Significance
The clearance of apoptotic cells is critical for maintaining tissue homeostasis, and myeloid C-type lectin receptors (CLRs) may be prominently involved in the clearance of dying cells and their debris. Here, we report that the CLR member LSECtin is expressed on macrophages, which are engaged in corpse clearance. This engulfment progress by macrophages through LSECtin is critical for intestinal healing. Loss of LSECtin promotes dextran sulfate sodium-induced colitis due to unrepaired intestinal epithelium. These results indicate that corpse clearance by macrophages can direct intestinal regeneration. These findings advance understanding of how homeostasis is maintained within the mucosa.
Keywords: macrophage, C-type lectin receptor, apoptotic cell clearance, intestinal regeneration
Abstract
Epithelial barrier disruption is a major cause of inflammatory bowel disease (IBD); however, the cellular and molecular regulation of intestinal epithelial homeostasis remains largely undefined. Here, we show that the C-type lectin receptor LSECtin (Clec4g) on macrophages is required for protection against dextran sulfate sodium-induced colitis. Mechanistically, LSECtin promotes apoptotic cell clearance by macrophages and induces the production of antiinflammatory/tissue repair factors in an engulfment-dependent manner, which in turn stimulates epithelial cell proliferation. Deletion of LSECtin results in defective engulfment by colon macrophages, leading to aberrant proresolving factor production and impaired intestinal epithelium repair. Collectively, our findings suggest that LSECtin-dependent corpse clearance by macrophages can direct intestinal regeneration and maintenance of the mucosal barrier after injury.
Unrepaired mucosal injury disrupts the epithelial barrier, resulting in inflammatory bowel disease (IBD), an inflammatory disorder of the gastrointestinal tract, which constitutes a major health problem in developed countries (1, 2). Regenerative responses are particularly important in the mammalian gastrointestinal tract, and mucosal healing has emerged as an important end point in clinical trials and as a key goal in IBD therapy (1, 2). The repair process is complex and influenced by numerous secreted factors including cytokines and growth factors, and the known cellular organizers of this process are mesenchymal cells and lymphocytes, especially innate lymphoid cells (3, 4). Macrophages are best known for acting as innate immune effector cells that interact with the microbiota and lymphoid cells (5). Little is known about the interaction between macrophages and intestinal epithelial cells (IECs), although previous works have addressed the topic (6, 7). It has been reported that the number of apoptotic colonic epithelial cells is considerably higher in the colons of IBD patients (8). Macrophages and nonprofessional phagocytes (9, 10) (such as epithelial cells) clear billions of apoptotic cells and particles on a daily basis (11). Although macrophages and epithelial cells reside in proximity in the intestine, whether they communicate with each other and how this might affect intestinal repair and remodeling are unclear.
Molecules associated with dead cells can be detected by receptors on macrophages. Myeloid C-type lectin receptors (CLRs) may be prominently involved in the clearance of dying cells and their debris (12), such as Lox-1 and Mgl-1 (Clec10a), which detect ligands in apoptotic cells (13, 14), or Mincle (Clec4e) (15) and DNGR-1 (Clec9a) (16), which sense proteins that are exposed or released by damaged cells. Our previous work has indicated that LSECtin is a C-type lectin receptor belonging to the DC-SIGN family and is expressed on liver resident macrophages [Kupffer cells (KCs)] (17). Gene array data have shown that LSECtin is expressed on intestinal macrophages (18). It has been reported that LSECtin is also expressed on macrophages generated in vitro (19). We have previously revealed that LSECtin negatively regulates T cell-mediated immunity via unknown receptors, thereby exposing a crucial mechanism for hepatic T cell immune suppression (20). It is unknown whether LSECtin has cell-intrinsic functions in macrophages. It is noteworthy that the intracellular tail of LSECtin contains a tyrosine and a di-acidic motif, both of which regulate endocytosis induced by antibody-mediated cross-linking (19). We also wondered whether LSECtin could participate in dead cell clearance in vivo and whether this clearance could contribute to tissue homeostasis.
In this study, we suggest a role of LSECtin-mediated apoptotic cell clearance by macrophages and in establishing the proresolving environment during dextran sulfate sodium (DSS)-induced colitis. We found that LSECtin-deficient mice were more susceptible to DSS-induced colitis, and the gut epithelial regenerative rate was decreased compared with WT mice. Mechanistically, the absence of LSECtin impairs the phagocytic abilities of macrophages, and the engulfment of dead cells induced macrophages to produce more tissue-healing factors, which in turn facilitated intestinal regeneration and ameliorated the colitis. These results represent an important advance in our understanding of how dead cell clearance by CLRs on macrophages orchestrates host intestinal healing.
Results
LSECtin-Deficient Mice Develop Severe DSS-Induced Colitis.
