10.1371/journal.ppat.1006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuzarte-Luís V, et al. Dietary alterations modulate susceptibility to Plasmodium infection. Nat Microbiol. 2017;2:1600–1607. doi: 10.1038/s41564-017-0025-2. [DOI] [PubMed] [Google Scholar]
- 22.Mejia P, et al. Dietary restriction protects against experimental cerebral malaria via leptin modulation and T-cell mTORC1 suppression. Nat Commun. 2015;6:6050. doi: 10.1038/ncomms7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon EB, et al. Targeting glutamine metabolism rescues mice from late-stage cerebral malaria. Proc Natl Acad Sci USA. 2015;112:13075–13080. doi: 10.1073/pnas.1516544112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira A, et al. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell. 2011;145:398–409. doi: 10.1016/j.cell.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 25.Cumnock K, et al. Host energy source is important for disease tolerance to malaria. Curr Biol. 2018;28:1635–1642.e3. doi: 10.1016/j.cub.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Olszewski KL, Llinás M. Central carbon metabolism of Plasmodium parasites. Mol Biochem Parasitol. 2011;175:95–103. doi: 10.1016/j.molbiopara.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wendel WB. Respiratory and carbohydrate metabolism of malaria parasites (Plasmodium knowlesi) J Biol Chem. 1943;148:21–34. [Google Scholar]
- 28.van Schalkwyk DA, Priebe W, Saliba KJ. The inhibitory effect of 2-halo derivatives of D-glucose on glycolysis and on the proliferation of the human malaria parasite Plasmodium falciparum. J Pharmacol Exp Ther. 2008;327:511–517. doi: 10.1124/jpet.108.141929. [DOI] [PubMed] [Google Scholar]
- 29.Nitcheu J, et al. Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J Immunol. 2003;170:2221–2228. doi: 10.4049/jimmunol.170.4.2221. [DOI] [PubMed] [Google Scholar]
- 30.Man K, Kallies A. Synchronizing transcriptional control of T cell metabolism and function. Nat Rev Immunol. 2015;15:574–584. doi: 10.1038/nri3874. [DOI] [PubMed] [Google Scholar]
- 31.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorovini-Zis K, et al. The neuropathology of fatal cerebral malaria in malawian children. Am J Pathol. 2011;178:2146–2158. doi: 10.1016/j.ajpath.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milner DA, Jr, et al. The systemic pathology of cerebral malaria in African children. Front Cell Infect Microbiol. 2014;4:104. doi: 10.3389/fcimb.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White VA, et al. Correlation of retinal haemorrhages with brain haemorrhages in children dying of cerebral malaria in Malawi. Trans R Soc Trop Med Hyg. 2001;95:618–621. doi: 10.1016/s0035-9203(01)90097-5. [DOI] [PubMed] [Google Scholar]
- 35.Moxon CA, et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood. 2013;122:842–851. doi: 10.1182/blood-2013-03-490219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francischetti IM, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15:81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angchaisuksiri P. Coagulopathy in malaria. Thromb Res. 2014;133:5–9. doi: 10.1016/j.thromres.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 38.Riedl J, et al. Alterations of blood coagulation in controlled human malaria infection. Malar J. 2016;15:15. doi: 10.1186/s12936-015-1079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: A systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2017;72:519–531. doi: 10.1111/anae.13765. [DOI] [PubMed] [Google Scholar]
- 40.Mohapatra S, Samantaray JC, Arulselvi S, Ghosh A. Disseminated intravascular coagulation following malaria due to Plasmodium vivax: A thromboelastography-based study. Malar J. 2013;12:336. doi: 10.1186/1475-2875-12-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117:953–960. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dmitrieva NI, Burg MB. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc Natl Acad Sci USA. 2014;111:6485–6490. doi: 10.1073/pnas.1404809111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galván-Peña S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan AT, et al. Constitutive glycolytic metabolism supports CD8+ T cell effector memory differentiation during viral infection. Immunity. 2016;45:1024–1037. doi: 10.1016/j.immuni.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao S, et al. Pathogen-mediated inhibition of anorexia promotes host survival and transmission. Cell. 2017;168:503–516.e12. doi: 10.1016/j.cell.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misztal T, Przesław K, Rusak T, Tomasiak M. Peroxynitrite–Altered platelet mitochondria–A new link between inflammation and hemostasis. Thromb Res. 2013;131:e17–e25. doi: 10.1016/j.thromres.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Waisberg M, Vickers BK, Yager SB, Lin CK, Pierce SK. Testing in mice the hypothesis that melanin is protective in malaria infections. PLoS One. 2012;7:e29493. doi: 10.1371/journal.pone.0029493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.