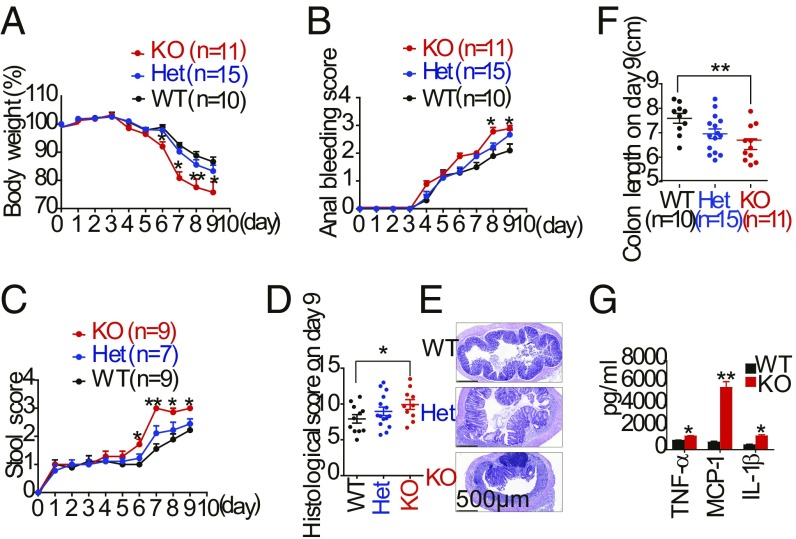
To determine the role of LSECtin in mucosal immune responses, we first checked the body weight and then the colons of mice for spontaneous colitis. LSECtin−/− mice had normal increases in body weight and colon length compared with WT mice (SI Appendix, Fig. S1 A–C). We then used DSS-induced colitis, a widely used model for experimental IBD, which reflects the contributions of the myeloid cell lineage in response to acute mucosal damage (21). We examined the colitis induced by the administration of 2.5% DSS for 5 d, followed by administration in drinking water for an additional 4 d, in LSECtin−/− and littermate control mice. After DSS treatment, LSECtin−/− mice developed more severe clinical symptoms of IBD, including rapid weight loss (Fig. 1A), bloody stools (Fig. 1B), severe diarrhea (Fig. 1C), and a lower survival rate (SI Appendix, Fig. S1D). LSECtin+/− mice showed intermediate body weight loss. Histological analysis of the colons revealed more severe disease, thickened walls, disruption of mucosal structures (Fig. 1 D and E), and a shortened colon (Fig. 1F and SI Appendix, Fig. S1E) in LSECtin−/− mice. We investigated the role of LSECtin in maintaining gut epithelial integrity by oral gavage of mice with fluorescein isothiocyanate (FITC)–dextran (22). We found a threefold increase in the FITC–dextran levels in sera of LSECtin−/− mice at day 9 of DSS treatment (SI Appendix, Fig. S1F), suggesting that LSECtin is important for the regulation of intestinal permeability during gut injury. The frequency of monocytes and neutrophils (SI Appendix, Fig. S1 G–J) and the production of TNF-α, MCP-1, and IL-1β (Fig. 1G) were significantly increased in the colons of LSECtin−/− mice during DSS-induced colitis, while there was no difference in cytokine expression of the colonic monocytes and polymorphonuclear (PMN) between WT and LSECtin−/− KO mice (SI Appendix, Fig. S1K). Thus, LSECtin deficiency exacerbated colon inflammation in DSS-treated mice.
Fig. 1.
LSECtin-deficient mice are more susceptible to DSS-induced colitis. (A–C) Body weight (A), anal bleeding score (B), and stool score (C) of DSS-treated LSECtin−/− (KO), littermate LSECtin+/+ (WT), and LSECtin+/− (Het) mice were measured every day during colitis development. (D) Histological scores of the colon on day 9 were measured. (E) Representative microscopic images of H&E-stained colons. (Scale bar: 500 μm.) (F) Colon length. (G) Cytokine secretion was measured in the supernatant after overnight culture of LPMCs. The number in parentheses represents the number of mice used in each group. The results shown are the mean ± SEM. A two-tailed unpaired t test was used to compare experimental groups. *P < 0.05; **P < 0.01 (WT vs. KO). Data are representative of seven independent experiments with similar results (A–F).
LSECtin on Macrophages Is Required for Protection Against DSS-Induced Colitis.
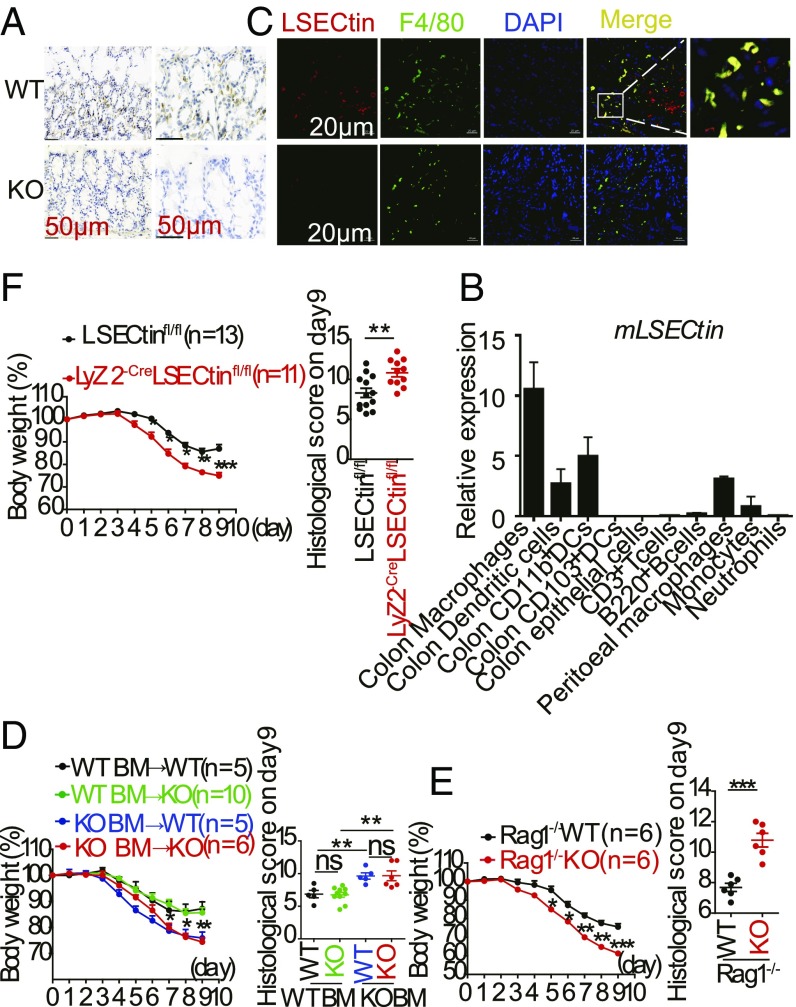
Although the gene array data have shown that LSECtin is expressed on intestinal macrophages (18), the exact expression patterns of LSECtin in the mouse intestine have not been clarified. We observed that LSECtin was strongly expressed in the mouse colon, as detected by immunohistochemical staining (Fig. 2A), which indicated to us that colonic epithelial cells did not express LSECtin. We isolated the different cell types from the mouse (SI Appendix, Fig. S2 A and B), and the qPCR data showed that LSECtin was primarily expressed in myeloid macrophages (Fig. 2B). To further confirm the mRNA data, immunofluorescence images showed coexpression of LSECtin and F4/80, suggesting that LSECtin could be detected on colon macrophages (Fig. 2C), and this coexpression was further confirmed in human colon tissue (SI Appendix, Fig. S2C). To assess the association of the colitis exacerbation in LSECtin−/− mice with hematopoietic cells, we reconstituted lethally irradiated WT mice with total bone marrow (BM)-derived cells from WT or LSECtin−/− mice, and we analyzed the contributions of the colonic macrophages (SI Appendix, Fig. S2D) in the chimaeras 8 wk after reconstitution. Weight loss was monitored daily in individual chimeric mice receiving 2.5% DSS in their drinking water over a period of 5 d. Chimeric mice lacking LSECtin in hematopoietic cells showed weight loss similar to that observed in WT mice reconstituted with LSECtin−/− BM-derived cells, demonstrating that LSECtin deficiency in hematopoietic cells could facilitate mouse susceptibility to acute DSS-induced colitis (Fig. 2D and SI Appendix, Fig. S2E).
Fig. 2.
LSECtin in hematopoietic macrophages is required for protection against DSS-induced colitis. (A) Immunohistochemical analysis of LSECtin expression in mouse colons. (B) Expression of LSECtin by different cell types was examined using qPCR; all genes are presented relative to the expression of Gapdh. (C) Immunofluorescence analysis of colon sections from WT/KO mice stained with anti-LSECtin and anti-F4/80 Abs. (Scale bar: 20 μm.) (D) Mean change in body weight and histological score of irradiated WT and KO mice that were reconstituted for 8 wk with WT or KO bone marrow and challenged with 2.5% DSS for 5 d. The results shown are the mean ± SEM. *P < 0.05 (WT BM vs. KO BM). (E) Body weight was calculated as a percentage, and the histological score of Rag1−/− WT or Rag−/− KO mice during DSS-mediated colitis is shown. The results shown are the mean ± SEM. *P < 0.05 (Rag1−/− WT vs. Rag−/− KO). (F) Mean change in body weight of control (LSECtinfl/fl) and LSECtin ΔMφ (Lyz2-creLSECtinfl/fl) mice challenged with DSS. The results shown are the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 (LSECtinfl/fl vs. Lyz2-creLSECtinfl/fl). Data are representative of three independent experiments with similar results (D–F). ns, not significant.
Our earlier work has shown that LSECtin is a regulator of T cell immune suppression (20) and that Th17 and Treg cells play a large role during IBD development. LSECtin−/− mice were crossed with Rag1−/− mice to generate LSECtin and Rag1 double-knockout mice. The weight loss of the double-knockout mice rapidly decreased compared with LSECtin+/+Rag1−/− mice (Fig. 2E and SI Appendix, Fig. S2 F and G), suggesting that the protective function of LSECtin occurred in a Rag1 (T or B cell)-independent manner. Then, we used mice lacking LSECtin in macrophages (Mφs) (LSECtin ΔMφ, Lyz2-Cre LSECtinfl/fl) to induce colitis, and we found that the LSECtin ΔMφ mice had severe pathological features like the systemic knockout mice (Fig. 2F and SI Appendix, Fig. S2 H and I). A recent study has shown that the macrophage pool in the intestines of adult mice is replenished from circulating monocytes (23), and we found that transferring WT monocytes could slightly help the LSECtin−/− mice to avoid developing serious colitis (SI Appendix, Fig. S2 J and K). Thus, we confirmed that LSECtin on hematopoietic cells, especially colonic macrophages, has a protective function during DSS-induced colitis.
LSECtin Promotes Repair of the Intestinal Epithelium.
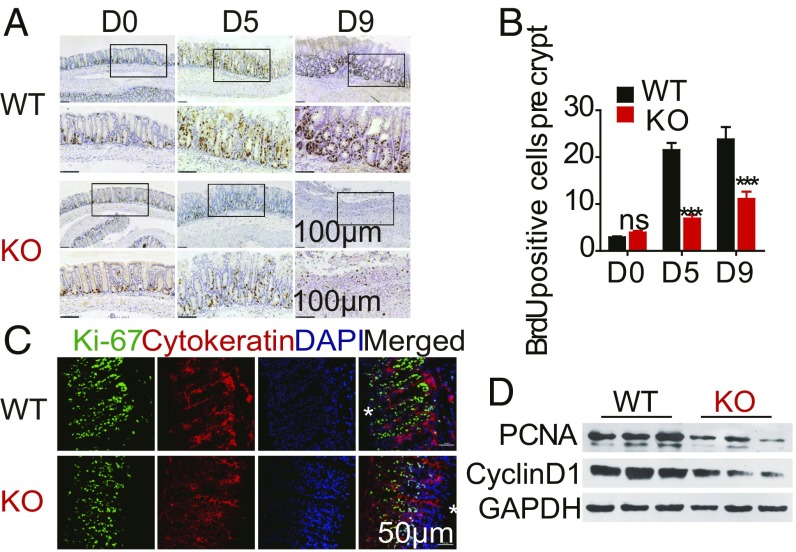
We analyzed the different CD4+ T cell populations in the colon and found no difference in the proportion of subpopulations of CD4+ T cells (SI Appendix, Fig. S3). Increased proliferation of epithelial cells in the crypt is a hallmark of the injury-induced repair response (24). To test this phenomenon, we investigated the rate of colon regeneration in WT and LSECtin−/− mice using Ki67 and 5-bromo-2′-deoxyuridine (BrdU) staining. Our data showed a similar baseline level (D0) of proliferation in WT and LSECtin−/− mice, but at days 5 and 9 after DSS challenge, the intestinal epithelium proliferation was markedly reduced in LSECtin−/− compared with WT mice (Fig. 3 A–C and SI Appendix, Fig. S4 A and B). Consistent with the increased rate of regeneration, proliferating cell nuclear antigen and cell-cycle genes encoding the late G1 to G1/S phase proteins Cyclin D1 (gene is known as CCND1) (Fig. 3D and SI Appendix, Fig. S4C) and CDK1 (SI Appendix, Fig. S4D) were expressed at higher levels in WT compared with LSECtin−/− mouse colon. We also observed a similar effect on epithelial proliferation in the LSECtin ΔMφ mouse (SI Appendix, Fig. S4 E and F). These data suggest that LSECtin can promote intestinal epithelium regeneration.
Fig. 3.
LSECtin promotes repair of the intestinal epithelium. (A) Representative images of BrdU staining from WT and KO mice treated or untreated with DSS for different days are shown. (Scale bars: 100 μm.) (B) The number of BrdU+ cells/colon crypt was quantified. (C) Immunofluorescence staining for cytokeratin+ (red) cells, Ki-67+ cells (green), and nuclei in colon tissues from DSS-challenged WT and KO mice. (Scale bar: 50 μm.) *Present the intestinal lumen. (D) Immunoblot analysis in colons of DSS-challenged WT or KO mice. Data are representative of three independent experiments (mean ± SEM in B). ***P < 0.001. ns, not significant.
LSECtin Controls the Expression of Antiinflammatory/Tissue Repair Factors in Colon Macrophages.
These findings raise questions concerning how the mechanisms by which LSECtin is expressed on macrophages affect intestinal epithelium regeneration. We tested the initial hypothesis that WT macrophages could promote tissue-healing phenotypic changes that enhanced intestinal epithelium regeneration. To address the characteristics of the colon macrophages, we isolated the colon macrophages, which were characterized as MHCII+CD11b+F4/80+Ly6C− (SI Appendix, Fig. S5A), and this population exhibited CD103–CD64+CD68+CCR2dim phenotypes, consistent with the markers used to identify colon macrophages (SI Appendix, Fig. S5B). Flow cytometry analysis of the inflamed colonic lamina propria revealed the progression of MHC-II+F4/80+ cells in frequency (SI Appendix, Fig. S5C), and the LSECtin expression level was not significantly altered during colitis progression (SI Appendix, Fig. S5D). There was no difference in the frequency of WT and LSECtin−/− macrophages regardless of whether they were untreated or treated with DSS (SI Appendix, Fig. S5 E and F).
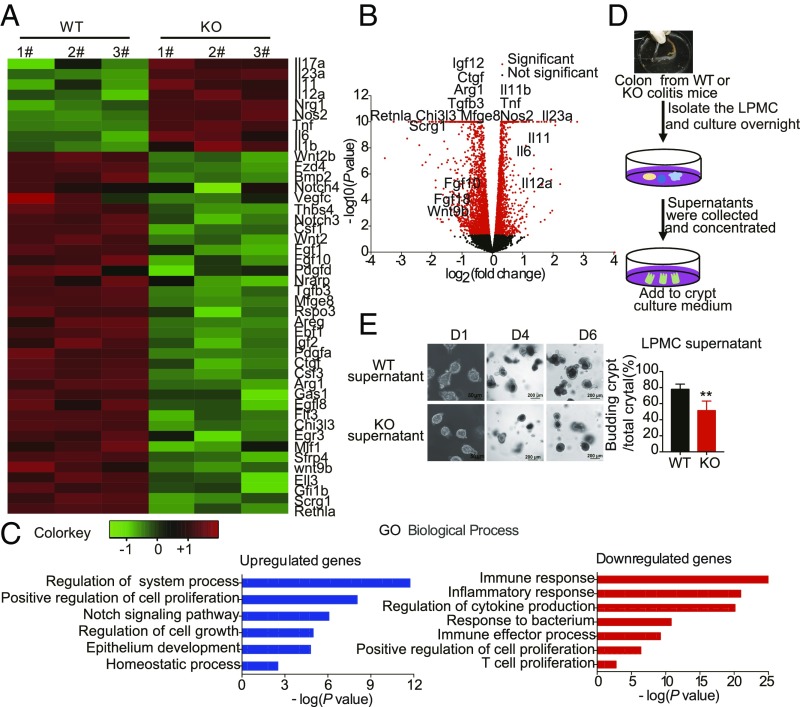
DSS colitis was induced in WT and LSECtin−/− mice for 5 d, and then colonic macrophages were sorted by fluorescence-activated cell sorting (FACS). RNA sequencing revealed that LSECtin deficiency induced a significant down-regulation of the gene expression program, including the genes Retnla, Chi3l3 (also known as Ym1), and Arg1 (Fig. 4 A and B). Gene Ontology (GO) analysis further highlighted that LSECtin up-regulated the genes that mediate the system process and cell proliferation while downregulating the genes that are involved in the inflammatory response (Fig. 4C). These observations prompted us to test whether LSECtin could promote the repair function of macrophages. LSECtin−/− macrophages exhibited a pronounced proinflammatory signature with high expression levels of Il6, Il23p19, Il11, and Il12a (SI Appendix, Fig. S5G). Moreover, WT macrophages preferentially exhibited alternatively activated (M2) and remodeling markers, such as Mrc1, Ym1, and Retnla (encoding Fizz1) (SI Appendix, Fig. S5H). However, a difference in gene expression was not observed in the isolated colon dendritic cells (SI Appendix, Fig. S6). These results suggest that the loss of LSECtin in colon macrophages leads to aberrant proresolving factor production and impaired epithelium repair.
Fig. 4.
LSECtin promotes the antiinflammatory/tissue repair function in colonic macrophages. (A) Macrophages sorted from WT and KO colitis mice were subjected to RNA-seq. Heatmap of RNA-seq data showing the up-regulation of known wound-healing genes by LSECtin. (B) Volcano plot of the RNA-seq data. (C) GO analysis of LSECtin up-regulated and down-regulated genes in colonic macrophages, respectively. (D) Schematic of the detection of crypt growth. (E) The intestinal organoids were photographed, and quantitative statistics of the number of budding crypts (D6) after incubation with the supernatant of LPMCs, which were isolated from colitis WT and KO mouse, respectively, were compiled. Data are representative of at least three independent experiments. Error bars, mean ± SEM. A two-tailed unpaired t test was used to compare experimental groups. **P < 0.01.
To investigate whether these antiinflammatory/tissue repair factors secreted from the WT macrophages could promote intestinal epithelium regeneration, we developed an in vitro culture system using primary crypt organoids and DSS-induced colitis colon lamina propria mononuclear cells (LPMCs) from WT and LSECtin−/− mice (Fig. 4D). We examined the effect of LPMC culture medium (CM) on crypt organoids and found that CM from WT LPMC could augment the organoid size and increase the number of budding crypts (Fig. 4E). The results suggest that the loss of LSECtin results in defective engulfment by colon macrophages, leading to aberrant proresolving factor production and impaired epithelial repair.
LSECtin Expression on Macrophages Promotes the Engulfment of Apoptotic Cells.
DSS-induced colitis leads to an epithelial barrier defect and the translocation of bacterial commensals (21). To assess the contribution of the microbiota to colon colitis development, we applied a mixture of antibiotics to the mouse colitis model. After 3 wk of antibiotic treatment, we detected the bacterial and fungal burden of fecal pellets, and most bacteria and fungi had been depleted (SI Appendix, Fig. S7A). However, depletion of the microbiota did not change the trend of colitis (SI Appendix, Fig. S7 B and C). Furthermore, when stimulated with different ligands, the expression levels of the proinflammatory cytokine TNF in WT and LSECtin−/− bone marrow-derived macrophages were not significantly different (SI Appendix, Fig. S7D), suggesting that LSECtin had a microbiota-independent protective function.
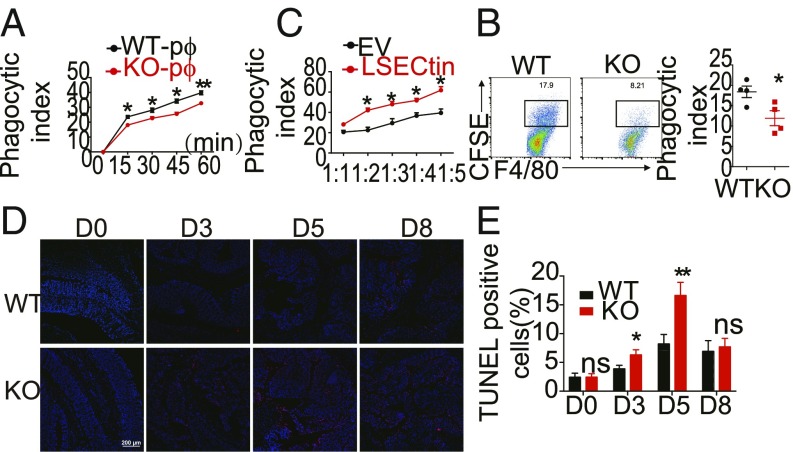
Macrophages act as professional phagocytes after engulfment of dead cells, and they can activate tolerogenic pathways (25), as we observed that WT mice exhibited antiinflammatory phenotypes (Fig. 4 A and C) and the intracellular tail of LSECtin could regulate endocytosis (19). We proposed that LSECtin expression on macrophages could promote the engulfment of dead cells during colitis development. To examine the functional role of LSECtin in the engulfment of dead cells, we used freshly isolated peritoneal macrophages (pMφs) from WT and LSECtin−/− mice and incubated them with dexamethasone-treated apoptotic thymocytes labeled with carboxyfluorescein succinimidyl ester (CFSE) (green). The phagocytic potential was assessed by flow cytometry-based and image-based analyses. LSECtin−/− pMφs were deficient in the uptake of CFSE-labeled apoptotic thymocytes compared with WT pMφs (Fig. 5A and SI Appendix, Fig. S8A). Phagocytosis analysis of pMφs incubated with synthetic beads (SI Appendix, Fig. S8B) or bacteria (SI Appendix, Fig. S8C) revealed that LSECtin had no defects in phagocytic ability, which suggested that LSECtin-mediated engulfment was dependent on the presence of dead cells. To study LSECtin on pMφs-mediated engulfment of dead cells in vivo, CFSE-labeled apoptotic cells were injected into the abdominal cavity, and a slight decrease in the amount of apoptotic cells was detected in the LSECtin−/− pMφs (Fig. 5B).
Fig. 5.
LSECtin on macrophages contributes to apoptotic cell clearance. (A) The CFSE-labeled apoptotic thymocytes were incubated with WT and KO peritoneal macrophages (pMφs), and phagocytosis was determined by measuring the positive CFSE-containing macrophages. The abscissa indicates the incubation time of dead thymocytes. (B) Percentage of peritoneal macrophages (F4/80+CD11b+) containing CFSE-related fluorescence was quantified by FACS 1 h after i.v. injection of CFSE-labeled apoptotic thymocytes (n = 4). (C) Raw 264.7 cells transfected with the EV control or LSECtin were incubated with CMTMR (red)–labeled apoptotic thymocytes, and phagocytosis was determined by measuring the number of positive CMTMR-containing macrophages. The abscissa indicates the ratio of dead thymocytes and Raw 264.7. (D) Cohoused WT and KO littermates were treated with DSS, and colon sections were studied for TUNEL-expressing cells by immunofluorescence. (Scale bars: 200 μm.) (E) The number of TUNEL+-positive cells were measured using Image. The graph presents the mean ± SD from three or five experiments. Error bars indicate SEM. *P < 0.05; **P < 0.01 by the Student’s t test. ns, not significant.
To further study the phagocytic function, we developed an LSECtin-overexpressing mouse macrophage cell line using Raw 264.7 cells, and LSECtin expression was detected using qPCR and Western blotting (SI Appendix, Fig. S8D). In accordance with the observations in pMφs, Raw 264.7 cells transfected with the empty vector (EV) were deficient in the uptake of CellTracker Orange dye 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR)-labeled apoptotic thymocytes (Fig. 5C and SI Appendix, Fig. S8E) or CT-26 cells (SI Appendix, Fig. S8F) compared with the Raw 264.7 cells transfected with LSECtin, while the phagocytosis of synthetic beads (SI Appendix, Fig. S8G) and bacteria (SI Appendix, Fig. S8H) showed no differences between EV and LSECtin overexpression Raw 264.7 cells. We further showed that deficiency of the LSECtin extracellular carbohydrate recognition domains (CRD) region (SI Appendix, Fig. S8I) abolished the ability to engulf dead cells (SI Appendix, Fig. S8J), and when macrophages were treated with cytochalasin D (CytoD), which inhibits engulfment but not binding, the engulfment ability was also abolished (SI Appendix, Fig. S8K). We further suggested that the recognition of apoptotic cells by LSECtin-expressing macrophages was phosphatidylserine (PtdSer)-independent, since the engulfment of apoptotic thymocytes by LSECtin was not blocked by purified recombinant Annexin V protein (SI Appendix, Fig. S8L).
To determine the exact role of LSECtin in the phagocytosis of apoptotic cells, we overexpressed LSECtin in the NIH 3T3 cell line (SI Appendix, Fig. S9A) and observed that NIH 3T3-LSECtin could promote the uptake of CMTMR-labeled apoptotic thymocytes compared with NIH 3T3-EV (SI Appendix, Fig. S9 B and C).
The specific localization of LSECtin in KCs (17) inspired us to investigate the role of LSECtin in the removal of apoptotic cells. In agreement with a role for LSECtin in phagocytosis in the overexpression cell line and pMφs, LSECtin−/− KCs internalized significantly fewer apoptotic cells compared with WT KCs in isolated KCs (SI Appendix, Fig. S10A) and in vivo (SI Appendix, Fig. S10B). Thus, LSECtin on macrophages participated in the engulfment of dead cells in vitro and in vivo.
We tested whether LSECtin deficiency caused defects in dead cell clearance in LSECtin-deficient mice during DSS-induced colitis. We examined the presence of apoptotic cells in whole mounts of the colon at different times during and after DSS exposure using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining (Fig. 5D). Quantification of the TUNEL-stained cells was calculated as a fraction of DAPI-positive cells using an imaging analysis program. The clearance of apoptotic cells in LSECtin−/− colon was delayed (Fig. 5E).
LSECtin Controls the Production of Antiinflammatory/Tissue Repair Factors and Epithelial Cell Proliferation in an Engulfment-Dependent Manner.
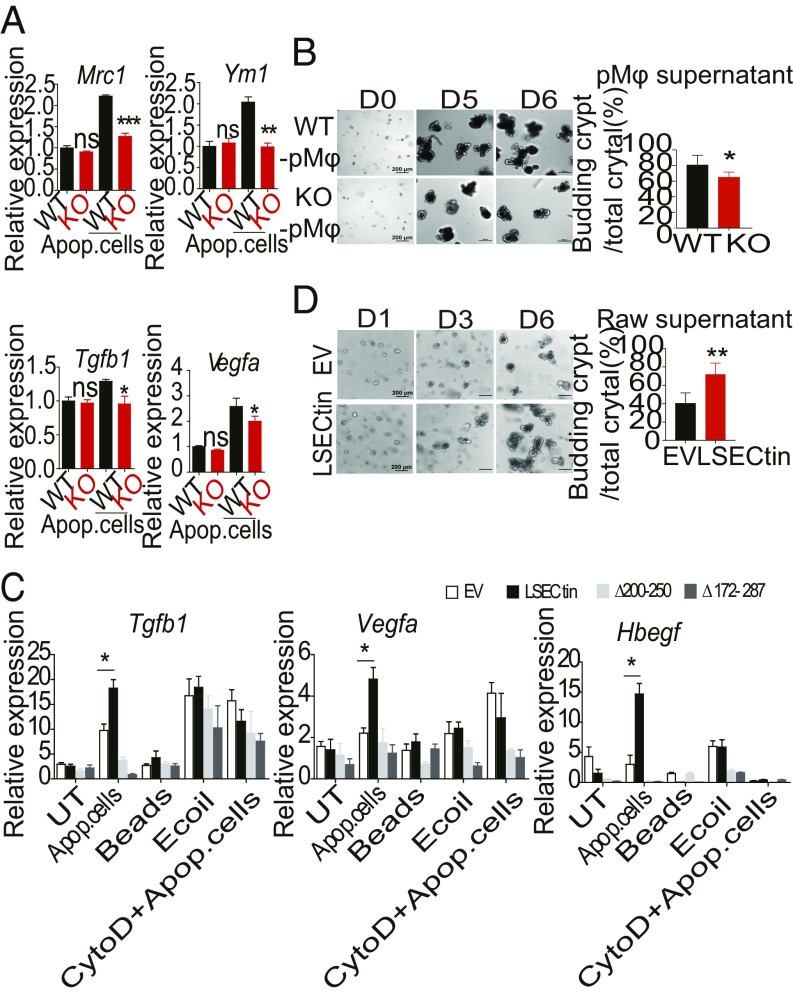
To investigate whether engulfment of the dead cells was sufficient to directly modulate gene expression in macrophages, we developed an in vitro stimulating system using either pMφs or Raw 264.7 cells transfected with EV or LSECtin. Treatment of LSECtin−/− pMφs with dead cells resulted in decreased levels of Mrc1, Ym1, Tgfb1, and Vegfa expression compared with WT macrophages (Fig. 6A). To further investigate the functions of culture supernatant in crypt growth, we mechanically dissociated small intestinal crypts, embedded them in Matrigel, and performed in vitro culture according to a previous report (26). We found that the supernatant of WT pMφs treated with dead cells markedly promoted the survival of intestinal crypts (Fig. 6B). The dead cells, but not beads, Escherichia coli, or CytoD plus dead cells were able to induce higher expression of Tgfb1, Vegfa, and Hbegf in Raw 264.7 cells transfected with LSECtin (Fig. 6C), indicating that LSECtin might be required for dead-cell engulfment to release soluble factors responsible for promoting macrophage polarization and wound healing. The supernatant of transfected Raw 264.7 cells that were treated with dead cells was used to stimulate the mouse intestinal crypts, and the supernatant of the LSECtin-overexpressing cell line promoted the survival of intestinal crypts (Fig. 6D). These results indicated that LSECtin promoted the phenotypic change and production of antiinflammatory/tissue repair factors in macrophages in an engulfment-dependent manner.
Fig. 6.
LSECtin promotes antiinflammatory/tissue repair factor production and epithelial cell proliferation in an engulfment-dependent manner. (A) Quantitative mRNA expression of Mrc1, Ym1, Tgfb1, and Vegfa in WT and KO pMφs treated or untreated with apoptotic thymocytes for 3 h. (B) The intestinal organoids were photographed and quantitative statistics of the number of budding crypts (D6) after incubation with the supernatant of pMφs treated with apoptotic thymocytes. (C) Quantitative mRNA expression of Tgfb1, Vegfa, and Hbegf in Raw 264.7 cells transfected with EV or LSECtin; the cells were either untreated or treated with apoptotic thymocyte cells, beads, E. coli, or cytochalasin D plus apoptotic thymocyte cells for 3 h. (D) The intestinal organoids were photographed, and quantitative statistics of the number of budding crypts (D6) after incubation with the supernatant of Raw 264.7 cells were transfected with EV or LSECtin, which had been treated with apoptotic thymocytes. Data are representative of at least two or three independent experiments. Error bars indicate SEM. *P < 0.05; **P < 0.01; ***P < 0.001 by the Student’s t test. ns, not significant.
Discussion
The data presented in this work establish a role for corpse clearance by macrophages in intestinal homeostasis and indicate that the CLR family member LSECtin is engaged in macrophage engulfment of apoptotic cells, which is critical for the regulation of intestinal regeneration.
Mucosal healing has emerged as an important end point in clinical trials and as a key goal in IBD therapy, predicting lower hospitalization rates, sustained clinical remission, and resection-free survival (1). Macrophages in the gastrointestinal mucosa represent the largest pool of tissue macrophages in the body. In the colon, activated macrophages in the wound bed of biopsy-injured mouse colons display the ability to heal wounds (27). Recently, Pollard and coworkers (28) have shown that macrophage-derived extracellular vesicle-packaged Wnt ligands can rescue intestinal stem cells and enhance survival after radiation injury. Our data presented here show that LSECtin on macrophages can promote intestinal healing after injury in mice. Furthermore, the effects of intestinal healing were dependent on the engulfment of dead cells, which stimulated the macrophages to secrete more tissue-healing factors. Thus, our study presents a mechanism for macrophages to contribute to tissue repair.
During infection and/or inflammation, additional cells undergo cell death, including cells that are native to the tissue, as well as recruited immune cells such as neutrophils and lymphocytes. The types of phagocytes that recognize and engulf apoptotic cells include professional phagocytes and nonprofessional phagocytes (such as epithelial cells and fibroblasts). Although dying cells in DSS-induced colitis can be cleared by neighboring colonic epithelial cells (29), little is known about how these are cleared by professional phagocytes, or their effects on intestinal inflammation. Our results showed that LSECtin expression was restricted to innate immune cells, particularly macrophages, and in situ data indicated that LSECtin-deficient macrophages showed decreased numbers of engulfed apoptotic cells with concomitantly fewer TUNEL-positive apoptotic nuclei. A recent study has also shown that beyond engulfment by nonprofessional phagocytes, innate immune recognition of apoptotic IECs is a critical component of the mechanisms that mediate gut physiological homeostasis (30).
Once a phagocyte recognizes an apoptotic cell, signaling occurs to rearrange the cytoskeleton and engulf the target. In our work, intestinal healing outcomes may rely on a versatile system for CLR signaling or endocytic adaptors that modulate the activation of other pathways, thereby reprogramming the tissue-healing function of macrophages. In the previous study (31), we showed that LSECtin couples to proinflammatory cytokine production through a DAP-12–dependent pathway in which LSECtin acts as a pattern recognition receptor (PRR) recognizing exogenous ligands. Here, we show that LSECtin mediates antiinflammatory/tissue repair responses acting as an engulfment receptor of apoptotic cells through recognizing endogenous ligands. We believe these findings reflect the involvement of LSECtin in different biological processes. However, the exact pathways involved in LSECtin-mediated clearance and tissue healing still need to be explored.
Members of the CLR family are conspicuous among the specialized receptors utilized by myeloid cells to orchestrate physiological responses to pathogen invasion or tissue damage (32). The CLR family member LSECtin acts as a coinhibitory molecule and limits T cell immunity to promote hepatitis B virus (HBV) tolerance (20, 33), and in a tumor microenvironment, LSECtin-mediated immunosuppression facilitates host immune escape (34). Here we found that LSECtin−/− mice were more susceptible to DSS-induced colitis, and the epithelial regenerative rate was decreased in the gut after DSS treatment. Mechanistically, the absence of LSECtin impaired the ability of macrophages to phagocytize dead cells, which in turn induced the repair program of colonic epithelial tissues. In this study, we suggest that LSECtin is a CLR that mediates dead-cell clearance, and we implicate LSECtin engagement in the engulfment of apoptotic cells by macrophages, which are critical regulators of tissue regeneration. Our study provides insights into the function of CLRs in sensing cell death and restoring tissue homeostasis.
Experimental Procedures
Study Approval.
Mice were housed in the specific pathogen-free Beijing Institute of Lifeomics animal facility and all animal experiments were approved by the institutional animal care and use committee at Beijing Institute of Lifeomics.
Statistical Analysis.
Data are expressed as the mean ± SEM. Statistical significance between two groups was evaluated using the Student’s t test (unpaired, two-tailed). All of the data were evaluated using Prism (GraphPad software). P <0.05 was considered statistically significant. ns, not significant. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Additional Materials and Methods.
Detailed information is provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Zhihua Liu for generously providing the Lyz2-Cre mice; Mingzhao Zhu for generously providing the B6.CD45.1 mice; Ruiqing Yan for isolation of murine colon lamina propria mononuclear cells; the Flow Cytometry Facility, Animal Facility, and Imaging Facility of the National Center for Protein Sciences, Beijing (Ms. Ping Wu), for their assistance. This work was supported by the Chinese National Natural Science Foundation Project (81500428); the Beijing Natural Science Foundation Project (7182035); the Chinese State Key Program in Basic Research (2013CB910802, 2014CBA02000); the National Key R&D Program of China (2018YFA0507500); and the Beijing Science Program for the Top Young (2015000021223TD04).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.M.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804094115/-/DCSupplemental.
References
- 1.Taniguchi K, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. doi: 10.1038/mi.2013.73. [DOI] [PubMed] [Google Scholar]
- 3.Chivukula RR, et al. An essential mesenchymal function for miR-143/145 in intestinal epithelial regeneration. Cell. 2014;157:1104–1116. doi: 10.1016/j.cell.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindemans CA, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80:802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 8.Di Sabatino A, et al. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Dis Colon Rectum. 2003;46:1498–1507. doi: 10.1007/s10350-004-6802-z. [DOI] [PubMed] [Google Scholar]
- 9.Mesa KR, et al. Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature. 2015;522:94–97. doi: 10.1038/nature14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juncadella IJ, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493:547–551. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory CD, Pound JD. Microenvironmental influences of apoptosis in vivo and in vitro. Apoptosis. 2010;15:1029–1049. doi: 10.1007/s10495-010-0485-9. [DOI] [PubMed] [Google Scholar]
- 12.Sancho D, Reis e Sousa C. Sensing of cell death by myeloid C-type lectin receptors. Curr Opin Immunol. 2013;25:46–52. doi: 10.1016/j.coi.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oka K, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci USA. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nauta AJ, et al. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur J Immunol. 2003;33:2853–2863. doi: 10.1002/eji.200323888. [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki S, et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 16.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, et al. Characterization of a novel C-type lectin-like gene, LSECtin: Demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J Biol Chem. 2004;279:18748–18758. doi: 10.1074/jbc.M311227200. [DOI] [PubMed] [Google Scholar]
- 18.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominguez-Soto A, et al. The DC-SIGN-related lectin LSECtin mediates antigen capture and pathogen binding by human myeloid cells. Blood. 2007;109:5337–5345. doi: 10.1182/blood-2006-09-048058. [DOI] [PubMed] [Google Scholar]
- 20.Tang L, et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology. 2009;137:1498–1508.e1-5. doi: 10.1053/j.gastro.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:Unit 15.25. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scandiuzzi L, et al. Tissue-expressed B7-H1 critically controls intestinal inflammation. Cell Rep. 2014;6:625–632. doi: 10.1016/j.celrep.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bain CC, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriwaki K, et al. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity. 2014;41:567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shklover J, Levy-Adam F, Kurant E. Apoptotic cell clearance in development. Curr Top Dev Biol. 2015;114:297–334. doi: 10.1016/bs.ctdb.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 27.Seno H, et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha S, et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun. 2016;7:13096. doi: 10.1038/ncomms13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CS, et al. Boosting apoptotic cell clearance by colonic epithelial cells attenuates inflammation in vivo. Immunity. 2016;44:807–820. doi: 10.1016/j.immuni.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings RJ, et al. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature. 2016;539:565–569. doi: 10.1038/nature20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao D, et al. The myeloid LSECtin is a DAP12-coupled receptor that is crucial for inflammatory response induced by Ebola virus glycoprotein. PLoS Pathog. 2016;12:e1005487. doi: 10.1371/journal.ppat.1005487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B, et al. Liver sinusoidal endothelial cell lectin inhibits CTL-dependent virus clearance in mouse models of viral hepatitis. J Immunol. 2013;190:4185–4195. doi: 10.4049/jimmunol.1203091. [DOI] [PubMed] [Google Scholar]
- 34.Xu F, et al. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014;74:3418–3428. doi: 10.1158/0008-5472.CAN-13-2690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.